Abstract
Background
Post-traumatic stress disorder (PTSD) is associated with atypical responses to emotional face stimuli with preferential processing given to threat-related facial expressions via hyperactive amygdalae disengaged from medial prefrontal modulation.
Method
We examined implicit emotional face perception in soldiers with (n = 20) and without (n = 25) PTSD using magnetoencephalography to define spatiotemporal network interactions, and a subsequent region-of-interest analysis to characterize the network role of the right amygdala and medial prefrontal cortex in threatening face perception.
Results
Contrasts of network interactions revealed the PTSD group were hyperconnected compared to controls in the phase-locking response in the 2–24 Hz range for angry faces, but not for happy faces when contrasting groups. Hyperconnectivity in PTSD was greatest in the posterior cingulate, right ventromedial prefrontal cortex, right parietal regions and the right temporal pole, as well as the right amygdala. Graph measures of right amygdala and medial prefrontal connectivity revealed increases in node strength and clustering in PTSD, but not inter-node connectivity. Additionally, these measures were found to correlate with anxiety and depression.
Conclusions
In line with prior studies, amygdala hyperconnectivity was observed in PTSD in relation to threatening faces, but the medial prefrontal cortex also displayed enhanced connectivity in our network-based approach. Overall, these results support preferential neurophysiological encoding of threat-related facial expressions in those with PTSD.
Keywords: Neurophysiology, Neural basis of fear, Biological psychiatry, Electrophysiological methods in neurobiology, Methods to study human brain function
1. Introduction
Post-traumatic stress disorder (PTSD) is serious psychiatric condition that involves re-experiencing of traumatic episodes, avoidance behaviours, emotional dysregulation, and hyper-arousal (American Psychiatric Association, 2013). The incidence of the disorder stands at around 5–10% in the general population, (Kessler et al., 2005), but the prevalence of PTSD is higher in military combat veterans (Boulos and Zamorski, 2013). Secondary sequelae are often evident, and studies report deficits in a number of psychological domains, including disturbance of executive function (Jenkins et al., 2000), inhibition (Leskin and White, 2007), and attentional control (Shucard et al., 2008). Moreover, emotional processing and threat perception are also often altered (Aupperle et al., 2012; Dalgleish et al., 2003). Functional MRI and PET studies suggest that the aetiology of these maladaptive processes in PTSD are due to atypical top-down modulation of the amygdalae (Simmons et al., 2011; Bruce et al., 2013) by the medial prefrontal cortex (Shin and Orr 2004; Shin and Wright, 2005).
In terms of face perception in PTSD, patients display enhanced activation in response to threat-related facial expressions, being neurophysiologically-biased toward angry or fearful faces (Simmons et al., 2011; Cisler et al., 2013; Fonzo et al., 2013; Bruce et al., 2013). It has been postulated that this is due to cognitive resources being prioritised to process threatening stimuli as a result of a hyperactive fear network, which is disengaged from an upstream inhibition circuit involving hypoactive frontal regions (for reviews, see Newport and Nemeroff, 2000; Pitman et al., 2012), with non-threatening information relegated to low-priority processing. Additionally, it has been shown that those with PTSD display lower reactivity in ventral striatal pathways to happy expressions (Felmingham et al., 2014). Despite imaging studies reporting atypical activity in PTSD (Morey et al., 2009; Tsoory et al., 2008), little is known about the impact of the disorder on the network dynamics of such processing, and particularly the neurophysiological connectivity that could potentially underlie psychopathology.
Network dynamics can be investigated through frequency-specific interactions among brain areas which have been demonstrated to play a critical role in the spatiotemporal organisation of information required for efficient goal-directed cognition (Buzsáki and Wang, 2012; Fries, 2005; Varela and Lachaux, 2001). Neurophysiological techniques (such as electroencephalography, EEG, and magnetoencephalography, MEG) have been crucial in this regard, given their exquisite temporal resolution and the ability to elucidate oscillatory synchronization and large-scale phase-phase interactions within and between regions of the brain (Palva and Palva, 2011).
Altered patterns of inter-regional synchrony have been observed in a number of psychiatric conditions, and studying these atypical networks has proven informative in understanding cortical pathophysiology (Montez et al., 2009; Tewarie et al., 2013). In PTSD, alterations to low-frequency spectral properties have been noted in left temporal, right frontal, and right parietal regions (Kolassa et al., 2007), and recently, we have shown that high-frequency synchronization during rest distinguishes PTSD from control soldiers, and is related to cognitive and affective sequelae as well as symptom severity in PTSD (Dunkley et al., 2014). These studies suggest abnormal synchrony across the brain might underlie some of the cognitive sequelae of the disorder.
1.1. Aims of the study
Here we investigated the role of inter-regional oscillatory phase-locking in an implicit emotional face processing task in soldiers with PTSD using MEG. The aims were twofold; first, to use a whole-brain, data-driven approach to examine task-dependent phase interactions in neuronal networks using MEG; and second, to use a region-of-interest (ROI) approach to test the hypothesis that the amygdalae would display enhanced connectivity related to angry face processing, whilst the medial prefrontal cortex would show comparative decreased connectivity. Regarding frequency-specific interactions that might be expected to distinguish the groups, we predicted that low- and medium-frequency phase synchrony (theta to beta range) would be differentially expressed. These particular frequency ranges are thought to reflect neuronal mechanisms that subserve large-scale cortical spatiotemporal integrative and segregative dynamics (Palva and Palva, 2007; Von Stein and Sarnthein, 2000; Siegel et al., 2012; Donner and Siegel, 2011). Given our previous observations in this population (Dunkley et al., 2014; Dunkley et al., 2015) and previous literature in this area, we anticipated that induced synchrony in the ‘fear circuit’ would be enhanced in our clinical group when viewing angry faces (particularly the amygdala seed regions and connected nodes), and connectivity in the ventromedial PFC would be reduced.
2. Materials and methods
2.1. Participants
MEG data were recorded from 45 Canadian Armed Forces soldiers, who were deployed in frontline roles in support of the Afghan mission. Twenty soldiers diagnosed with PTSD (all male, mean age = 37.67, SD = 1.39) and 25 combat-exposed soldiers without PTSD (all male, mean age = 33.97, SD = 0.98) were recruited. All participants underwent cognitive-behavioural testing and completed a number of other cognitive and behavioural tasks in the scanner during the session, as part of a wider study into PTSD, including a test of mental flexibility (Dunkley et al., 2015; Pang et al., 2014), a test of rapid serial visual attention (Todd et al., 2015), as well as a task-free resting-state recording (Dunkley et al., 2014; Dunkley et al., 2015b). All participants had normal or corrected-to-normal visual acuity. All participants gave prior informed consent and were initially approached by a military clinician if they wished to participate in the study. Their names were then passed to a research assistant, who established contact to see if they were still willing to participate in the study. All procedures were approved by the Hospital for Sick Children and Canadian Armed Forces Research Ethics Boards, and the soldiers gave informed written consent.
