Abstract
The activity of the deep cerebellar nuclei (DCN) neurons conveys the bulk of the output of the cerebellum. To generate these motor signals, DCN neurons integrate synaptic inputs with their own spontaneous activity. We have previously reported that N-type voltage-gated Ca2+ channels modulate the spontaneous activity of the majority of juvenile DCN neurons in vitro. Specifically, pharmacologically blocking N-type Ca2+ channels increases their firing rate causing DCN cells to burst. Adult DCN neurons however, behaved differently. To further investigate this change, we have studied here the effect of cadmium on the firing rate of DCN neurons in acute cerebellar slices obtained from adult ( > 2 months old) or juvenile (12–21 days old) rats and mice. Strikingly, and in contrast to juvenile DCN cells, cadmium did not affect the pacemaking of adult DCN cells. The activity of Purkinje cells (PCs) however was transformed into high-frequency bursting, regardless the age. Further, we questioned whether these findings could be due to an artifact associated with the added difficulty of preparing adult DCN slices. Hence we proceeded to examine the spontaneous activity of DCN neurons in anesthetized juvenile and adult rats and mice in vivo. When cadmium was injected into the DCN in vivo no significant change in firing rate was observed, conversely to most juvenile DCN neurons which showed high-frequency bursts after cadmium injection. In these same animals, PCs pacemaking showed no developmental difference. Thus our results demonstrate a remarkable age-dependent functional modification in the regulation of DCN neurons pacemaking.
Keywords: cerebellum, calcium channels, pacemaking, development
INTRODUCTION
One of the main functions of the cerebellum is to provide timing signals to precisely coordinate movement and to maintain the body posture and balance (Ito, 1984). The basic neuronal compartments of the cerebellum are the cerebellar cortex (that receives inputs from all over the Central Nervous System), and the deep cerebellar nuclei (DCN), which comprise its main output. DCN neurons integrate inhibitory signals from Purkinje cells (PCs) located in the cerebellar cortex, and excitatory inputs from mossy and climbing fibers’ collaterals arriving from other sites in the brain (Ito, 1984), in order to generate final motor signals.
DCN neurons are spontaneously active in absence of synaptic inputs (Jahnsen, 1986; Llinas and Muhlethaler, 1988; Raman et al., 2000). This indicates that DCN neurons possess the conductances required to sustain the intrinsic firing of action potentials, even in conditions of extensive inhibition from their afferent PCs (Ito et al., 1970; Sastry et al., 1997; Telgkamp and Raman, 2002). We have previously shown that voltage-gated Ca2+ channels (VGCCs) are in fact one of the main conductances contributing to the regulation of the intrinsic activity in the majority of juvenile rat DCN neurons (Alvina and Khodakhah, 2008). Moreover, with the use of specific toxins we found that only N-type VGCCs are responsible for sustaining normal firing in DCN neurons. In addition, N-type VGCCs were found to be coupled to small-conductance Ca2+-activated K+ channels (SK channels), which after being blocked with apamin also cause avid bursting in DCN neurons (Aizenman and Linden, 1999; Alvina and Khodakhah, 2008).
Interestingly however, DCN neurons in more mature developmental stages ( > 2 months-old), did not respond to the blockade of VGCCs as the juvenile cells did, suggesting a possible developmental change in the expression and/or function of VGCCs in DCN neurons. Indeed, it has been shown that in cerebellar PCs the expression of VGCCs during the first three postnatal weeks changes significantly (Gruol et al., 1992), suggesting that the developmental expression of Ca2+ conductances plays a prominent role in the physiological maturation of these cells. Namely, high-threshold Ca2+ conductance is expressed early in development (contributing to repetitive simple spike firing of both the young and old neurons) while low-threshold Ca2+ conductance appears later in development, coincident with dendritic expression, and plays a major role in the complex spike generation (Gruol et al., 1992). Consequently, to further investigate functional differences in the regulation of cerebellar cells’ pacemaking here we recorded the activity of DCN neurons and PCs in vivo and in acute slices, from adult rats and mice, at different developmental stages.
