Abstract
Objective
To evaluate rheumatoid arthritis (RA) and mortality risk among women followed prospectively in the Nurses’ Health Study (NHS).
Methods
We analyzed 119,209 women in the NHS who reported no connective tissue disease at enrollment in 1976. Comorbidity and lifestyle data were collected through biennial questionnaires. Incident RA cases were validated by medical records review. Cause of death was determined by death certificate and medical records review. Cox regression models estimated hazard ratios (HRs) and 95% confidence intervals (95% CIs) for all-cause, cardiovascular disease (CVD), cancer, and respiratory disease mortality for women with RA compared to those without RA.
Results
We validated 964 incident RA cases and identified 28,808 deaths during 36 years of prospective follow-up. Of 307 deaths among women with RA, 80 (26%) were from cancer, 70 (23%) were from CVD, and 44 (14%) were from respiratory causes. Women with RA had increased total mortality (HR 1.40, 95% CI 1.25–1.57) compared to those without RA, independent of mortality risk factors, including smoking. RA was associated with significantly increased respiratory disease mortality (HR 2.06, 95% CI 1.51–2.80) and cardiovascular disease mortality (HR 1.45, 95% CI 1.14–1.83), but not cancer mortality (HR 0.93, 95% CI 0.74–1.15). For women with seropositive RA, respiratory disease mortality was nearly 3-fold higher than among non-RA women (HR 2.67, 95% CI 1.89–3.77).
Conclusion
Women with RA had significantly increased mortality compared to those without RA. Respiratory disease and cardiovascular disease mortality were both significantly elevated for women with RA. The nearly 3-fold increased relative risk of respiratory disease mortality was observed only for those with seropositive RA.
INTRODUCTION
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by inflammatory polyarthritis, affecting approximately 1% of the population and associated with increased morbidity (1). Prior studies suggest that RA patients may be at increased risk for cardiovascular disease, cancer, respiratory disease, and serious infections, compared to the general population (2-5). Despite advances in RA treatment with disease-modifying antirheumatic drugs (DMARDs), a mortality gap between RA patients and the general population may persist (6-8).
Most previous studies investigating RA and mortality were derived from RA-only cohorts and compared observed RA mortality rates to age- and sex-standardized general population estimates (6,9,10). However, mortality rates vary based on factors such as temporal trends, geographic location of cohort, and RA duration, perhaps explaining the variability of prior standardized mortality ratios (SMRs) for RA (ranging from 0.87 to 2.03) (11,12). The use of age standardization and sex standardization alone does not account for unmeasured confounders such as body mass index (BMI) and smoking, which may influence both RA susceptibility and mortality (13-16). While excess cardiovascular disease has been demonstrated for RA, cause-specific mortality has not been evaluated among cohorts that include both RA and non-RA individuals (2,6,17). To evaluate the association of RA with mortality, detailed follow-up data on mortality risk factors are necessary. Traditional mortality risk factors assessed in the study cohort (both RA and non-RA) should include sociodemographic and clinical factors. We aimed to determine whether RA was associated with increased mortality among women followed prospectively during 36 years of follow-up in the Nurses’ Health Study (NHS), with adjustment for time-varying confounders.
SUBJECTS AND METHODS
Study population
In 1976, the NHS enrolled 121,700 female registered nurses in the US, ages 30–55 years. Women in the NHS completed questionnaires at baseline and every 2 years, providing data on sociodemographics, anthropometrics, behaviors, medications, diet, and diseases. Only 4.4% of person-years in the NHS have been lost to follow-up (18). The study protocol was approved by the Partners HealthCare Institutional Review Board.
Incident RA cases
Women who self-reported a physician diagnosis of RA were mailed a validated questionnaire (19). For those who screened positive, medical records were obtained and independently reviewed by 2 rheumatologists to confirm RA according to the 1987 American College of Rheumatology classification criteria (20). In addition, the date of RA diagnosis and serologic subtype (seropositive: presence of rheumatoid factor [RF] and/or anti–cyclic citrul-linated peptide) were obtained from medical records review. For these analyses, participants who reported prevalent RA or another connective tissue disease (CTD) prior to enrollment in the NHS in 1976 were excluded. Women were followed from cohort entry until death, censoring for loss to follow-up or self-reported CTD that was not subsequently validated as RA upon medical records review, or end of follow-up (May 31, 2012). The final analysis consisted of 119,209 women who were followed from 1976 to 2012.
Identification and cause of death
Deaths were identified by systematic searches of the National Death Index and state vital records, and supplemented by postal authority and family reports, as previously described (21). This method ascertains >98% of deaths in the NHS (22). Death certificates and medical records were reviewed by trained NHS study physicians in order to classify into major categories the primary cause of death in accordance with the 8th and 9th revisions of the International Classification of Diseases. For each study participant, reviewers systematically determined a single cause of death as the underlying condition that resulted in death. For example, the death of a patient who developed sepsis and subsequently died and whose death certificate listed respiratory failure as the primary cause of death would be classified as death due to infection by the NHS reviewers. A study investigator further reviewed medical records of women with RA classified as having died from RA or respiratory causes, but the classification by NHS reviewers was not changed.
Study covariates
Time-varying data on potential confounders were obtained from questionnaires mailed every 2 years beginning in 1976. We selected covariates based on prior mortality studies in the NHS, as well as established risk factors for RA or death (21,23-30).
