Abstract
A study was conducted on the transformation of SnO to SnO2 using X-ray diffraction and subjecting the SnO to heat treatments between 300 °C < T < 600 °C in two different atmospheres, argon and air. The intermediary oxide that appears in the disproportionation process was identified as Sn2O3. In an argon atmosphere, decomposition occurs in three stages: (1) a direct transformation of SnO to SnO2, (2) the formation of some intermediary Sn2O3 from SnO, and (3) the conversion of the Sn2O3 to SnO2 with the formation of metallic tin, Sn (l). When an atmosphere of air is used, however, a reaction occurs, concurrent with the decomposition reactions, that relates to the specific oxidation of the metallic tin produced in the course of the three process stages.
Keywords: Engineering, Materials science, Inorganic chemistry
1. Introduction
As a result of their many technological applications, tin oxides − SnOx have received much recent attention. Of these, cassiterite (from the Greek word for tin, Kassiteros − SnO2) is the best known, having a rutile-type, tetragonal crystal structure with lattice parameters a = b = 4.737 Å y c = 3.185 Å, where each tin atom is linked to six oxygen atoms and each oxygen atom to three nearest neighbor tin atoms in an approximately equilateral triangle [1]. The heat of formation of this structure is 1.9×103 J mol−1 K−1 and its heat capacity 52.59 J mol−1 K−1, with a melting point of 1650 °C and a boiling point of 1900 °C. SnO2 is an n-type semiconductor with a wide band gap (3.5 eV). It boasts a combination of chemical, electronic and optical properties that make it suitable for a range of applications [1, 2], particularly in the ceramics industry as a flattening agent for glass and enamel [3] as well as a catalyst [4], gas sensor [5, 6, 7], varistor [8, 9], and as a transparent electrode for solar cells [10].
Tin monoxide, SnO, meanwhile, occurs in a range of modifications [11]. Blue-black SnO, α-SnO has a lithorge tetragonal structure (α-PbO, tP4, P4/nmm, SG No. 129, Z = 2) with quadrant pyramids of {SnO4} arranged in parallel layers with Sn(II) at the apex and alternating itself above and below the layer of oxygen atoms [11]. The structure may also be described as fluorite type with alternating layers of lost anions. If the blue-black SnO is ground in an agate mortar, a brownish-green powder is obtained, but if the initial α-SnO is heated in vacuum at about 550 °C, a greyish-green powder, β-SnO is obtained.
SnO is technologically important as a p-type semiconductor [12] as well as a coating for storing solar energy [13] and in organic synthesis, for example in the cyclization of maleic acid, lactic acid polymerization and the degradation of polylactic acid to lactic acid, among others [14]. Specifically, SnO is recognized for its potential as anode material for lithium ion batteries, since during the charging of the battery, the Li+ ions react with the SnO via the following reaction [15]:
| (1) |
While during the subsequent discharge-charge cycles the following reaction occurs [15]:
| (2) |
The advantage using SnO rather than SnO2 is that the loss of Li ions is reduced.
SnO always contains some Sn(IV), so that traces of oxygen are sufficient to oxidize it, and it is metastable in ambient conditions, a reaction that in the presence of air can be expressed as [16, 17]:
| (3) |
characterized by a ΔG°298(1) = −262.9 KJ mol−1. Even in the absence of oxygen, this oxide undergoes a disproportionation reaction of a spontaneous nature with a ΔG°298(2) = −5.9 KJ mol−1 [16, 17]; given by:
| (4) |
where the subscripts (s) and (l) indicate the solid and liquid states, respectively. The above two expressions correspond to the transformation of SnO a SnO2 and involve the incorporation of oxygen atoms, Eq. (3), or the removal of Sn atoms, Eq. (4). It is expected that during this transformation an intermediate tin oxide, SnxO, is albeit transiently. The literature reports two stoichiometries: one with x = 0.67 and another with x = 0.75 [18, 19], such that recently, from the latter, single crystals were obtained using a carbide-thermal reduction process [20].
