Abstract
We previously reported that polymethoxyflavones (PMFs) in black ginger (Kaempferia parviflora) extract (KPE) increased energy production by activating AMP-activated protein kinase (AMPK) in C2C12 myoblasts. We herein evaluated the effects of KPE on physical fitness performance and muscular endurance in mice. Male mice were orally administered KPE for 4 weeks, and then forced swimming test, open-field test, inclined plane test, and wire hanging test were performed. KPE significantly increased the swimming time, motility after swimming, and grip strength. IL-6 and TNF-α mRNA expression levels were decreased in the soleus muscle, whereas peroxisome proliferator-activated receptor γ coactivator (PGC)-1α and glycogen synthase mRNA expression levels, mitochondrial number, and glycogen content were increased. These results were in agreement with those obtained for KPE and PMFs in C2C12. Therefore, the activation of AMPK by PMFs may be one of the mechanisms by which KPE improves physical fitness performance and muscular endurance.
Keywords: Biological sciences, Plant biology, Biochemistry
1. Introduction
Black ginger, the rhizome of Kaempferia parviflora (Zingiberaceae), has traditionally been used as food and a folk medicine for more than 1000 years in Thailand. The dried rhizome is generally pulverized and used as tea bags, while the fresh one is utilized to brew wine. The wine preparation is increasingly used in Thailand as a tonic and as an aphrodisiac. As dietary supplements, it has been made into various preparations such as medicinal liquor or liquor plus honey, pills (powdered rhizome with honey), capsules and tablets. In Thai traditional medicine, black ginger has been claimed to cure allergy, asthma, impotence, gout, diarrhea, dysentery, peptic ulcer and diabetes. A large number of recent studies have demonstrated the biological activities of black ginger extract (Kaempferia parviflora extract: KPE) and polymethoxyflavones (PMFs) including anti-oxidative activity, etc [1, 2, 3, 4, 5, 6, 7]. KPE has been shown to improve physical fitness performance in clinical studies [6, 7]. The anti-oxidative activity of KPE has been implicated in its beneficial effects. We previously reported that PMFs in KPE increased energy production through AMPK activation induced improvements of metabolism in myocytes [8]. For the beneficial effects of black ginger on health, we have developed a powdered black ginger extract as a healthy food ingredient and put it on the market, which is known as Black Ginger Extract that has been standardized to contain not less than 2.5% of 5,7-dimethoxyflavone and 10% of total PMFs. The healthy function of black ginger has been continuously being investigated.
Physical exhaustion decreases physical fitness performance and muscular endurance. Fatigue was previously thought to decrease in intracellular pH (acidosis) by the accumulation of lactic acid (LA) [9]. However, recent findings suggest that the accumulation of LA is not a direct cause of fatigue, but rather a fatigue-recovering factor [10, 11, 12, 13]. Furthermore, various factors including ATP metabolism, acidosis, and oxidative stress have been suggested to play a role in the complex processes of fatigue [14, 15, 16, 17, 18, 19, 20, 21].
AMP-activated protein kinase (AMPK) is known to be critically involved in the regulation of energy homeostasis, [22, 23, 24] and its activation has been shown to enhance the metabolism of glucose and lipids [25, 26]. Therefore, AMPK has been an attracting target for the discovery of anti-diabetic or anti-obesity treatments. AMPK is linked to physical activity and muscular endurance. 5-Aminoimidazole-4-carboxyamide ribonucleotide (AICAR), an agonist of AMPK, was previously reported to increase running endurance by up to 44% and decrease body fat in mice when orally administered for 4 weeks [27]. Consequently, the activation of AMPK has been suggested to improve physical fitness performance, muscular endurance, and fat metabolism. Therefore, we hypothesized that KPE may improve physical fitness performance not only by its anti-oxidative activity, but also through other mechanisms including the activation of AMPK.
The beneficial effects of black ginger on fatigue or muscular endurance have not yet been investigated. Based on the findings described above, we herein evaluated the effects of KPE on physical fitness performance and muscular endurance in mice. In order to clarify the underlying mechanisms of action, the soleus muscle and blood were collected from mice administered KPE, and various parameters related to physical fitness performance, muscular endurance, inflammation, metabolism in mitochondria, and the accumulation of glycogen were evaluated. Furthermore, in an attempt to identify active compounds in KPE, PMFs were evaluated in mouse myoblasts (C2C12).
