Abstract
The repeats-in-toxin (RTX) family of toxins includes proteins produced by Gram negative bacteria such as Escherichia coli (α-hemolysin), Bordetella pertussis (adenylate cyclase toxin), and Aggregatibacter actinomycetemcomitans (LtxA), which contribute to the pathogenesis of these organisms by killing host cells. In the case of LtxA produced by A. actinomycetemcomitans, white blood cells are targeted, allowing the bacteria to avoid clearance by the host immune system. In its association with target cells, LtxA binds to a receptor, lymphocyte function-associated antigen-1 (LFA-1), as well as membrane lipids and cholesterol, before being internalized via a lysosomal-mediated pathway. The motivation for this project comes from our discovery that DRAQ5™, a membrane-permeable nuclear stain, prevents the internalization of LtxA in a Jurkat T cell line. We hypothesized that DRAQ5™, in crossing the plasma membrane, alters the properties of the membrane to inhibit LtxA internalization. To investigate how DRAQ5™ interacts with the lipid membrane to prevent LtxA internalization, we used studied DRAQ5™-mediated membrane changes in model membranes using a variety of techniques, including differential scanning calorimetry (DSC) and fluorescence spectroscopy. Our results suggest that DRAQ5™ inhibits the activity of LtxA by decreasing the fluidity of the cellular lipid membrane, which decreases LtxA binding. These results present an interesting possible anti-virulence strategy; by altering bacterial toxin activity by modifying membrane fluidity, it may be possible to inhibit the pathogenicity of A. actinomycetemcomitans.
Keywords: Bacterial toxin, membrane fluidity, virulence, anthracycline
Introduction
Aggregatibacter actinomycetemcomitans, the causative agent for localized juvenile periodontitis (Zambon, 1985) and systemic diseases such as endocarditis (Paturel et al., 2004), produces a number of virulence factors that aid the bacterium in colonizing its host. One of these virulence factors is leukotoxin (LtxA), a member of the repeats-in-toxin (RTX) (Welch, 1991) family of proteins that are produced by pathogens including Escherichia coli, Bordetella pertussis, and Vibrio cholerae. LtxA contributes to the pathogenesis of the organism by the targeted killing of white blood cells, allowing the bacteria to avoid clearance by the host immune system (Taichman et al., 1984; Taichman et al., 1980; Taichman et al., 1987).
The specificity of LtxA for human white blood cells is due to the toxin’s affinity for lymphocyte function-associated antigen-1 (LFA-1) (Kieba et al., 2007; Lally et al., 1997), a β2 integrin expressed by human immune cells. While a strong affinity has been shown between LtxA and LFA-1 (Kieba et al., 2007; Lally et al., 1997), we have also shown that the toxin interacts strongly with receptor-free membranes, including binding to cholesterol-rich lipid rafts (Fong et al., 2006) via a cholesterol recognition amino acid consensus (CRAC) motif (Brown et al., 2013; Miller et al., 2014). Once bound to the membrane, LtxA undergoes conformational changes (Walters et al., 2013) and disrupts the bilayer structure of the membrane, before being internalized in a lysosomal-mediated pathway (Balashova et al., 2015; DiFranco et al., 2012). Aspects of this mechanism, including association with lipid rafts and binding to a β2 integrin, are shared by the RTX toxins, B. pertussis adenylate cyclase toxin and Mannheima haemolytica leukotoxin (Atapattu and Czuprynski, 2007; Bumba et al., 2010).
In a study to characterize the lysosomal-mediated pathway of LtxA, we discovered that the commonly used nuclear stain, DRAQ5™, altered the internalization of LtxA in target cells (Fig. 1). DRAQ5™ is a membrane-permeable, anthracycline derivative originally developed as a chemotherapy drug. Because other anthracycline derivatives have been demonstrated to alter membrane fluidity (Jedrzejczak et al., 1999; Kaye and Merry, 1985; Pacilio et al., 1998), we hypothesized that DRAQ5™, upon crossing the plasma membrane, might alter the structure of this membrane in a manner that prevents LtxA internalization. Thus, we undertook this project to determine the mechanism by which DRAQ5™ inhibits LtxA internalization for the future identification of less toxic molecules to inhibit LtxA and RTX toxin activity. Our results demonstrate that at low concentrations, DRAQ5™ decreases the fluidity of the membrane, which inhibits LtxA binding and bilayer disruption, leading to decreased activity of the toxin.
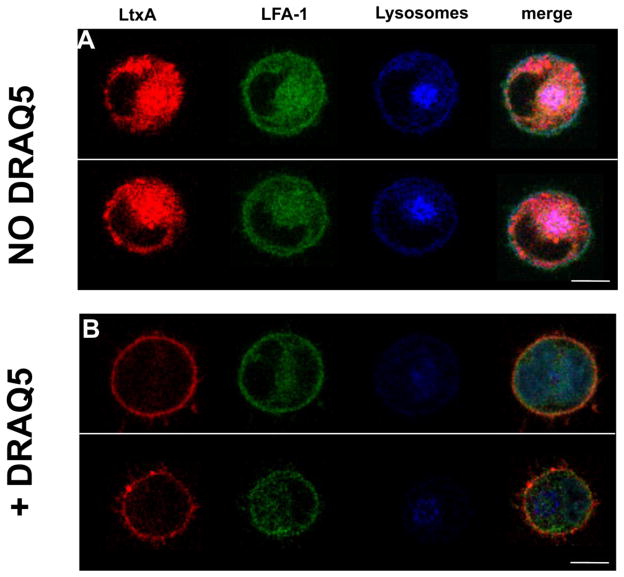
Fig. 1. DRAQ5™-mediated inhibition of LtxA internalization in Jn.9 cells.