Sociodemographic and lifestyle factors
Race, ethnicity, and education level were self-reported. Median household income was based on home address and US Census tract-level data. BMI was classified as underweight, normal, overweight, or obese (31). Smoking pack-years were calculated as number of years smoked multiplied by number of cigarette packs smoked per day (25). Physical activity was measured with a validated survey and converted into weekly hours of moderate to vigorous activity (32). Menopausal status and postmenopausal hormone use were self-reported. Dietary factors, including alcohol intake, were assessed using semiquantitative food-frequency questionnaires and categorized according to the Alternate Healthy Eating Index that classifies adherence to the US Department of Agriculture’s Dietary Guidelines for Americans (33,34).
Clinical factors
Chronic conditions associated with mortality were self-reported and included cancer, cardiovascular disease, hyperlipidemia, and diabetes mellitus. Aspirin use and family history of cancer and diabetes mellitus in first-degree relatives, and parental myocardial infarction at <60 years of age were self-reported. The validity of self-report in the NHS compared to medical records review has been demonstrated (35).
Statistical analysis
The primary analysis assessed the association of RA diagnosis with total mortality. Secondary analyses investigated RA serologic subtypes and cause-specific mortality. Person-years of follow-up accrued from the return date of the baseline questionnaire to the end of follow-up, date of death, or date of censor. We defined the exposure as RA starting at date of diagnosis. Prior to RA diagnosis for RA cases, women contributed person-years to the non-RA reference group. Therefore, person-time was assigned based on the presence or absence of RA at each 2-year follow-up point.
We used the Cox proportional hazards regression model to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for the association between RA and mortality, with non-RA as the reference. Analyses were also performed for each RA serologic subtype, with non-RA as the reference. Multivariable models controlled for age and period (defined as each questionnaire cycle), as well as time-varying sociodemographic, lifestyle, and clinical confounders that were updated until death, end of study, or censoring. Covariates remained in the final model if their inclusion changed the age-adjusted HR by ≥5%. The final model included age, cycle, education, household income, cigarette smoking, BMI, physical activity, post-menopausal status and hormone use, alcohol intake, Alternate Healthy Eating Index quintile, aspirin use, and diagnosis of cardiovascular disease.
In addition to total mortality, we evaluated the associations of RA with cause-specific mortality (cancer, cardiovascular disease, and respiratory disease) in separate models using similar methods as those previously described. To check for violations of the proportional hazards assumption, we compared nested models with and without interaction terms of age by RA status using likelihood ratio tests. Age and follow-up time are collinear, since all women entered the NHS in 1976. The proportional hazards assumption was met in all analyses.
We plotted standardized mortality rates for women with and without RA, as well as seropositive and seronegative RA during follow-up in the NHS. We standardized mortality rates to the age-specific mortality rates of women using US Census data for each period of observations. Curves were created using cubic smoothing splines with 5 degrees of freedom. We chose this number of degrees of freedom to balance the smoothing and stability of mortality rate curves. Since we only included incident RA occurring after the NHS baseline, there were few deaths among women with RA in the early years of the NHS follow-up. After 16 years of follow-up in the NHS, there were sufficient outcomes among women with RA to report stable mortality rates.
We investigated survival after RA diagnosis using Kaplan-Meier curves. For each woman with incident RA, we chose up to 10 random controls without RA matched on age and calendar year at the index date of RA diagnosis. A total of 964 incident RA cases were matched to 9,499 controls for these analyses. We plotted curves for all RA, seropositive RA, and seronegative RA, and their matched non-RA controls and tested for differences using log rank tests.
Analyses were performed using SAS, version 9.3. For all analyses, 2-sided P values less than 0.05 were considered statistically significant.
RESULTS
Among 119,209 women, we identified 964 incident RA cases. The characteristics of the study population in 1976 (shown in Table 1) are presented according to whether a subject developed RA during follow-up. At the inception of the NHS, women who later developed RA had a mean ± SD age of 42.8 ± 6.8 years and a mean ± SD BMI of 23.7 ± 3.8 kg/m2. At baseline, women who developed RA consumed less alcohol (5.8 ± 9.4 gm/day) than women who did not develop RA (6.4 ± 10.6 gm/day). Women who were subsequently diagnosed with RA were heavier smokers at baseline (44.9% with >10 pack-years) than were women who did not (35.3% with >10 pack-years).
Table 1.
Baseline characteristics of participants in the Nurses’ Health Study in 1976 according to incident RA diagnosis occurring during follow-up*
| Characteristic | RA (n = 964) | No RA (n = 118,245) |
|---|---|---|
| Age, mean ± SD years | 42.8 ± 6.8 | 42.4 ± 7.2 |
| Census-tract median family income, mean ± SD thousands of dollars | 64.2 ± 24.3 | 64.4 ± 25.9 |
| Moderate to vigorous physical activity, mean ± SD hours per week† | 3.9 ± 2.9 | 3.8 ± 2.9 |
| Body mass index, mean ± SD kg/m2 | 23.7 ± 3.8 | 23.8 ± 4.1 |
| Alternate Healthy Eating Index, mean ± SD score (excluding alcohol)† | 42.8 ± 10.2 | 42.7 ± 10.2 |
| Alcohol intake, mean ± SD gm/day‡ | 5.8 ± 9.4 | 6.4 ± 10.6 |
| White race | 97.3 | 96.8 |
| US region of residence | ||
| West | 14.0 | 17.8 |
| Midwest | 20.0 | 18.3 |
| Mid-Atlantic | 46.2 | 47.2 |
| New England | 17.2 | 14.1 |
| Southeast | 2.6 | 2.7 |
| Education level | ||
| Registered nurse | 74.3 | 78.4 |
| Bachelor’s degree | 17.3 | 14.5 |
| Master’s degree and above | 8.4 | 7.0 |
| Pack-years of cigarette smoking | ||
| Never to ≤10 | 52.7 | 63.2 |
| >10–20 | 17.2 | 14.3 |
| >20 | 27.7 | 21.0 |
| Postmenopausal hormone use | ||
| Premenopausal | 80.6 | 80.1 |
| Postmenopausal, never hormone use | 7.2 | 9.2 |
| Postmenopausal, current hormone use | 8.0 | 6.5 |
| Postmenopausal, past hormone use | 3.8 | 3.5 |
| Cancer | 1.8 | 2.7 |
| Cardiovascular disease | 1.8 | 1.3 |
| Diabetes mellitus | 1.0 | 1.8 |
| Family history of cancer | 13.7 | 11.9 |
| Parental myocardial infarction <60 years | 19.0 | 18.7 |
| Family history of diabetes mellitus | 31.7 | 25.6 |
| Aspirin use† | 45.6 | 37.5 |
Values are percentages unless otherwise indicated. Missing values are not reported.