2. Materials and methods
2.1. Animals and feeding
Animal experiments were performed in accordance with the Guidelines for the Proper Conduct of Animal Experiments (Special Council of Japan, June 1, 2006). Male ddY mice aged 10 weeks old (Japan SLC, Co., Ltd., Hamamatsu, Japan) were kept at 23 ± 1 °C with a humidity of 60 ± 5% under a 12-h light/dark cycle. Mice were freely fed a standard solid feed (CE-2, Oriental Yeast Co., Ltd., Tokyo, Japan). They were divided into two groups with 15 mice in each group. The KPE group was orally administered KPE (45 mg/kg/day) suspended in water for 4 weeks. The control group was orally administered the vehicle of KPE.
2.2. Regents
NucleoSpin® Tissue, the kit used to extract total DNA from the soleus muscle, was purchased from Takara Bio Inc. (Kusatsu, Japan). Lipopolysaccharide (LPS) from Escherichia coli 0127: B8 was purchased from Sigma-Aldrich Co. LLC (St. Louis, MA, USA). LA was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Lactate colorimetric assay kit 2 was purchased from BioVision (Milpitas, CA, USA). Other reagents used in the in vitro test and various evaluations of mice were prepared according to our previous study [8].
2.3. Preparation of KPE and PMFs
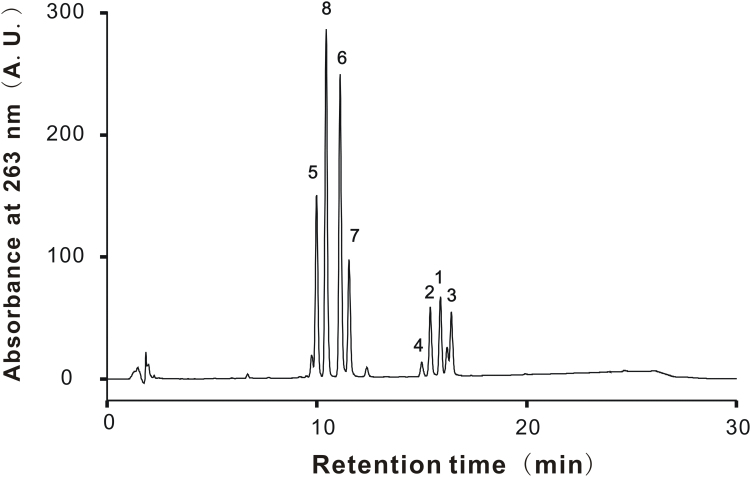
KPE was obtained from dried rhizomes of black ginger by extracting with aqueous alcohol (yield 15.9%). KPE was mixed with modified starch at a ratio of 3:7 (KPE: modified starch) and then powdered by spray drying. The KPE suspension for in vivo experiment use was prepared by suspending 600 mg of powdered KPE in 40 mL water. Regarding the vehicle, 420 mg of modified starch was dissolved in 40 mL water. These solutions were ingested to mice. PMF standards including 5-hydroxy-3,7-dimethoxyflavone (1), 5-hydroxy-7-methoxyflavone (2), 5-hydroxy-3,7,4'-trimethoxyflavone (3), 5-hydroxy-3,7,3',4'-tetramethoxyflavone (4), 3,5,7,3',4'-pentamethoxyflavone (5), 5,7,4'-trimethoxyflavone (6), 3,5,7,4'-tetramethoxyflavone (7), and 5,7-dimethoxyflavone (8) were the same ones as used in our previous study [8]. The contents of all PMFs in powdered KPE were determined by reverse-phase HPLC using a Prominence HPLC system (Shimadzu, Kyoto, Japan) equipped with a photodiode array detector (Model SPD-M20A) and a Develosil RPAQUEOUS-AR-5 column (4.6 × 150 mm, 5 μm particle size, Nomura Chemical Co., Ltd., Japan). The mobile phase was a binary gradient and consisted of a mixture of acetonitrile, water and acetic acid (35:62.5:2.5, v/v) as solvent A and a mixture of acetonitrile and acetic acid (97.5:2.5, v/v) as solvent B. The flow rate was fixed at 1.0 mL/min and column temperature was set at 35 °C. The gradient condition was as follows: 0–20 min (solvent A: 99–1%). A UV detection at 263 nm was used. The contents of the determined PMFs were 1 (0.66%), 2 (0.52%), 3 (0.81%), 4 (0.30%), 5 (2.94%), 6 (3.14%), 7 (1.75%) and 8 (2.5%), respectively (Fig. 1).