(A) TR-LtxA (red) was incubated with K562 cells expressing CFP- and YFP-labeled LFA-1 (green). After 30 mins, TR-LtxA was found to be colocalized with lysosomes (blue). (B) K562 cells were first pretreated with 5 μM DRAQ5™ according to manufacturer’s instructions before addition of TR-LtxA. After 30 mins, LtxA was found on the surface of the cell, but not in the cytosol. Scale bar = 10 μm
Materials and Methods
Chemicals
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) were purchased from Avanti Polar Lipids (Alabaster, AL). Cholesterol was purchased from Sigma-Aldrich (St. Louis, MO), and N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)-1,2-Dihexadecanoyl-sn-Glycero-3-Phosphoethanolamine (NBD-PE) was purchased from Molecular Probes (Eugene, OR). DRAQ5™ and 6-dodecanoyl-2-dimethylaminonaphthalene (laurdan) were purchased from Thermo Fisher Scientific (Waltham, MA).
LtxA Purification
A. actinomycetemcomitans strain JP2 was grown overnight in AAGM (Fine et al., 1999) supplemented with 12.5 μg/ml vancomycin and 75 μg/ml bacitracin, and LtxA was purified from the culture supernatant as described previously (Kachlany et al., 2002). SDS-PAGE and Western blot were used to confirm the purity of the toxin, and a cytotoxicity assay was used to confirm the activity.
LtxA was labeled with Alexa Fluor® 555 NHS Ester (Molecular Probes, Eugene, OR) according to the manufacturer’s instructions, with a slight modification. Specifically, after labeling, the toxin was purified from excess dye using a 40k MWCO Zeba™ Spin Desalting column (Pierce Biotechnology, Rockford, IL).
Cell Culture
Jn.9 cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 0.1 mM MEM non-essential amino acids, 1x MEM vitamin solution, and 2 mM L-glutamine, and 0.5 μg/mL gentamicin. K562 cell lines were grown in RPMI 1640 medium containing 20% FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.5 mg/ml G418. THP-1 cells were grown in RPMI medium containing 10% FBS and 0.01% 2-mercaptoethanol. All cell lines were grown at 37 °C under 5% CO2.
Liposome Preparation
The lipid film technique was used to prepare liposomes composed of DMPC or POPC. Stock solutions of each lipid (both at 25 mg/mL) were prepared in chloroform and then added to a glass vial in the required amounts. The chloroform was fully evaporated under a stream of nitrogen, followed by vacuum-induced evaporation to create a thin lipid film on the glass surface. The resulting film was hydrated with liposome buffer (150 mM NaCl, 5 mM CaCl2, 5 mM HEPES, and 3 mM NaN3, pH 7.4) or DSC buffer (100 mM NaCl, 10 mM HEPES, pH 7.4) to create multilamellar liposomes (MLVs). Unilamellar vescicles (LUVs) were prepared from the MLV solutions by extrusion (MacDonald et al., 1991) through a 100 nm polycarbonate Whatman membrane (GE Healthcare BioSciences, Pittsburgh, PA) with a LiposoFast® extruder (AVESTIN Inc., Ottawa, ON).
Giant unilamellar vesicles (GUVs) were formed from a mixture of POPC/Chol/NBD-PE (79/20/1 mol%) dissolved in chloroform/acetonitrile (90/10 vol%) for a final lipid concentration of 4 mg/mL. The mixture was spin-coated onto indium tin oxide (ITO)-coated glass slides (SPI, West Chester, PA) using a Laurell WS-650-23 spin coater. The slides were then placed under vacuum for 30 minutes to remove any remaining solvent (Estes and Mayer, 2005). Two spin-coated slides were separated by a polydimethylsiloxane (PDMS) spacer and filled with 18.2 MΩ/cm ultrapure water from a Milli-Q® Advantage A10 system (EMD Millipore, Billerica, MA) and sealed. An electric field was applied for 3 hours at 23 °C to form the GUVs (Angelova et al., 1992). GUVs were used within the same day.
Confocal Microscopy
Purified LtxA was fluorescently labeled with Texas Red-X Succinimidyl Ester (Thermo Fisher Science) in the following manner. LtxA was hydrated in PBS at a concentration of 0.125 μg/μL. The dye, dissolved in DMSO at 50 mg/mL, was mixed with the toxin at a concentration of 1.0 μg dye/μL protein. The mixture was shaken gently for one hour in the dark, then dialyzed overnight against 4 L of PBS at 4°C in the dark.
K562 cells (Lozzio and Lozzio, 1975) expressing a cyan fluorescent protein (CFP)-tagged αL cytosolic domain and a yellow fluorescent protein (YFP)-tagged β2 domain (Kim et al., 2003) at a concentration of 0.6 × 106 cells/mL were labeled with Lysotracker® blue DND-22 (Thermo Fisher Scientific) for 30 min at 37 °C and then washed. Texas Red-labeled LtxA (TR-LtxA) was added at a concentration of 1 × 10−8 M; the cells were incubated for 30 min at 37 °C and then washed twice in PBS. In a second experiment, the cells were first incubated with 5 μM DRAQ5™ for 30 mins, followed by Lysotracker® blue and TR-LtxA.
Membrane Fluidity
DMPC or POPC liposomes containing 0.1% laurdan were incubated without DRAQ5™ or with DRAQ5™ at a concentration of 1, 5, 10 or 20 μM (lipid:dye ratios of 250, 50, 25, and 12.5, respectively). Steady-state emission spectra were recorded on a Quantamaster 400 spectrofluorometer (PTI Horiba, Edison, NJ) using an excitation wavelength of 340 nm. The fluidity of the membrane was quantified using the generalized polarization (GP):
where I440 and I480 are the fluorescence intensities at wavelengths of 440 nm and 480 nm, respectively.
