Abstract
The synthesis of multiple amine neurotransmitters, such as dopamine, norepinephrine, serotonin, and trace amines, relies in part on DOPA decarboxylase (DDC, AADC), an enzyme that is required for normative neural operations. Because rare, loss-of-function mutations in the DDC gene result in severe enzymatic deficiency and devastating autonomic, motor, and cognitive impairment, DDC common genetic polymorphisms have been proposed as a source of more moderate, but clinically important, alterations in DDC function that may contribute to risk, course, or treatment response in complex, heritable neuropsychiatric illnesses. However, a direct link between common genetic variation in DDC and DDC activity in the living human brain has never been established. We therefore tested for this association by conducting extensive genotyping across the DDC gene in a large cohort of 120 healthy individuals, for whom DDC activity was then quantified with [18F]-FDOPA positron emission tomography (PET). The specific uptake constant, Ki, a measure of DDC activity, was estimated for striatal regions of interest and found to be predicted by one of five tested haplotypes, particularly in the ventral striatum. These data provide evidence for cis-acting, functional common polymorphisms in the DDC gene and support future work to determine whether such variation might meaningfully contribute to DDC-mediated neural processes relevant to neuropsychiatric illness and treatment.
Introduction
DOPA decarboxylase (DDC; aromatic L-amino acid decarboxylase) is a pyridoxal 5′-phosphate-reliant enzyme that facilitates the synthesis of several key neuroactive biogenic amines in the brain. DDC-mediated decarboxylation represents the last committed step in both dopamine and serotonin synthesis, the penultimate step in norepinephrine synthesis, and a critical process in the synthesis of several centrally expressed trace amines, including 2-phenylethylamine, for which it is rate limiting (Zhu and Juorio, 1995). The essential nature of this enzyme for central nervous system (CNS) physiology is evident in cases of rare, loss-of-function mutations in the DDC gene that lead to a devastating neurodevelopmental syndrome (DDC deficiency, OMIM #608643). In this condition, dramatic DDC enzymatic insufficiency results in a constellation of severe autonomic, motor, and cognitive impairments that manifest early in life (Brun et al, 2010).
The hypothesis that more common DDC genetic variation might induce subtler but nonetheless biologically meaningful alterations in DDC enzymatic activity has formed the basis for many previous investigations. In clinical genetic association studies, for example, DDC variation has been linked to age of onset in schizophrenia (Borglum et al, 2001), and implicated in risk for a range of other conditions, including attention-deficit hyperactivity disorder (ADHD) (Guan et al, 2009; Lasky-Su et al, 2008), autism (Toma et al, 2012), nicotine dependence (Ma et al, 2005), and migraine (Corominas et al, 2010). Similarly, recent reports have linked single-nucleotide polymorphisms (SNPs) in DDC with clinically relevant neurocognitive and behavioral phenotypes, such as alerting attention (Zhu et al, 2013), suicidal behavior (Giegling et al, 2008), and alcohol consumption patterns (Pan et al, 2013). In Parkinson's disease, an illness involving nigrostriatal dopaminergic neuronal degeneration, L-3,4-dihydroxyphenylalanine (L-DOPA) is a gold-standard treatment whose efficacy is thought to depend largely on its conversion to dopamine by DDC. Preliminary data indicate that common DDC polymorphisms may lead to alterations in therapeutic response to L-DOPA (Devos et al, 2014). However, whether common genetic variation in DDC is in fact predictive of DDC activity measured in the living human brain remains untested.
