Abstract
Background
Exposure to gun violence and African ancestry have been separately associated with increased risk of asthma in Puerto Rican children.
Objective
The objective of this study was to examine whether African ancestry and gun violence interact on asthma and total IgE in school-aged Puerto Rican children.
Methods
This is a case-control study of 747 Puerto Rican children aged 9 to 14 years living in San Juan, Puerto Rico (n = 472), and Hartford, Connecticut (n = 275). Exposure to gun violence was defined as the child’s report of hearing gunshots more than once, and the percentage of African ancestry was estimated using genome-wide genotypic data. Asthma was defined as parental report of physician-diagnosed asthma and wheeze in the previous year. Serum total IgE (IU/mL) was measured in study participants. Multivariate logistic and linear regressions were used for the analysis of asthma and total IgE, respectively.
Results
In multivariate analyses, there was a significant interaction between exposure to gun violence and African ancestry on asthma (P = .001) and serum total IgE (P = .04). Among children exposed to gun violence, each quartile increase in the percentage of African ancestry was associated with approximately 45% higher odds of asthma (95% CI, 1.15-1.84; P = .002) and an approximately 19% increment in total IgE (95% , 0.60-40.65, P = .04). In contrast, there was no significant association between African ancestry and asthma or total IgE in children not exposed to gun violence.
Conclusions
Our results suggest that exposure to gun violence modifies the estimated effect of African ancestry on asthma and atopy in Puerto Rican children.
Key Words: African ancestry, asthma, children, gun violence, Puerto Rico, total IgE
Abbreviations: ETS, environmental tobacco smoke; SES, socioeconomic status; SNP, single-nucleotide polymorphism
Asthma is the most common chronic disease of childhood in the United States.1, 2 In this country, the prevalence of asthma is higher in Puerto Ricans (16.1%) or African Americans (11.2%) than in non-Hispanic whites (7.7%) or Mexican Americans (5.4%).2 Both Puerto Rican children living in the mainland United States and those living in the island of Puerto Rico are disproportionately affected with asthma.3 The reasons behind racial or ethnic disparities in asthma prevalence are unclear, but likely result from complex interactions between genetics and environmental factors.4
Puerto Ricans and African Americans have a higher percentage of African ancestry than non-Hispanic whites or Mexican Americans,5, 6, 7 and previous findings suggest that African ancestry partly explains the higher burden of asthma in these populations.7, 8 Indeed, several studies have shown that African ancestry is associated with an increased risk of asthma and/or reduced lung function in Puerto Ricans and African Americans.7, 8, 9, 10, 11, 12, 13, 14
Exposure to violence is common among Puerto Rican and African American children4, 15, 16, 17, 18 and could thus partly explain the observed link between African ancestry and asthma. Exposure to violence is increasingly being recognized as a potentially modifiable risk factor for childhood asthma.17, 19, 20, 21, 22, 23 We recently showed that hearing gunshots (a marker of exposure to a more severe type of violence) is associated with asthma in Puerto Rican children.21
On the basis of these findings, we hypothesized that African ancestry is associated with asthma in Puerto Rican children, but that this association would differ according to exposure to gun violence. To test this hypothesis, we analyzed data from a case-control study of asthma in 747 Puerto Rican children aged 9 to 14 years, living in San Juan (Puerto Rico) and Hartford (Connecticut).
Materials and Methods
Subject Recruitment
From March 2009 to June 2010, children were chosen from randomly selected households in San Juan. As previously described,24 households in the metropolitan area of San Juan were selected by a multistage probability sampling design. Primary sampling units were randomly selected neighborhood clusters based on the 2000 US census, and secondary sampling units were randomly selected households within each primary sampling unit. A household was eligible if ≥ 1 resident was a child aged 6 to 14 years old. In households with > 1 eligible child, only one child was randomly selected for screening. On the basis of the sampling design, 7,073 households were selected and 6,401 (90.5%) were contacted. Of these 6,401 households, 1,111 had ≥ 1 child within the age range of the study who met other eligibility criteria (see the following section). In an effort to reach a target sample size of approximately 700 children, we attempted to enroll a random sample (n = 783) of these 1,111 children. Parents of 105 of these 783 eligible households refused to participate or could not be reached. There were no significant differences in age, sex, or area of residence between eligible children who did (n = 678 [86.6%]) and did not (n = 105 [13.4%]) agree to participate. Of the 592 participants from whom blood samples were collected, 583 (98.5%) had sufficient DNA for genotyping (e-Fig 1).
From September 2003 to July 2008, children were recruited from 15 public elementary and middle schools in Hartford (a US city with a relatively large Puerto Rican population) that enrolled a significant proportion (42%-94%) of Puerto Rican children.25 As previously described,7 informational flyers with a study description were distributed to all parents of children in grades kindergarten to 8 in participating schools by mail (n = 10,881) or in person during different school activities (n = 885). Parents of 640 children completed a screening questionnaire. Of these children, 585 (91.4%) were eligible for inclusion (see the following section). Parents of 136 of these 585 eligible children refused to participate or could not be reached. There were no significant differences in age, sex, or area of residence between eligible children who did (n = 449 [76.8%]) and did not (n = 136 [23.2%]) agree to participate. Of the 425 participating children in whom blood samples were collected, 416 (97.9%) had sufficient DNA for genotyping (e-Fig 2).