The disproportionation reaction of SnO has been studied conducting experiments in-situ and ex-situ using various techniques such as, for example, Mössbauer [17]. This reaction depends on the temperature, reaction time and the way the starting material has been obtained, and the in-situ experiments provided the most information on the details of the progress of the reaction, specifically the speed of the reaction [18].
In the Lawson’s work [19], it is indicated that the SnO disproportionation reaction is first order and in the Moreno’s work [21] it is reported that the reaction expressed in Eq. (4) appears to occur more rapidly than that for Eq. (3). Additionally, in order to know more about this reaction, it was carried out at high pressure (up to 15 GPa), revealing that at high pressures and high temperatures Sn2O3 is not formed, since the reaction mechanism changes from nucleation and growth, at ambient pressure, to be controlled by diffusion when the pressure is increased; finally, it returns to the mechanism of nucleation and growth on reaching pressures greater than 10 GPa [18].
Depending on the pieces being analyzed, different techniques have been used to study the process of oxidation of SnO to SnO2, among them the thin film technique [22, 23]. The results from these studies indicate that the decomposition of deposited SnO to Sn and SnO2, at temperatures above 300 °C, led to the formation of oxygen-deficient SnOx (x < 2) films that were annealed in O2 to obtain stoichiometric SnO2 films [24, 25]. During annealing, the films reached the final oxidation state through a simple oxidation of the SnO (direct transition) or via intermediate oxidation states (indirect transition) − mainly Sn2O3 or Sn3O4, depending on deposition parameters. In the work carried out by Choi et al. [26]. the mechanism of oxidation of SnO to SnO2 using undoped substoichiometric tin oxide thin films on Si (100) formed by two distinct methods were studied: (1) using oxygen ion-assisted deposition, with a subsequent annealing at 400–600 °C in a vacuum for 1 h; (2) depositing the films with simultaneous heating of the substrate at 400–500 °C. They found that the oxidation of SnO to SnO2 greatly depended on the deposition method, the initial oxygen content and the annealing temperature; furthermore, the process began with internal disproportionation with a subsequent direct/indirect transformation. During the indirect phase transformation, one of the phases that appeared in the sample was distorted o-SnO, while in the direct transformation the SnO films were easily transformed to SnO2 such that the latter showed a preferential orientation along the axis similar to the atomic distance of the tin matrix.
As well as the interest the study presents in the SnO disproportionation reaction, in order to clarify the contradictions that arise in the design of the O-Sn phase diagram [27], there is a particular technological interest related to optimization of the gas sensor operation [28]. The stoichiometry of oxygen in the tin oxide particle surface varies between SnO and SnO2, for their different conditions of synthesis, so that the charge transfer process is different for SnO than for SnO2. It is necessary to control the changes in stoichiometry during synthesis and thereby be able to optimize the functionality of the sensors. To achieve this, it is very important to understand well the details of the SnO disproportiation reaction.
With the aim of obtaining more information about the SnO disproportionation reaction, in this work the effect of the atmosphere in which it takes place was studied. To do this, SnO synthesized by controlled precipitation was taken. It was then suitably characterized and subjected to a heating program to enable the transformation of the SnO into SnO2 in air and in an atmosphere of argon. The nature of the crystalline phases present in the sample for the two atmospheres was determined using X-ray diffraction and with differential scanning calorimetry the principal physicochemical events that occur during the reaction were determined.
2. Experimental
2.1. SnO synthesis
The tin oxide SnO was obtained by the controlled precipitation method according to the following procedure. A 0.4 M solution of SnCl2.2H2O (Merck, 98–100%) was prepared in a 0.1 M HNO3 solution and stirred at room temperature for 30 min. Concentrated ammonium hydroxide was then added to 28% NH4OH (Ashland reagent, 28–30%) via an automatic feeder (Dosimat 685 Metrohm) at a rate of 25 μL s−1. The pH of the reaction was simultaneously monitored using a glass electrode (Hanna Instruments, pHmeter). The addition of ammonium hydroxide was halted when the system reached a pH value between 6.25–6.5. The suspension formed was stirred constantly for 60 min at a temperature of 100 °C, and left to sit for 24 h. The suspension was subsequently filtered and the obtained solid was dried at 100 °C for 12 h.