The positron emission tomography (PET) radiopharmaceutical L-3,4-Dihydroxy-6-[18F]fluorophenylalanine ([18F]-FDOPA) is a fluorinated analog of L-DOPA and therefore a DDC substrate whose specific uptake in the brain and subsequent vesicular storage as its decarboxylation product, [18F]fluorodopamine, is driven by DDC activity and can be reliably estimated in vivo (Gjedde et al, 1991; Hoshi et al, 1993). Twin studies indicate that a substantial portion of interindividual variation in striatal DDC activity is heritable (Stokes et al, 2013). Developing an understanding of the precise genetic factors underlying this heritability has become increasingly important in view of accumulating evidence supporting PET-quantified striatal DDC activity as a significant correlate of heritable neuropsychiatric illnesses. Exaggerated striatal DDC activity has been a replicated finding not only in groups of individuals with schizophrenia (Howes et al, 2012; Meyer-Lindenberg et al, 2002) but also in those with clinical or genetic risk factors for schizophrenia (Egerton et al, 2013; Howes et al, 2011; Huttunen et al, 2008), suggesting it may be an endophenotype (Gottesman and Gould, 2003) valuable for molecular discovery and biological validation of identified risk genes. Preliminary reports have noted abnormalities of [18F]-FDOPA uptake in the striatum of individuals with autism spectrum conditions (Nieminen-von Wendt et al, 2004), alcohol dependence (Kumakura et al, 2013), and ADHD (Ludolph et al, 2008) but await further corroboration. Here, in a large cohort of healthy volunteers, we employed [18F]-FDOPA PET and extensive genotyping across DDC to determine whether and what portion of interindividual variation in striatal DDC activity might be explained by common DDC polymorphisms.
Materials and methods
Subjects
A total of 120 Caucasian healthy volunteers (ages 20–55 years, mean 34.8±10.4 years; 61 women) participated after providing informed consent as approved by the National Institute of Mental Health Institutional Review Board and National Institutes of Health (NIH) Radiation Safety Committee. Participants were recruited from the local community and had no psychiatric, neurological, or major medical illness, including any substance use disorder, as determined by clinician-administered standardized clinical interview (SCID) (First et al, 1996), structural MRI, routine laboratory tests, qualitative urine toxicology (including amphetamines, cocaine, opiates, cannabinoids, sedative hypnotics), medical history, and physical examination.
Genetics
A total of 23 markers with minor allele frequencies >5% were selected from across the DDC gene providing coverage of HapMap annotated common variants between 20 kb upstream and 2 kb downstream of DDC in the CEU sample (at r2 >0.7 by 2- and 3-marker tagging as implemented by Haploview). All markers were intronic variants, except for rs11575575, a downstream SNP, and rs11575542, a nonsynonymous coding variant resulting in an arginine-to-glutamine amino acid change.
All subjects were genotyped using the TaqMan 5′ exonuclease assay (Applied Biosystems, Foster City, CA). DDC haplotypes were generated using PHASE software (http://stephenslab.uchicago.edu/software.html). Haplotypes with frequencies of at least 5% were selected for study, and the estimated number of each of the haplotypes carried by each individual were calculated based on the probabilities provided by PHASE. Hardy–Weinberg equilibrium exact testing was performed in R (Graffelman, 2014; Wigginton et al, 2005).
Neuroimaging
PET sessions were conducted in a fasting state (at least 6 h) to prevent competitive inhibition of radiotracer transport across the blood–brain barrier by ingested large amino acids. Caffeine and nicotine were not permitted for 4 h before scanning, and the absence of any psychotropic or other confounding substance exposure was confirmed before PET procedures. To reduce peripheral radiotracer metabolism, a 200 mg dose of carbidopa was administered by mouth 1 h before [18F]-FDOPA injection. Volunteers underwent PET imaging with a General Electric Advance 3D PET camera (32 planes, 6.5 mm FWHM) while wearing a thermoplastic mask to limit head motion and remaining in an awake, resting state. An 8-min transmission scan for attenuation correction and—immediately following intravenous injection of 7–16 mCi (mean mass 3.16±0.78 mg, specific activity 1082.96±358.23 mCi/mmol) [18F]-DOPA—a 90-min series of 27 dynamically acquired emission scans were collected for each session.