At both study sites, the main recruitment tool was a screening questionnaire given to parents of children aged 6 to 14 years to obtain information about the child’s general and respiratory health and the birthplace of his or her grandparents. We selected as cases children who had parental report of physician-diagnosed asthma and wheeze in the previous year. We selected as control subjects children who had neither parental report of physician-diagnosed asthma nor wheeze in the prior year. All study participants had to have four Puerto Rican grandparents to ensure their Puerto Rican descent. Children who were aged 9 years and older (and thus completed the Exposure to Violence Survey, see the following section) were included in the current analysis (composing a total of 747 participants or 74.8% of the 999 participants with DNA for genotyping at both sites).
Study Procedures
At both study sites, participants completed a protocol that included questionnaires and collection of blood samples (for DNA extraction and measurement of serum total IgE). One of the child’s parents (usually [> 93%] the mother) completed a questionnaire that was slightly modified from the one used in the Collaborative Study of the Genetics of Asthma.26 This questionnaire was used to obtain information about the child’s general and respiratory health, sociodemographic characteristics, and maternal history of asthma.
Exposure to gun violence was assessed by the child’s response to the question: “Have you ever heard a gunshot?” and (if so) “never,” “once,” or “more than once.” A positive exposure to gun violence was defined as hearing a gunshot more than once during the child’s lifetime, as previously described.21 This question was administered to participating children aged 9 years and older as part of the Exposure to Violence Survey.19, 27, 28, 29 Internal consistency, test-retest reliability, and validity have been established for the English and Spanish versions of the Exposure to Violence Survey.27
Genotyping of approximately 2.5 million markers was conducted in DNA from study subjects using the HumanOmni2.5 BeadChip (Illumina, Inc.), as previously described.7 We removed single-nucleotide polymorphisms (SNPs) that were not in Hardy-Weinberg equilibrium (P < 10-6) and had minor allele frequency lower than 1% or a failure rate greater than 2%. Ancestry was estimated using the Local Ancestry in adMixed Populations method and software.30, 31 The analysis was restricted to SNPs that were present in all three ancestral populations and that were not in tight linkage disequilibrium (using the software default of r2 ≥ 0.1), leaving a final sample of 85,059 SNPs. The algorithm uses ancestral proportions from prior studies (in this case, Tang et al32) and data from reference panels to estimate ancestral proportions for racially admixed populations. Puerto Ricans are an admixture of European, African, and Native Americans populations.7 To approximate this admixture, we used reference panels from HapMap33 for Europeans (Utah residents from Western and Central Europe and Tuscans) and Africans (Yorubans from West Africa) as well as from the Human Genome Diversity Project34 for Native Americans.
Serum total IgE (IU/mL) was measured using the UniCAP 100 system (Pharmacia & Upjohn). Levels were then transformed to a log10 scale for data analysis.
Written parental consent was obtained for participating children, from whom written assent was also obtained. The study was approved by the Institutional Review Boards of Connecticut Children’s Medical Center (Hartford, CT; protocol #03-011), the University of Puerto Rico (San Juan, PR; protocol #0160507), Brigham and Women’s Hospital (Boston, MA; protocol #2007-P-001174/9), and the University of Pittsburgh (Pittsburgh, PA; protocol #PRO-10030498).
Statistical Analysis
Our exposure and effect modifier of interest were the percentage of African ancestry and exposure to gun violence, respectively. Our main outcome of interest was asthma (defined as physician-diagnosed asthma and wheeze in the previous year). We analyzed serum total IgE as a secondary outcome, given that atopy is common in Puerto Rican children with asthma.7, 35
Because of sample size and statistical power, the analysis of African ancestry and asthma or total IgE was first conducted for the combined cohort and then conducted separately for each study site. Bivariate analyses were conducted using Student t test or one-way analysis of variance for continuous variables, and Pearson χ2 test or Cochran-Armitage trend for categorical variables. For the multivariate analysis, we used a stepwise approach to build the logistic (for asthma) or linear (for total IgE) regression models. Because of their previously established association with the exposure or outcomes of interest, all models included age,2 sex,36 household income (< vs ≥ $15,000/y [near the median income for households in Puerto Rico in 2008-200937]),38 maternal history of asthma,39 early-life exposure (in utero or before 2 years of age) to environmental tobacco smoke (ETS),40 exposure to gun violence,21 and (for the analysis of the combined cohort) study site.3 The model for total IgE was further adjusted for case-control status. The following covariates were also included in the initial multivariate models if they were associated with asthma or total IgE at P ≤ .20 in bivariate analyses: BMI as a z score (based on 2000 US Centers for Disease Control and Prevention growth charts41), obesity (defined as a BMI ≥ 95th percentile for age and sex [ie, a z score ≥ 1.96]), breastfeeding, prematurity, low birth weight (< 2,500 g), mode of delivery (cesarean vs vaginal birth), current exposure to ETS, parental education (≥ 1 parent completed high school vs none), and type of health insurance (private or employer-based health insurance vs others). These additional covariates remained in the final models if they were associated with asthma at P < .05 or changed the parameter estimate (β) by ≥ 10%. After the final models were built, we tested for a first-order interaction between African ancestry and exposure to gun violence. Statistical significance was defined as a P < .05. All statistical analyses were performed using SAS, version 9.3 (SAS Institute).