Inclusion criteria for the PTSD group were: a clinical diagnosis of PTSD at a Canadian operational trauma stress support centre (OTSSC) as determined by a psychiatrist or psychologist specializing in trauma-related mental health injuries; PTSD symptoms present between 1 and 4 years prior to taking part in the study; regular mental health follow-ups; and current PTSD check list (PCL-Military version) scores of >50, indicating the presence of moderate to severe PTSD.
The diagnosis was determined through a comprehensive, semi-structured interview with a clinician based upon DSM-IV-TR diagnostic criteria (American Psychiatric Association, 2000), along with Canadian Armed Forces (CAF) standardized psychometric testing. All participants in the PTSD group were recruited from one of the CAF OTSSCs. There were usually more than one DSM-IV-TR ‘A1’ stressor-related criteria (American Psychiatric Association, 2000) identified as a traumatic event contributing to the development of PTSD (direct personal experience of an event that involves actual or threatened death or injury), with diagnosis related to operational exposure. Control soldiers were combat-exposed, frontline troops in similar military roles, and selected from cohorts of comparable rank, education level, handedness and military experience. An additional inclusion criterion applied to both groups was no history of a traumatic brain injury (TBI), as screened by a psychiatrist through a review of their electronic health record, telephone interview, and administration of the Defence and Veteran’s Brain Injury Centre (DVBIC) screening tool.
Exclusion criteria for both groups included ferrous metal inside the body or implanted medical devices that might be MRI contraindications or interfere with MEG data acquisition; seizures or other neurological disorders; certain ongoing medications (anticonvulsants, and/or benzodiazepines, or other GABA antagonists) known to directly or significantly influence M/EEG findings. This was a naturalistic study and we accepted PTSD participants undergoing treatment including evidenced-based psychotropic medication(s), such as selective serotonin reuptake inhibitors (SSRIs), serotonin-norephedrine reuptake inhibitors (SNRIs), and Prazosin.
2.2. Cognitive-behavioural evaluation
All subjects completed short cognitive-behavioural assessments, including the Generalized Anxiety Disorder 7-item Scale (GAD7) for anxiety, Patient Health Questionnaire (PHQ9) for depression, and those with PTSD, the Post Traumatic Stress Disorder Check List Military Version (PCL-M). PTSD soldiers had increased anxiety (p < 0.001) and depression (p < 0.001) (Table 1), compared to Control soldiers, consistent with their PTSD diagnosis.
Table 1.
Mean and standard deviation for cognitive-behavioural outcome measures in PTSD and Control Soldiers.
| PTSD | Control | Test statistic | |
|---|---|---|---|
| n | 20 | 25 | |
| Age | 37.67 (1.39) | 33.97 (0.98) | |
| Handedness (R:L) | 18:02 | 22:03 | |
| GAD-7 | 15.25 (4.23) | 2.24 (2.31) | t = 13.14, df = 43, p < 0.001 |
| PHQ9 | 16.90 (4.19) | 2.24 (2.59) | t = 14.41, df = 43, p < 0.001 |
| PCL | 63.0 (7.58) | NA |
2.3. Procedure
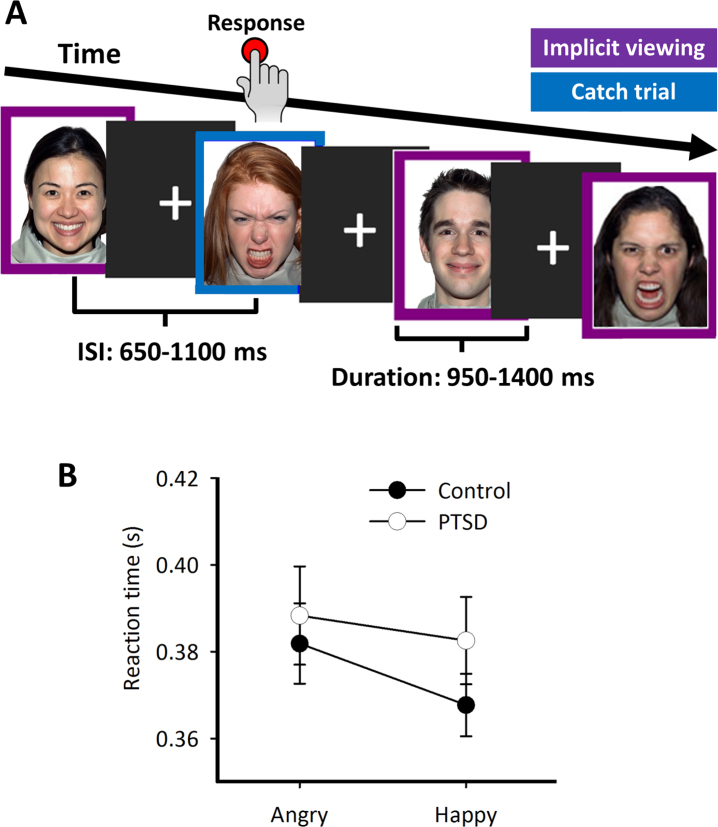
Participants completed an implicit emotional face processing task (Fig. 1A) that contained 26 different faces taken from the NimStim Set of Facial Expressions (http://www.macbrain.org/resources.htm); Tottenham et al., 2009). Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Please contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set. Each face was shown for both types of emotion (happy and angry, giving 52 faces in total), which were contained within a purple or blue border. Participants were instructed, both outside the scanner during a short practice run (up to 10 trials), and inside the scanner just before the experimental run, to ignore the faces, and concentrate on the border around a face. They were directed to press a button as quickly as possible each time their pre-defined target colour was displayed, which they were told for the practice run and reminded before the experimental run. These ‘catch trials’ were included to maintain the participants’ attention (comprising 25% of the total trial count). Catch trials were only used for the analysis of reaction time to behaviourally-categorise participants’ responses to emotional faces, and only correct (i.e., no response) implicit/passive trials were used in the imaging analysis; the rationale for this was to avoid large evoked motor responses which occur to the catch trials and would obscure more subtle cognitive activity related to implicit face processing.
Fig. 1.
Experimental schematic and catch-trial reaction times. (A) During the implicit emotional face processing task, participants were instructed to ignore the faces and attend to the colour of the frame surrounding the face. In this example, a purple border signifies a passive viewing trial, and the blue border signifies a catch trial, for which responses were to made as fast as possible. (B) Reaction time for correct hits on catch trials. No significant main effects or interactions were observed, although there was a slight trend for faster responses in the control group, especially when presented with happy faces.