Filtered back-projection reconstruction was performed with corrections for radioactivity decay, dead time, and scatter after injection, and a registered attenuation correction algorithm was applied that included realignment for the purpose of motion correction. All emission scans were individually realigned to the twenty-first frame with a rigid body transformation using FLIRT (http://fsl.fmrib.ox.ac.uk/fsl/) to correct for interscan motion. Because the first three frames have relatively lower signal with which to guide registration, these images were realigned to the reference frame by adopting the same transformation matrix as the fourth frame. Using the mean of these frames as a target, each individual's separately acquired, nonparametric nonuniform intensity normalized (Sled et al, 1998), T1-weighted structural MRI image was coregistered to his or her PET data with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/). Freesurfer-assisted MRI segmentation (http://freesurfer.net/) followed by manual editing using ITKSnap (Yushkevich et al, 2006), which was conducted blinded to genetic status, delineated three striatal regions of interest (ROIs) representing known areas of highest tracer uptake signal-to-noise ratios: bilateral dorsal caudate, bilateral dorsal putamen, and bilateral ventral striatum. A standard reference region, the cerebellum (Calne et al, 1985), was defined in a manner similar to ROIs above and excluded both the vermis, an area of potential specific binding, as well as lateral/superior regions abutting the transverse venous sinuses.
ROI and reference time-activity curves were extracted from the emission frames, and the kinetic rate constant, Ki, representing specific tracer uptake and a measurement of DDC activity, was calculated for each ROI with PMOD software (http://www.pmod.com/), using the previously validated, noninvasive graphical linearization method established by Patlak and Gjedde (Hoshi et al, 1993; Patlak and Blasberg, 1985). For post hoc, voxel-wise analyses, ANTS software (http://stnava.github.io/ANTs/) was used to warp each individual's PET data into standardized Montreal Neurological Institute (MNI) space using the transform between the individual's coregistered MRI and an MNI space DARTEL-generated target image derived from 240 healthy subjects. To improve signal-to-noise ratios, voxel-wise smoothing (10 mm isotropic Gaussian kernel) was applied as implemented in SPM. Estimation of Ki for each voxel in the striatum was then conducted on the anatomically normalized PET data with PMOD software, using the native-space cerebellar reference region time-activity curve as the input function.
Statistical Analyses
Statistical analyses for demographic and ROI Ki association testing were performed in SPSS (http://www-01.ibm.com/software/analytics/spss/) with standard nonparametric tests and general linear modeling. To better characterize effects, post hoc statistical analyses of voxel-wise Ki were conducted in SPM within high signal voxels (1 SD above the whole-brain mean voxel value) and with an uncorrected threshold of p<0.005.
Results
Haplotypes and Demographics
Five common haplotypes were identified (Table 1). The frequencies for haplotypes 1–5 were: 35%, 15%, 11%, 7%, and 5%, respectively. None of the haplotypes showed distribution differences across sex or handedness, except haplotype 3 that showed evidence for unequal distribution in males vs females (Mann–Whitney U=2112.5, p=0.020). None of the haplotypes showed association with age (all p's ⩾0.1). None of the studied SNPs showed deviation from Hardy–Weinberg equilibrium (all p's >0.1).
Table 1. Genotyped Markers and Identified Common Haplotypes.