RA = rheumatoid arthritis.
Category was first assessed in 1980.
Category was first assessed in 1984.
There were 307 deaths during follow-up (31.8%) among women with RA and 28,501 deaths among women without RA (24.1%). Causes of death for women with and without RA during the NHS follow-up as determined by NHS study physicians are listed in Table 2. The most common cause of death was cancer (26.1% for women with RA and 40.6% for women without RA). There were 70 cardiovascular disease–related deaths (22.8%) among women with RA and 6,203 (21.8%) among women without RA. Respiratory disease–related causes accounted for 14.3% of deaths in women with RA, compared to 7.2% of deaths in women without RA.
Table 2.
Total deaths and specific causes of death according to incident RA diagnosis occurring during follow-up in the Nurses’ Health Study (1976–2012)*
| RA (n = 964) | No RA (n = 118,245) | |
|---|---|---|
| Total deaths, no. (%) | 307 (31.8) | 28,501 (24.1) |
| Cause of death, no. (% of total deaths) | ||
| Cancer | 80 (26.1) | 11,570 (40.6) |
| Cardiovascular disease† | 70 (22.8) | 6,203 (21.8) |
| Respiratory disease† | 44 (14.3)‡ | 2,050 (7.2) |
| RA | 27 (8.8)§ | 0 (0.0) |
| Dementia | 15 (4.9) | 1,998 (7.0) |
| Injury | 11 (3.6) | 1,120 (3.9) |
| Infection | 4 (1.3) | 405 (1.4) |
| Cirrhosis | 4 (1.3) | 278 (1.0) |
| Renal disease | 4 (1.3) | 346 (1.2) |
| Gastrointestinal disease | 6 (2.0) | 339 (1.2) |
| Diabetes mellitus | 2 (0.7) | 373 (1.3) |
| Parkinson’s disease | 1 (0.3) | 283 (1.0) |
| Other | 26 (8.5) | 2,430 (8.5) |
| Unable to classify by single diagnosis | 13 (4.2) | 1,106 (3.9) |
RA = rheumatoid arthritis.
For cardiovascular disease–related mortality, International Classification of Diseases, Eighth Revision (ICD-8) codes 390–458 were combined into a single category, which included coronary heart disease, stroke, aortic aneurysm, valvular heart disease, cardiomyopathy, hypertensive heart disease, pericarditis, peripheral vascular disease, and pulmonary embolism. For respiratory disease–related mortality, ICD-8 codes 490–519 were combined into a single category, which included chronic obstructive pulmonary disease (COPD), asthma, pleurisy, lung abscess, bronchiectasis, and pulmonary fibrosis. This did not include lung cancer, pneumonia, influenza, or pulmonary tuberculosis.
Of 44 respiratory deaths in women with RA, 25 were due to COPD, 9 were due to pneumonia, 6 were due to interstitial lung disease, 1 was due to asthma, and 3 were from other diseases of the respiratory system.
Of 27 deaths attributed to RA, 6 were due to RA-related factors, 6 were due to interstitial lung disease, 5 were due to sepsis, 2 were from pulmonary embolism, 1 was from myocardial infarction, and 7 were due to other causes.
SMRs during the NHS follow-up for women with and without RA in the NHS are shown in Figure 1. These rates were standardized to US Census mortality rates for women in the US during each time period. After 16 years of follow-up, women with RA, particularly seropositive RA, had higher mortality rates than women without RA. Women with seronegative RA had similar mortality rates as non-RA women throughout NHS follow-up.
Figure 1.
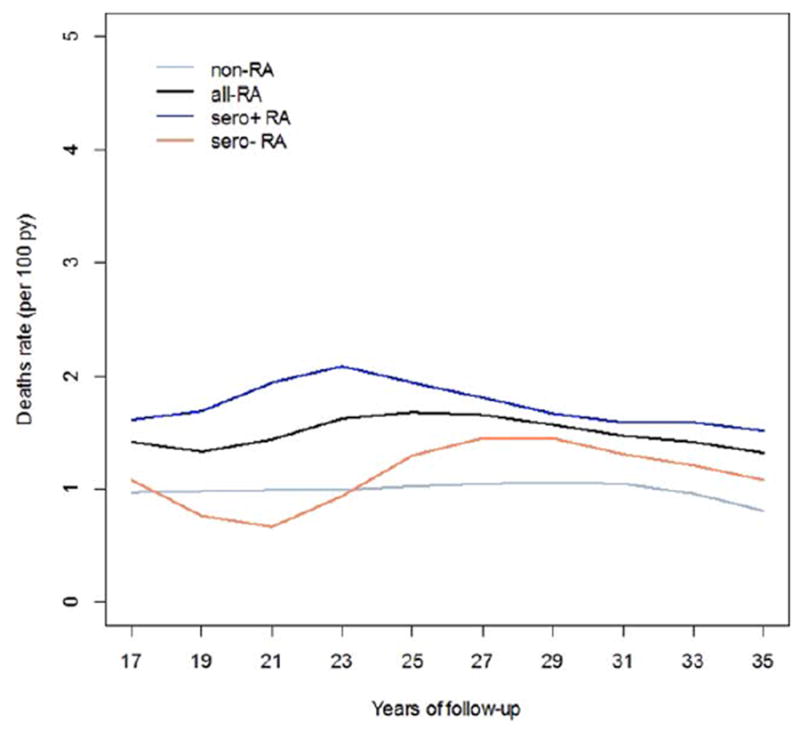
Standardized mortality rates during 36 years of follow-up in the Nurses’ Health Study (NHS) for women with all rheumatoid arthritis (RA; all-RA), seropositive RA (sero + RA), seronegative RA (sero– RA), and no RA (non-RA). Mortality rates were standardized to the age-specific US female population mortality rate by calendar year. There were too few deaths among women with RA diagnosed in the first 16 years of NHS follow-up to provide stable estimates of mortality rates during this period. py = person-years.