Results
The baseline characteristics of study participants are summarized in Table 1. At each study site and in the combined cohort, cases were significantly more likely to have current exposure to ETS, a maternal history of asthma, and a higher serum total IgE. Compared with control subjects in the combined cohort, cases were also significantly more likely to be obese and to have early-life exposure to ETS, a higher BMI, a history of prematurity, lower parental education, no private or employer-based health insurance, and exposure to gun violence.
Table 1.
Baseline Characteristics of Participating Puerto Rican Children According to Study Site and Case-Control Status
| Covariate | San Juan (n = 472) |
Hartford (n = 275) |
Combined (n = 747) |
|||
|---|---|---|---|---|---|---|
| Control Cases (n = 235) |
Cases (n = 237) |
Control Cases (n = 108) |
Cases (n = 167) |
Control Cases (n = 343) |
Cases (n = 404) |
|
| Age, y | 11.8 (1.9) | 11.5 (1.8)a | 11.5 (2.3) | 11.6 (2.3) | 11.7 (2.0) | 11.5 (2.0) |
| Female | 126 (53.4%) | 103 (43.5%)a | 57 (52.8%) | 89 (53.3%) | 183 (53.4%) | 192 (47.5%) |
| BMI (z score) | 0.5 (1.0) | 0.7 (1.1) | 0.7 (1.3) | 1.0 (1.2) | 0.6 (1.1) | 0.9 (1.2)a |
| Obesityb | 18 (8.4%) | 31 (14.7%)a | 24 (22.2%) | 41 (24.7%) | 42 (13.0%) | 72 (19.1%)a |
| Current exposure to ETS | 78 (33.2%) | 101 (42.6%)a | 44 (41.1%) | 88 (54.0%)a | 122 (35.7%) | 189 (47.3%)a |
| Exposure to ETS in utero or before 2 years of age | 91 (38.9%) | 116 (49.0%)a | 54 (50.5%) | 99 (60.7%) | 145 (42.5%) | 215 (53.8%)a |
| Household income < $15,000/y | 131 (57.5%) | 149 (63.7%) | 66 (71.7%) | 92 (62.6%) | 197 (61.6%) | 241 (63.3%) |
| Neither parent graduated from high school | 38 (16.2%) | 41 (17.3%) | 25 (23.2%) | 57 (34.3%)a | 63 (18.4%) | 98 (24.3%)a |
| No private or employer-based health insurance | 134 (57.0%) | 160 (67.5%)a | 74 (81.3%) | 109 (76.8%) | 208 (63.8%) | 269 (71.0%)a |
| Maternal history of asthma | 50 (21.6%) | 117 (49.6%)a | 33 (30.8%) | 75 (45.5%)a | 83 (24.5%) | 192 (47.9%)a |
| Any breastfeeding | 129 (55.6%) | 127 (53.6%) | 46 (43.0%) | 74 (45.4%) | 175 (51.6%) | 201 (50.3%) |
| Prematurity | 11 (4.8%) | 22 (9.4%) | 5 (4.7%) | 12 (7.4%) | 16 (4.8%) | 34 (8.5%)a |
| Low birth weight (< 2500 g) | 10 (4.5%) | 16 (6.8%) | 7 (6.7%) | 5 (3.2%) | 17 (5.2%) | 21 (5.3%) |
| Birth by cesarean section | 66 (28.6%) | 85 (35.9%) | 17 (21.8%) | 25 (21.4%) | 83 (26.9%) | 110 (31.1%) |
| Percentage of African ancestry | 24.7 (12.4) | 25.4 (11.8) | 21.1 (10.0) | 22.1 (8.3) | 23.6 (11.8) | 24.0 (10.6) |
| Exposure to gun violencec | 122 (52.1%) | 156 (67.2%)a | 25 (32.9%) | 46 (42.2%) | 147 (47.4%) | 202 (59.2%)a |
| Serum total IgE (IU/mL) | 158.5 (5.0) | 316.2 (5.0)a | 63.1 (4.0) | 125.9 (5.0)a | 125.9 (5.0) | 199.5 (5.0)a |
Data presented as No. (%) for binary variables or mean (SD) for continuous variables (other than serum total IgE, shown as geometric mean [SD]). Percentages were calculated for children with complete data. ETS = Environmental tobacco smoke.
P < .05 for linear trend.
Obesity defined as a BMI ≥ 95th percentile for age and sex.
Heard a gunshot more than once during his or her lifetime.
Table 2 shows the baseline characteristics of study participants across quartiles of percentage of African ancestry. In this analysis, children with a higher proportion of African ancestry were more likely to have a lower household income, to lack private or employer-based health insurance, or to have been exposed to gun violence, but less likely to have been born by cesarean section.
Table 2.