| SNP | Haplotype 1 | Haplotype 2 | Haplotype 3 | Haplotype 4 | Haplotype 5 |
|---|---|---|---|---|---|
| rs11575575 | G | G | G | T | G |
| rs2060762 | G | A | G | G | G |
| rs11575542 | G | G | G | G | G |
| rs4947535 | A | T | A | T | A |
| rs11575536 | C | C | C | C | T |
| rs11761683 | T | C | C | T | T |
| rs11238134 | C | A | A | C | C |
| rs745043 | C | A | C | C | C |
| rs10899736 | A | G | G | G | G |
| rs11575404 | T | T | C | T | T |
| rs3807558 | C | C | C | C | C |
| rs11575387 | A | A | A | C | A |
| rs17634958 | G | A | G | G | G |
| rs6592961 | G | G | A | A | G |
| rs6950777 | G | A | G | G | G |
| rs998850 | G | C | C | G | C |
| rs17133877 | G | G | G | G | G |
| rs3779078 | G | A | G | G | G |
| rs2044859 | T | C | T | T | C |
| rs2876830 | G | G | A | G | G |
| rs4947644 | C | T | T | C | T |
| rs6593010 | A | G | G | A | A |
| rs6593011 | C | C | C | C | A |
Neuroimaging
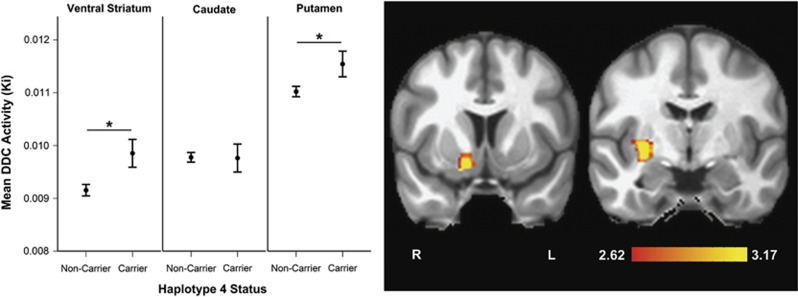
Estimation of five simple multivariate general linear models (one for each haplotype predictor) with regional striatal Ki values (caudate, putamen, ventral striatum) as the dependent variables revealed a specific and significant effect of the estimated number of haplotype 4 copies carried (Pillai's trace=0.1; F(3, 116)=4.31; p=0.006). None of the other haplotypes approached significance (all p's >0.2). Follow-up univariate tests indicated that this finding was strongest for the ventral striatum (F(1, 118)=5.28; p=0.023) and dorsal putamen (F(1, 118)=3.98; p=0.048), but was not present for dorsal caudate (p>0.9). Repeat multivariate and follow-up univariate testing of haplotype 4 effects on Ki were conducted with haplotype 4 recoded as a binary variable (carrier (estimated number of copies >0.5) vs noncarrier (all others)), and the results were nearly identical (multivariate, ventral striatum univariate, putamen univariate p's= 0.006, 0.024, and 0.061 respectively). A simple multivariate linear analysis confirmed no significant relationship between the amount of activity injected and the Ki parameter (Pillai's trace=0.016; F(3, 116)=0.62; p=0.60). In addition, a simple linear regression analysis confirmed no significant relationship between the estimated number of haplotype 4 copies carried and terminal frame cerebellar activity (p>0.3), a result that was unchanged when including the amount of activity injected in the model.
The post hoc voxel-wise analysis suggested that the strongest effects of haplotype 4 localized to foci in right medial ventral striatum and mid postcommissural putamen (peak voxel MNI coordinates: x, y, z=16.5, 1.5, −24; p=2.4 × 10-4 and 30, −6, −3; p=4.9 × 10-4 respectively), where the haplotype predicted greater uptake rates (Figure 1, Table 2).
Figure 1.
DDC haplotype 4 effects. The graph shows region of interest (ROI) analyses with mean DDC activity values and standard errors for each ROI by haplotype 4 status. Multivariate Pillai's trace=0.1; F(3, 116)=4.31; p=0.006. Asterisks indicate p<0.05 for univariate comparisons. Coronal slices show results of post hoc voxel-wise analysis indicating locations of DDC effects in the striatum (at MNI Y=9 for ventral striatum and Y=−6 for caudate). Colored clusters represent areas where more copies of the DDC haplotype predicted greater DDC activity. Color bar indicates t-values, and data are displayed by radiological convention at a voxel-wise, uncorrected threshold of p<0.005.