To measure DRAQ5™-induced alterations in membrane transition temperatures, the experiment was repeated as a function of temperature. DMPC liposomes containing 0.1% laurdan were incubated with without DRAQ5™ or with DRAQ5™ (2.5 μM, 10 μM, and 50 μM; lipid:dye ratios of 100, 25, and 5, respectively) at 10 °C, and the GP was recorded. The temperature was then increased in 5° increments, and the GP of each sample was collected at each temperature.
Differential Scanning Calorimetry
DRAQ5™ was added in varying amounts (2.5 μM, 10 μM, and 50 μM) to DMPC liposomes in DSC buffer, corresponding to lipid:dye ratios of 40,000, 10,000, and 2,000, respectively. The samples were degassed and loaded into the sample cell of a differential scanning calorimeter (TA Instruments). Heating scans were run from 5 to 35 °C with a heating rate of 0.5 °C/min.
Membrane Disruption
The laurdan experiment described previously was modified to measure membrane disruption by LtxA. Because the fluorescence emission spectra of laurdan are sensitive to the presence of water (Sanchez et al., 2007), changes in the packing of the bilayer induced by LtxA result in changes in GP. POPC liposomes containing 0.1% laurdan were incubated with increasing concentrations of LtxA (0 μM, 0.083 μM, and 0.167 μM), and the GP was recorded for each sample. The experiment was then repeated with POPC liposomes that had been preincubated with 1 μM, 5 μM, or 10 μM DRAQ5™ (lipid:dye ratios of 250, 50, and 25). The fluorescence emission spectra were recorded from 400 to 500 nm, with an excitation wavelength of 340 nm.
DRAQ5™ Binding
GUVs were incubated for 30 minutes with or without DRAQ5™ at a final concentration of 2.5 μM. The GUVs were washed three times using ultrapure 18.2 MΩ/cm water and then deposited onto ibiTreat μ-Dishes (Ibidi, Martinsried, Germany) coated with poly-L-lysine (Sigma-Aldrich. St. Louis, MO) for 30 minutes. Each μ-Dish was then incubated with 30 ng of Alexa Fluor® 555-labeled LtxA for 30 min. Imaging was performed using a Nikon C2si+ confocal microscope utilizing a LU-N4S laser unit and a 60x oil objective (N.A. 1.4). The images were processed using Elements v4.3, Nikon’s imaging software suite.
Cytotoxicity
2.5 μM of DRAQ5™ was preincubated with THP-1 or Jn.9 cells (1 × 106 cells/mL) for 15 min. LtxA (10–20 μg) was added to DRAQ5™-treated THP-1 or Jn.9 cells and to cells not treated with DRAQ5™. Untreated cells were used as a control. The number of live cells initially (N0) was recorded and then the samples were incubated for 3 h in 5% CO2 at 37 °C. A final cell count (N3) was then recorded. The percent viability after each treatment was calculated:
Statistical Analysis
Statistical analysis of the data was performed using Sigma Plot 13 (Systat Software, Inc. San Jose, CA) using two-way ANOVA or a student’s t-test. In cases where P > 0.05, we reported no statistically significant difference between the two data sets in question.
Results
DRAQ5™ Inhibits LtxA Internalization in Target Cells
This work was initiated after an unexpected discovery in a confocal microscopy experiment. In this initial experiment, TR-LtxA was incubated with K562 cells expressing a CFP-tagged αL cytosolic domain and a YFP-tagged β2 domain (Kim et al., 2003). After 30 mins, TR-LtxA was found to be colocalized with the lysosomes, which had been prestained with a LysoTracker dye (Fig. 1a). However, when the cells were first incubated with DRAQ5™ for 30 mins, following the manufacturer’s directions, to stain the nucleus, LtxA was found on the cell membrane, but not in the lysosomes (Fig. 1b). DRAQ5™ is a nuclear stain from the anthracycline family, which crosses both the plasma and nuclear membranes before binding to DNA in the cell nucleus. Because other anthracyclines have reported effects on membrane packing (Jedrzejczak et al., 1999; Kaye and Merry, 1985; Pacilio et al., 1998) and some DRAQ5™ can be seen to be associated with the plasma membrane, we hypothesized that DRAQ5™ alters the fluidity of the plasma membrane when it crosses, thereby inhibiting the ability of LtxA to initiate the internalization process.
DRAQ5™ Decreases Membrane Fluidity
To investigate the effect of DRAQ5™ on membrane structure, we conducted a series of polarization studies in membranes composed of DMPC or POPC in the presence of DRAQ5™ at concentrations ranging from 0 μM to 20 μM. For both types of membranes, the generalized polarization (GP) increased with increasing DRAQ5™ concentration, as shown in Fig. 2a, indicating that DRAQ5™ decreases the fluidity of both types of membranes.
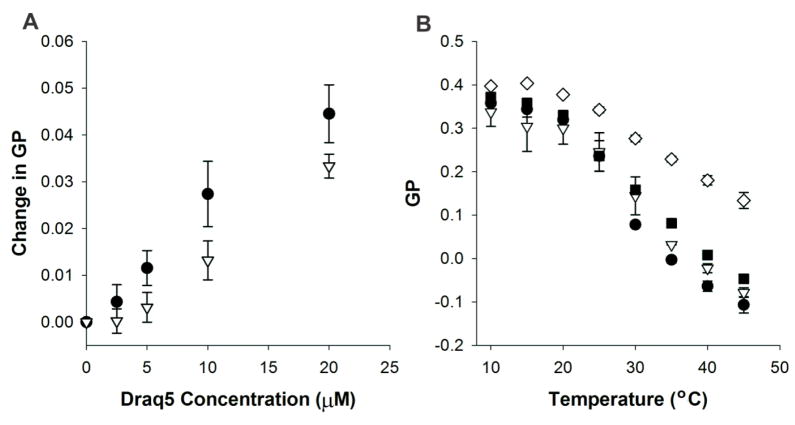
Fig. 2. DRAQ5™ decreases membrane fluidity.