Baseline Characteristics of Participating Puerto Rican Children According to African Ancestry
| Covariate | Quartiles of Percentage of African Ancestry |
|||
|---|---|---|---|---|
| Q1 (0.1%-15.7%) | Q2 (15.8%-21.2%) | Q3 (21.3%-29.8%) | Q4 (29.9%-67.7%) | |
| Age (y) | 11.8 (2.0) | 11.7 (2.0) | 11.3 (2.0) | 11.4 (1.9) |
| Female | 78 (49.7%) | 69 (44.0%) | 83 (53.2%) | 79 (51.0%) |
| BMI (z score) | 0.7 (1.1) | 0.8 (1.2) | 0.8 (1.2) | 0.6 (1.2) |
| Obesityb | 24 (15.3%) | 24 (15.3%) | 33 (21.2%) | 22 (14.3%) |
| Current exposure to ETS | 62 (39.5%) | 59 (37.8%) | 78 (50.3%) | 69 (44.5%) |
| Exposure to ETS in utero or before 2 years of age | 74 (47.1%) | 79 (50.6%) | 82 (53.3%) | 68 (43.9%) |
| Household income < $15,000/y | 80 (54.5%) | 91 (62.3%) | 97 (65.5%) | 114 (75.0%)a |
| Neither parent graduated from high school | 31 (19.8%) | 34 (21.7%) | 36 (23.1%) | 38 (24.5%) |
| No private or employer-based health insurance | 88 (59.1%) | 103 (68.2%) | 111 (73.5%) | 116 (75.3%)a |
| Maternal history of asthma | 61 (39.4%) | 57 (36.5%) | 63 (41.2%) | 50 (32.3%) |
| Any breastfeeding | 83 (52.9%) | 74 (47.7%) | 76 (49.7%) | 76 (49.0%) |
| Prematurity | 10 (6.4%) | 20 (12.9%) | 7 (4.6%) | 8 (5.2%) |
| Low birth weight (< 2500 g) | 9 (5.8%) | 10 (6.8%) | 5 (3.3%) | 9 (6.0%) |
| Birth by cesarean section | 52 (36.4%) | 43 (30.1%) | 40 (28.8%) | 31 (20.7%)a |
| Exposure to gun violencec | 66 (46.5%) | 69 (49.3%) | 70 (50.4%) | 92 (63.9%)a |
| Asthma | 77 (49.0%) | 87 (55.4%) | 90 (57.7%) | 87 (56.1%) |
| Serum total IgE (IU/mL) | 158.5 (5.0) | 125.9 (5.0) | 199.5 (5.0) | 199.5 (5.0) |
Data presented as No. (%) for binary variables or mean (SD) for continuous variables (other than serum total IgE, shown as geometric mean [SD]). Percentages were calculated for children with complete data. See Table 1 legend for expansion of abbreviation.
P < .05 for linear trend.
Obesity defined as a BMI ≥ 95th percentile for age and sex.
Heard a gunshot more than once during his or her lifetime.
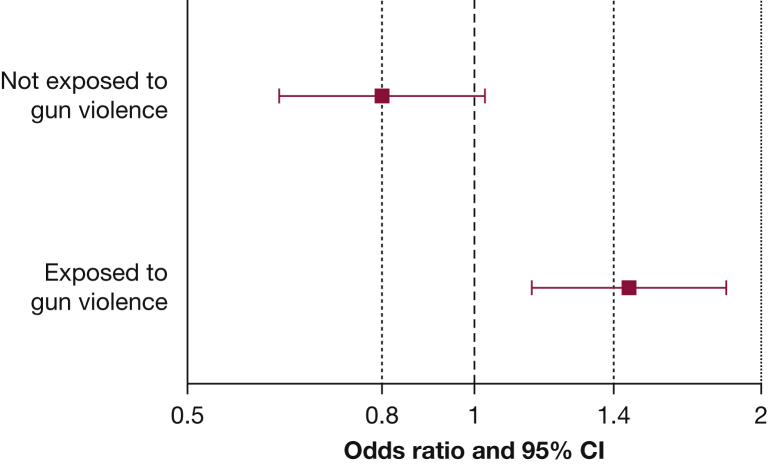
Table 3 shows the results of the multivariate analysis of African ancestry and asthma. In this analysis, there was significant modification of the estimated effect of African ancestry on asthma by exposure to gun violence (P for the interaction term = .001). Thus, we repeated the multivariate analysis after stratification by exposure to gun violence. In this stratified analysis, each quartile increment in the percentage of African ancestry was significantly associated with 45% increased odds of asthma in children exposed to gun violence (95% CI for OR, 1.15-1.84; P = .002). In contrast, there was no significant association between African ancestry and asthma in children not exposed to gun violence (OR, 0.80; 95% CI, 0.62-1.03; P = .08) (Fig 1).
Table 3.