The inter-stimulus interval (ISI) and stimulus duration were adjusted in real-time using a modified staircase procedure (based on global/long-term and local/short-term accuracy, calculated by hits on target trials and false alarms on non-target trials) to maintain a stable error rate (∼5% for catch trials). The procedure was run until 80 correct non-catch face trials (implicit face processing) were collected, 40 for each type of emotion. Between trials, participants fixated a centrally-presented cross. The experimental protocol was programmed using Presentation® software (www.neurobs.com) and projected via a back projection screen (42w × 32 h cm) placed 78 cm from the participants’ eyes. The stimuli were foveal, with a size of 7.4w × 9 h cm (with a 2 cm thick border), and subtended ∼14 × 16° of visual angle. This protocol lasted for approximately 2–3 min.
2.4. MEG data acquisition
MEG data were collected inside a magnetically-shielded room on a CTF Omega 151 channel system (CTF Systems, Inc., Coquitlam, Canada) at 600 Hz with third-order spatial gradient noise cancellation, at the Hospital for Sick Children. Throughout the run, head position was continuously recorded by three fiducial coils placed on the nasion, and left and right pre-auricular points. Moreover, sensor time series data were visually inspected and significant artefacts related to head-motion resulted in the removal of a trial from subsequent analysis. This visual inspection was supplemented by head-movement recordings to confirm such observations, with trials displaying >5 mm head motion being excluded from subsequent analysis (any potential system-related artefacts are investigated before any experimental MEG data is recorded, with bad channels being omitted from any recordings). MEG data were band-pass filtered offline at 1–150 Hz using a finite impulse response (FIR) filter, with a bandstop notch filter applied at the 60 Hz powerline frequency.
After the MEG session, anatomical 3T MRI images were acquired (Magnetom Tim Trio, Siemens AG, Erlangen, Germany), which were T1-weighted magnetic resonance images using high-resolution 3D MPRAGE sequences on a 12 channel head coil. MEG data were coregistered to the MRI structural images using the reference fiducial coil placements.
2.5. MEG processing
2.5.1. Connectivity analysis
This study used a seed-based approach to connectivity, where the Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) was used to identify 90 sources (seeds) in cortical and subcortical regions. Defining the source solution to these locations provides reasonable coverage of anatomically-parcellated regions and has shown reliability in studying large-scale network dynamics for functional connectivity analyses (Doesburg et al., 2013; Dunkley et al., 2015). These coordinates defined locations for time-series to be extracted and analysed. These standardised coordinates were unwarped from Montreal Neurological Institute (MNI) space into individual space using Advanced Normalization Tools (ANTs; http://stnava.github.io/ANTs/), and broadband (1–150 Hz) time-series from these 90 voxels were reconstructed using an implementation of the Linearly Constrained Minimum-Variance (LCMV) vector beamformer (Van Veen et al., 1997; Sekihara et al., 2001), with noise normalization implemented by conversion of the signal from physical units (Ampere-meter) to pseudo-z. This beamformer implementation is a type of adaptive spatial filter, or inverse source modeling method, that minimizes total brain power (i.e., suppresses the contribution of signal from areas beyond the region-of-interest), whilst being optimally sensitive to activity in a given brain location (in this case, each of the 90 AAL seed locations). Individual weight vectors are applied to each sensor measurement and summated to derive estimated source activity at the seed location. This output, often called a ‘virtual electrode’ or ‘virtual sensor’, can be envisaged as source-level signals (that is, from the brain), and are analogous to what one might expect if there were an electrode in that particular cortical location. Furthermore, because MEG beamformers are spatial filters, they are effective at suppressing ocular artefacts generated by eye movements (in particular, blinks), as well as other non-ocular physiological artefacts, such as cardiac and muscle activity (Muthukumaraswamy, 2013), therefore rejection of trials showing these specific artefacts is not required in this case. These time-series were then filtered into 2.344 Hz frequency bins (64 bins in total covering the 1–150 Hz signal).
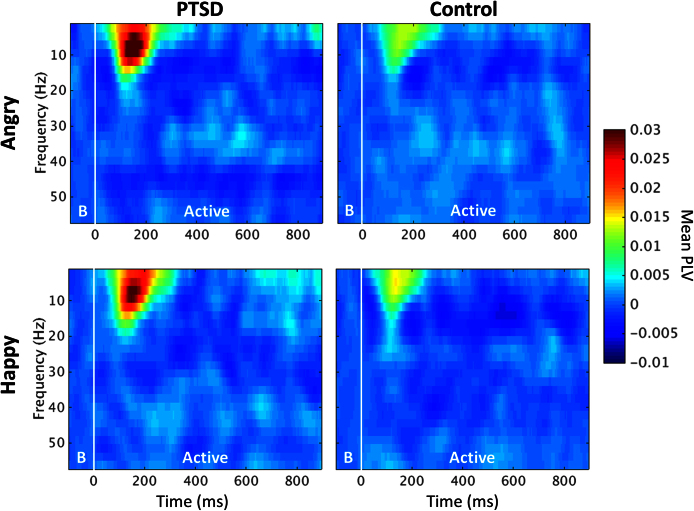
The instantaneous phase of each sample from the filtered time-series bins was calculated using a short-time Fourier Transform (STFT) over a 200 ms sliding window from -200 ms to 1000 ms in 5 ms steps using the time-frequency decomposition implementation in the EEGLAB toolbox (Delorme and Makeig, 2004); hence Fig. 2 and Fig. 3 show phase synchrony from -100 ms to 900 ms, as the PLV estimate was calculated at the centre point of the moving time window. Each time-series of the instantaneous phase estimate for each frequency bin of the filtered waveforms was then submitted to functional connectivity analysis by calculating the cross-trial phase-locking value (PLV; Lachaux et al., 2012). The PLV was derived for each frequency-bin phase angle time-series from the degree of phase synchronization for every sample point between all pairwise combinations of the pre-defined seed regions. In other words, the PLV estimates the regularity or consistency of the phase angle of the oscillating time-series from two brain regions; brain regions that oscillate together are thought to be communicating-through-coherence (Fries, 2005), and in this fashion, the brain is transferring information between areas. The PLV ranges between 0 and 1, and these values quantify the degree of phase-locking between two sources (‘0’ being non-phase locked, or no phase relationship; ‘1’ being phase-locked, or oscillating in perfect harmony), which is referred to as functional connectivity.
Fig. 2.
Time-frequency spectrograms of phase-locking values for implicit face processing trials for PTSD (left column) and control soldiers (right column), in the angry (top row) and happy (bottom row) conditions. PLV values during the ‘Baseline’ period (denoted by the B, -100 to 0 ms) were subtracted from the ‘Active’ window (0 to 900 ms). Warm colours indicate an increase in phase locking relative to baseline, or ‘ongoing’, phase synchrony. Both groups showed stimulus-dependent (presented at 0 ms) increases in inter-regional phase locking that peak around 175 ms, evident when viewing both types of emotional faces, although these phase-locking responses are particularly pronounced in the PTSD group.