2.2. Characterization techniques
2.2.1. TGA-DSC
The thermal behavior of the SnO was determined using thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) in two different atmospheres: air and argon. This analysis was performed in a temperature range between 300 and 600 °C at a heating rate of 2 °C min−1, with a gas flow of 50 cm3 min−1. The equipment used was a SDT Q600 V8.1.
2.2.2. X-ray diffraction
To understand the evolution of the crystalline phases when heating the SnO synthesized in the different atmospheres, X-ray diffraction was used (Siemens, model D-5000) with a heating probe, using the radiation CuKα = 1.54056 Å in a step of 0.02 ° and a fixed time of 0.3 s. This assay allowed us to determine the phases that are formed during the heat treatment of SnO, in situ, in the temperature range 300 < T < 600 °C. For this, the SnO was heated in an atmosphere of air, or of argon, to a temperature of 300 °C. Subsequently, was raised by increases of 25 °C until a final temperature of 600 °C was reached. At each temperature increment, including for the 300 °C value, X-ray diffractograms were recorded in a range of angles of 15 < 2θ < 70.
Additionally, IR spectroscopy was used to discover which functional groups were present in the sample. This was carried out using Nicolet-500 equipment. Finally, to obtain information about the textural characteristics of the solid, analyses of the adsorption and desorption of nitrogen at 77 K were carried out, based on the BET and BJH model using TRISTAR 3000 instrumentation.
3. Results and discussion
3.1. Characterization of synthesized SnO
3.1.1. IR spectroscopy
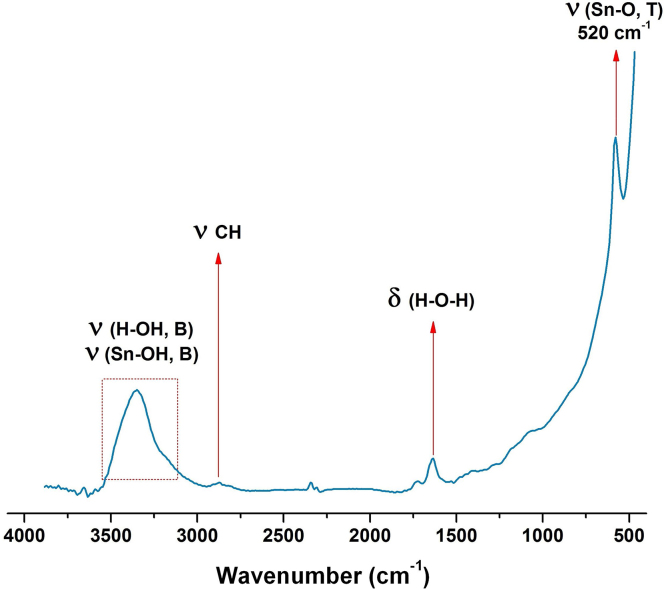
The absorption spectrum (FT-IR) in Fig. 1, corresponding to a solid sample treated at 300 °C, shows a band at 3415 cm−1 that corresponds to the fundamental vibration mode characteristic of stretching ט(Sn-OH, B). It can also be associated with stretching ט(H-OH, B) of the water molecules adsorbed on the surface of the solid, which is repeated with the bending vibration δ(H-O-H) at 1636 cm−1. At a smaller wave number an absorption band occurs at 520 cm−1 which can be attributed to the vibration mode ט(Sn-O,T) [29].
Fig. 1.
FT-IR spectrum of the tin oxide SnO at 300 °C.