Analysis of African Ancestry and Asthma in Participating Puerto Rican Childrena
| OR (95% CI), P Value | |
|---|---|
| Unadjusted | |
| Each quartile increase in the percentage of African ancestry | 1.10 (0.96-1.27), .2 |
| Adjustedb,c | |
| Each quartile increase in the percentage of African ancestry | 1.10 (0.93-1.29), .3 |
| Exposure to gun violenced | 1.84 (1.25-2.71), .002 |
| Exposure to ETS in utero or before 2 years of age | 1.81 (1.24-2.63), .002 |
| Household income < $15,000/year | 1.34 (0.90-1.99), .1 |
See Table 1 legend for expansion of abbreviation.
Asthma defined as parental report of physician-diagnosed asthma and wheeze in the previous year.
Multivariate models were adjusted for age, sex, maternal asthma, and study site in addition to the covariates listed in the first column.
There was a significant interaction between African ancestry and exposure to gun violence on asthma in this model (P for interaction term = .001).
Heard a gunshot more than once during his or her lifetime.
Figure 1.
Results of the multivariate logistic regression analysis of African ancestry and asthma in participating Puerto Rican children after stratification by exposure to gun violence (defined as child’s report of hearing a gunshot more than once during his or her lifetime). Multivariate models were adjusted for age, sex, exposure to environmental tobacco smoke in utero or before 2 years of age, household income, maternal asthma, and study site.
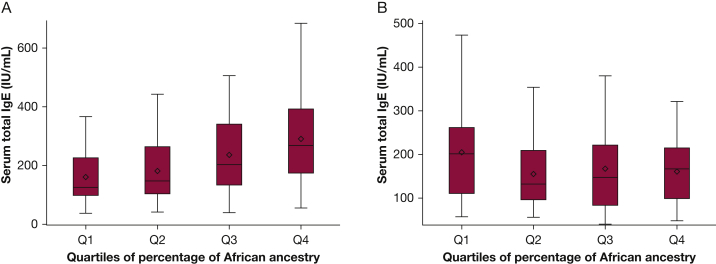
We then conducted a multivariate analysis of African ancestry and total IgE. In this analysis, there was significant modification of the estimated effect of African ancestry on total IgE by exposure to gun violence (P for interaction term = .04). Thus, we conducted a multivariate analysis after stratification for exposure gun violence, obtaining similar results to those for African ancestry and asthma (ie, significant results in children exposed to gun violence but nonsignificant results in unexposed children [Fig 2]). In a multivariate analysis adjusted for age, sex, early-life exposure to ETS, household income, maternal asthma, case-control status, and study site, each quartile increment in the percentage of African ancestry was significantly associated with higher total IgE (β = 18.95 IU/mL; 95% CI, 0.60-40.65 IU/mL; P = .04) in children exposed to gun violence. In contrast, there was no significant association between African ancestry and total IgE in children not exposed to gun violence (β = −16.88 IU/mL; 95% CI, −33.38 to 3.72 IU/mL; P = .10).
Figure 2.
Box plots of predicted serum total IgE according to quartiles of percentage of African ancestry in Puerto Rican children with (A) and without (B) exposure to gun violence. Exposure to gun violence was defined as child’s report of hearing a gunshot more than once during his or her lifetime. Estimates obtained from multivariate models adjusted for age, sex, exposure to environmental tobacco smoke in utero or before 2 years of age, household income, maternal asthma, case-control status, and study site. Serum total IgE transformed to a logarithmic (log10) scale for analyses. Results were back transformed for ease of interpretation.
As a final step, we repeated the multivariate analyses of African ancestry and asthma or total IgE, stratified by exposure to gun violence, separately for the San Juan and Hartford study sites. At both study sites, African ancestry was significantly associated with asthma in children exposed to gun violence but not in children unexposed to gun violence (e-Table 1). Although the observed association between African ancestry and total IgE in children exposed to violence was not statistically significant at either study site, they were of similar magnitude and in the same direction across study sites (e-Table 2).
Discussion
We show that exposure to gun violence (a preventable factor) modifies the estimated effect of African ancestry on asthma or total IgE in Puerto Rican children (an ethnic group with high prevalence of asthma).2, 4 Among children exposed to gun violence, African ancestry was significantly associated with asthma and total IgE. In contrast, we found no significant association between African ancestry and asthma or total IgE in children unexposed to gun violence.
African ancestry has been previously associated with asthma or asthma-related outcomes in Puerto Ricans and non-Puerto Ricans.7, 8, 9, 10, 11, 12, 13 In a recent study of 5,493 Hispanic children and adults (including Puerto Ricans), each 20% increment in African ancestry increased the odds of asthma by 40% (95% CI, 1.14-1.72; P = .001).8 Similar associations have been demonstrated in African American, Colombian, and Brazilian adults.9, 10, 11 For example, each 10% increment in African ancestry was associated with a 16% increment in the odds of asthma in a case-control study of 796 African American adults (95% CI, 1.06-1.28; P = .002).11 African ancestry has been associated with higher total or allergen-specific IgEs in some but not all previous reports.7, 9, 10, 42 Of note, none of the studies referenced previously examined whether exposure to violence explains or modifies the observed association between African ancestry and asthma.