EXPERIMENTAL PROCEDURES
Preparation of acute cerebellar slices
All procedures employed were in accordance with the policies established by the Animal Institute Committee of the Albert Einstein College of Medicine. P12–21 or > 2 months-old Wistar rats (Charles River Laboratories), and C57Bl/6 mice (The Jackson Laboratory) were anesthetized with halothane and decapitated. The brain was quickly removed and placed in cold recording solution containing (in mM): 125NaCl, 2.5KCl, 26NaHCO3, 1.25NaH2PO4, 1MgCl2, 2CaCl2, and 10glucose. The cerebellum was mounted on a modified Oxford vibratome and 300-μm-thick sagittal slices were obtained. Before use, the slices were kept on oxygenated recording solution at 34 °C for one hour, and then at room temperature.
Extracellular recording in cerebellar slices
Slices were placed in a recording chamber on a Zeiss Axioskop microscope stage. DCN neurons were visually identified using a 40× water-immersion objective with infrared optics. Slices were superfused with recording solution (1.5 ml/min flow rate) and temperature adjusted to ~35 °C. To isolate the intrinsic activity of the cell fast synaptic blockers were used: 100 μM picrotoxin (GABAA receptor antagonist), and 5 mM kynurenic acid (broad-spectrum ionotropic glutamate receptor antagonist).
Extracellular recordings were obtained from single DCN neurons using a home-made differential amplifier and glass pipette electrodes filled with recording solution. Data were sampled at 10 kHz using an analog-to-digital converter (PCI-MIO-16XE-10; National Instruments, Austin, TX, USA), and acquired and analyzed using custom software written in LabView (National Instruments). Picrotoxin, apamin and cadmium chloride were obtained from Sigma Aldrich (St. Louis, MO, USA). Kynurenic acid was obtained from Spectrum Chemical MFG Corp., Gardena, CA, USA.
Extracellular recordings in vivo
In vivo recordings were obtained from both male and female juvenile (P13–15) and adult ( > 2 months-old) Wistar rats, and adult ( > 2 months-old) C57Bl/6 mice. Subjects were placed on a stereotaxic apparatus under isofluorane anesthesia, and a 1.5-mm-diameter recording chamber was drilled in the skull using the following coordinates, for adult rats: Bregma −11.1 to −11.7 mm and 1.5–2.5 mm lateral, taken from the L.W. Swanson Brain Maps Atlas (Elsevier, 1992). For adult mice: Bregma −5.9 to −6.2 mm and 2 mm lateral, according to K.B.J Franklin and G. Paxinos Mouse Brain Atlas (Elsevier, 2008). For juvenile rats we adjusted the coordinates obtained from the same Mouse Brain Atlas used for adult mice. In pilot experiments we confirmed the position of the electrode by injecting saline solution containing methylene blue and subsequently dissecting the cerebellum.
For the experiments performed in juvenile and adult rats, single DCN cells and PCs were recorded using platinum quartz electrodes (Thomas Recording system). A minimum of 5 min of baseline activity was recorded before the application of a blocker. To block VGCCs in vivo we used a ~120-μm-diameter quartz-glass micropipette filled with 10 mM cadmium chloride (dissolved in (mM): 125NaCl, 2.5KCl, 1MgCl2, 10HEPES and 0.25% Methylene blue), positioned 300 μm next to the recording electrode and connected to a picospritzer apparatus. Pulse pressure and duration were adjusted to deliver ~1 μl per pulse. In pilot experiments we observed that 10 pulses (one pulse every 30 s, injecting ~10 μl in total) diffused into a ~1-mm-diameter sphere. Signals were band-pass filtered (80 Hz to 10 kHz), amplified and fed into a Plexon Neuro-technology A/D system (40-kHz sampling per channel).
For adult mice recordings a three-barrel pipette (Carbostar-3, Kation Scientific) was used for both single-unit recording and delivery of blockers. One of the barrels contained a carbon fiber, while the other two barrels were filled with solutions containing either 100 μM apamin or 10 mM cadmium (both prepared as mentioned above). The drug-filled barrels were then connected to a picospritzer set to deliver single 100-ms long pulses. Pressure was calibrated while recording from PCs, i.e. changes in PCs activity were observed immediately following a single pulse and lasted several seconds. For each cell, baseline activity was recorded for 5 min and then single puffs of cadmium chloride were delivered every 3 s during 5 min. The cell was allowed to recover to baseline (~5 min), after which the response to apamin was recorded. As a control, the same experiment was performed on a PC at the beginning and end of each DCN experiment. Signals were amplified using a homemade amplifier and band-pass filtered (80 Hz to 10 kHz).