(A) DRAQ5™-mediated effect on membrane fluidity. DRAQ5™ decreased the fluidity of DMPC (black circles) and POPC (white triangles) membranes, as measured by an increase in GP. A two-way ANOVA indicates that the effect of DRAQ5™ depends on the lipid composition (p = 0.005). (B) Effect of DRAQ5™ on the transition temperature of DMPC. DMPC was incubated with 0 μM (black circles), 2.5 μM (white triangles), 10 μM (black squares), or 50 μM (white diamonds) DRAQ5™, and changes in the GP, as the sample undergoes a transition from the gel (high GP) to the bilayer phase (low GP), were recorded. A two-way ANOVA indicates that the effect of temperature depends on the DRAQ5™ concentration (p < 0.001).
The previous experiment, which was conducted at room temperature (23 °C), where POPC exists in the liquid phase and DMPC in the gel phase, suggests that DRAQ5™ decreases the fluidity in both gel and liquid phase membranes. To investigate the role of membrane phase behavior on the DRAQ5™-mediated change in fluidity, we conducted an additional experiment using DMPC with DRAQ5™ concentrations ranging from 0 μM to 50 μM, at temperatures ranging from 10°C to 45°C, as shown in Fig. 2b. In this experiment, we found that DRAQ5™ broadens the gel-to-liquid transition (Tm) of DMPC but does not change the transition temperature (24°C). This finding was verified using differential scanning calorimetry (DSC). As shown in Table 1, DSC demonstrated that the transition temperature of DMPC does not change significantly upon the addition of DRAQ5™. In addition, no changes in the pre-transition temperature of DMPC were detected. Together, these results indicate that DRAQ5™ alters membrane fluidity without affecting the transition temperature of the membrane. The greatest effect on membrane fluidity occurs in fluid-phase DMPC bilayers, suggesting that the dye may have a preference for this lipid over POPC, and is better able to induce order in the fluid-phase DMPC membrane relative to the gel-phase DMPC membrane.
Table 1. Transition temperature (Tm) of DMPC in the absence and presence of Draq5™.
DMPC liposomes were incubated with increasing amounts of DRAQ5™ and the transition temperature of the lipid was measured using DSC.
| DRAQ5™ Concentration (μM) | Transition Temperature (Tm, °C) |
|---|---|
| 0 | 23.78 |
| 5 | 23.74 |
| 10 | 23.73 |
| 50 | 23.73 |
DRAQ5™ Inhibits LtxA Binding
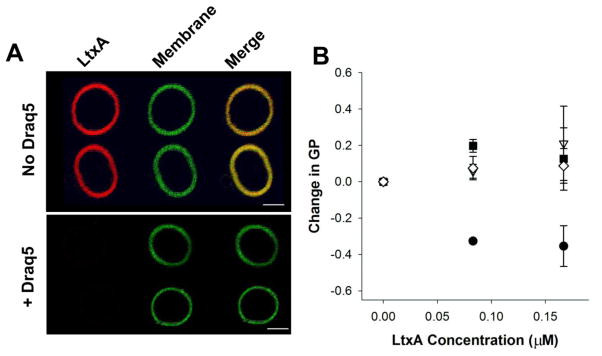
We next investigated whether DRAQ5™-mediated alterations in membrane structure affected LtxA binding to membranes. Active LtxA was labeled with Alexa Fluor™ 555 (AF555, red) and incubated with GUVs composed of POPC and cholesterol and fluorescently labeled with NBD (green). When active AF555-LtxA (30 ng) was added to GUVs in the absence of DRAQ5™, the toxin was found to be associated with the membranes within 30 minutes, as shown in Fig. 3a. In contrast, no binding was detected when the same amount of active AF555-LtxA was incubated with vesicles that had been pretreated with DRAQ5™, as shown in Fig. 3b. These images suggest that DRAQ5™ inhibits the ability of LtxA to bind to membrane lipids. In this experiment, the DRAQ5™ remains associated with the membrane throughout the experiment (data not shown).
Fig. 3. DRAQ5™-induced changes in LtxA interactions with POPC membranes.
(A) LtxA affinity for POPC membranes. GUVs composed of POPC/Chol, labeled with 1% NBD (green) were pretreated with DRAQ5™ or left untreated. LtxA (red) was then added to the GUVs. LtxA showed a strong affinity for untreated membranes, but binding was abolished in DRAQ5™-pretreated GUVS. Scale bar = 5 μm. (B) LtxA-mediated membrane disruption. The disruption of bilayer packing induced by LtxA was recorded using laurdan fluorescence. POPC liposomes were preincubated with DRAQ5™ at a concentration of 0 μM (black circles), 1 μM (white diamonds), 5 μM (black squares), or 10 μM (white triangles) before addition of LtxA to measure inhibition of LtxA-mediated membrane disruption. A two-way ANOVA indicates that the effect of LtxA depends on the DRAQ5™ concentration (p = 0.005).
DRAQ5™ Inhibits LtxA Membrane Disruption
Previously, we demonstrated that LtxA disrupts the bilayer structure of target cells (Brown et al., 2012). To investigate the effect that DRAQ5™-mediated membrane fluidity changes have on the ability of LtxA to disrupt the membrane, we conducted another set of GP experiments, in which membrane disruption by LtxA was quantified as a decrease in GP. As the toxin disrupts membrane packing, more water penetrates to the interior of the membrane, resulting in a decrease in GP, as shown in Fig. 3B. The experiment was repeated in POPC liposomes that had been preincubated with one of three different DRAQ™ concentrations. After DRAQ5™ incubation, the GP remains constant with increasing LtxA concentrations, indicating that LtxA is unable to disrupt bilayer packing under these conditions. This experiment demonstrates that LtxA penetration into the bilayer is inhibited by DRAQ5™.