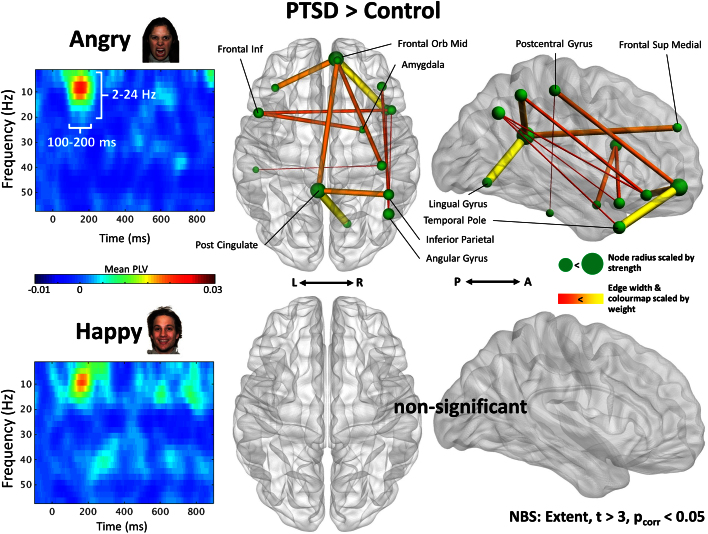
Fig. 3.
Group difference (PTSD minus control) spectrograms (left) and functional connectivity contrasts (right). Time-frequency representations for PTSD minus control in the angry (top) and happy (bottom) trials show relatively enhanced 2–24 Hz increase in PLV for PTSD in the 100–200 ms time window, more prominent for angry faces. Connectivity analysis of temporally- and frequency-averaged adjacency matrices revealed significant increases in PLV in the PTSD group compared to controls for angry (t > 3, pcorr < 0.05), but not happy (pcorr > 0.05), facial expressions. These elevated interactions were particularly evident for connections involving the posterior cingulate, and the right medial frontal orbital cortex. The node radius denotes the strength of the connections for the vertices, whilst the connection width and colour warmth (yellow is greater, red is less) signifies the edge weight between regions.
Adjacency matrices with PLV values acting as edge weights for all sources were constructed for every frequency bin and at each phase angle sample point. This resulted in a 90 × 90 [×64 frequencies] [×200 samples] weighted undirected adjacency graph for each participant. For the generation of statistically-thresholded functional connectivity images, temporally-averaged adjacency matrices over time windows of interest were generated, and from these the elementwise mean baseline (-100 to 0 ms, for each frequency bin) adjacency matrix PLV value was subtracted, to give a baseline-corrected estimate of synchrony for each connection/edge specifically related to face processing.
Statistical analyses were performed on the resulting baseline-corrected matrices using the Network Based Statistic (NBS; Zalesky et al., 2010). NBS first applies an initial univariate threshold to each analyzed edge. The extent of connectivity components, defined as contiguous groups of nodes connected by suprathreshold connections, is then obtained. Group membership is then shuffled and the extent of the largest component which occurs in this surrogated data is recorded, and this process is repeated 5000 times to generate a null distribution. The ranking of connectivity components from the unshuffled data in the surrogate distribution is used to determine statistical confidence; as the surrogate distribution considers the largest connectivity component that could occur, assuming the null hypothesis, across the entire analyzed network. This approach controls for false positives due to multiple comparisons at any threshold. In the present analysis, the initial univariate threshold was set at a moderate t-value of 3 (Zalesky et al., 2012; Zalesky et al., 2010). Further measures of interest, such as graph theoretical/brain connectivity metrics, were derived from the Brain Connectivity Toolbox (Rubinov and Sporns, 2010), and functional brain networks were visualized using BrainNet Viewer (Xia et al., 2013).
3. Results
3.1. Task performance
Reaction times (RT) for correct hits on target trials are shown in Fig. 1B. RT measures were submitted to a 2 × 2 mixed factorial ANOVA with ‘Group’ (PTSD and control) as the between-participant variable and ‘Condition’ (Angry and Happy faces) as the within-participant variable. There were no significant main effects (Group effect: F(1,43) = 0.75, p = 0.39; Condition effect: F(1,43) = 3.76, p = 0.059) or interaction effects (F = 0.67, p = 0.417).
3.2. Spectral characteristics of connectivity
A time-frequency analysis of mean, whole-brain inter-regional phase-locking values for implicit face processing (i.e., attention was not directed to the faces) was conducted to identify frequency- and time-specific bins to be used in an exploratory between-groups connectivity analysis. Spectrograms showing whole-brain, mean trial PLV (-100 to 900 ms) can be seen in Fig. 2 for PTSD and control soldiers, for both emotions. Each 2.34 Hz PLV times-series frequency bin was independently baseline-corrected using the mean PLV in the -200 to 0 ms pre-stimulus baseline time interval for that frequency bin.
This analysis revealed global increases in inter-regional phase-locking below 20 Hz from 100–200 ms following stimulus onset. Visual assessment suggests relative increases in PLV for the PTSD group compared to control soldiers, but no apparent specificity for facial expression, appearing approximately equal for happy and angry faces when comparing within groups.
A formal connectivity analysis of temporally- (100–200 ms) and frequency- (2–24 Hz) averaged adjacency matrices revealed significant increases in phase locking in the PTSD group compared to controls for angry (pcorr < 0.05), but not happy (pcorr > 0.05), facial expressions. For angry faces, increased interactions between a variety of network nodes was observed for the soldiers with PTSD, including the posterior cingulate, right parietal and temporal regions, and the right amygdala; areas within the frontal cortex, and especially the medial prefrontal cortex, also showed increased connectivity, contrary to our initial hypothesis that these regions would be hypoconnected in PTSD.
In Fig. 3, edge weights are scaled by connection width and colour warmth (highest in yellow, lowest in red); in other words, the greater the difference in strength between PTSD and control groups (PTSD > controls), the thicker and warmer the edge. Notably large weight differences were observed for the posterior cingulate-lingual gyrus connection, and the right temporal pole-medial frontal orbital connection. Additional contrasts were conducted for Control > PTSD, but revealed no significant differences in network interactions (pcorr > 0.05). The size of nodes in Fig. 3 reflect connectivity strength, which is the sum of the connection weights binding a region to the extant network component; the higher the connectivity strength of a node, the greater the radius. A list of significant nodes identified by the NBS analysis, as well as their MNI coordinates and component strength, can be found in Table 2.
Table 2.