Table 2. DDC Activity (K i) by Haplotype 4 Status.
|
DDC activity (Ki) |
||||
|---|---|---|---|---|
| Region | Haplotype 4 status | Mean | SEM | SD |
| Ventral striatum | Noncarrier | 9.15 × 10−3 | 1.09 × 10−4 | 1.12 × 10−3 |
| Carrier | 9.85 × 10−3 | 2.63 × 10−4 | 1.02 × 10−3 | |
| Caudate | Noncarrier | 9.78 × 10−3 | 9.12 × 10−5 | 9.34 × 10−4 |
| Carrier | 9.76 × 10−3 | 2.64 × 10−4 | 1.02 × 10−3 | |
| Putamen | Noncarrier | 1.10 × 10−2 | 9.85 × 10−5 | 1.01 × 10−3 |
| Carrier | 1.15 × 10−2 | 2.43 × 10−4 | 9.41 × 10−4 | |
| Ventral striatum peak | Noncarrier | 3.00 × 10−3 | 4.56 × 10−5 | 4.67 × 10−4 |
| Carrier | 3.42 × 10−3 | 1.18 × 10−4 | 4.57 × 10−4 | |
| Putamen peak | Noncarrier | 7.61 × 10−3 | 8.79 × 10−5 | 9.01 × 10−4 |
| Carrier | 8.38 × 10−3 | 2.89 × 10−4 | 1.12 × 10−3 | |
Mean, standard error (SEM), and SD values for Ki measured for each ROI and peak ventral striatum and putamen foci (see Figure 1) from the voxel-wise analyses. Peak foci values for each subject were defined as the mean Ki within a sphere, centered at the peak voxel coordinates and of radius 4.5 mm.
Discussion
The present work provides novel evidence that common variation in the DDC gene predicts measureable differences in striatal DDC activity. Unlike rare, loss-of-function mutations responsible for clinical DDC deficiency syndrome, DDC haplotype 4 was associated with moderate and clinically silent effects within the normal range of DDC functioning as indexed by specific [18F]-FDOPA uptake. In previous studies, striatal DDC activity, so measured, shows variability across healthy individuals that is both heritable and linked to cognitive, personality, and neuropsychiatric illness risk (Borglum et al, 2001; Corominas et al, 2010; Guan et al, 2009; Lasky-Su et al, 2008; Ma et al, 2005; Pan et al, 2013; Toma et al, 2012; Zhu et al, 2013); the present data suggest the possibility that this variability may be under genetic control via cis-acting polymorphisms. Whether the haplotypic effects denoted here might mechanistically contribute to or, alternatively, lend noise to some of these past association findings cannot be confidently determined by these results, but both possibilities highlight the importance of understanding the sources of variance in this widely used measure.
The most robust genetic association was localized to the ventral striatum, a region closely allied with limbic circuitry and dysfunctional in disorders affecting motivated behaviors, such as addictions, mood disorders, and schizophrenia (Everitt and Robbins, 2005; Haber and Knutson, 2009; Russo and Nestler, 2013; Schlagenhauf et al, 2014). Patient studies will be required to determine whether the identified haplotype may be of particular significance in these conditions. By the same token, the observed haplotype effects were not diffusely observed (eg, caudate ROI analyses did not show a statistically significant effect), suggesting regional specificity in the impact of this genetic variation, as has been observed with other dopamine-related proteins, in which gene polymorphisms predict the expression of certain splice variants in select brain structures (Kunii et al, 2014). This regional specificity may reflect engagement of unique molecular mechanisms (eg, preferential DDC isoform transcription) induced by particular intracellular signaling characteristics of dopaminergic neurons projecting to ventral striatum and putamen or, alternatively, it may be that the impact of DDC sequence variation on DDC activity in select caudate projecting neurons is negated or dwarfed by differential local regulatory constraints.