3.1.2. Crystalline structure
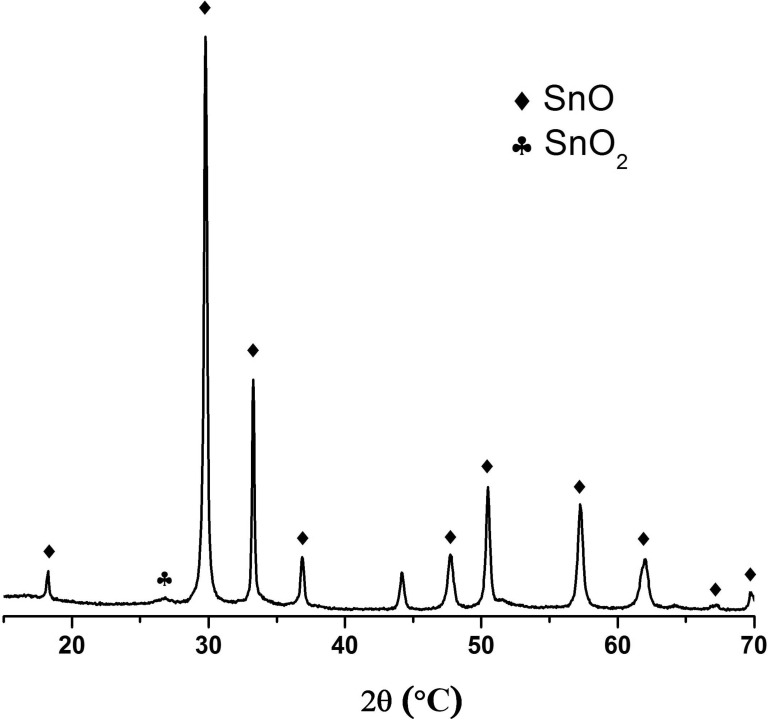
The X-ray diffractogram in Fig. 2 demonstrates the formation of romarchite phase SnO (PDF 72–1012), marked with the symbol ♦ in the precipitated solid dried at 100 °C and heat treated at 300 °C. Cassiterite, SnO2 (PDF 72–448) also exists as a secondary phase, although in very small amounts, and is marked with the symbol ♣.
Fig. 2.
DRX of the tin oxide SnO thermally treated at 300 °C.
3.1.3. Textural characterization
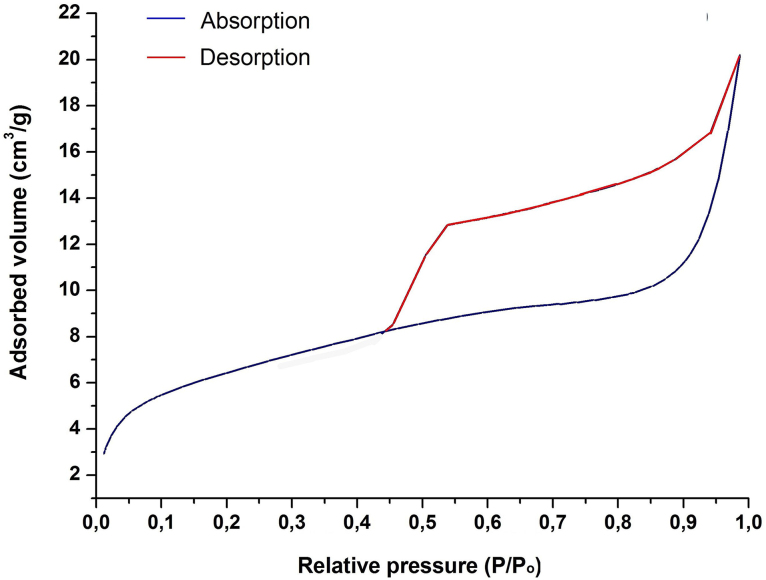
Fig. 3 presents the N2 physisorption isotherm for the SnO synthesized, while in Table 1 its main textural characteristics are summarized. The N2 adsorption isotherm can be classified, according to IUPAC, as type (IV) with an H2 hysteresis loop in a relative pressure interval of P/P0 = 0.45–1.0, characteristic behavior of materials that have bottleneck-type pores on their surface. It should be noted that the oxide obtained has a significant surface area of 22.39 m2 g−1.
Fig. 3.
N2 physisorption isotherm for the tin oxide SnO at 300 °C.
Table 1.