DRAQ5™ Inhibits LtxA Toxicity
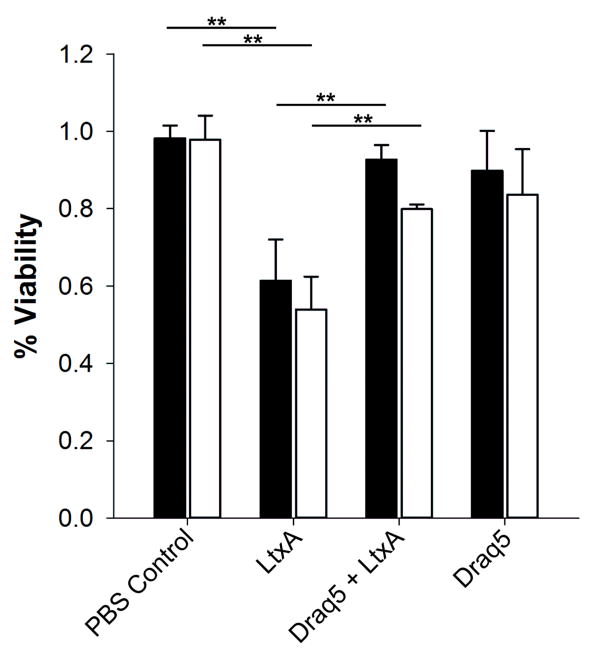
The previous results demonstrate that DRAQ5™-mediated alterations to membrane fluidity have a significant effect on the ability of the toxin to interact with the target cell membrane. To investigate whether these changes inhibit LtxA-induced toxicity, we observed the effects of low concentrations of DRAQ5™ on target cell viability using both THP-1 and Jn.9 cells exposed to LtxA. In both the THP-1 (Fig. 4a) and Jn.9 (Fig. 4b) cells, the viability of the LtxA-treated cells decreased significantly relative to PBS-treated controls. The THP-1 and Jn.9 cells that were pretreated with DRAQ5™ were less susceptible to LtxA-mediated cytotoxicity compared to cells not treated with DRAQ5™. Cells treated only with DRAQ5™ remained viable through the time course of the experiment. These results demonstrate that in both types of cells, DRAQ5™ inhibits LtxA-mediated toxicity.
Fig. 4. Inhibition of LtxA toxicity in target cells.
THP-1 (black bars) and Jn.9 cells (white bars) were preincubated with 10 μM DRAQ5™ for 30 min followed by LtxA for three hours. The percentage of living cells initially and after the three-hour incubation were used to calculate percent cell viability. A student’s t-test indicates a significant difference between untreated and LtxA-treated cells and between LtxA-treated cells and LtxA- and DRAQ5™-treated cells. **, p < 0.01.
Discussion
DRAQ5™ is a membrane-permeable, anthracycline derivative, which has been used as a chemotherapeutic drug (Smith et al., 2000). The members of this family of molecules have wide-ranging effects on both the cell membrane and chromatin and have been used as antitumor agents because of their ability to cause cell cycle arrest (Chen et al., 2004; Hellin et al., 2000; Kim et al., 2009). DRAQ5™ is often used as a nuclear stain because of its DNA binding as well as its ability to cross the plasma and nuclear membranes. This membrane permeation suggests that the dye may change the structure of the membrane to facilitate translocation. Several anthracycline derivatives have been shown to cause drastic, complex changes in the cell membrane phase behavior. For example, emodin was shown to promote the formation of the nonbilayer, inverted hexagonal (HII) phase, while barbaloin and daunomycin were found to promote the stable lamellar phase (Alves et al., 2004; Escriba et al., 1995). Doxorubicin promotes the formation of the liquid phase, but inhibits the formation of this phase in the presence of cardiolipin (Tritton et al., 1978). In addition, mitoxantrone, doxorubicin, daunorubicin, and idarubicin were found to cross the membrane through a flip-flop mechanism that was inhibited by cholesterol and promoted by increased temperature, suggesting that these molecules may induce HII phase formation (Regev and Eytan, 1997; Regev et al., 2005).
We discovered that LtxA is unable to enter DRAQ5™-treated cells; after DRAQ5™ treatment, LtxA was found on the plasma membrane but not within the cytosol. The association of LtxA with the cell membrane has been shown to require two essential elements: membrane lipids and an integrin receptor, LFA-1. We therefore hypothesized that DRAQ5™ changes the structure of the cell membrane, which prevents the LtxA-mediated membrane interactions that are necessary for internalization through endocytosis; the localization of LtxA on the membrane of DRAQ5™-treated cells is due to its binding to LFA-1, which is unaltered by DRAQ5™. Here, we demonstrated that DRAQ5™ does induce changes in the plasma membrane structure, which prevent LtxA internalization and binding to the plasma membrane. As a result of these changes, DRAQ5™-treated cells are resistant to LtxA.
Cells must maintain their membrane integrity and structure to function properly, and one way that they combat changes to membrane fluidity is to alter the membrane composition by adjusting the ratio of certain lipids, particularly cholesterol (to decrease fluidity) and phosphatidylethanolamine (PE, to increase fluidity) (Dawaliby et al., 2016). We have previously shown that, like other bacterial toxins, e.g., (Alonso et al., 2000; Boesze-Battaglia et al., 2009; Farrand et al., 2010; Lai et al., 2013; Maxfield and Tabas, 2005; Patel et al., 2002; Schwiering et al., 2013; Zitzer et al., 2001), LtxA requires cholesterol in the plasma membrane to bind and kill target cells (Brown et al., 2013; Brown et al., 2015; Fong et al., 2006). Thus, it is possible that the cell counteracts DRAQ5™-mediated decreases in the plasma membrane fluidity by decreasing the membrane cholesterol composition and that this change inhibits LtxA internalization. Studies to investigate this possibility are currently underway, along with experiments to generalize the mechanism by which changes in membrane fluidity by small molecules inhibits toxin activity.