Spike waveforms were sorted offline using commercial software (Plexon Offline Sorter). Recordings were selected for analysis based on the electrode position and whether the signals could be clearly distinguished as individual cells. After experiments concluded the cerebellum was dissected and electrode location was confirmed.
All data are presented as mean ± S.E.M. Statistical analysis was performed by a one-way ANOVA. Statistical significance was accepted if p < 0.05.
RESULTS
Adult DCN neurons are insensitive to blockade of VGCCs in vitro
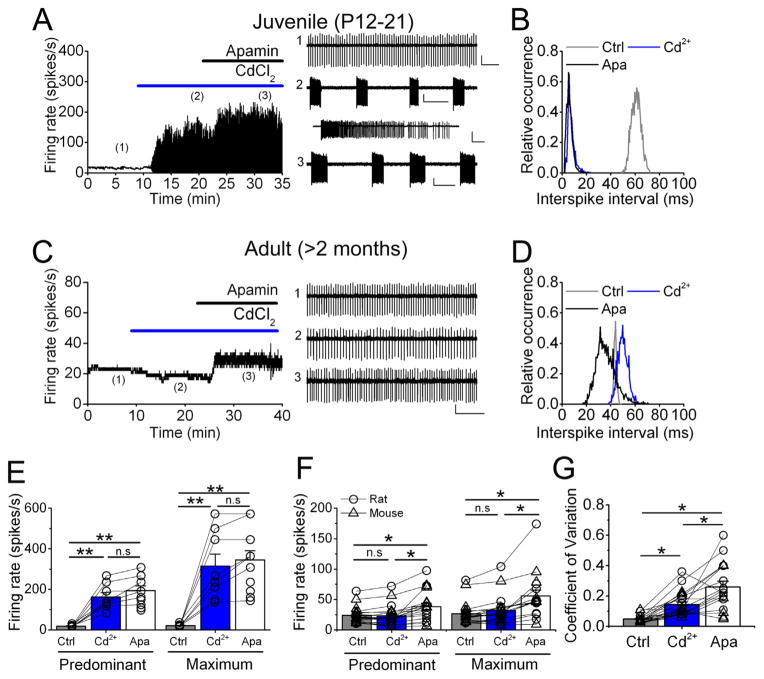
N-type Ca2+ channels control the pacemaking in the majority of juvenile DCN neurons by coupling to SK channels (Alvina and Khodakhah, 2008). We first corroborated these findings by performing extracellular recordings from rat juvenile DCN cells (P12–P21) in the presence of synaptic blockers. Fig. 1A shows an example juvenile DCN cell firing quite regularly at an average of 15.6 spikes/s (trace#1), in baseline conditions. After bath-applying 100 μM cadmium chloride the tonic activity changed to high-frequency bursting (trace#2), reaching 129.3 spikes/s. Addition of 100 nM apamin (SK channels specific blocker) did not change the avid bursting (trace#3), the average firing rate in this condition was 148.5 spikes/s. On average (Fig. 1E), the firing rate in control was 18.1 ± 1.7 spikes/s (n = 9 cells), in cadmium 162.3 ± 22.2 (p < 0.001 vs. control) and after apamin 194.2 ± 22.9 spikes/s (p < 0.001 vs. control, p > 0.5 vs. cadmium). The interspike interval (ISI) distribution (Fig. 1B) illustrates that cadmium and apamin had comparable effects, confirming our previous report.
Fig. 1.
Blockade of VGCCs does not change the spontaneous activity of adult DCN neurons in vitro. (A) Bath-application of cadmium and subsequent apamin on a juvenile DCN cell. Raw traces are displayed on the right. (B) Interspike interval (ISI) histogram of cell in A. (C) Similar treatment with cadmium followed by apamin to an adult DCN cell. Examples of raw traces displayed on the right. (D) ISI histogram of cell shown in C. (E) Average predominant and maximum firing rates for juvenile rat DCN cells recorded in vitro. Each open circle shows a single cell. (**) represents p < 0.01. (F) Combined data from adult rat and mice slices showing average predominant and maximum firing rate in each condition. Open circles depict rat cells and open triangles mouse cells. (*) p < 0.05, (n.s.) not significant. (G) Average coefficient of variation from same cells in E. Calibration bar in A, trace #1, 300 μV, 0.5 s; traces #2 and 3, 250 μV, 2 s (inset in #2, 500 μV, 0.1 s). Calibration bar in C 200 μV, 0.5 s. (*) represents p < 0.001.