Compared to women without RA, women with RA had significantly increased total mortality, adjusting for age, period, income, smoking pack-years, BMI, menopausal status and hormone use, physical activity, Alternate Healthy Eating Index, alcohol consumption, cardiovascular disease, and aspirin use (HR 1.40, 95% CI 1.25–1.57) (Table 3). Results were similar when adjusted for time-varying ever/ never smoking status (HR 1.43, 95% CI 1.27–1.60) or never/past/current smoking status (HR 1.42, 95% CI 1.27–1.59) other than cumulative smoking pack-years. Women with seropositive RA had an HR for total mortality of 1.51 (95% CI 1.31–1.74), while women with seronegative RA had no significant association with total mortality (HR 1.15, 95% CI 0.95–1.39), both compared to non-RA women.
Table 3.
Hazard ratios for total mortality for women with RA by serologic phenotype compared to women without RA in the Nurses’ Health Study (1976–2012)*
| Deaths | Person-years | Mortality rate† | Age-adjusted HR (95% CI) | Multivariable HR (95% CI)‡ | |
|---|---|---|---|---|---|
| All RA | |||||
| No RA | 28,501 | 3,678,801 | 775 | 1.00 (reference) | 1.00 (reference) |
| RA | 307 | 17,983 | 1,707 | 1.45 (1.30–1.63) | 1.40 (1.25–1.57) |
| Seropositive RA | |||||
| No RA | 28,492 | 3,425,924 | 832 | 1.00 (reference) | 1.00 (reference) |
| RA | 202 | 10,869 | 1,858 | 1.59 (1.38–1.82) | 1.51 (1.31–1.74) |
| Seronegative RA | |||||
| No RA | 28,501 | 3,666,676 | 777 | 1.00 (reference) | 1.00 (reference) |
| RA | 105 | 7,113 | 1,476 | 1.18 (0.97–1.43) | 1.15 (0.95–1.39) |
RA = rheumatoid arthritis; HR = hazard ratio; 95% CI = 95% confidence interval.
Per 100,000 person-years.
Model adjusted for age, questionnaire cycle, census-tract family income (<$40,000 or ≥$40,000 per year), body mass index (<18.5, 18.5–24.9, 25–29.9, or ≥30 kg/m2), cigarette smoking (never–10, 10.1–20, or >20 pack-years), postmenopausal hormone use (premenopausal, postmenopausal: never postmenopausal hormone use, postmenopausal: past postmenopausal hormone use, or postmenopausal: current postmenopausal hormone use), moderate to vigorous physical activity (0, 0.01–0.99, 1.00–3.49, 3.50–5.99, or ≥6 hours per week), cumulative average of Alternate Healthy Eating Index excluding alcohol component (quintiles), alcohol consumption (0, 0.1–4.9, 5.0–14.9, or ≥15.0 gm/day), cardiovascular disease (yes/no), and aspirin use (yes/no). Covariates were updated up to death, end of study period, censor, or loss to follow-up, whichever came first.
Cause-specific mortality is shown in Table 4. The HR for respiratory disease–related mortality for women with RA was 2.06 (95% CI 1.51–2.80) compared to women without RA. Women with seropositive RA had a nearly 3-fold increased risk of respiratory disease–related death compared to those without RA (HR 2.67, 95% CI 1.89–3.77), which remained significant after adjusting for confounders, including smoking. There was no association of seronegative RA (HR 0.98, 95% CI 0.49–1.99) with respiratory disease–related mortality.
Table 4.
Hazard ratios for cause-specific mortality for women with incident RA by serologic phenotype compared to women without RA in the Nurses’ Health Study (1976–2012)*
| Cancer mortality
|
Cardiovascular mortality
|
Respiratory mortality
|
||||
|---|---|---|---|---|---|---|
| Age-adjusted HR (95% CI) | Multivariable HR (95% CI)† | Age-adjusted HR (95% CI) | Multivariable HR (95% CI)† | Age-adjusted HR (95% CI) | Multivariable HR (95% CI)† | |
| All RA | ||||||
| No RA | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| RA | 1.00 (0.80–1.25) | 0.93 (0.74–1.15) | 1.49 (1.18–1.89) | 1.45 (1.14–1.83) | 2.57 (1.91–3.48) | 2.06 (1.51–2.80) |
| Seropositive RA | ||||||
| No RA | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| RA | 0.95 (0.71–1.26) | 0.86 (0.65–1.15) | 1.39 (1.02–1.90) | 1.41 (1.03–1.93) | 3.55 (2.55–4.95) | 2.67 (1.89–3.77) |
| Seronegative RA | ||||||
| No RA | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| RA | 1.01 (0.72–1.42) | 0.96 (0.68–1.35) | 1.55 (1.08–2.21) | 1.41 (0.98–2.02) | 1.09 (0.54–2.19) | 0.98 (0.49–1.99) |
RA = rheumatoid arthritis; HR = hazard ratio; 95% CI = 95% confidence interval.