The use of small molecules to inhibit toxin activity has been demonstrated recently (Bender et al., 2015) and represents an exciting alternative to antibiotics for the treatment of bacterial disease. Some of these molecules act by precipitating the targeted toxin from solution (Choi et al., 2007; Hisano et al., 2003; Verhelst et al., 2013). We found that Draq5 inhibits toxin activity by directly modifying membrane structure, a finding that is similar to a previously reported finding that resveratrol inhibits cholera toxin (CT) activity by disrupting internalization of the toxin but not binding to the cell membrane (Morinaga et al., 2010). Another recent study demonstrated that sumac tannins decrease membrane fluidity, as we found for DRAQ5™, and as a result, decrease hemolysis of erythrocytes by Bacillus cereus and hemolysin II (Olchowik-Grabarek et al., 2014).
Together, these findings suggest that small alterations in membrane organization may represent a novel antivirulence strategy. Rather than targeting bacteria with antibiotics, an antivirulence approach that inhibits toxin activity would specifically reduce the pathogenicity of the organism. By characterizing the mechanism by which DRAQ5™ inhibits LtxA internalization and toxicity, we are now able to pursue alternative molecules that induce similar changes in membrane structure, with the goal of identifying therapeutic candidates for anti-toxin methods to treat A. actinomycetemcomitans infections.
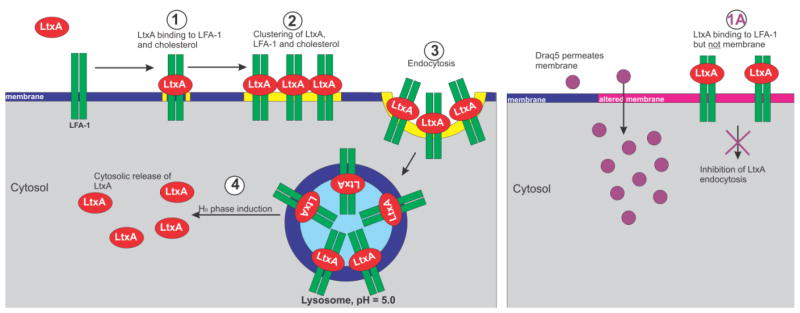
Fig. 5. Proposed mechanism of DRAQ5™-mediated inhibition of LtxA internalization.
In the absence of DRAQ5™, LtxA binds to the plasma membrane, specifically cholesterol and LFA-1 (1). This causes the formation of large-scale clusters containing LtxA, LFA-1, and cholesterol (2). LtxA is then endocytosed and localized to lysosomes (3). LtxA disrupts the lysosomal membrane (HII phase induction), to be released to the cytosol (4). In the presence of DRAQ5™, the membrane structure is altered, which prevents LtxA from binding to the membrane lipids, but does not affect the toxin’s affinity for LFA-1. This inhibition of toxin binding prevents the necessary interactions of LtxA with the membrane that promote endocytosis
Acknowledgments
This work was funded by the National Institutes of Health (R00DE022795, ACB). The authors wish to acknowledge the University of Pennsylvania, School of Dental Medicine live cell confocal imaging core for assistance in the confocal microscopy experiments.
References
- Alonso A, Goni FM, Buckley JT. Lipids Favoring Inverted Phase Enhance the Ability of Aerolysin To Permeabilize Liposome Bilayers. Biochemistry. 2000;39:14019–14024. doi: 10.1021/bi001739o. [DOI] [PubMed] [Google Scholar]
- Alves DS, Perez-Fons L, Estepa A, Micol V. Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochemical Pharmacology. 2004;68:549–561. doi: 10.1016/j.bcp.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Angelova MI, Soleau S, Meleard P, Faucon F, Bothorel P. Trends in Colloid and Interface Science VI. Darmstadt: Steinkopff; 1992. Preparation of Giant Vesicles by External AC Electric Fields. Kinetics and Applications; pp. 127–131. [Google Scholar]
- Atapattu DN, Czuprynski CJ. Mannheimia haemolytica leukotoxin binds to lipid rafts in bovine lymphoblastoid cells and is internalized in a dynamin-2- and clathrin-dependent manner. Infection and Immunity. 2007;75:4719–4727. doi: 10.1128/IAI.00534-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashova N, Dhingra A, Boesze-Battaglia K, Lally ET. Aggregatibacter actinomycetemcomitans leukotoxin induces cytosol acidification in LFA-1 expressing immune cells. Mol oral Microbiol. 2015 doi: 10.1111/omi.12136. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KO, Garland M, Ferreyra JA, Hryckowian AJ, Child MA, Puri AW, et al. A small-molecule antivirulence agent for treating Clostridium difficile infection. Science Translational Medicine. 2015;7 doi: 10.1126/scitranslmed.aac9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Brown A, Walker L, Besack D, Zekavat A, Wrenn S, et al. Cytolethal Distending Toxin-induced Cell Cycle Arrest of Lymphocytes Is Dependent upon Recognition and Binding to Cholesterol. Journal of Biological Chemistry. 2009;284:10650–10658. doi: 10.1074/jbc.M809094200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Boesze-Battaglia K, Du Y, Stefano FP, Kieba IR, Epand RF, et al. Aggregatibacter actinomycetemcomitans leukotoxin cytotoxicity occurs through bilayer destabilization. Cellular Microbiology. 2012;14:869–881. doi: 10.1111/j.1462-5822.2012.01762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Balashova NV, Epand RM, Epand RF, Bragin A, Kachlany SC, et al. Aggregatibacter actinomycetemcomitans Leukotoxin Utilizes a Cholesterol Recognition/Amino Acid Consensus Site for Membrane Association. Journal of Biological Chemistry. 2013;288:23607–23621. doi: 10.1074/jbc.M113.486654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Koufos E, Balashova N, Boesze-Battaglia K, Lally ET. Inhibition of LtxA Toxicity by Blocking Cholesterol Binding With Peptides. Mol oral Microbiol. 2015 doi: 10.1111/omi.12133. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumba L, Masin J, Fiser R, Sebo P. Bordetella adenylate cyclase toxin mobilizes its β2 integrin receptor into lipid rafts to accomplish translocation across target cell membrane in two steps. PLoS Pathogens. 2010;6 doi: 10.1371/journal.ppat.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Hsieh WT, Chang WC, Chung JG. Aloe-emodin induced in vitro G2/M arrest of cell cycle in human promyelocytic leukemia HL-60 cells. Food and Chemical Toxicology. 2004;42:1251–1257. doi: 10.1016/j.fct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Choi O, Yahiro K, Morinaga N, Miyazaki M, Noda M. Inhibitory effects of various plant polyphenols on the toxicity of Staphylococcal α-toxin. Microbial Pathogenesis. 2007;42:215–224. doi: 10.1016/j.micpath.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Dawaliby R, Trubbia C, Delporte C, Noyon C, Ruysschaert JM, Van Antwerpen P, Govaerts C. Phosphatidylethanolamine is a Key Regulator of Membrane Fluidity in Eukaryotic Cells. Journal of Biological Chemistry. 2016;291:3658–3667. doi: 10.1074/jbc.M115.706523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco KM, Gupta A, Galusha LE, Perez J, Nguyen TV, Fineza CD, Kachlany SC. Leukotoxin (Leukothera) Targets Active Leukocyte Function Antigen-1 (LFA-1) Protein and Triggers a Lysosomal Mediated Cell Death Pathway. Journal of Biological Chemistry. 2012;287:17618–17627. doi: 10.1074/jbc.M111.314674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriba PV, Sastre M, Garcia-Sevilla JA. Disruption of cellular signaling pathways by daunomycin through destabilization of nonlamellar membrane structures. Proceedings of the National Academy of Sciences. 1995;92:7595–7599. doi: 10.1073/pnas.92.16.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes DJ, Mayer M. Electroformation of Giant Liposomes from Spin-Coated Films of Lipids. Colloids and Surfaces B: Biointerfaces. 2005;42:115–123. doi: 10.1016/j.colsurfb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proceedings of the National Academy of Sciences. 2010;107:4341–4346. doi: 10.1073/pnas.0911581107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine DH, Furgang D, Schreiner HC, Goncharoff P, Charlesworth J, Ghazwan G, et al. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth. Implications for virulence. Microbiology. 1999;145:1335–1347. doi: 10.1099/13500872-145-6-1335. [DOI] [PubMed] [Google Scholar]
- Fong KP, Pacheco CMF, Otis LL, Baranwal S, Kieba IR, Harrison G, et al. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cellular Microbiology. 2006;8:1753–1767. doi: 10.1111/j.1462-5822.2006.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellin AC, Bentires-Alj M, Verlaet M, Benoit V, Gielen J, Bours V, Merville MP. Roles of Nuclear Factor-kB, p53, and p21/WAF1 in Daunomycin-Induced Cell Cycle Arrest and Apoptosis. Journal of Pharmacology and Experimental Therapeutics. 2000;295:870–878. [PubMed] [Google Scholar]
- Hisano M, Yamaguchi K, Inoue Y, Ikeda Y, Iijima M, Adachi M, Shimamura T. Inhibitory effect of catechin against the superantigen staphylococcal enterotoxin B (SEB) Archives of Dermatological Research. 2003;295:183–189. doi: 10.1007/s00403-003-0411-x. [DOI] [PubMed] [Google Scholar]
- Jedrzejczak M, Koceva-Chyla A, Gwozdzinski K, Jozwiak Z. Changes in Plasma Membrane Fluidity of Immortal Rodent Cells Induced by Anticancer Drugs Doxorubicin, Aclarubicin and Mitoxantrone. Cell Biology International. 1999;23:497–506. doi: 10.1006/cbir.1999.0399. [DOI] [PubMed] [Google Scholar]
- Kachlany SC, Fine DH, Figurski DH. Purification of secreted leukotoxin (LtxA) from Actinobacillus actinomycetemcomitans. Protein Expression and Purification. 2002;25:465–471. doi: 10.1016/s1046-5928(02)00037-2. [DOI] [PubMed] [Google Scholar]
- Kaye S, Merry S. Tumour cell resistance to anthracyclines - A review. Cancer Chemotherapy and Pharmacology. 1985;14:96–103. doi: 10.1007/BF00434344. [DOI] [PubMed] [Google Scholar]
- Kieba IR, Fong KP, Tang HY, Hoffman KE, Speicher DW, Klickstein LB, Lally ET. Aggregatibacter actinomycetemcomitans leukotoxin requires β-sheets 1 and 2 of the human CD11a β-propeller for cytotoxicity. Cellular Microbiology. 2007;9:2689–2699. doi: 10.1111/j.1462-5822.2007.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Lee YS, Kim DK. Doxorubicin Exerts Cytotoxic Effects through Cell Cycle Arrest and Fas-Mediated Cell Death. Pharmacology. 2009;84:300–309. doi: 10.1159/000245937. [DOI] [PubMed] [Google Scholar]