We then performed same experiments in adult DCN cells from cerebellar slices from > 2 months-old rats and mice. Surprisingly, we found that cadmium did not cause any significant change in any cell tested (Fig. 1C, D, example of a rat cell). Fig. 1C shows the activity of an adult rat DCN neuron. The very regular baseline activity is comparable to juvenile cells (trace#1). After cadmium there was no significant change in the firing rate (trace#2). Moreover, apamin added after cadmium only increased the firing rate, without causing high-frequency bursts (trace#3). Fig. 1D shows the ISI in these three conditions, which was only slightly affected, particularly with apamin. We repeated similar experiments in slices obtained from adult mice and no significant differences were observed therefore the data were pooled in Fig. 1E, F (see Table 1 for a detailed quantification per species). On average, baseline firing reached a predominant firing rate of 23.9 ± 3.1 and a maximum firing rate of 26.7 ± 3.7 spikes/s (n = 23 cells in total, mouse cells n = 13, rat cells n = 10, Fig. 1F). After cadmium the predominant firing rate was 23.4 ± 3.7 spikes/s and the maximum firing rate was 32.2 ± 5.4 spikes/s (both p > 0.7 vs. control). The coefficient of variation (CV) of the ISI was slightly but significantly increased upon VGCC blockade (Fig. 1G, CV in control = 0.049 ± 0.005 and after cadmium = 0.14 ± 0.015, p < 0.01). Apamin caused a significant increase in firing rate, predominant firing rate was 38.3 ± 5.8 spikes/s (p < 0.05 vs. control and vs. cadmium), and maximum firing rate 55.8 ± 9.7 spikes/s (p < 0.05 vs. control and cadmium). Apamin further increased the CV to 0.26 ± 0.04 (p < 0.001 vs. control and cadmium).
Table 1.
In vitro DCN cell firing rate (in spikes per second) per species and experimental condition. No significant differences were observed when comparing mouse vs rat data (p > 0.5)
| Mouse (n = 13 cells) | Rat (n = 10 cells) | ||
|---|---|---|---|
| Control | Predominant | 21.9 ± 3.8 | 24.3 ± 3.8 |
| Maximum | 25.3 ± 5.4 | 27.4 ± 4.8 | |
| CV | 0.057 ± 0.007 | 0.04 ± 0.009 | |
| Cd2+ | Predominant | 23.6 ± 5.4 | 23.2 ± 5.4 |
| Maximum | 33.7 ± 7.9 | 30.3 ± 7.3 | |
| CV | 0.153 ± 0.027 | 0.132 ± 0.021 | |
| Apamin | Predominant | 36.9 ± 7.8 | 44.7 ± 10.2 |
| Maximum | 57.4 ± 14.1 | 52.9 ± 12.5 | |
| CV | 0.293 ± 0.045 | 0.198 ± 0.067 |
Blockade of VGCCs in vivo does not alter the spontaneous activity of adult DCN neurons
The results shown in the prior section suggest an age-dependent functional change in Ca2+ conductances contributing to the intrinsic activity in DCN neurons. However, we had previously reported the existence of a minor population of juvenile DCN cells that did not burst after cadmium (Alvina and Khodakhah, 2008). Therefore, considering the difficulties in obtaining cerebellar slices from adult animals, we questioned whether the differences observed could be due to tissue damage and consequent death of a specific population of DCN cells, leaving intact only the ones insensitive to cadmium. To address this issue we recorded in vivo the activity of DCN neurons in anesthetized juvenile rats and adult rats and mice while blocking Ca2+ channels (see schematic in Fig. 2A and methods).