Model adjusted for age, questionnaire cycle, census-tract family income (<$40,000 or ≥$40,000 per year), body mass index (<18.5, 18.5–24.9, 25–29.9, or ≥30 kg/m2), cigarette smoking (never–10, 10.1–20, or >20 pack-years), postmenopausal hormone use (premenopausal, postmenopausal: never postmenopausal hormone use, postmenopausal: past postmenopausal hormone use, or postmenopausal: current postmenopausal hormone use), moderate to vigorous physical activity (0, 0.01–0.99, 1.00–3.49, 3.50–5.99, or ≥6 hours per week), cumulative average of Alternate Healthy Eating Index excluding alcohol component (quintiles), alcohol consumption (0, 0.1–4.9, 5.0–14.9, or ≥15.0 gm/day), cardiovascular disease (yes/ no), and aspirin use (yes/no). Covariates were updated up to death, end of study period, censor, or loss to follow-up, whichever came first.
Women diagnosed with RA had a significantly increased risk of cardiovascular disease–related mortality (HR 1.45, 95% CI 1.14–1.83) compared to women without RA. Seropositive RA (HR 1.41, 95% CI 1.03–1.93) significantly increased the risk of cardiovascular disease–related mortality, while seronegative RA trended toward statistical significance with a similar point estimate (HR 1.41, 95% CI 0.98–2.02) for cardiovascular disease–related mortality. RA was not associated with a risk of cancer mortality (HR 0.93, 95% CI 0.74–1.15).
Kaplan-Meier curves showed significantly worse survival after the index date of RA diagnosis for all women with RA (P <, 0.001) and for seropositive RA (P <, 0.001), but not for seronegative RA (P = 0.066), compared to age- and period-matched controls without RA (Figure 2).
Figure 2.
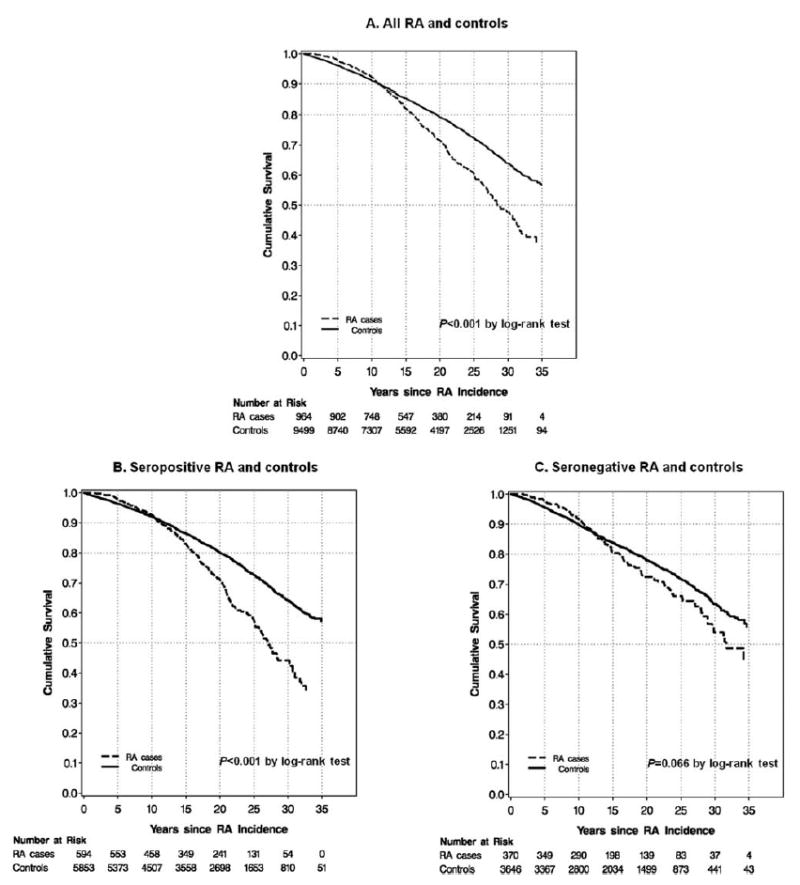
Kaplan-Meier curves for survival after incident rheumatoid arthritis (RA) diagnosis and age- and period-matched controls at index date of RA diagnosis for women in the Nurses’ Health Study comparing (A) all RA and controls (P < 0.001 by log rank test), (B) seropositive RA and controls (P < 0.001 by log rank test), and (C) seronegative RA and controls (P = 0.066 by log rank test).
DISCUSSION
For women diagnosed with RA during 36 years of prospective follow-up, there was a 40% increased total mortality risk compared to women without RA. We observed a nearly 3-fold elevated risk of respiratory disease–related mortality among women with seropositive RA. Cardiovascular disease–related mortality was increased for women with RA, but there was no association of RA with cancer mortality. Mortality risk was highest for women with seropositive RA, and there was no association between seronegative RA and mortality. Due to the relatively low incidence of RA and the long follow-up required for death outcomes, previous prospective cohorts have not been able to evaluate RA mortality longitudinally from incidence until death on a large scale while also including individuals without RA. This study extends findings of other RA cohorts to demonstrate the independent association of RA with mortality after adjustment for time-varying sociodemographic, lifestyle, and clinical confounders (36,37).