Fig. 1.
HPLC chromatogram of KPE for determination of PMFs. PMFs in KPE were separated on a Develosil RPAQUEOUS-AR-5 column. The chromatographic mobile phase consisted of A (acetonitrile: water: acetic acid = 35: 62.5: 2.5, v/v) and B (acetonitrile: acetic acid = 97.5: 2.5, v/v). The gradient program was set as 0–20 min (solvent A: 99–1%). Use of 263 nm as a selective wavelength allowed identification of the 8 known PMFs. Peak 1, 5-hydroxy-3,7-dimethoxyflavone; 2, 5-hydroxy-7-methoxyflavone; 3, 5-hydroxy-3,7,4'-trimethoxyflavone; 4, 5-hydroxy-3,7,3',4'-tetramethoxyflavone; 5, 3,5,7,3',4'-pentamethoxyflavone; 6, 5,7,4'-trimethoxyflavone; 7, 3,5,7,4'-tetramethoxyflavone; 8, 5,7-dimethoxyflavone.
2.4. Test schedule in mice
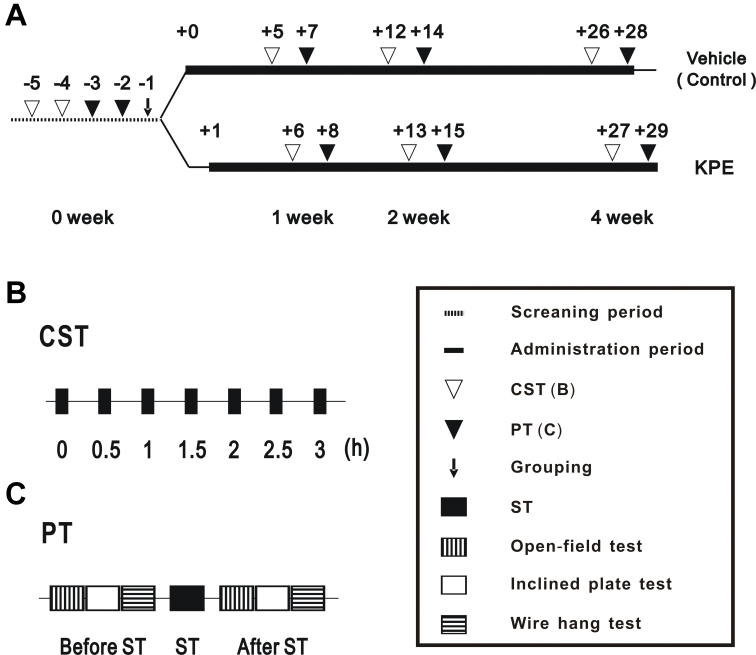
In vivo tests were performed based on the schedule described in Fig. 2A. At screening period, consecutive forced swimming test (CST) was performed on day −5 and −4. On day −3 and −2, physical fitness measurement tests (PT) were performed and mice were divided into 2 groups according to the data obtained from CST and PT to insure the total physical fitness performance of the two groups to be almost same. After grouping (day 0), mice were orally administered KPE or vehicle, and the same paired tests (CST and PT) conducted at 0 week were performed at the 1-, 2-, and 4-week periods. After the test at the 4-week period, the soleus muscle was collected from the right hind leg in order to investigate the expression of various mRNAs, mitochondrial number, and glycogen content. Blood was collected from the ventral aorta under anesthesia in order to determine blood LA levels.
Fig. 2.