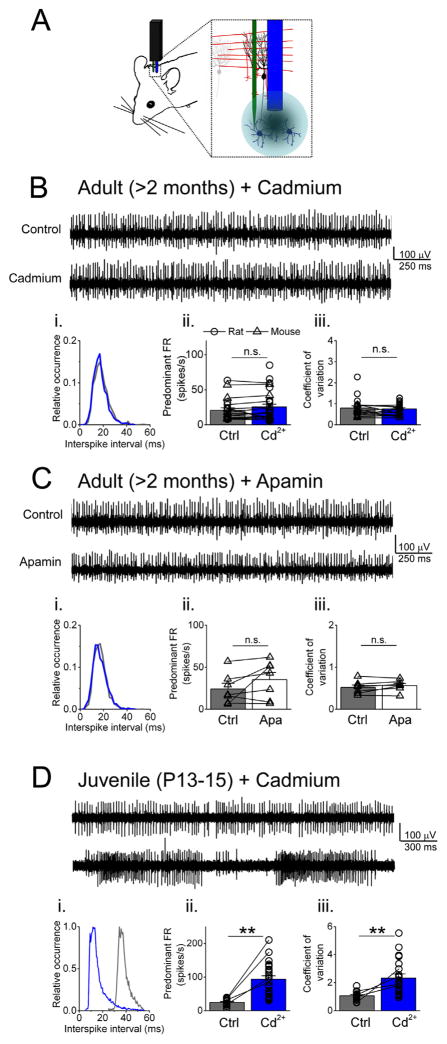
Fig. 2.
Spontaneous activity of adult DCN neurons is not altered by VGCC blockade in vivo. (A) Schematic of in vivo recording conditions. Cadmium or apamin were pressure-injected close to the recorded cell via a cannula or a barrel pipette (shown in blue), attached to the recording electrode (green). (B) Example of an adult DCN cell in control and after cadmium and its ISI histogram (i). Average (combining rat and mouse cells, as depicted) predominant firing rate (FR) and coefficient of variation are shown in ii and iii respectively. (C) and (D) Similar to B but examples of an adult mouse DCN cell before and after apamin, and juvenile rat DCN cell before and after cadmium. (**) represents p < 0.001 and (n.s.) not significant.
In control conditions (Fig. 2B, upper trace, mouse cell in the example) the majority of adult DCN cells recorded showed tonic activity (n = 20 cells in total obtained from N = 3 > 2 months old adult rats, and N = 4 adult mice, > 2 months old). After recording baseline activity for several minutes we applied cadmium chloride. Upon blockade of VGCCs (Fig. 2B, lower trace), no significant changes were observed (histogram in Fig. 2Bi, control in gray and cadmium in blue). On average DCN cells fired at 20.7 ± 3.5 spikes/s (n = 13 rat cells and n = 7 mouse cells combined, Fig. 2Bii) in control and at 25.6 ± 5.3 spikes/s after cadmium chloride (n = 19 rat cells and n = 7 mouse cells, p = 0.29 vs. control). The CV was also not affected by blockade of VGCCs (Fig. 2Biii), CV in control was 0.80 ± 0.10 and after cadmium 0.76 ± 0.06 (p = 0.65 vs. control, same cells as in Fig. 2Bii). This discrepancy with our data obtained in slices might reflect the fact that cadmium chloride is likely blocking synaptic transmission within the DCN (mediated by P/Q- and N-type Ca2+ channels) (Takahashi and Momiyama, 1993). A reduction in synaptic inputs will also reduce the irregularity of the ISI (Hausser and Clark, 1997).
After the cells recovered from cadmium we tested the SK channel blocker apamin (Fig. 2C) in adult mice. Similarly to acute cerebellar slices, blockade of SK channels did increase the spontaneous activity although not to significant levels (n = 7 cells, control = 24.2 ± 6.8 spikes/s, in apamin = 35.3 ± 8.4 spikes/s, p = 0.35, Fig. 2Cii). The CV however did not change (control = 0.52 ± 0.06 vs apamin = 0.57 ± 0.05, p = 0.57, Fig. 2Ciii). This inconsistency with juvenile DCN cells might be explained by developmental changes in SK channels expression, which might occur simultaneously with changes observed in Ca2+ channel expression (Gruol et al., 1992).
The spontaneous activity of juvenile rat DCN neurons was also tested in vivo upon blockade of VGCCs with cadmium. As expected, the firing pattern did change from tonic to high-frequency bursting (Fig. 2D). In control conditions most juvenile DCN neurons fired spontaneously in a tonic fashion, similar to adult individuals (Fig. 2D, upper trace). Rapidly after cadmium, this tonic activity switched to avid bursting (Fig. 2D, lower trace). As shown in Fig. 2Di the distribution of the ISI shifted to the left (blue trace for cadmium, gray trace for control conditions). Moreover, the long tail of the histogram toward longer intervals reflects the presence of pauses between bursts (blue trace, Fig. 2Di). On average the firing rate in control was 24.6 ± 2.4 spikes/s (n = 18 cells obtained from N = 5 juvenile rats), after cadmium this tonic activity changed to bursting, reaching 93.7 ± 9.9 spikes/s (Fig. 2Dii, n = 26 cells, p < 0.001 vs. control). As expected from the bursting, the CV increased from 1.07 ± 0.07 in control to 2.32 ± 0.32 after cadmium (Fig. 2Diii, p < 0.001).