Textural properties of SnO at 300 °C.
| Total surface area (m2g−1) | Total pore volume 10−3 (cm3 g−1) |
Mesopore volume 10−3(cm3 g−1) | Pore diameter (Å) |
|---|---|---|---|
| 22.39 | 22.96 | 22.18 | 65.25 |
3.1.4. SnO phase transformation
3.1.4.1. XRD study with heating probe
It is known that SnO is a thermodynamically unstable phase that when subjected to a relatively low temperature leads to the formation of the more stable rutile-type SnO2 structure and β-Sn [1, 17, 18, 27].
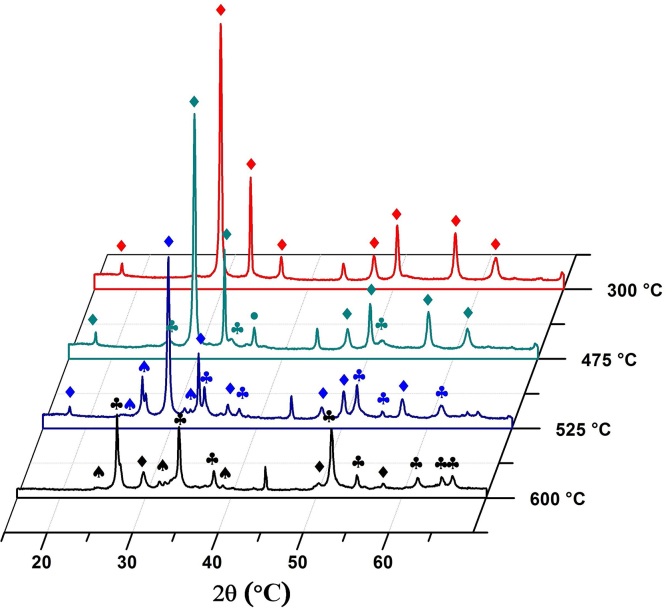
Fig. 4 shows the XRD taken of the SnO subjected to heat treatment in situ, in an atmosphere of air to ensure the presence of oxygen. It can be seen that, in the temperature range between 300 and 475 °C, the sample has romarchite SnO (PDF 72–1012) as its major phase, to whose characteristic peaks the symbol (♦) was assigned. Cassiterite SnO2 (PDF 41–1445) is also present, with its peaks in the diffractogram represented by the symbol (♣).
Fig. 4.
In situ diffractograms for the disproportionation reaction of SnO to SnO2 in an atmosphere of air.
In Fig. 4, at 525 °C in the diffractogram the onset of the Sn2O3 phase (PDF 25–1259) with characteristic peaks labeled with the symbol (♠), as the intermediary of the transformation.
Finally, the diffractogram of the solid treated at 600 °C shows the presence of the SnO, Sn2O3 and SnO2 phases, the tetragonal rutile-type SnO2 phase being the majority phase, given its greater thermodynamic stability.
Based on the above results, the following disproportionation reactions can be proposed:
| (5) |
| (6) |
| (7) |
Very consistent with the O-Sn phase diagram established by Cahen and Mohn [27, 30] except for the stoichiometry of the intermediate oxide: Sn3O4 for Mohn and Sn2O3 in this study.
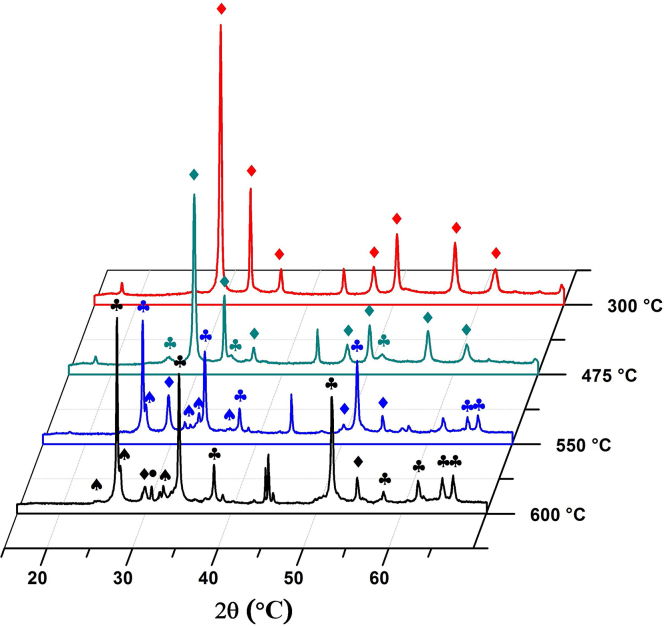
To determine how the transformation of SnO occurs in a non-oxidizing atmosphere, the experiment was repeated but in an atmosphere of argon. Fig. 5 shows the diffractograms obtained in situ for a SnO sample subjected to a heat treatment in an atmosphere of argon. In these, it can be seen that, up to a temperature of 475 °C romarchite SnO (PDF 72–1012) is found as the majority phase along with some cassiterite SnO2 (PDF 41–1445).