Regions showing significant increases in 2–24 Hz connectivity in PTSD in response to angry faces. Node strength is derived from connectivity measures from the seed region to the differential network/connected graph component identified by the NBS analysis and shown in Fig. 3.
| Region name | x | y | z | Node strength |
|---|---|---|---|---|
| L Post Cingulate | -6 | -43 | 25 | 0.155 |
| R Medial Frontal Orbital | 7 | 52 | -7 | 0.150 |
| R Angular Gyrus | 45 | -60 | 39 | 0.098 |
| R Middle Temporal Pole | 43 | 15 | -32 | 0.089 |
| R Inferior Parietal | 45 | -46 | 50 | 0.088 |
| L Inferior Frontal Operculum | -49 | 13 | 19 | 0.084 |
| R Postcentral Gyrus | 40 | -25 | 53 | 0.074 |
| R Inferior Frontal Orbital | 40 | 32 | -12 | 0.072 |
| R Superior Temporal Pole | 47 | 15 | -17 | 0.072 |
| R Lingual Gyrus | 15 | -67 | -4 | 0.058 |
| L Inferior Frontal Orbital | -37 | 31 | -12 | 0.050 |
| R Medial Superior Frontal | 8 | 51 | 30 | 0.047 |
| R Amygdala | 26 | 1 | -18 | 0.044 |
| L Inferior Temporal | -51 | -28 | -23 | 0.031 |
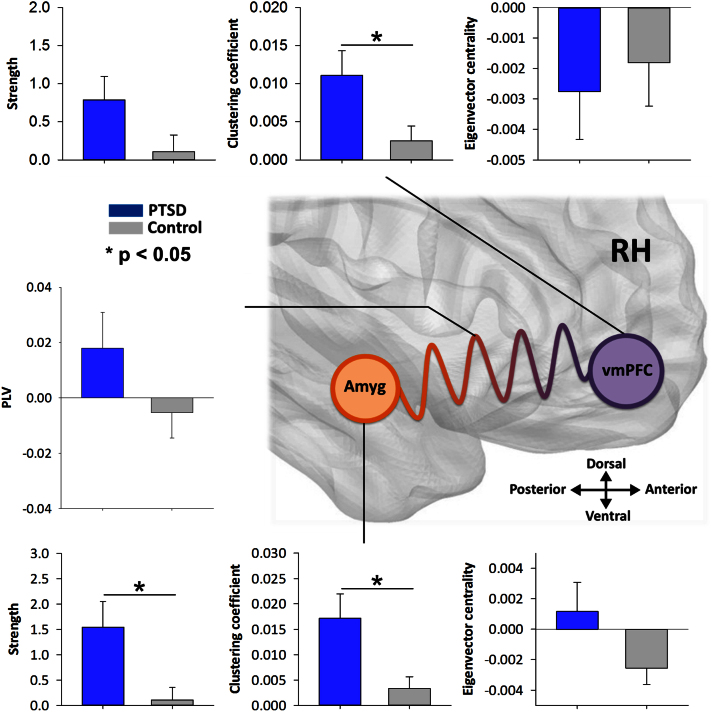
3.3. Graph analysis of right amygdala and medial prefrontal connectivity for threatening faces
In addition to our data-driven results showing increased interactions during threat-related face perception in PTSD, we characterised the network role of amygdala - medial prefrontal connectivity in the right hemisphere in emotional face processing (see Fig. 4). This was achieved by calculating a number of graph theoretic measures of node properties for these regions, including overall strength (the sum of the edge weights connecting a node to the rest of the whole-brain network), clustering coefficient (the node’s degree of functional embeddedness within the network), and eigenvector centrality (the role of the node as a communication hub, or how many of its connected nodes also have many connections). These graph measures were derived from the baseline-corrected PLV estimate for a node’s cross-trial phase synchronisation across the entire network (seed-to-whole-brain connectivity). Independent two-tailed t-tests revealed increased strength in the right amygdala in PTSD compared to controls (t(43) = 2.71, p < 0.05), as well as clustering (t(43) = 2.78, p < 0.05), but no significant difference in eigenvector centrality (t(43) = 1.79, p = 0.081). For the medial prefrontal cortex, there was significantly greater clustering in this region for the PTSD group compared to controls (t(43) = 2.37, p = 0.022), but not for strength (t(43) = 1.84, p = 0.07), or centrality (t(43) = -0.45, p = 0.66). Finally, the single, direct edge weight (PLV estimate) connecting the right amygdala and the medial prefrontal seed was compared, and no significant difference was found between the PTSD and control group, (t(43) = 1.50, p = 0.14).
Fig. 4.
Region-of-interest graph analysis of right amygdala and medial prefrontal cortex seeds for the viewing of angry faces, in PTSD (blue bars) and controls (grey bars), as well as the degree of oscillatory synchronisation between those two regions. Values are baseline-corrected against the pre-stimulus window. Significant differences denoted by *p < 0.05.
3.4. Angry-face dynamic network topology and comorbid symptom severity
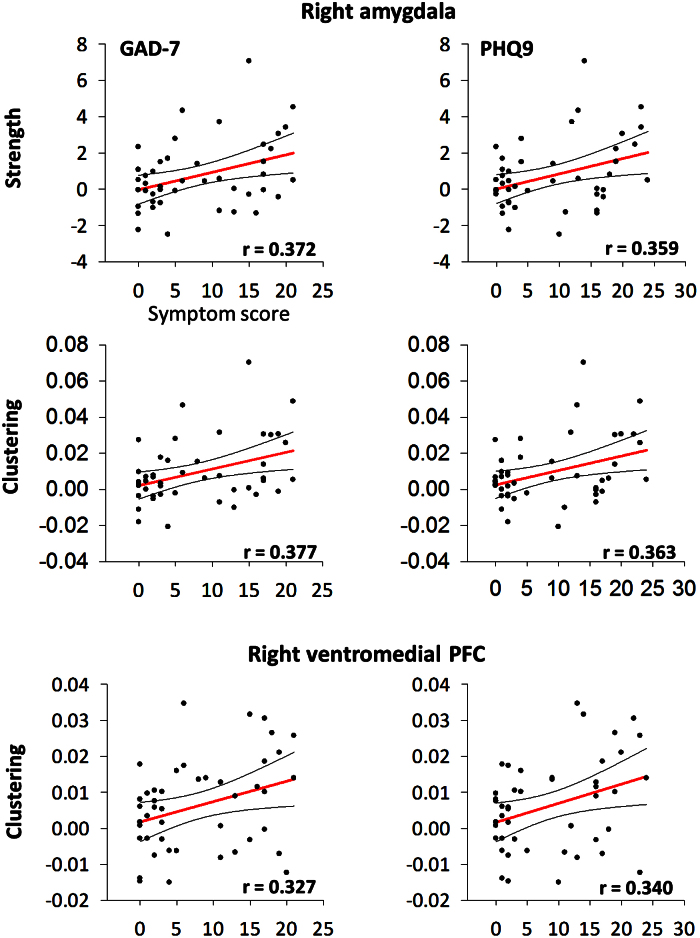
To investigate whether the network topology in the right amygdala and right ventromedial prefrontal cortex was related to symptoms, correlations of PTSD severity (PCL, PTSD group only), anxiety (GAD-7, combined groups), and depression (PHQ9, combined groups) versus node strength, clustering and eigenvector centrality were computed (see Fig. 5). No significant relations were found for any PCL correlation (either by node or graph measure; all p’s > 0.05). For anxiety, significant correlations were found for right amygdala strength (r = 0.372, p = 0.012) and clustering (r = 0.377, p = 0.011), as well as the right vmPFC clustering (r = 0.327, p = 0.029). For depression, significant correlations were observed for right amygdala strength (r = 0.359, p = 0.016) and clustering (r = 0.363, p = 0.014), and the right vmPFC clustering (r = 0.340, p = 0.022).