VGCCs blockade causes bursting in both juvenile and adult PCs
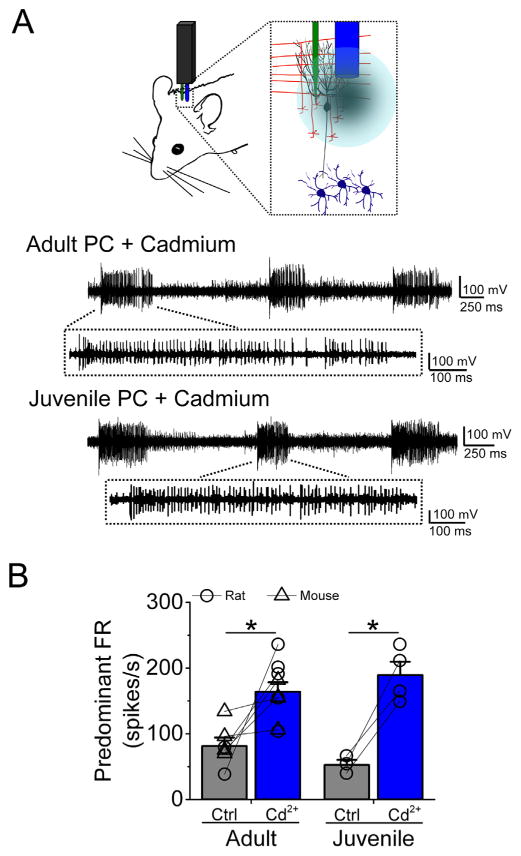
We have previously shown that P/Q-type Ca2+ channels are fundamental to sustain the spontaneous activity in cerebellar PCs in vitro (Womack et al., 2004). Therefore, as a control for our in vivo recordings in DCN neurons, we also recorded from PCs in the same animals (see schematic on Fig. 3A) and injected cadmium chloride to block VGCCs. As shown in Fig. 3A, in both adult rat and mouse (upper trace, this particular example corresponds to a rat PC), and juvenile rat (lower trace) PC, cadmium caused high-frequency bursting. In adult PCs the firing rate in control was 81.2 ± 12.9 spikes/s and after cadmium it reached 164.1 ± 14.4 spikes/s (Fig. 3B, n = 9 cells total, five cells from adult rat and four from adult mice, p < 0.005 vs control). Similarly, the firing rate of juvenile rat PCs increased from an average of 52.5 ± 7.7 spikes/s in control to 189.55 ± 20.2 spikes/s after cadmium (Fig. 3B, n = 4 cells, p < 0.005 vs control).
Fig. 3.
Both juvenile and adult Purkinje cells are sensitive to VGCCs blockade in vivo. (A) Top, schematic of the recording conditions. Bottom, raw traces of adult and juvenile Purkinje cells (PC) showing avid bursting in the presence of cadmium. (B) Average of predominant firing rate (FR) of both juvenile (rat only) and adult (rat and mice) PCs in control and after cadmium. (*) represents p < 0.005.
In conclusion, our results here provide evidence of a remarkable functional change in the contribution of VGCCs to the intrinsic firing of DCN neurons.
DISCUSSION
Particular types of VGCCs are essential to sustain the normal intrinsic activity of the two principal neuronal groups in the cerebellum, namely P/Q-type Ca2+ channels in PCs, and N-type Ca2+ channels for DCN neurons (Raman and Bean, 1999; Raman et al., 2000; Womack and Khodakhah, 2004; Alvina and Khodakhah, 2008). However we have observed a major developmental change in DCN neurons. Blockade of VGCCs did not change the spontaneous activity of any DCN cell recorded from rats or mice older than 8 weeks, in marked contrast to PCs whose activity was sensitive to VGCCs blockade at all developmental stages examined.