Exposure to violence (either at the individual or community level) has been linked to asthma, particularly in populations of African descent.17, 19, 20, 21, 22, 23 In a prior study of 1,213 Puerto Rican children, we reported that a history of child abuse was associated with 2.5 times increased odds of asthma (95% CI, 1.27-5.00; P = .009).20 These findings were later replicated in a large longitudinal cohort of adult African American women.22 In a more recent case-control study of 466 children in Puerto Rico, we found that exposure to gun violence increases the odds of asthma by 80% (95% CI, 1.10-2.70; P = .01).21
To our knowledge, this is the first study to present the separate effects of African ancestry on childhood asthma or total IgE according to exposure to a severe type of violence. In a prior case-control study of 291 Puerto Rican children and adults, there was an interaction between African ancestry and socioeconomic status (SES).6 In models stratified by SES, African ancestry was only associated with asthma among individuals with a high SES. In contrast, we found no significant interaction between African ancestry and SES (assessed by household income, parental education, or type of health insurance). The discrepant results between our study and those of the prior report may be explained by differences in study design (as participants in the prior study were selected from health-care centers), study population (both children and adults participated in the prior study), and nonassessment of certain covariates in the prior study (including exposure to gun violence, early-life exposure to ETS, and maternal asthma).
The mechanisms underlying the link between African ancestry or exposure to violence and asthma are incompletely understood. Inherited alleles or haplotypes may have been protective against certain infectious diseases among Africans living in Africa, but could now increase the risk of allergic airway inflammation in westernized populations of African descent.8, 43 Exposure to violence may lead to childhood asthma through increased psychosocial stress, which could cause dysregulation of the hypothalamic-pituitary-adrenocortical axis, enhanced Th2 immune responses, autonomic nervous system dysfunction, and/or abnormal expression of asthma-susceptibility genes.16, 17, 44, 45
Gun violence is an important and modifiable public health problem among all children in the United States, regardless of race or ethnicity. Prior studies have shown that Puerto Ricans are particularly susceptible to the detrimental effects of traumatic events such as violence.46, 47 Our findings suggest that a Puerto Rican child with high African ancestry may be more susceptible to the detrimental effects of gun violence than a Puerto Rican child with lower African ancestry. If our results are confirmed in longitudinal studies, further examination of genetic variation or environmental exposures correlated with African ancestry could offer novel insights into the pathogenesis of asthma, particularly stress-related asthma.
Our study has considerable strengths such as sample size, availability of data on ancestry and gun violence, and a predefined approach to formally test for interaction between African ancestry and asthma or total IgE (thus reducing the risk of obtaining spurious results). However, we also recognize several limitations to our findings. First, we cannot infer causality or temporal relationships because of the cross sectional design of our observational study. Second, selection bias is possible in any observational study such as ours. However, the participation rates among all eligible children in Hartford and San Juan were approximately 77% and approximately 87%, respectively, which are high for a study of an ethnic minority group in the United States. Third, our results for total IgE were not significant at each individual site (likely from insufficient power). However, our findings were consistent in direction and magnitude across sites, despite differences in recruitment approach and sample size. Fourth, there could be residual confounding by SES or unmeasured variables (such as child abuse, intimate partner violence, or type of gun violence [eg, at the individual, family, or community level]). Nonetheless, our analysis was adjusted for household income, and we obtained similar results when the analysis was adjusted for other indicators of SES (parental education or type of health insurance; data not shown). Child abuse is also an unlikely major confounder of our analysis because it was relatively uncommon in a prior study in Puerto Rican children.20 Fifth, recall bias is a potential but improbable explanation for our results, because misclassification of exposure to gun violence by participating children would likely be nondifferential regarding case-control status (and thus bias our results toward the null hypothesis). Moreover, we obtained almost identical results when defining exposure to violence as hearing a gunshot at least once instead of at least twice (data not shown). Last, our results may not be generalizable to non-Puerto Rican children but are likely to be relevant to members of racial or ethnic groups that are often exposed to gun violence.
In conclusion, our results suggest that exposure to gun violence modifies the estimated effect of African ancestry on asthma and atopy in Puerto Rican children. Our findings merit replication in longitudinal studies of Puerto Rican children and in children from other racial or ethnic groups with a high proportion of African ancestry and frequent exposure to gun violence (such as African Americans).
Acknowledgments
Author contributions: J. C. C. is the guarantor of the manuscript. C. R.-S., Y.-Y. H., J. M. B., and E. F. participated in data analysis. E. A.-P., M. M. C., M. A., and A. C.-S. participated in data collection. G. C. and J. C. C. participated in study design and implementation and obtained the necessary funding. All authors participated in manuscript preparation and approved the final version of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. M. C. served as a consultant for GlaxoSmithKline on a topic unrelated to this manuscript. None declared (C. R.-S., Y.-Y. H., J. M. B., E. F., E. A.-P., M. A., A. C.-S., G. C., J. C. C.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank all the participating children and their families for their invaluable participation in the study.
Additional information: The e-Tables and e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [grants HL079966 and HL117191] and by The Heinz Endowments.