Fig. 5.
Scatterplots showing significant correlations (all p’s < 0.05) of comorbird symptom severity for anxiety (GAD-7; left column) and depression (PHQ9; right column), versus dynamic network topology (strength and clustering measures) in the right amygdala (top and middle) and right ventromedial prefrontal cortex (bottom) for both groups. Red line shows linear least squares, curved black lines show 95% confidence interval estimates.
4. Discussion
We present evidence of increased neurophysiological network interactions in PTSD when compared to a group of matched control soldiers during the perception of threatening faces. Specifically, soldiers with PTSD, compared with combat-matched control soldiers, exhibited increased 2–24 Hz phase locking between regions when viewing affective, angry faces, a differential response that was absent when viewing happy faces. As predicted and in line with previous findings, the right amygdala showed enhanced connectivity compared to our trauma-exposed control group, but unexpectedly, we also observed increased connectivity in the prefrontal medial cortex when using a network-based approach. These observations were confirmed by a region-of-interest analysis on these a priori seeds, in which we examined the dynamic network topology of these nodes; additionally, significant relationships between graph measures and comorbid symptoms were also observed (that of anxiety and depression).
fMRI studies of affective face processing in PTSD previously reported elevated amygdala responses and decreased medial prefrontal cortex activation (Shin and Orr, 2004; Shin and Wright, 2005), which were consistent with the neurobiological models of the disorder that postulated that disengaged, hypoconnected frontal circuits (top-down control) fail to inhibit hyperresponsive amygdalae (Shin et al., 2006; Simmons et al., 2011), critical in the fear circuitry. This was theorised to be one of the principal reason for maladaptive threat responses and emotional dysregulation in the disorder, and similar findings were reported in a combat PTSD population recently (Simmons et al., 2011). Simmons and colleagues reported that fMRI connectivity was relatively greater within prefrontal-amygdala interactions in the combat-exposed group compared to patients, supporting the widespread model of PTSD fronto-limbic disinhibition. It has also been reported that a group of female intimate-partner violence PTSD patients exhibit increased amygdala and medial prefrontal fMRI responses to angry faces, but only when matching to a male versus female target (Fonzo et al., 2010). In contrast to the data reported here, Fonzo and colleagues did report decreased amygdala connectivity with the insula, but greater connectivity with the ACC; in other words, they observed hyperactivity in affective and limbic regions, but bidirectional alterations in amygdala connectivity throughout other areas of the cortex.
Other studies using implicit and unconscious emotional face processing tasks, however, have shown elevated amygdala responses in response to affective facial stimuli, but also increased prefrontal activation patterns (Bryant et al., 2008; Fani et al., 2012; Bruce et al., 2013), which, similar to our data, would be largely inconsistent with the hypothesised fear circuitry model of PTSD. It has been proposed that these unexpected medial prefrontal responses might be due to processing of unconscious facial expressions, which might explain these findings given the nature of the task (ignore the faces and attend to the border, react to the 25% of trials; however, it is difficult it ignore emotional faces, and many studies use implicit face processing tasks (e.g., Stefanics et al., 2012; Brennan et al., 2014; Batty et al., 2011; Frühholz et al., 2011). Bryant et al. (2008) suggests that the fronto-limbic theory of disinhibition and attentional control in PTSD therefore may only apply to consciously perceived threats. Despite this, certain caveats must be remembered. These previous studies used fMRI, which while affording excellent spatial resolution, offers little temporal sensitivity with connectivity at very low frequencies of <0.1 Hz, as well as only measuring indirect neural function by way of associated haemodynamics. Critically, the current MEG results reflect neurophysiological interactions which fMRI is unable to image directly. Accordingly, we believe this study contributes a significant advance to our understanding of the cortical substrates of threatening face perception in PTSD.
As well as differences between groups in our whole-brain network-level analyses, we also observed significant differences in contrasts between groups for graph measures of network topology in ROI regions, the right amygdala and vmPFC. Moreover, significant positive correlations with secondary symptoms (specifically, anxiety and depression) were also observed in these areas. The right vmPFC seed showed significantly higher clustering in our PTSD group during the perception of angry faces. In terms of the functional significance of this, the clustering coefficients reflects the local connectedness or embededness of a node in the network as a whole, and measures the fraction of the node’s neighbours that are also neighbours of each other; in other words, this is the regional segregation of that node. This means that the vmPFC is locally highly connected during this time, which could reflect the engagement of neural circuits required for inhibitory control/suppressions of responses during emotion processing (Hänsel and von Känel, 2008), vigilance and/or overt attentional control for face recognition (Wolf et al., 2014), and decision making (Bechara et al., 2000).
Interestingly, there was no difference in centrality in this seed, but both groups showed decreased centrality relative to baseline measures. Centrality refers to a node’s relative importance in the network, and indicates the degree to which that vertex acts as a ‘hub’. Important structural brain regions (in terms of centrality) mediate interactions between anatomically distinct, but functionally coupled, brain regions, facilitating the integration of information, and thus switching to a less ‘hub-like’ state might indicate a shift in the organisation principles of the network. In other words, the organisation of information in frontal regions sees the vmPFC go from acting as a centralised relay station, to more diffuse, parallelised, processing principles (Rubinov and Sporns, 2010).
As expected, the right amygdala showed increased strength and clustering in our PTSD group when viewing angry faces. In terms of the functional interpretation, clustering signifies local connectivity increases, and strength has a relatively straightforward neurobiological interpretation: nodes with increased high strength are more functionally interactive with other nodes in the network. In essence, the PTSD group showed both local and large-scale/global increases in communication during angry face processing, measured by clustering and strength, respectively.
4.1. Limitations
These findings should been interpreted considering some limitations. First, we cannot completely exclude the influence of confounding symptoms on our results; the high incidence of anxiety, depression and attention problems in our PTSD group could have contributed to the results shown here. However, given that PTSD was the primary diagnosis in all cases and that these associated sequelae are part of the PTSD symptom clusters (especially anxiety, given that the DSM-IV considered PTSD an anxiety-related disorder), we believe that PTSD is likely the principle factor in the emotional face processing connectivity alterations we observed.