Fig. 5.
X-ray diffractograms relating to SnO subjected to a heat treatment in an atmosphere of argon.
As the temperature increases to 550 °C, the SnO peaks are observed to diminish, whereas the characteristic peaks of the SnO2 phase are more evident, with greater intensity. Peaks of low intensity corresponding to the Sn2O3 phase (PDF 25–1259) are also seen.
Finally, at a temperature of 600 °C, the diffractogram of this sample shows peaks corresponding to the phases: SnO, Sn2O3 and SnO2, the latter being the major phase. The diffractogram also shows characteristic peaks of the metallic tin β-Sn (PDF 1–926), which are identified by the symbol (●). In this case, the disproportionation reactions indicated above remain valid except that the reaction in Eq. (4) would occur spontaneously.
3.1.4.2. Thermal analysis
To complete the XRD study of, thermal analysis was performed on SnO in the presence of the atmospheres of interest, air and argon.
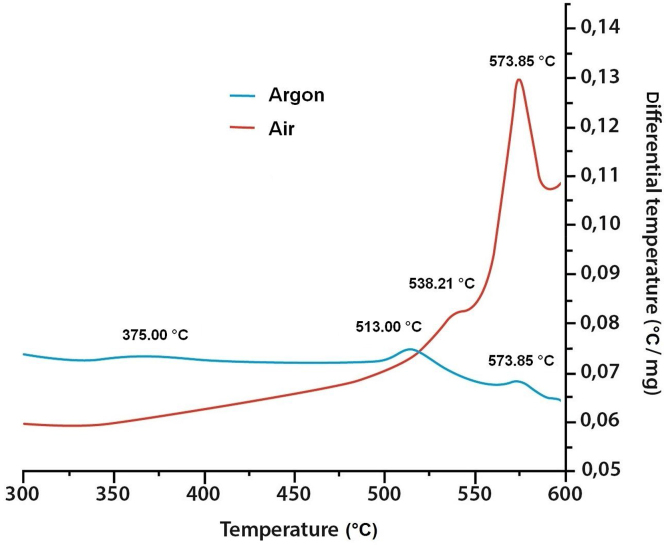
Fig. 6 shows the results of the DSC analysis carried out on tin oxide SnO in atmospheres of air and argon. In an atmosphere of air, the exothermic peak that occurs at a temperature of ≅ 538 °C could be associated with the decomposition reaction, Eq. (6), in other words the formation of the intermediate oxide Sn2O3. The process that occurs at ≅ 574 °C may correspond both to the decomposition reaction of Eq. (7), the formation of SnO2, and the oxidation of the metallic tin, Sn, by the oxygen present in the medium, by way of the following equation:
| (8) |
Fig. 6.
DSC curves corresponding to SnO samples subjected to heat treatments, in situ, in atmospheres of air and argon.
Furthermore, the SnO subjected to heat treatment in an atmosphere of argon showed an exothermic peak at a ≅ 376 °C, which might be associated with the decomposition reaction in Eq. (4) that is able to take place in the absence of oxygen (ΔG(298°C) = −5.9 kJ mol−1) [18].
The second exothermic peak in the curve ≅ 513 °C may be associated with the formation of the intermediate oxide Sn2O3 (the reaction in Eq. (6)) whose presence in the sample is demonstrated by the XRD results (Fig. 5).