Supplementary Data
References
- 1.Malveaux F.J. The state of childhood asthma: introduction. Pediatrics. 2009;123(Suppl 3):S129–S130. doi: 10.1542/peds.2008-2233B. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami L.J., Moorman J.E., Bailey C. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;(94):1–8. [PubMed] [Google Scholar]
- 3.Cohen R.T., Canino G.J., Bird H.R., Shen S., Rosner B.A., Celedón J.C. Area of residence, birthplace, and asthma in Puerto Rican children. Chest. 2007;131(5):1331–1338. doi: 10.1378/chest.06-1917. [DOI] [PubMed] [Google Scholar]
- 4.Rosser F.J., Forno E., Cooper P.J., Celedón J.C. Asthma in Hispanics. An 8-year update. Am J Respir Crit Care Med. 2014;189(11):1316–1327. doi: 10.1164/rccm.201401-0186PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tishkoff S.A., Reed F.A., Friedlaender F.R. The genetic structure and history of Africans and African Americans. Science. 2009;324(5930):1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhry S., Burchard E.G., Borrell L.N. Ancestry-environment interactions and asthma risk among Puerto Ricans. Am J Respir Crit Care Med. 2006;174(10):1088–1093. doi: 10.1164/rccm.200605-596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brehm J.M., Acosta-Pérez E., Klei L. African ancestry and lung function in Puerto Rican children. J Allergy Clin Immunol. 2012;129(6):1484–1490. doi: 10.1016/j.jaci.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pino-Yanes M., Thakur N., Gignoux C.R. Genetic ancestry influences asthma susceptibility and lung function among Latinos. J Allergy Clin Immunol. 2015;135(1):228–235. doi: 10.1016/j.jaci.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergara C., Murray T., Rafaels N. African ancestry is a risk factor for asthma and high total IgE levels in African admixed populations. Genet Epidemiol. 2013;37(4):393–401. doi: 10.1002/gepi.21702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergara C., Caraballo L., Mercado D. African ancestry is associated with risk of asthma and high total serum IgE in a population from the Caribbean Coast of Colombia. Hum Genet. 2009;125(5-6):565–579. doi: 10.1007/s00439-009-0649-2. [DOI] [PubMed] [Google Scholar]
- 11.Flores C., Ma S.F., Pino-Yanes M. African ancestry is associated with asthma risk in African Americans. PLoS One. 2012;7(1):e26807. doi: 10.1371/journal.pone.0026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin A.M., Wang Y., Wells K.E. Nocturnal asthma and the importance of race/ethnicity and genetic ancestry. Am J Respir Crit Care Med. 2014;190(3):266–273. doi: 10.1164/rccm.201402-0204OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rumpel J.A., Ahmedani B.K., Peterson E.L. Genetic ancestry and its association with asthma exacerbations among African American subjects with asthma. J Allergy Clin Immunol. 2012;130(6):1302–1306. doi: 10.1016/j.jaci.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R., Seibold M.A., Aldrich M.C. Genetic ancestry in lung-function predictions. N Engl J Med. 2010;363(4):321–330. doi: 10.1056/NEJMoa0907897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Taboas A., Canino G., Wang M.Q., García P., Bravo M. Prevalence and victimization correlates of pathological dissociation in a community sample of youths. J Trauma Stress. 2006;19(4):439–448. doi: 10.1002/jts.20144. [DOI] [PubMed] [Google Scholar]
- 16.Yonas M.A., Lange N.E., Celedón J.C. Psychosocial stress and asthma morbidity. Curr Opin Allergy Clin Immunol. 2012;12(2):202–210. doi: 10.1097/ACI.0b013e32835090c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W., Boutaoui N., Brehm J.M. ADCYAP1R1 and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2013;187(6):584–588. doi: 10.1164/rccm.201210-1789OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams D.R., Sternthal M., Wright R.J. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123(suppl 3):S174–S184. doi: 10.1542/peds.2008-2233H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sternthal M.J., Jun H.J., Earls F., Wright R.J. Community violence and urban childhood asthma: a multilevel analysis. Eur Respir J. 2010;36(6):1400–1409. doi: 10.1183/09031936.00003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen R.T., Canino G.J., Bird H.R., Celedón J.C. Violence, abuse, and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2008;178(5):453–459. doi: 10.1164/rccm.200711-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramratnam S.K., Han Y.Y., Rosas-Salazar C. Exposure to gun violence and asthma among children in Puerto Rico. Respir Med. 2015;109(8):975–981. doi: 10.1016/j.rmed.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coogan P.F., Wise L.A., O'Connor G.T., Brown T.A., Palmer J.R., Rosenberg L. Abuse during childhood and adolescence and risk of adult-onset asthma in African American women. J Allergy Clin Immunol. 2013;131(4):1058–1063. doi: 10.1016/j.jaci.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu Y.H., Coull B.A., Sternthal M.J. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2014;133(3):713–722. doi: 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bird H.R., Canino G.J., Davies M. A study of disruptive behavior disorders in Puerto Rican youth: I. Background, design, and survey methods. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1032–1041. doi: 10.1097/01.chi.0000227878.58027.3d. [DOI] [PubMed] [Google Scholar]
- 25.Ennis SR, Ríos-Vargas M, Albert NG. The Hispanic population: 2010. 2010 census brief. US Census Bureau. http://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf. Accessed May 3, 2016.