Haplotype 4 has not previously been shown to be functional at the enzymatic level, and further work is needed to understand the extent to which it may not only bias striatal [18F]-FDOPA measurements, which are of crucial importance for a range of basic and clinical PET research applications, but also affect clinically meaningful outcomes. For instance, in conditions treated with L-DOPA, a DDC substrate, DDC enzymatic properties may be important determinants of L-DOPA dose–response relationships, as hypothesized in recent work in Parkinson's disease that identified preliminary associations between common genetic variation and motor responses to L-DOPA therapy (Devos et al, 2014). In that study, the major allele of rs921451 predicted greater clinical response but not peripheral pharmacokinetic measurements. Haplotype 4 contains the major allele of rs6593010, which is in linkage disequilibrium with rs921451 in the CEU HapMap sample (r2=0.805; data not shown). The direction of those findings appears to agree (ie, greater central DDC activity would be assumed to facilitate greater L-DOPA treatment response); however, as the haplotype is not uniquely defined by this marker, any inferences about genetically driven L-DOPA efficacy based on the present findings from healthy individuals must await future studies of DDC genetics in Parkinson's disease and treatment response.
As DDC is not only involved in monoaminergic synthesis, but is also the rate-limiting enzyme for several neuro- and vaso-active trace amines, such as 2-phenylethylamine, tyramine, and tryptamine that have been implicated in multiple neurological and psychiatric conditions, these data may have clinical relevance beyond Parkinson's disease therapeutics (Berry, 2007). Accordingly, DDC has been identified as a candidate risk gene for multiple CNS conditions (Corominas et al, 2010; Guan et al, 2009; Lasky-Su et al, 2008; Ma et al, 2005; Toma et al, 2012). However, past association studies have proceeded without evidence that common polymorphisms in DDC are associated with differential DDC protein function. Here, we provide such evidence that offers some guidance for future work. For instance, a four-marker DDC haplotype well aligned with haplotype 4, but not with the other haplotypes studied, has been linked recently with autism (Toma et al, 2012) and may merit follow-up experiments.
Although the markers tested provide substantial coverage of the common polymorphisms in the DDC gene, these data are not exhaustive and cannot exclude other cis-acting, untested variants. By the same token, in view of the relatively limited frequency of haplotype 4 and the measured Ki variance, it is clear that this genetic factor is not the only important source of interindividual variability in striatal [18F]-FDOPA uptake, and additional potential sources should be investigated. Though this haplotype was independent of age and sex in our cohort, the current work does not exclude the possibility that other nongenetic variables, such as menstrual cycle phase or adequacy of pyridoxal 5′-phosphate levels, affected the reported results, and independent replication to confirm these findings is warranted. The Gjedde–Patlak modeling of the macroparameter, Ki, requires negligible differences in K1, the rate constant reflecting tracer perfusion and extraction from the arterial compartment, between regions of interest and reference. The remote possibility that there exists a spurious or systematic relationship between regional K1 across individuals and DDC haplotype, therefore, poses a potential limitation of the current approach. Arterial sampling was not conducted in this large cohort, precluding empirical comparisons between Ki measurements obtained with the present methods and similar parameters derived using an arterial blood input function. However, past research has identified a strong correlation between reference region delineated Ki (also referred to as k3s elsewhere) and estimates of k3, the rate constant reflecting tracer entrance to the specific compartment, using arterial sampling and kinetic modeling (Hoshi et al, 1993). In addition, because the expression of DDC in the cerebellum is extremely low in post-mortem assays (eg, as documented by the Allen Brain Atlas; http://www.brain-map.org), it is not expected that cis-acting variation would affect tracer kinetics in this region, as supported by the lack of haplotype 4 effects on terminal frame cerebellar activity in the current data. Thus, results are unlikely to reflect genetically driven reference region biases. Finally, it is not known by what mechanism haplotype 4—or variation with which it is in linkage disequilibrium—is associated with greater in vivo enzymatic activity, a question that can be best answered with future molecular studies.