Once the Sn2O3 is formed, it must undergo a decomposition reaction such as that shown in Eq. (7), to which can be attributed the exothermic peak at a ≅ 574 °C. In this reaction, the Sn(l) that is produced cannot interact with oxygen via Eq. (8), to form the SnO2, so that metallic tin remains in the sample, as indicated by the XRD results (Fig. 5).
Interestingly, in the DSC curves for the SnO sample, in both argon and air, the temperature at which Sn2O3 decomposition occurs is the same: exothermic peak at ≅ 574 °C while the formation of this intermediate oxide does occur at different temperatures, at a lower temperature when the SnO is heat-treated in argon. Transformation of the SnO phase to SnO2 was also evident, independent of the type of atmosphere in which the treatment was carried out.
4. Conclusions
Analyzing the results, it can be concluded that the phase transformation from SnO to SnO2 occurs in atmospheres both which contain and do not contain oxygen − i.e. air and argon − meaning that it is not a simple oxidation process.
The X-ray diffraction studies, in situ, indicate that the nature of the atmosphere in which the transformation takes place does determine the presence or absence of metallic tin, β-Sn, but not the existence of the intermediate oxide Sn2O3, which is present in the samples whether treated in argon or air. The only difference is the temperature at which Sn2O3 begins to form.
Even though at 300 °C the solid has an only crystal phase, even at 600 °C a mixture of phases occurs in the sample. The physicochemical events are more exothermic, in other words they involve greater energy.
Declarations
Author contribution statement
Carlos M. Campo: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jorge E. Rodríguez, Alfonso E. Ramírez: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Universidad del Cauca.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thanks to the Universidad of Cauca.
References
- 1.Smith P.J. Blackie Academic & Professional; London: 1998. Chemistry of Tin. [Google Scholar]
- 2.Jorzebsk J.M., Marton J.P. Physical Properties of Tin(IV) Oxide Materials. J. Electrochem. Soc. 1976;123:299c–310c. [Google Scholar]
- 3.Vogel W. The American Ceramic Society, Inc.; 1985. Chemistry of Glass. [Google Scholar]
- 4.Fierro J.L.G. CRC Press Taylor & Francis Group; Boca Raton: 2006. Metal oxides: Chemistry and Applications. [Google Scholar]
- 5.Barsan N., Koziej D., Weimar U. Metal oxide-based gas sensor research: How to? Sensor. Actuat. B-Chem. 2007;121:18–35. [Google Scholar]
- 6.Yamazoe N., Shimanoe K. Theory of power laws for semiconductor gas sensors. Sensor. Actuat. B-Chem. 2008;128:566–573. [Google Scholar]
- 7.Eranna G. CRC Press Taylor & Francis Group; Boca Raton: 2012. Metal oxide: Nanostructures as gas sensing devices. [Google Scholar]
- 8.Pianaro S.A., Bueno P.R., Olivi P., Tongo E., Varela J.A. A new SnO2-based varistor system. J. Mater. Sci. Mater. Electron. 1998;9:158–165. [Google Scholar]
- 9.Mosquera A., Rodríguez-Páez J.E., Varela J.A., Bueno P.R. Synthesis of SnO2 by chemical routes and its use in varistors production. J. Eur. Ceram. Soc. 2007;27:3893–3896. [Google Scholar]
- 10.Okuya M., Kaneko S., Hiroshima K., Yaggi I., Murakami K. Low temperature deposition of SnO2 thin films as transparent electrodes by spray pyrolysis of tetra-n-butyltin (IV) J. Eur. Ceram. Soc. 2001;21:2099–2102. [Google Scholar]