- Kim M, Carman CV, Springer TA. Bidirectional Transmembrane Signaling by Cytoplasmic Domain Separation in Integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- Lai CH, Lai CK, Lin YJ, Hung CL, Chu CH, Feng CL, et al. Characterization of Putative Cholesterol Recognition/Interaction Amino Acid Consensus-Like Motif of Campylobacter jejuni Cytolethal Distending Toxin C. PLos ONE. 2013;8:e66202. doi: 10.1371/journal.pone.0066202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally ET, Kieba IR, Sato A, Green CL, Rosenbloom J, Korostoff J, et al. RTX toxins recognize a β2 integrin on the surface of human target cells. Jouranl of Biological Chemistry. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- MacDonald RC, MacDonald RI, Menco BP, Takeshita K, Subbarao NK, Hu Lr. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- Miller CM, Brown AC, Mittal J. Disorder in Cholesterol-Binding Functionality of CRAC Peptides: A Molecular Dynamics Study. J Phys Chem B. 2014;118:13169–13174. doi: 10.1021/jp5106423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga N, Yahiro K, Noda M. Resveratrol, a natural polyphenolic compound, inhibits cholera toxin-induced cyclic AMP accumulation in Vero cells. Toxicon. 2010;56:29–35. doi: 10.1016/j.toxicon.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Olchowik-Grabarek E, Swiecicka I, Andreeva-Kovaleskaya Z, Solonin A, Bonarska-Kujawa D, Kleszczynska H, et al. Role of Structural Changes Induced in Biological Membranes by Hydrolysable Tannins from Sumac Leaves (Rhus typhina L.) in their Antihemolytic and Antibacterial Effects. Journal of Membrane Biology. 2014;247:533–540. doi: 10.1007/s00232-014-9664-x. [DOI] [PubMed] [Google Scholar]
- Pacilio C, Florio S, Pagnini U, Crispino A, Claudio PP, Pacilio G, Pagnini G. Modification of membrane fluidity and depolarization by some anthracyclines in different cell lines. Anticancer Research. 1998;18:4027–4034. [PubMed] [Google Scholar]
- Patel HK, Willhite DC, Patel RM, Ye D, Williams CL, Torres EM, et al. Plasma Membrane Cholesterol Modulates Cellular Vacuolation Induced by the Helicobacter pylori Vacuolating Cytotoxin. Infection and Immunity. 2002;70:4112–4123. doi: 10.1128/IAI.70.8.4112-4123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturel L, Casalta JP, Habib G, Nezri M, Raoult D. Actinobacillus actinomycetemcomitans endocarditis. Clinical Microbiology and Infection. 2004;10:98–118. doi: 10.1111/j.1469-0691.2004.00794.x. [DOI] [PubMed] [Google Scholar]
- Regev R, Eytan GD. Flip-Flop of Doxorubicin across Erythrocyte and Lipid Membranes. Biochemical Pharmacology. 1997;54:1151–1158. doi: 10.1016/s0006-2952(97)00326-2. [DOI] [PubMed] [Google Scholar]
- Regev R, Yeheskely-Hayon D, Katzir H, Eytan GD. Transport of anthracyclines and mitoxantrone across membranes by a flip-flop mechanism. Biochemical Pharmacology. 2005;70:161–169. doi: 10.1016/j.bcp.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Sanchez SA, Tricerri MA, Gunther G, Gratton E. Laurdan Generalized Polarization: from cuvette to microscope. In: Mendez-Vilas A, Diaz J, editors. Modern Research and Educational Topics in Microscopy. Formatex; 2007. [Google Scholar]
- Schwiering M, Brack A, Stork R, Hellman N. Lipid and phase specificity of α-toxin from S. aureus. Biochimica et Biophysica Acta. 2013;1828:1962–1972. doi: 10.1016/j.bbamem.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blunt N, Wiltshire M, Hoy T, Teesdale-Spittle P, Craven MR, et al. Characteristics of a novel deep red/infrared fluorescent cell-permeant DNA probe, DRAQ5, in intact human cells analyzed by flow cytometry, confocal and multiphoton microscopy. Cytometry. 2000;40:280–291. doi: 10.1002/1097-0320(20000801)40:4<280::aid-cyto4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Taichman NS, Shenker BJ, Tsai CC, Glickman LT, Baehni PC, Stevens R, Hammond BF. Cytopathic effects of Actinobacillus actinomycetemcomitans on monkey blood leukocytes. Journal of Periodontal Research. 1984;19:133–145. doi: 10.1111/j.1600-0765.1984.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Taichman NS, Dean RT, Sanderson CJ. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infection and Immunity. 1980;28:258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman NS, Simpson DL, Sakurada S, Cranfield M, DiRienzo J, Slots J. Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiology and Immunology. 1987;2:97–104. doi: 10.1111/j.1399-302x.1987.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Tritton TR, Murphree SA, Sartorelli AC. Adriamycin: A proposal on the specificity of drug action. Biochemical and Biophysical Research Communications. 1978;84:802–808. doi: 10.1016/0006-291x(78)90775-1. [DOI] [PubMed] [Google Scholar]
- Verhelst R, Schroyen M, Buys N, Niewold TA. E. coli heat labile toxin (LT) inactivation by specific polyphenols is aggregation dependent. Veterinary Microbiology. 2013;163:319–324. doi: 10.1016/j.vetmic.2012.12.039. [DOI] [PubMed] [Google Scholar]
- Walters MJ, Brown AC, Edrington TC, Baranwal S, Du Y, Lally ET, Boesze-Battaglia K. Membrane association and destabilization by Aggregatibacter actinomycetemcomitans leukotoxin requires changes in secondary structures. Mol oral Microbiol. 2013;28:342–353. doi: 10.1111/omi.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RA. Pore-forming cytolysins of gram-negative bacteria. Molecular Microbiology. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Zambon JJ. Actinobacillus actinomycetemcomitans in human periodontal disease. Journal of Clinical Periodontology. 1985;12:1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Zitzer A, Bittman R, Verbicky CA, Erukulla RK, Bhakdi S, Weis S, et al. Coupling of Cholesterol and Cone-shaped Lipids in Bilayers Augments Membrane Permeabilization by the Cholesterol-specific Toxins Streptolysin O and Vibrio cholerae Cytolysin. Journal of Biological Chemistry. 2001;276:14628–14633. doi: 10.1074/jbc.M100241200. [DOI] [PubMed] [Google Scholar]