Despite these caveats, by offering novel biological evidence for functional effects of common DDC polymorphisms in the living human brain, this study identifies a genetic source of variation in a heritable and widely relied upon neurochemical phenotype and lays groundwork upon which to pursue hypotheses linking this candidate gene and aspects of neuropsychiatric conditions with ventral striatal involvement.
Funding and disclosure
This work was funded by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (Bethesda, MD) under project MH002717 (NCT00024622). The authors declare no conflict of interest with respect to this work.
Acknowledgments
We thank the staff of the NIH PET Center for their assistance in data acquisition.
References
- Berry MD (2007). The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials 2: 3–19. [DOI] [PubMed] [Google Scholar]
- Borglum AD, Hampson M, Kjeldsen TE, Muir W, Murray V, Ewald H et al (2001). Dopa decarboxylase genotypes may influence age at onset of schizophrenia. Mol Psychiatry 6: 712–717. [DOI] [PubMed] [Google Scholar]
- Brun L, Ngu LH, Keng WT, Ch'ng GS, Choy YS, Hwu WL et al (2010). Clinical and biochemical features of aromatic L-amino acid decarboxylase deficiency. Neurology 75: 64–71. [DOI] [PubMed] [Google Scholar]
- Calne DB, Langston JW, Martin WR, Stoessl AJ, Ruth TJ, Adam MJ et al (1985). Positron emission tomography after MPTP: observations relating to the cause of Parkinson's disease. Nature 317: 246–248. [DOI] [PubMed] [Google Scholar]
- Corominas R, Sobrido MJ, Ribases M, Cuenca-Leon E, Blanco-Arias P, Narberhaus B et al (2010). Association study of the serotoninergic system in migraine in the Spanish population. Am J Med Genet B Neuropsychiatr Genet 153B: 177–184. [DOI] [PubMed] [Google Scholar]
- Devos D, Lejeune S, Cormier-Dequaire F, Tahiri K, Charbonnier-Beaupel F, Rouaix N et al (2014). Dopa-decarboxylase gene polymorphisms affect the motor response to L-dopa in Parkinson's disease. Parkinsonism Relat Disord 20: 170–175. [DOI] [PubMed] [Google Scholar]
- Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P et al (2013). Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry 74: 106–112. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW (1996) User's Guide for the SCID-I for DSM-IV Axis I Disorders - Research Version. Biometrics Research: New York. [Google Scholar]
- Giegling I, Moreno-De-Luca D, Rujescu D, Schneider B, Hartmann AM, Schnabel A et al (2008). Dopa decarboxylase and tyrosine hydroxylase gene variants in suicidal behavior. Am J Med Genet B Neuropsychiatr Genet 147: 308–315. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Reith J, Dyve S, Leger G, Guttman M, Diksic M et al (1991). Dopa decarboxylase activity of the living human brain. Proc Natl Acad Sci USA 88: 2721–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD (2003). The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry 160: 636–645. [DOI] [PubMed] [Google Scholar]
- Graffelman J (2014). HardyWeinberg: Graphical Tests for Hardy-Weinberg Equilibrium. R package version 1.5.4 http://CRAN.R-project.org/package=HardyWeinberg.