Protocol for in vivo test. The time schedule of the in vivo test (A). Each number shows the day before and after the first oral administration of KPE or vehicle. The allow (↓), open triangle (∇) and closed triangle (▼) mean grouping, consecutive forced swimming test (CST) and physical fitness measurement tests (PT), respectively. Bold lines indicate the administration period of the vehicle (upper line) for the control and KPE (lower line). The time schedules of the CST and PT are shown in B. Each closed rectangle (■) means the forced swimming test (ST). The vertically lined rectangle (▥), open rectangle (▭), and horizontally lined rectangle (▤) mean the open-field test, inclined plate test, and wire hanging test, respectively.
2.5. CST
In a round water pool (φ 25 cm, 30 cm in depth filled with warmed water at 37 °C to 40 °C), mice were bound by their tails to a weight that was approximately 10% of their body weights and forced to swim. In order to exclude air on the surface of the hair, measurements were started after the body had been submerged once below the water surface. The floating time was defined as the time until the mouse was unable to float for 5 seconds [or 7 seconds in case of forced swimming (ST) in PT] on the surface of the water. Moreover, in the CST, secondary ST was performed 30 min after the first ST under the same conditions. ST was subsequently performed a total of 7 times at 0, 0.5, 1, 1.5, 2, 2.5 and 3 h (Fig. 2B).
2.6. PT
PT were performed using the following methods: ST, open-field test, inclined plane test, and wire hanging test (Fig. 2C). ST was performed under almost the same conditions as the CST. The other tests were performed twice (Fig. 2C), before and after ST, as described below. Open-field test: A mouse was placed in a square field (30 × 30 cm) divided into 9 areas. The number of times the mouse moved to other areas was measured for 3 min. Inclined plate test: after the open-field test, the mouse was placed on a wooden board covered with a canvas, which was leaned gradually at a constant speed. The angle at which the mouse dropped from the board was measured. Wire hanging test: after the inclined plane test, mice were bound by their tails to a weight that was 15% of their body weight. They were then placed on a square wire mesh (30 × 30 cm, mesh size 1 cm, wire diameter φ 1 mm). The wire mesh with the mouse on was turned upside down and placed at a height of 50 cm. The sides were shielded and the time until the mouse dropped off was measured.
2.7. Measurement of mitochondrial DNA
Mitochondrial DNA was determined by the method of Henagan et al. [28] Total DNA was extracted from 25 mg of the soleus muscle by NucleoSpin® Tissue. The DNA obtained was used to determine the expression of mitochondrial DNA (mtDNA) and genomic DNA (gDNA) by using real-time PCR. The specific primers used for the nuclear gene of lipoprotein lipase were 5'-GGATGGACGGTAAGAGTGATTC-3' (forward) and 5'-ATCCAAGGGTAGCAGACAGGT-3' (reverse). The specific primers used for the mitochondrial gene of NADH dehydrogenase subunit I were 5'-CCCATTCGCGTTATTCTT-3' (forward) and 5'-AAGTTGATCGTAACGGAAGC-3' (reverse). The ratio of mitochondrial numbers was calculated from the relative expression of the mitochondrial gene to the nuclear gene using the ΔΔCt method.
2.8. Determination of glycogen content
Glycogen content was determined by the method of Lo et al. [29]. The soleus muscle (20 mg) or C2C12 were soaked in 1 mL of 30% (w/v) KOH and incubated at 100 °C for 15 min. After the incubation, the extract was added 2 mL of EtOH and incubated at 4 °C for 5 min. Then, the extract was centrifuged (500 × g, 4 °C, 7 min) and the supernatant was removed. After sufficient drying of the pellets, glycogen contents were measured using the phenol-sulfuric acid method and then normalized by the weight of the soleus muscle or cell number.
2.9. Determination of blood LA level
After PT at the 4-week period, blood was collected and centrifuged (200 × g, room temperature, 3 min). The supernatant obtained was used to measure blood LA levels by lactate colorimetric assay kit 2.