Second, we observed no difference in reaction times to emotional faces between groups, yet we observed distinct brain connectivity profiles. This is at odds with literature which suggests those with PTSD exhibit behavioural-biases to emotional face perception. It could be that the task demands of concentrating visuo-spatial attention on the bordering stimuli negated perceptual biases to the faces. Such effects are more often reported with directed rather than implicit processing of emotional faces; recent research also suggests inconsistent reports of emotional face biases might be explained by stimulus selection (Savage et al., 2015)
Third, the observation that angry faces induces hyperconnectivity in PTSD compared with matched controls does not equate to angry faces inducing increased connectivity compared to happy faces. Such inference would require formally comparing these conditions within subjects, or using a factorial design, this caveat remains, and should be remembered in the interpretation of these results (Gelman and Stern, 2006).
Finally, given that the phase angle is estimated over a 200 ms moving time-window, it likely only captures spectral changes reliably that occur at 5 Hz and above. Therefore, apparent changes phase synchrony occurring below this threshold should be interpreted with caution.
5. Conclusions
From these results we conclude that 1) inter-regional phase synchronization, a mechanism known to be directly involved in cognition, mediates implicit emotional face processing by regulating the integration of cognitive contents across functionally-distinct brain regions; and 2) combat-experienced soldiers with PTSD exhibit elevated neurophysiological network interactions in response to threatening face stimuli when compared to a trauma-exposed control group. Right amygdala hyperconnectivity was observed in PTSD, but contrary to expectations, the medial prefrontal cortex also displayed enhanced connectivity. Overall, these results support some previous findings proposing preferential encoding of threat-related facial expressions. They also question, however, previous hypotheses positing that an underactive, disinhibited medial prefrontal circuit is responsible for heightened amygdala responses and emotional dysregulation, and that in fact this atypical fronto-limbic connectivity may only apply for the explicit processing of threat-related stimuli. The sensitivity of MEG network synchronization provides a fresh perspective on cortical processing in PTSD; the application of graph theory and network science to test the frontal-limbic circuitry model of PTSD has generated novel information previously inaccessible to conventional hemodynamic measures.
Declarations
Author contribution statement
Paul A. Sedge: Conceived and designed the experiments.
Rakesh Jetly: Conceived and designed the experiments; Wrote the paper.
Margot J. Taylor and Elizabeth W. Pang: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Benjamin T. Dunkley and Sam M. Doesburg: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by funding from Defence Research and Development Canada (DRDC) (Contract #: W7719-135182/001/TOR) to MJT and EWP, and the Canadian Forces Health Services.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Thank you to Marc Lalancette, Amanda Robertson and Richard Grodecki for help in the data collection, Daniel Cassel for help with data analyses, and Simeon Wong for his helpful comments.
References
- American Psychiatric Association Diagnostic and statistical manual of mental disorders. 2000 [Google Scholar]
- American Psychiatric Association Diagnostic and statistical manual of mental disorders. 2013 [Google Scholar]
- Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. Executive function and PTSD: disengaging from trauma. Neuropharmacol. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty M., Meaux E., Wittemeyer K., Rogé B., Taylor M.J. Early processing of emotional faces in children with autism. J. Exp. Child Psychol. 2011;109(4):430–444. doi: 10.1016/j.jecp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Bechara A., Tranel D., Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Brennan A.M., Harris A.W., Williams L.M. Neural processing of facial expressions of emotion in first onset psychosis. Psychiat. Res. 2014;219(3):477–485. doi: 10.1016/j.psychres.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Boulos D., Zamorski M.A. Deployment-related mental disorders among Canadian Forces personnel deployed in support of the mission in Afghanistan, 2001–2008. Can. Med. Assoc. J. 2008;185:E545–E552. doi: 10.1503/cmaj.122120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce S.E., Buchholz K.R., Brown W.J., Yan L., Durbin A., Sheline Y.I. Altered emotional interference processing in the amygdala and insula in women with Post-Traumatic Stress Disorder. Neuroimage Clin. 2013;2:43–49. doi: 10.1016/j.nicl.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant R.A., Kemp A.H., Felmingham K.L., Liddell B., Olivieri G., Peduto A., Williams L.M. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum. Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Wang X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Scott Steele J., Smitherman S., Lenow J.K., Kilts C.D. Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: a network-level analysis among adolescent girls. Psychiat. Res. 2013;214(3):238–246. doi: 10.1016/j.pscychresns.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish T., Taghavi R., Neshat-Doost H., Moradi A., Canterbury R., Yule W. Patterns of processing bias for emotional information across clinical disorders: a comparison of attention, memory, and prospective cognition in children and adolescents with depression, generalized anxiety, and posttraumatic stress disorder. J. Clin. Child Adolesc. 2003;32(1):10–21. doi: 10.1207/S15374424JCCP3201_02. Division 53. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Meth. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Doesburg S.M., Vidal J., Taylor M.J. Reduced Theta Connectivity during Set-Shifting in Children with Autism. Front. Hum. Neurosci. 2013;7:785. doi: 10.3389/fnhum.2013.00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner T.H., Siegel M. A framework for local cortical oscillation patterns. Trends Cogn. Sci. 2011;15(5):191–199. doi: 10.1016/j.tics.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Dunkley B.T., Doesburg S.M., Sedge P.A., Grodecki R.J., Shek P.N., Pang E.W., Taylor M.J.J. Resting-state hippocampal connectivity correlates with symptom severity in post-traumatic stress disorder. Neuroimage Clin. 2014;5:377–384. doi: 10.1016/j.nicl.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley B.T., Sedge P.A., Doesburg S.M., Grodecki R.J., Jetly R., Shek P.N., Taylor M.J., Pang E.W. Theta, mental flexibility, and post-traumatic stress disorder: connecting in the parietal cortex. PLoS ONE. 2015;10(4):e0123541. doi: 10.1371/journal.pone.0123541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley B.T., Doesburg S.M., Jetly R., Sedge P.A., Pang E.W., Taylor M.J. Characterising intra- and inter-intrinsic network synchrony in combat-related post-traumatic stress disorder. Psychiatry Res. Neuroimaging. 2015;234(2):172–181. doi: 10.1016/j.pscychresns.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Fani N., Jovanovic T., Ely T.D., Bradley B., Gutman D., Tone E.B., Ressler K.J. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol. Psychol. 2012;90:134–142. doi: 10.1016/j.biopsycho.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K.L., Falconer E.M., Williams L., Kemp A.H., Allen A., Peduto A., Bryant R.A. Reduced Amygdala and Ventral Striatal Activity to Happy Faces in PTSD Is Associated with Emotional Numbing. PLoS ONE. 2014;9:e103653. doi: 10.1371/journal.pone.0103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G.A., Simmons A.N., Thorp S.R., Norman S.B., Paulus M.P., Stein M.B. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo G.A., Flagan T.M., Sullivan S., Allard C.B., Grimes E.M., Simmons A.N., Paulus M.P., Stein M.B. Neural functional and structural correlates of childhood maltreatment in women with intimate-partner violence-related posttraumatic stress disorder. Psychiatry Res. 2013;211(2):93–103. doi: 10.1016/j.pscychresns.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Frühholz S., Jellinghaus A., Herrmann M. Time course of implicit processing and explicit processing of emotional faces and emotional words. Biol. Psychol. 2011;87(2):265–274. doi: 10.1016/j.biopsycho.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Gelman A., Stern H. The Difference Between Significant and Not Significant is not Itself Statistically Significant. Am. Stat. 2006;60(4):328–331. [Google Scholar]