We found a nearly 3-fold increased risk of respiratory disease–related mortality for women with seropositive RA compared to women without RA, independent of smoking throughout follow-up. There was no association of seronegative RA and respiratory disease–related mortality. Similar findings of increased respiratory disease–related mortality for seropositive RA have been reported (38,39). In a prior study using an RA-only cohort, the SMR for RF-positive RA was 3.49 (38). Since smoking is associated with seropositive RA, excess smoking could have explained the increased respiratory disease–related mortality in that previous study, but smoking data for controls were unavailable for adjustment (14). Similarly, a 3-fold increased risk of respiratory disease–related deaths was described for men with RA, but smoking data were unavailable for controls, and cause of death was determined by the National Death Index (39). In this study, the association of seropositive RA with respiratory disease–related mortality persisted after adjustment for time-varying smoking. This suggests that the excess respiratory disease–related mortality may be due to other RA-related factors, such as interstitial lung disease (ILD). A previous study reported a 9-fold increased mortality rate for RA-related ILD, compared to RA patients without ILD (5). However, of 44 respiratory disease–related deaths in women with RA in this study, only 6 were identified as being due to ILD, while most (25 of 44) were due to chronic obstructive pulmonary disease (COPD). Some deaths due to ILD may have been misclassified as COPD if subjects were not diagnosed with ILD by their physician, if available medical records did not include advanced chest imaging or pathology, or if ILD did not appear on the death certificate. In this study, the cause of death could be classified more accurately because medical records were available, whereas previous studies used death certificates. This study indicates that respiratory disease–related death may be an important contributor to the excess mortality of RA. Since this finding was independent of cigarette smoking, inflammation may specifically worsen airway obstruction, leading to a worse disease course for patients with COPD and RA. Since we observed a specific association between seropositive RA and respiratory disease–related mortality, specific factors related to seropositivity may also worsen respiratory disease. In particular, immune tolerance loss and production of RA-specific autoantibodies may be initiated in the small airways (40). Therefore, local altered anatomy and inflammation of small airways involved in the pathogenesis of seropositive RA may portend worsened respiratory disease–related outcomes after diagnosis.
In this study, women with RA, particularly seropositive RA, had an excess risk of mortality compared to women without RA. Other studies reported increased mortality when comparing RA-only cohorts to general population estimates (10,12,17). Past studies consisting of early RA cohorts have reported modestly increased mortality rates for RA, perhaps due to shorter follow-up (9,41). The lack of data on clinical, behavioral, and lifestyle factors for comparison groups in these studies, limited follow-up duration, and design differences (prevalent versus incident RA cohorts) may account for varying mortality estimates in previous studies. A meta-analysis of 11 previous studies reported an SMR of 1.47 for RA, similar to the finding that we report (HR 1.40) (11). There is a suggestion that SMRs for women with and without RA were both decreasing in later years of follow-up (Figure 1).
Previous studies examined mortality prospectively by directly comparing individuals with and without RA in the same population (42-44). In the Iowa Women’s Health Study, women with validated incident RA had increased mortality compared to women without RA; however, covariates were only available for adjustment at baseline, and cause-specific mortality could not be investigated due to limited sample size (43). Among a subset of postmenopausal women in the Women’s Health Initiative who self-reported RA, those identified with RA had excess all-cause mortality compared to women with self-reported RA who were not confirmed to have RA (42). Among patients in the UK, RA cases matched to controls had a significantly increased risk of death compared to controls, adjusted for age and sex, but not for other confounders such as smoking (44). This study is unique in that it provides a long duration of prospective follow-up in a large population of women with and without RA, time-varying repeated measures of confounders, and detailed assessment of cause-specific mortality.
We found that seropositive RA had excess all-cause mortality and that there was no association between seronegative RA and mortality. The association of seropositive RA with excess mortality has been reported in other studies (9,38,42,43). A lack of association of seronegative RA with total mortality has also been reported (38,43,45).
This study found similar cardiovascular disease–related mortality estimates for seropositive and seronegative RA subtypes. Previous studies hypothesized that excess mortality burden in seropositive RA might be due to accelerated cardiovascular disease from chronic inflammation and autoimmunity (8,38,41,46). However, we found a 45% increased risk for cardiovascular disease mortality regardless of RA serologic subtype, which may imply that inflammation, even in the absence of autoantibodies, may be related to cardiovascular disease–related mortality risk. Despite cancer being the leading cause of death in women with RA, there was no association between RA and cancer mortality. Prior studies investigating RA and cancer mortality have had conflicting results (3,38). Women with RA in the NHS are more likely to receive cancer screening, such as mammograms, compared to women without RA (47). In addition to shorter follow-up, healthcare utilization factors may explain prior reports of only slightly increased mortality in early RA cohorts (9,41).
Given the sample size and length of follow-up of this study, we were able to identify many incident RA cases and deaths. The high follow-up rate in the NHS minimizes bias. Since all women in these analyses were free of RA at onset and were censored when RA or another CTD was reported but not confirmed, we were able to consistently compare women who developed RA to women free of RA or other CTDs. Repeated measures for confounders were available, including demographics, clinical factors, and lifestyle factors, including smoking. In addition, we were able to adjust for comorbid conditions, such as cardiovascular disease, occurring before or after RA diagnosis. Previous RA mortality studies had limited or unavailable data on mortality risk factors in the comparison group. The NHS provided detailed data on the timing and cause of mortality outcomes, determined systematically by death certificate and medical records review. Since a single underlying cause of death was determined for all deaths, cause-specific mortality analyses may not fully capture the complexity of concurrent disease processes that ultimately led to death. However, reviewers in the NHS classified deaths based on all available medical records and did not rely solely on the primary cause of death listed on death certificates. Therefore, these analyses have advantages over prior studies in which medical records were unavailable.