2.10. Analysis of mRNA in the mouse soleus muscle and C2C12
Total RNA was extracted from the soleus muscle (25 mg) or C2C12 and relative mRNA expression levels were evaluated using our previously described method [8]. The specific primers used were as follows: IL-6, 5'- TCTATACCACTTCACAAGTCGGA −3' (forward) and 5'- GAATTGCCATTGCACAACTCTTT −3' (reverse), TNF-α, 5'- GACGTGGAAGTGGCAGAAGAG −3' (forward) and 5'- TGCCACAAGCAGGAATGAGA −3' (reverse); peroxisome proliferator-activated receptor γ coactivator (PGC)-1α, 5'- TATGGAGTGACATAGAGTGTGCT −3' (forward) and 5'- CCACTTCAATCCACCCAGAAAG −3' (reverse); peroxisome proliferative activated receptor (PPAR)-γ1, 5'- GGAAGACCACTCGCATTCCTT −3' (forward) and 5'- GTAATCAGCAACCATTGGGTCA −3' (reverse); glycogen synthase (Gsy) 1, 5'- TCCTGGCCCAGAACGAAGA −3' (forward) and 5'- TGAGTGGTGAAGATGGTTGCC −3' (reverse); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5'- ACCTCAACTACATGGTCTAC −3' (forward) and 5'- TTGTCATTGAGAGCAATGCC −3' (reverse). mRNA expression levels were evaluated by the relative expression ratio of the gene to GAPDH using the ΔΔCt method.
2.11. Inflammation in C2C12 induced by LPS
The experiment was performed as described previously [28, 30]. Briefly, C2C12 were induced to differentiate with KPE (10 μg/mL) or PMFs (compound 1–8, 10 μM) for one week. Cells were then cultured with 1 μg/mL LPS or LA for 1 h. Cells were collected and total RNA was extracted. The relative mRNA expression levels of total interleukin (IL)-6 and tumor necrosis factor (TNF)-α were examined using RT-PCR.
2.12. Statistical analysis
Data are presented as the mean ± S.E. A one-way analysis of variance (ANOVA) followed by Welch’s t-test was performed for statistical comparisons of in vivo experimental data. The significance of differences from the initial data (0 weeks) was indicated as *: P < 0.05, **: P < 0.01, while that from the control was indicated as †: P < 0.05, ††: P < 0.01, respectively. The in vivo test was performed on 15 mice in each group. However, one mouse treated with KPE drowned in ST at week 4. Therefore, the statistical analysis was performed on 15 mice for the control group and 14 mice for the KPE group. The same method as that described above for in vivo experimental data was used for statistical comparisons of in vitro experimental data.
3. Results
3.1. KPE suppressed decrease in muscular endurance induced by fatigue in CST
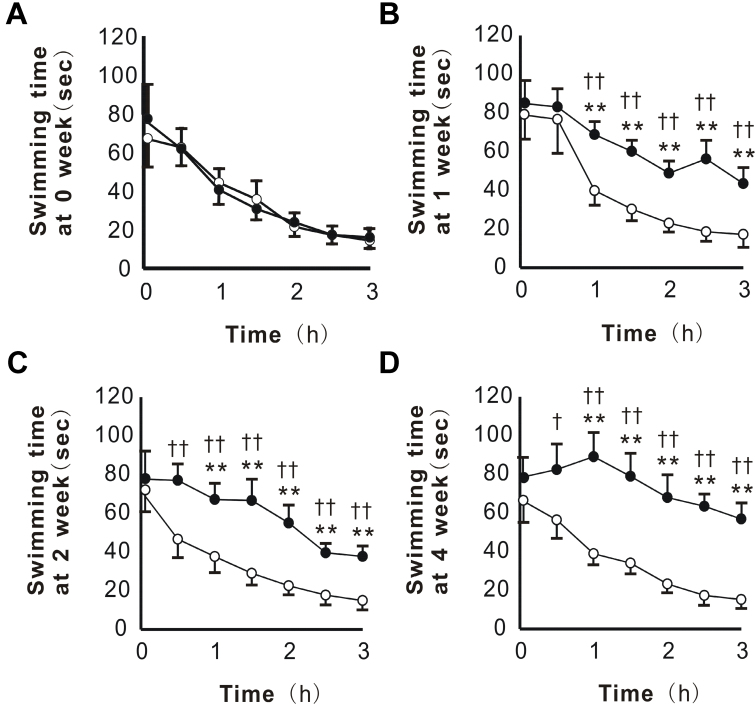
KPE was orally administered to mice for 4 weeks and muscular endurance was evaluated at 0-, 1-, 2-, and 4-week periods. In each week, no significant differences between the control and KPE groups were observed in the initial values determined (0 h) (Fig. 3). However, from the second and third measurements (0.5 or 1 h), the decreases in the swimming time were smaller in the KPE group than in the control group (Fig. 3B-D). This suppression was confirmed from the 1-week period and became clearer in a time-dependent manner (Fig. 3B-D). In the 4-week period determination, the swimming time duration for the last measurement (3 h) shortened only 27% against the first measurement in the KPE group, while that shortened 78% in the control group (Fig. 3D), indicating the enforcement of the muscular endurance or the rapid recovery from swimming-induced fatigue in the KPE group.