Expression of VGCCs in DCN neurons
Several studies have examined the expression of poreforming α-subunits of VGCCs in the cerebellum (Tanaka et al., 1995; Ludwig et al., 1997; Barclay et al., 2001; Schlick et al., 2010). Unfortunately, a detailed quantification was performed primarily in the cerebellar cortex (i.e. PCs) and limited information was provided regarding DCN neurons. In situ hybridization of the α1-subunit mRNA showed that the gene expression of most VGCC subunits is elevated in adult DCN cells (~7 weeks old), being P/Q- and N-type the most abundant (Tanaka et al., 1995). Confirming previous findings (Tanaka et al., 1995; Ludwig et al., 1997; Schlick et al., 2010) reported that the α1A-subunit (P/Q-type channel) is the dominant pore-forming subunit in the adult cerebellum, followed by 1B and 1C (N- and L-type). However, this study analyzed whole cerebellum samples thus being inconclusive about DCN cells in specific. Nevertheless DCN neurons do express N-type VGCCs in adult stages therefore the lack of effect of blocking these channels must result from a different mechanism (see below).
Developmental changes in VGCCs and SK channels
Functional VGCCs are formed by one α-subunit (pore), and two auxiliary subunits: β and α2δ (Catterall, 2000). These auxiliary subunits regulate the activity of the channel and can dramatically alter its pharmacological properties (Obermair et al., 2008). In contrast to other brain areas, it remains unknown whether a postnatal change in expression of these subunits happens in the cerebellum. For instance, Schlick et al. (2010) reported a sharp decrease in the expression of subunits β1 and β3 and a concomitant increase in β2 and β4 subunits from embryonic stage to 8-week-old in the hippocampus and neocortex. It is tempting to speculate that comparable changes could occur in the cerebellum since β4 is the most abundant β-subunit found in DCN neurons (Tanaka et al., 1995; Schlick et al., 2010). If this were the case, the α1B subunit (N-type channel) could form a complex with different β-subunits throughout development thus the sensitivity to cadmium would change. β-subunits regulate diverse channel properties (Dolphin, 2003), therefore, it is possible that a switch in auxiliary subunit could explain differences in pharmacological sensitivity (Moreno et al., 1997). The β4-subunit seems to be important for cerebellar function since mice lacking this subunit show the lethargic phenotype, which involves ataxia and epileptic seizures (McEnery et al., 1998; Burgess et al., 1999). Strikingly in this lethargic mouse the mutation results not only in the absence of β4-subunit but also in the over expression of β1-subunit which is abundant during early developmental stages, thus the lethargic mouse have “immature” N-type channels in adult stages (McEnery et al., 1998).
Pathophysiological considerations
Our results here show that in contrast to the insensitivity to VGCCs blockade observed in adult DCN neurons, adult PCs’ spontaneous activity is dramatically affected by cadmium. This is important in light of P/Q-type Ca2+ channel mutations that affect the pacemaking of PCs (Hoebeek et al., 2005; Walter et al., 2006). Pacemaking in P/Q-type mutant PCs is highly irregular. Such erratic activity in P/Q-type mutant PCs will carry poorly encoded signals to their target DCN cells, therefore contributing to generate erroneous cerebellar motor commands. The fact that blocking VGCCs in adult DCN neurons does not affect their pacemaking suggests that exclusive PC malfunction (i.e. irregular pacemaking derived, for example, from said P/Q-type channel mutations) is sufficient to generate abnormal cerebellar output that ultimately results in motor deficits.
We have also shown here that SK channels do not play a major role in shaping adult DCN neurons intrinsic activity whereas in PCs these channels change the pacemaking from tonic to bursting across developmental ages. Even though modeling studies showed that SK channels in particular could regulate synaptically evoked changes in DCN neurons activity (Feng et al., 2013), such measurements took into account intrinsic conductances found in young (P15–20) DCN cells, thus suggesting that the mechanisms involved in such computations might change over time.
Acknowledgments
This work was funded by NIH – United States grant 6R01NS050808 (K.K.).