- Guan L, Wang B, Chen Y, Yang L, Li J, Qian Q et al (2009). A high-density single-nucleotide polymorphism screen of 23 candidate genes in attention deficit hyperactivity disorder: suggesting multiple susceptibility genes among Chinese Han population. Mol Psychiatry 14: 546–554. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2009). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi H, Kuwabara H, Leger G, Cumming P, Guttman M, Gjedde A (1993). 6-[18F]fluoro-L-dopa metabolism in living human brain: a comparison of six analytical methods. J Cereb Blood Flow Metab 13: 57–69. [DOI] [PubMed] [Google Scholar]
- Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR et al (2011). Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry 168: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A et al (2012). The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry 69: 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J, Heinimaa M, Svirskis T, Nyman M, Kajander J, Forsback S et al (2008). Striatal dopamine synthesis in first-degree relatives of patients with schizophrenia. Biol Psychiatry 63: 114–117. [DOI] [PubMed] [Google Scholar]
- Kumakura Y, Gjedde A, Caprioli D, Kienast T, Beck A, Plotkin M et al (2013). Increased turnover of dopamine in caudate nucleus of detoxified alcoholic patients. PLoS One 8: e73903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunii Y, Hyde TM, Ye T, Li C, Kolachana B, Dickinson D et al (2014). Revisiting DARPP-32 in postmortem human brain: changes in schizophrenia and bipolar disorder and genetic associations with t-DARPP-32 expression. Mol Psychiatry 19: 192–199. [DOI] [PubMed] [Google Scholar]
- Lasky-Su J, Neale BM, Franke B, Anney RJ, Zhou K, Maller JB et al (2008). Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. Am J Med Genet B Neuropsychiatr Genet 147B: 1345–1354. [DOI] [PubMed] [Google Scholar]
- Ludolph AG, Kassubek J, Schmeck K, Glaser C, Wunderlich A, Buck AK et al (2008). Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: a 3,4-dihdroxy-6-[18F]fluorophenyl-l-alanine PET study. Neuroimage 41: 718–727. [DOI] [PubMed] [Google Scholar]
- Ma JZ, Beuten J, Payne TJ, Dupont RT, Elston RC, Li MD (2005). Haplotype analysis indicates an association between the DOPA decarboxylase (DDC) gene and nicotine dependence. Hum Mol Genet 14: 1691–1698. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M et al (2002). Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci 5: 267–271. [DOI] [PubMed] [Google Scholar]
- Nieminen-von Wendt TS, Metsahonkala L, Kulomaki TA, Aalto S, Autti TH, Vanhala R et al (2004). Increased presynaptic dopamine function in Asperger syndrome. Neuroreport 15: 757–760. [DOI] [PubMed] [Google Scholar]
- Pan Y, Luo X, Liu X, Wu LY, Zhang Q, Wang L et al (2013). Genome-wide association studies of maximum number of drinks. J Psychiatr Res 47: 1717–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG (1985). Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 5: 584–590. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ (2013). The brain reward circuitry in mood disorders. Nat Rev Neurosci 14: 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F, Huys QJM, Deserno L, Rapp MA, Beck A, Heinze H-J et al (2014). Striatal dysfunction during reversal learning in unmedicated schizophrenia patients. Neuroimage 89: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998). A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17: 87–97. [DOI] [PubMed] [Google Scholar]
- Stokes PR, Shotbolt P, Mehta MA, Turkheimer E, Benecke A, Copeland C et al (2013). Nature or nurture? Determining the heritability of human striatal dopamine function: an [18F]-DOPA PET study. Neuropsychopharmacology 38: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma C, Hervas A, Balmana N, Salgado M, Maristany M, Vilella E et al (2012). Neurotransmitter systems and neurotrophic factors in autism: association study of 37 genes suggests involvement of DDC. World J Biol Psychiatry.. [DOI] [PubMed]
- Wigginton JE, Cutler DJ, Abecasis GR (2005). A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet 76: 887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC et al (2006). User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31: 1116–1128. [DOI] [PubMed] [Google Scholar]
- Zhu B, Chen C, Moyzis RK, Dong Q, He Q, Li J et al (2013). The DOPA decarboxylase (DDC) gene is associated with alerting attention. Prog Neuropsychopharmacol Biol Psychiatry 43: 140–145. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Juorio AV (1995). Aromatic L-amino acid decarboxylase: biological characterization and functional role. Gen Pharmacol 26: 681–696. [DOI] [PubMed] [Google Scholar]