These findings may not be generalizable to other populations, since the NHS is composed mostly of US white women who were healthy and working in advanced nursing professions at the inception of the study, when they were ages 30–55 years. While detailed data were available for many covariates, data on RA disease activity and DMARDs were unavailable. Immunosuppressive medications may affect mortality in RA patients (48,49). Medical records concerning RA were reviewed only around the time of RA diagnosis, so we have limited information on RA sequelae, such as ILD and infections. All participants were in the same birth cohort and entered the NHS in 1976, limiting our ability to directly compare RA-associated mortality rates in different periods based on long-term trends, such as biologic DMARD availability. Data on biologic DMARD use were not available in this study. Health care utilization resulting in preventive services may have been different between women with and without RA. However, a prior study in the NHS showed more preventive care among RA cases, so health care utilization differences are unlikely to explain worsened mortality for women with RA (47). Since all women were nurses at enrollment, they may have more access to medical care during follow-up, so these findings may not be representative of health care for women in the general population.
In conclusion, this study demonstrates that RA is associated with increased all-cause mortality independent of traditional mortality risk factors. Seronegative RA was not associated with total mortality, but both seropositive and seronegative RA subtypes were associated with increased cardiovascular disease–related mortality. Respiratory disease–related mortality was 3-fold higher for seropositive RA independent of smoking. Clinicians should be aware of the increased mortality risk among RA patients, particularly respiratory disease–related mortality in seropositive RA. Further research is warranted to determine factors that may reduce the excess mortality in RA, particularly from cardiovascular and respiratory causes.
Significance & Innovations.
Rheumatoid arthritis (RA) has been associated with excess mortality in studies comparing mortality rates in RA cohorts to standardized general population estimates, but they were unable to adjust for traditional mortality risk factors.
We investigated RA and mortality among 119,209 women ages 30–55 years without RA or another connective tissue disease at baseline who were followed from 1976 until 2012 in the Nurses’ Health Study.
Women with RA had significantly increased total mortality (hazard ratio [HR] 1.40), respiratory disease–related mortality (HR 2.06), and cardiovascular disease–related mortality (HR 1.45) compared to women without RA, independent of traditional mortality risk factors, including smoking.
Clinicians should be aware that patients with RA are at increased risk for respiratory disease–related and cardiovascular disease–related mortality, particularly those with seropositive RA.
Acknowledgments
The authors thank the participants of the NHS for their dedicated participation in this longitudinal study, as well as the NHS staff members at the Channing Division of Network Medicine (Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School) for their crucial assistance. The authors also thank Murray Mittleman, MD, DrPH, Walter Willett, MD, DrPH, Meir Stampfer, MD, DrPH, and Frank Hu, MD, PhD, for critical advice on study design and analysis. The authors also thank the following state cancer registries for their help: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Mississippi, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming.
Supported by the NIH (grants CA-087969, CA-049449, CA-050385, and CA-067262), by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants AR-049880, AR-052403, AR-047782, AR-061362, and AR-059073), by the Harvard Catalyst ∣ Harvard Clinical and Translational Science Center, the National Center for Advancing Translational Sciences (grant 1UL1-TR-001102-01), and by Harvard University and its affiliated academic health care centers. Dr. Sparks’ work was supported by a Rheumatology Research Foundation Scientist Development Award and by the NIH Loan Repayment Award (grant L30-AR-066953).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, the Harvard Catalyst, or Harvard University and its affiliates.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Sparks had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sparks, Liao, Lu, Solomon, Costenbader, Karlson.
Acquisition of data. Sparks, Solomon, Costenbader, Karlson.
Analysis and interpretation of data. Sparks, Chang, Liao, Lu, Fine, Solomon, Costbenbader, Karlson.
References
- 1.Gabriel SE, Crowson CS, O’Fallon WM. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955–1985. Arthritis Rheum. 1999;42:415–20. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 3.Mellemkjaer L, Linet MS, Gridley G, Frisch M, Moller H, Olsen JH. Rheumatoid arthritis and cancer risk. Eur J Cancer. 1996;32A:1753–7. doi: 10.1016/0959-8049(96)00210-9. [DOI] [PubMed] [Google Scholar]
- 4.Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287–93. doi: 10.1002/art.10524. [DOI] [PubMed] [Google Scholar]
- 5.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62:1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez A, Maradit Kremers H, Crowson CS, Nicola PJ, Davis JM, III, Therneau TM, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum. 2007;56:3583–7. doi: 10.1002/art.22979. [DOI] [PubMed] [Google Scholar]
- 7.Gabriel SE, Crowson CS, O’Fallon WM. Mortality in rheumatoid arthritis: have we made an impact in 4 decades? J Rheumatol. 1999;26:2529–33. [PubMed] [Google Scholar]
- 8.Bergstrom U, Jacobsson LT, Turesson C. Cardiovascular morbidity and mortality remain similar in two cohorts of patients with long-standing rheumatoid arthritis seen in 1978 and 1995 in Malmo, Sweden. Rheumatology (Oxford) 2009;48:1600–5. doi: 10.1093/rheumatology/kep301. [DOI] [PubMed] [Google Scholar]
- 9.Humphreys JH, Warner A, Chipping J, Marshall T, Lunt M, Symmons DP, et al. Mortality trends in patients with early rheumatoid arthritis over 20 years: results from the Norfolk Arthritis Register. Arthritis Care Res (Hoboken) 2014;66:1296–301. doi: 10.1002/acr.22296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radovits BJ, Fransen J, Al Shamma S, Eijsbouts AM, van Riel PL, Laan RF. Excess mortality emerges after 10 years in an inception cohort of early rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62:362–70. doi: 10.1002/acr.20105. [DOI] [PubMed] [Google Scholar]