Abbreviations
- CV
coefficient of variation
- DCN
deep cerebellar neurons
- ISI
interspike interval
- PCs
Purkinje cells
- VGCCs
voltage-gated calcium channels
References
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- Alvina K, Khodakhah K. Selective regulation of spontaneous activity of neurons of the deep cerebellar nuclei by N-type calcium channels in juvenile rats. J Physiol. 2008;586:2523–2538. doi: 10.1113/jphysiol.2007.148197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, Perez-Reyes E, Lander ES, Frankel WN, Gardiner RM, Dolphin AC, Rees M. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J Neurosci. 2001;21:6095–6104. doi: 10.1523/JNEUROSCI.21-16-06095.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess DL, Biddlecome GH, McDonough SI, Diaz ME, Zilinski CA, Bean BP, Campbell KP, Noebels JL. Beta subunit reshuffling modifies N- and P/Q-type Ca2+ channel subunit compositions in lethargic mouse brain. Mol Cell Neurosci. 1999;13:293–311. doi: 10.1006/mcne.1999.0748. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Beta subunits of voltage-gated calcium channels. J Bioenerg Biomembr. 2003;35:599–620. doi: 10.1023/b:jobb.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- Feng SS, Lin R, Gauck V, Jaeger D. Gain control of synaptic response function in cerebellar nuclear neurons by a calcium-activated potassium conductance. Cerebellum. 2013;12:692–706. doi: 10.1007/s12311-013-0476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruol DL, Deal CR, Yool AJ. Developmental changes in calcium conductances contribute to the physiological maturation of cerebellar Purkinje neurons in culture. J Neurosci. 1992;12:2838–2848. doi: 10.1523/JNEUROSCI.12-07-02838.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Hoebeek FE, Stahl JS, van Alphen AM, Schonewille M, Luo C, Rutteman M, van den Maagdenberg AM, Molenaar PC, Goossens HH, Frens MA, De Zeeuw CI. Increased noise level of purkinje cell activities minimizes impact of their modulation during sensorimotor control. Neuron. 2005;45:953–965. doi: 10.1016/j.neuron.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Ito M. The cerebellum and neural control. New York: Raven Press; 1984. [Google Scholar]
- Ito M, Yoshida M, Obata K, Kawai N, Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10:64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Jahnsen H. Electrophysiological characteristics of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986;372:129–147. doi: 10.1113/jphysiol.1986.sp016001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Muhlethaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem-cerebellar preparation. J Physiol. 1988;404:241–258. doi: 10.1113/jphysiol.1988.sp017288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Flockerzi V, Hofmann F. Regional expression and cellular localization of the alpha1 and beta subunit of high voltage-activated calcium channels in rat brain. J Neurosci. 1997;17:1339–1349. doi: 10.1523/JNEUROSCI.17-04-01339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEnery MW, Copeland TD, Vance CL. Altered expression and assembly of N-type calcium channel alpha1B and beta subunits in epileptic lethargic (lh/lh) mouse. J Biol Chem. 1998;273:21435–21438. doi: 10.1074/jbc.273.34.21435. [DOI] [PubMed] [Google Scholar]
- Moreno H, Rudy B, Llinas R. Beta subunits influence the biophysical and pharmacological differences between P- and Q-type calcium currents expressed in a mammalian cell line. Proc Natl Acad Sci USA. 1997;94:14042–14047. doi: 10.1073/pnas.94.25.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermair GJ, Tuluc P, Flucher BE. Auxiliary Ca(2+) channel subunits: lessons learned from muscle. Curr Opin Pharmacol. 2008;8:311–318. doi: 10.1016/j.coph.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20:9004–9016. doi: 10.1523/JNEUROSCI.20-24-09004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry BR, Morishita W, Yip S, Shew T. GABA-ergic transmission in deep cerebellar nuclei. Prog Neurobiol. 1997;53:259–271. doi: 10.1016/s0301-0082(97)00033-6. [DOI] [PubMed] [Google Scholar]
- Schlick B, Flucher BE, Obermair GJ. Voltage-activated calcium channel expression profiles in mouse brain and cultured hippocampal neurons. Neuroscience. 2010;167:786–798. doi: 10.1016/j.neuroscience.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Tanaka O, Sakagami H, Kondo H. Localization of mRNAs of voltage-dependent Ca(2+)-channels: four subtypes of alpha 1- and beta-subunits in developing and mature rat brain. Brain Res Mol Brain Res. 1995;30:1–16. doi: 10.1016/0169-328x(94)00265-g. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Raman IM. Depression of inhibitory synaptic transmission between Purkinje cells and neurons of the cerebellar nuclei. J Neurosci. 2002;22:8447–8457. doi: 10.1523/JNEUROSCI.22-19-08447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci. 2004;24:3511–3521. doi: 10.1523/JNEUROSCI.0290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24:8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]