Fig. 3.
KPE enhanced muscular endurance in the consecutive forced swimming test (CST). The CST was performed at the 0- (A), 1- (B), 2- (C), and 4- (D) week periods. According to Fig. 1B, ST was repeated at 30-min intervals, and the swimming time was measured for a total of 7 times. Each point represents the mean with the S.E. (control; n = 15, KPE; n = 14). Open circle (○) for the control group and closed circle (●) for the KPE group. Asterisks denote significant differences from the initial value (0 week) at *: P<0.05, **: P<0.01, respectively. Daggers denote significant differences from the control at †: P<0.05, ††: P<0.01, respectively.
3.2. KPE enhanced physical fitness performance in PT
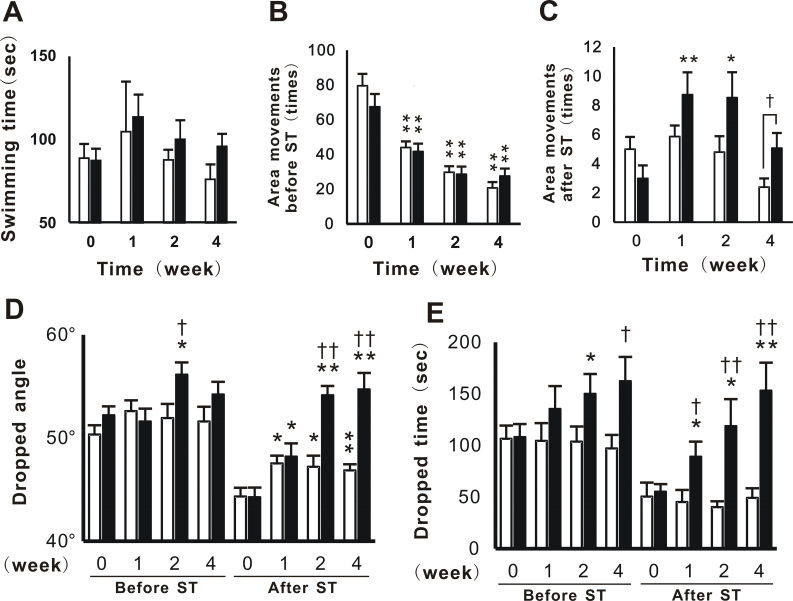
Physical fitness performance in PT was examined in order to evaluate the effects of KPE 2 days after the CST. In ST, which induced sufficient fatigue in mice, swimming times were slightly longer in the KPE group than in the control group from the 1-week period (Fig. 4A). In the open-field test performed before ST (Fig. 4B), the number of movements in control and KPE in 1-, 2- and 4-week periods were significantly decreased compared to the values at 0-week period. However, the number of movements after ST was significantly increased in the KPE group, but not in the control group at the 1- and 2-week periods (Fig. 4C). Furthermore, the number of movements after ST was significantly increased in the KPE group at the 4-week period (Fig. 4C). In the inclined plate test, dropped angles were significantly greater in the KPE group than in the control group at the 2-week period (Fig. 4D). The dropped angles in KPE group after ST were similar to those before ST at the 2- and 4-week periods (Fig. 4D). In the wire hanging test, similar results to those obtained in the inclined plate test were observed from the 1- to 4-week periods. From the ratio calculated for the dropped time after and before ST, the ratio in the KPE group (96%) was almost twice at the 4-week period (Fig. 4E), indicating the enhancement of the grip strength in the KPE group.
Fig. 4.