- 11.Greenwood N.N., Earnshaw A. second ed. Elsevier Butterwoith-Heinemann Ltd.; UK: 1984. Chemistry of the elements. [Google Scholar]
- 12.Wang S., Xie S., Lie H., Yan S., Fan K., Qiao M. Solution route to single crystalline SnO platelets with tunable shapes. Chem. Comm. 2005:507–509. doi: 10.1039/b414913k. [DOI] [PubMed] [Google Scholar]
- 13.Foster M. Theoretical Investigation of the Svstem. SnOx/Sn for the Thermochemical Storage of Solar Energy. Energy. 2004;29:789–799. [Google Scholar]
- 14.Sinha P., Roy S., Barbier J. Barbier Reaction in the Regime of Metal Oxide: Carbonyl Allylation over β-SnO/Cu2O and Surface Diagnostics. Organometallics. 2004;23:67–71. [Google Scholar]
- 15.Wu D., Han C., Wang S., Wu N., Rusakova I.A. Microwave assisted solution synthesis of SnO nanocrystallites. Mater. Lett. 2002;53:155–159. [Google Scholar]
- 16.Bard A.J., Parsons R., Jordan J. Marcel Dekker, Inc.; New York: 1985. Standard potentials in aqueous solution. [Google Scholar]
- 17.Moreno M.S., Punte G., Rigotti G., Mercader R.C., Weisz A.D., Blessa M.A. Kinetic study of the disproportionation of tin monoxide. Solid State Ionics. 2001;144:81–86. [Google Scholar]
- 18.Giefers H., Porsch F., Wortmann G. Kinetics of the disproportionation of SnO. Solid state ionics. 2005;176:199–207. [Google Scholar]
- 19.Lawson F. Tin Oxide-Sn3O4. Nature. 1967;215:955–956. [Google Scholar]
- 20.Damaschio C.J., Berengue O.M., Stroppa D.G., Simon R.A., Ramirez A.J., Schreiner W.H., Chiquito A.J., Leite E.R. Sn3O4 single crystal nanobelts grown by carbothermal reduction. J. Cryst. Growth. 2010;312:2881–2886. [Google Scholar]
- 21.Moreno M.S., Mercader R.C., Bibiloni A.G. Study of intermediate oxides in SnO thermal decomposition. J. Phys. Condens. Matter. 1992;4:351–356. [Google Scholar]
- 22.Uen T.M., Huang K.F., Chen M.S., Gou Y.S. Preparation and characterization of some tin oxide films. Thin Solid Films. 1988;158:69–80. [Google Scholar]
- 23.Soares M.R., Dionisiâ P.H., Baumvol I.J.R., Schreiner W.H. Influence of sputtering parameters on the composition and crystallinity of tin oxide. Thin solids films. 1992;214:6–16. [Google Scholar]
- 24.Geuets J., Rau S., Richter W., Schmitte F.J. SnO films and their oxidation to SnO2: Raman scattering, IR reflectivity and X-ray diffraction studies. Thin solids films. 1984;121:217–225. [Google Scholar]
- 25.Reddy M.H.M., Jawalekar S.R., Chandorkar A.N. The effect of heat treatment on the structural properties of electron-beam-evaporated SnO2 films. Thin Solids Films. 1989;169:117–126. [Google Scholar]
- 26.Choi W.K., Sung H., Kim K.H., Cho J.S., Choi S.C., Jung H.-J., Koh S.K. Oxidation process from SnO to SnO2. J. Mater. Sci. Lett. 1997;16:1551–1554. [Google Scholar]
- 27.Cahen S., David N., Fiorani J.M., Maitre A., Vilasi M. Thermodynamic modelling of the O-Sn system. Thermochim. Acta. 2003;403:275–285. [Google Scholar]
- 28.Ramamoorthy R., Kennedy M.K., Nienhaws H., Lorke A., Kruis F.E., Fissan H. Surface Oxidation of Monodisperse SnOx Nanoparticles. Sensor. Actuat. B-Chem. 2003;88:281–285. [Google Scholar]
- 29.Ararat-Ibarguen C., Mosquera A., Parra R., Castro M., Rodríguez-Páez J.E. Synthesis of SnO2 nanoparticles through the controlled precipitation route. Mater. Chem. Phys. 2007;101:433–440. [Google Scholar]
- 30.Mohn G.H. Chemie der Erde; Berlin: 1974. Tin-containing mineral systems. Part I. The Sn-Fe-S-O system and mineral assemblages in ores. [Google Scholar]