- Hänsel A., von Känel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? BioPsychoSoc. Med. 2008;2(1):21. doi: 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.A., Langlais P.J., Delis D.A., Cohen R.A. Attentional dysfunction associated with posttraumatic stress disorder among rape survivors. Clin. Neuropsychol. 2000;14:7–12. doi: 10.1076/1385-4046(200002)14:1;1-8;FT007. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kolassa I.-T., Wienbruch C., Neuner F., Schauer M., Ruf M., Odenwald M., Elbert T. Altered oscillatory brain dynamics after repeated traumatic stress. BMC Psychiatry. 2007;7:56. doi: 10.1186/1471-244X-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux J.-P., Axmacher N., Mormann F., Halgren E., Crone N.E. High-frequency neural activity and human cognition: past, present and possible future of intracranial EEG research. Prog. Neurobiol. 2012;98:279–301. doi: 10.1016/j.pneurobio.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskin L.P., White P.M. Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology. 2007;21:275–284. doi: 10.1037/0894-4105.21.3.275. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D. High-frequency brain activity and muscle artifacts in MEG/EEG: a review and recommendations. Front. Hum. Neurosci. 2013;7(April):138. doi: 10.3389/fnhum.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montez T., Poil S.-S., Jones B.F., Manshanden I., Verbunt J.P.A., van Dijk B.W., Linkenkaer-Hansen K. Altered temporal correlations in parietal alpha and prefrontal theta oscillations in early-stage Alzheimer disease. P. Natl. Acad. Sci. USA. 2009;106:1614–1619. doi: 10.1073/pnas.0811699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey R.A., Dolcos F., Petty C.M., Cooper D.A., Hayes J.P., LaBar K.S., McCarthy G. The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J. Psychiat. Res. 2009;43:809–817. doi: 10.1016/j.jpsychires.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport D.J., Nemeroff C.B. Neurobiology of posttraumatic stress disorder. Curr. Opin. Neurobiol. 2000;10(2):211–218. doi: 10.1016/s0959-4388(00)00080-5. [DOI] [PubMed] [Google Scholar]
- Palva S., Palva J.M. New vistas for α-frequency band oscillations. Trends Neurosci. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Palva S., Palva J.M. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front. Psychol. 2011;2:204. doi: 10.3389/fpsyg.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang E.W., Sedge P., Grodecki R., Robertson A., MacDonald M.J., Jetly R., Taylor M.J. Colour or shape: examination of neural processes underlying mental flexibility in posttraumatic stress disorder. Transl. Psychiatry. 2014;4(8):e421. doi: 10.1038/tp.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Liberzon I. Biological studies of post-traumatic stress disorder. Nature Rev. Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Savage R.A., Becker S.I., Lipp O.V. Visual search for emotional expressions: Effect of stimulus set on anger and happiness superiority. Cogn. Emot. 2015:1–18. doi: 10.1080/02699931.2015.1027663. [DOI] [PubMed] [Google Scholar]
- Sekihara K., Nagarajan S.S., Poeppel D., Marantz A., Miyashita Y. Reconstructing spatio-temporal activities of neural sources using an MEG vector beamformer technique. IEEE Trans. Biomed. Eng. 2001;48(7):760–771. doi: 10.1109/10.930901. [DOI] [PubMed] [Google Scholar]
- Shin L., Orr S. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch. Gen. Psychiat. 2004:61. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin L., Wright C. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiat. 2005:62. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Rauch S.L., Pitman R.K. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. NY Acad. Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shucard J.L., McCabe D.C., Szymanski H. An event-related potential study of attention deficits in posttraumatic stress disorder during auditory and visual Go/NoGo continuous performance tasks. Biol. Psychol. 2008;79:223–233. doi: 10.1016/j.biopsycho.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M., Donner T.H., Engel A.K. Spectral fingerprints of large-scale neuronal interactions. Nat. Rev. Neurosci. 2012;13(February):20–25. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Simmons A.N., Matthews S.C., Strigo I.A., Baker D.G., Donovan H.K., Motezadi A., Stein M.B., Paulus M.P. Altered amygdala activation during face processing in Iraqi and Afghanistani war veterans. Biol. Mood Anxiety Disord. 2011;1(1):6. doi: 10.1186/2045-5380-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanics G., Csukly G., Komlósi S., Czobor P., Czigler I. Processing of unattended facial emotions: a visual mismatch negativity study. Neuroimage. 2012;59(3):3042–3049. doi: 10.1016/j.neuroimage.2011.10.041. [DOI] [PubMed] [Google Scholar]
- Tewarie P., Schoonheim M.M., Stam C.J., van der Meer M.L., van Dijk B.W., Barkhof F., Hillebrand A. Cognitive and clinical dysfunction, altered MEG resting-state networks and thalamic atrophy in multiple sclerosis. PloS One. 2013;8:e69318. doi: 10.1371/journal.pone.0069318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R.M., MacDonald M.J., Sedge P., Robertson A., Jetly R., Taylor M.J., Pang E.W. Soldiers with Posttraumatic Stress Disorder See a World Full of Threat: Magnetoencephalography Reveals Enhanced Tuning to Combat-Related Cues. Biol. Psychiat. 2015:1–9. doi: 10.1016/j.biopsych.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C. The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M.M., Vouimba R.M., Akirav I., Kavushansky A., Avital A., Richter-Levin G. Amygdala modulation of memory-related processes in the hippocampus: potential relevance to PTSD. Prog. Brain Res. 2008;167:35–51. doi: 10.1016/S0079-6123(07)67003-4. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Varela F., Lachaux J. The brainweb: phase synchronization and large-scale integration. Nature Rev. Neurosci. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Van Veen B.D., van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Von Stein A., Sarnthein J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000;38(3):301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Wolf R.C., Philippi C.L., Motzkin J.C., Baskaya M.K., Koenigs M. Ventromedial prefrontal cortex mediates visual attention during facial emotion recognition. Brain: A Journal of Neurology. 2014;137(Pt 6):1772–1780. doi: 10.1093/brain/awu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PloS One. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Cocchi L., Fornito A., Murray M.M., Bullmore E. Connectivity differences in brain networks. NeuroImage. 2012;60:1055–1062. doi: 10.1016/j.neuroimage.2012.01.068. [DOI] [PubMed] [Google Scholar]