- 26.Blumenthal M.N., Banks-Schlegel S., Bleecker E.R., Marsh D.G., Ober C. Collaborative studies on the genetics of asthma–National Heart, Lung and Blood Institute. Clin Exp Allergy. 1995;25(suppl 2):29–32. doi: 10.1111/j.1365-2222.1995.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 27.Selner-O'Hagan M.B., Kindlon D.J., Buka S.L., Raudenbush S.W., Earls F.J. Assessing exposure to violence in urban youth. J Child Psychol Psychiatry. 1998;39(2):215–224. [PubMed] [Google Scholar]
- 28.Suglia S.F., Ryan L., Wright R.J. Creation of a community violence exposure scale: accounting for what, who, where, and how often. J Trauma Stress. 2008;21(5):479–486. doi: 10.1002/jts.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson C.C., Roberts K., Curran A., Ryan L., Wright R.J. Caretaker-child concordance for child's exposure to violence in a preadolescent inner-city population. Arch Pediatr Adolesc Med. 2002;156(8):818–823. doi: 10.1001/archpedi.156.8.818. [DOI] [PubMed] [Google Scholar]
- 30.Sankararaman S., Sridhar S., Kimmel G., Halperin E. Estimating local ancestry in admixed populations. Am J Hum Genet. 2008;82(2):290–303. doi: 10.1016/j.ajhg.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pasaniuc B., Sankararaman S., Kimmel G., Halperin E. Inference of locus-specific ancestry in closely related populations. Bioinformatics. 2009;25(12):i213–i221. doi: 10.1093/bioinformatics/btp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang H., Choudhry S., Mei R. Recent genetic selection in the ancestral admixture of Puerto Ricans. Am J Hum Genet. 2007;81(3):626–633. doi: 10.1086/520769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorisson G.A., Smith A.V., Krishnan L., Stein L.D. The International HapMap Project Web site. Genome Res. 2005;15(11):1592–1593. doi: 10.1101/gr.4413105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J.Z., Absher D.M., Tang H. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319(5866):1100–1104. doi: 10.1126/science.1153717. [DOI] [PubMed] [Google Scholar]
- 35.Rosas-Salazar C., Ramratnam S.K., Brehm J.M. Prematurity, atopy, and childhood asthma in Puerto Ricans. J Allergy Clin Immunol. 2014;133(2):357–362. doi: 10.1016/j.jaci.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss S.T., Gold D.R. Gender differences in asthma. Pediatr Pulmonol. 1995;19(3):153–155. doi: 10.1002/ppul.1950190302. [DOI] [PubMed] [Google Scholar]
- 37.Noss A. Household income for states: 2008 and 2009. American community survey briefs. US Census Bureau. http://www.census.gov/prod/2010pubs/acsbr09-2.pdf. Accessed May 3, 2016.
- 38.Forno E., Celedón J.C. Asthma and ethnic minorities: socioeconomic status and beyond. Curr Opin Allergy Clin Immunol. 2009;9(2):154–160. doi: 10.1097/aci.0b013e3283292207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guilbert T.W., Stern D.A., Morgan W.J., Martinez F.D., Wright A.L. Effect of breastfeeding on lung function in childhood and modulation by maternal asthma and atopy. Am J Respir Crit Care Med. 2007;176(9):843–848. doi: 10.1164/rccm.200610-1507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaakkola J.J., Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health. 2004;94(1):136–140. doi: 10.2105/ajph.94.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuczmarski R.J., Ogden C.L., Grummer-Strawn L.M. CDC growth charts: United States. Adv Data. 2000;8(314):1–27. [PubMed] [Google Scholar]
- 42.Yang J.J., Burchard E.G., Choudhry S. Differences in allergic sensitization by self-reported race and genetic ancestry. J Allergy Clin Immunol. 2008;122(4):820–827.e9. doi: 10.1016/j.jaci.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fumagalli M., Sironi M., Pozzoli U., Ferrer-Admetlla A., Pattini L., Nielsen R. Signatures of environmental genetic adaptation pinpoint pathogens as the main selective pressure through human evolution. PLoS Genet. 2011;7(11):e1002355. doi: 10.1371/journal.pgen.1002355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright R.J. Epidemiology of stress and asthma: from constricting communities and fragile families to epigenetics. Immunol Allergy Clin North Am. 2011;31(1):19–39. doi: 10.1016/j.iac.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller G.E., Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and beta2-adrenergic receptor in children with asthma. Proc Natl Acad Sci U S A. 2006;103(14):5496–5501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ortega A.N., Rosenheck R. Posttraumatic stress disorder among Hispanic Vietnam veterans. Am J Psychiatry. 2000;157(4):615–619. doi: 10.1176/appi.ajp.157.4.615. [DOI] [PubMed] [Google Scholar]
- 47.Galea S., Vlahov D., Tracy M., Hoover D.R., Resnick H., Kilpatrick D. Hispanic ethnicity and post-traumatic stress disorder after a disaster: evidence from a general population survey after September 11, 2001. Ann Epidemiol. 2004;14(8):520–531. doi: 10.1016/j.annepidem.2004.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.