- 11.Dadoun S, Zeboulon-Ktorza N, Combescure C, Elhai M, Rozenberg S, Gossec L, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine. 2013;80:29–33. doi: 10.1016/j.jbspin.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–94. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 13.Qin B, Yang M, Fu H, Ma N, Wei T, Tang Q, et al. Body mass index and the risk of rheumatoid arthritis: a systematic review and dose-response meta-analysis. Arthritis Res Ther. 2015;17:86. doi: 10.1186/s13075-015-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69:70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med. 2012;55:163–70. doi: 10.1016/j.ypmed.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Bjornadal L, Baecklund E, Yin L, Granath F, Klareskog L, Ekbom A. Decreasing mortality in patients with rheumatoid arthritis: results from a large population based cohort in Sweden, 1964–95. J Rheumatol. 2002;29:906–12. [PubMed] [Google Scholar]
- 18.Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA. 2011;306:1884–90. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5:297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 20.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 21.Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369:2001–11. doi: 10.1056/NEJMoa1307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–9. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 23.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172:555–63. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119:503e1–9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 26.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses’ Health Study Arthritis Rheum. 2004;50:3458–67. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 27.Baer HJ, Glynn RJ, Hu FB, Hankinson SE, Willett WC, Colditz GA, et al. Risk factors for mortality in the Nurses’ Health Study: a competing risks analysis. Am J Epidemiol. 2011;173:319–29. doi: 10.1093/aje/kwq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparks JA, Chen CY, Hiraki LT, Malspeis S, Costenbader KH, Karlson EW. Contributions of familial rheumatoid arthritis or lupus and environmental factors to risk of rheumatoid arthritis in women: a prospective cohort study. Arthritis Care Res (Hoboken) 2014;66:1438–46. doi: 10.1002/acr.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu B, Solomon DH, Costenbader KH, Karlson EW. Alcohol consumption and risk of incident rheumatoid arthritis in women: a prospective study. Arthritis Rheum. 2014;66:1998–2005. doi: 10.1002/art.38634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu B, Hiraki LT, Sparks JA, Malspeis S, Chen CY, Awosogba JA, et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis. 2014;73:1914–22. doi: 10.1136/annrheumdis-2014-205459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64:650–8. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 34.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–71. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 35.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 36.Michaud K, Vera-Llonch M, Oster G. Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J Rheumatol. 2012;39:54–9. doi: 10.3899/jrheum.110491. [DOI] [PubMed] [Google Scholar]
- 37.Wolfe F, Michaud K. Effect of body mass index on mortality and clinical status in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:1471–9. doi: 10.1002/acr.21627. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez A, Icen M, Kremers HM, Crowson CS, Davis JM, III, Therneau TM, et al. Mortality trends in rheumatoid arthritis: the role of rheumatoid factor. J Rheumatol. 2008;35:1009–14. [PMC free article] [PubMed] [Google Scholar]
- 39.England BR, Sayles H, Michaud K, Caplan L, Davis LA, Cannon GW, et al. Cause-specific mortality in male US veterans with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:36–45. doi: 10.1002/acr.22642. [DOI] [PubMed] [Google Scholar]
- 40.Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis–related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64:1756–61. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young A, Koduri G, Batley M, Kulinskaya E, Gough A, Norton S, et al. Mortality in rheumatoid arthritis: increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford) 2007;46:350–7. doi: 10.1093/rheumatology/kel253. [DOI] [PubMed] [Google Scholar]
- 42.Kuller LH, Mackey RH, Walitt BT, Deane KD, Holers VM, Robinson WH, et al. Determinants of mortality among post-menopausal women in the Women’s Health Initiative who report rheumatoid arthritis. Arthritis Rheum. 2014;66:497–507. doi: 10.1002/art.38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikuls TR, Saag KG, Criswell LA, Merlino LA, Kaslow RA, Shelton BJ, et al. Mortality risk associated with rheumatoid arthritis in a prospective cohort of older women: results from the Iowa Women’s Health Study. Ann Rheum Dis. 2002;61:994–9. doi: 10.1136/ard.61.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogdie A, Haynes K, Troxel AB, Love TJ, Hennessy S, Choi H, et al. Risk of mortality in patients with psoriatic arthritis, rheumatoid arthritis and psoriasis: a longitudinal cohort study. Ann Rheum Dis. 2014;73:149–53. doi: 10.1136/annrheumdis-2012-202424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Schaardenburg D, Hazes JM, de Boer A, Zwinderman AH, Meijers KA, Breedveld FC. Outcome of rheumatoid arthritis in relation to age and rheumatoid factor at diagnosis. J Rheumatol. 1993;20:45–52. [PubMed] [Google Scholar]
- 46.Goodson N, Marks J, Lunt M, Symmons D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann Rheum Dis. 2005;64:1595–601. doi: 10.1136/ard.2004.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solomon DH, Karlson EW, Curhan GC. Cardiovascular care and cancer screening in female nurses with and without rheumatoid arthritis. Arthritis Rheum. 2004;51:429–32. doi: 10.1002/art.20418. [DOI] [PubMed] [Google Scholar]
- 48.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 49.Listing J, Kekow J, Manger B, Burmester GR, Pattloch D, Zink A, et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNF alpha inhibitors and rituximab. Ann Rheum Dis. 2015;74:415–21. doi: 10.1136/annrheumdis-2013-204021. [DOI] [PMC free article] [PubMed] [Google Scholar]