KPE enhanced physical fitness performance with or without fatigue loading. Physical fitness measurement tests (PT) consisting of forced swimming test (ST, A), open-field test (B and C), inclined plate test (D) and wire hanging test (E) were performed. Columns represented open column (□): control group and closed column (■): KPE group. Open-field test, inclined plate test, and wire hanging test were performed twice: before and after ST. The values in the open-field test before (B) and after (C) ST were indicated separately. Each column represents the mean with the S.E. (control; n = 15, KPE; n = 14). Asterisks denote significant differences from the initial result (0 week) at *: P<0.05, **: P<0.01, respectively. Daggers denote significant differences from the control at †: P<0.05, ††: P<0.01, respectively.
3.3. KPE and PMFs suppressed muscular inflammation in vitro and in vivo
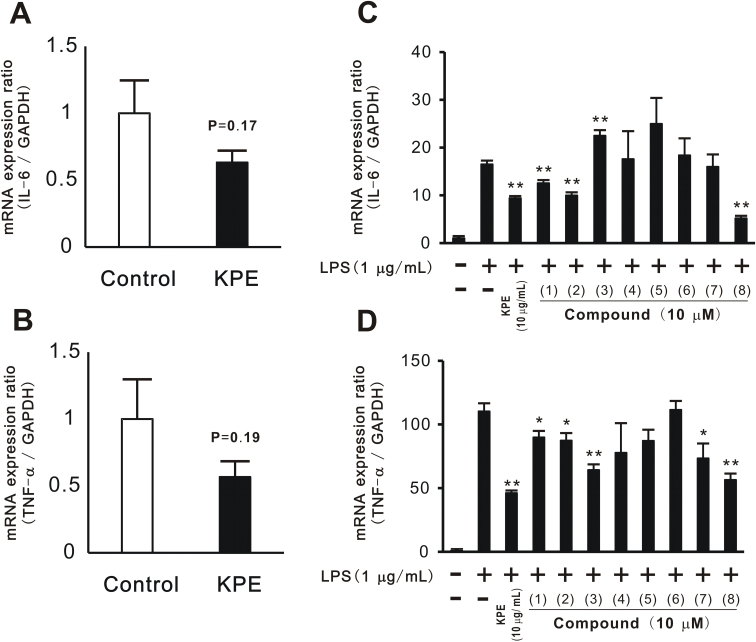
In previous experiments (Fig. 3 and Fig. 4), KPE was shown to improve physical fitness performance and muscular endurance in vivo. Therefore, we attempted to clarify the mechanisms responsible for the effects of KPE. mRNA expression was evaluated in relation to inflammation, namely, that of IL-6 and TNF-α, in the soleus muscle. The results showed that IL-6 (Fig. 5A) and TNF-α (Fig. 5B) mRNA expression levels tended to be lower in the KPE group than in the control group. Therefore, the anti-inflammatory effects of KPE may contribute to improvements in physical fitness performance and muscular endurance.
Fig. 5.
KPE and PMFs suppressed muscular inflammation in vivo and in vitro.Total RNA was extracted from mouse soleus muscles after physical fitness measurement tests had been completed. mRNA expressions of IL-6 (A) and TNF-α (B) were evaluated using real-time PCR. C2C12 differentiated with KPE (10 μg/mL) and compounds 1–8 (10 μM) were cultured with LPS (final concentration: 1 μg/mL) for 1 h. Total RNA was extracted from cells and the mRNA expression of IL-6 (C) and TNF-α (D) was evaluated. Each column represents the mean with the S.E. (A and B: n = 14, C and D: n = 4). Asterisks denote significant differences from the control (A and B) or the control only treated with LPS (C and D) at *: p<0.05, **: p<0.01, respectively.
The anti-inflammatory effects of KPE and PMFs were investigated in vitro using a previously reported method [30]. LPS and LA were used to induce muscular inflammation. IL-6 and TNF-α mRNA expression levels were increased by LPS, but not by LA (data not shown). The increases induced in mRNA expression levels by LPS were slightly suppressed in the cells cultured with KPE. PMFs, compounds 1–8 isolated from KPE, were examined by a similar procedure. KPE and compounds 1, 2, and 8 significantly suppressed the increases induced by LPS in the mRNA expression levels of IL-6 (Fig. 5C) and TNF-α (Fig. 5D). Compounds 3, 7 and 8 suppressed the mRNA expression of TNF-α.
3.4. KPE increased mitochondrial number and decreased blood LA level in vivo
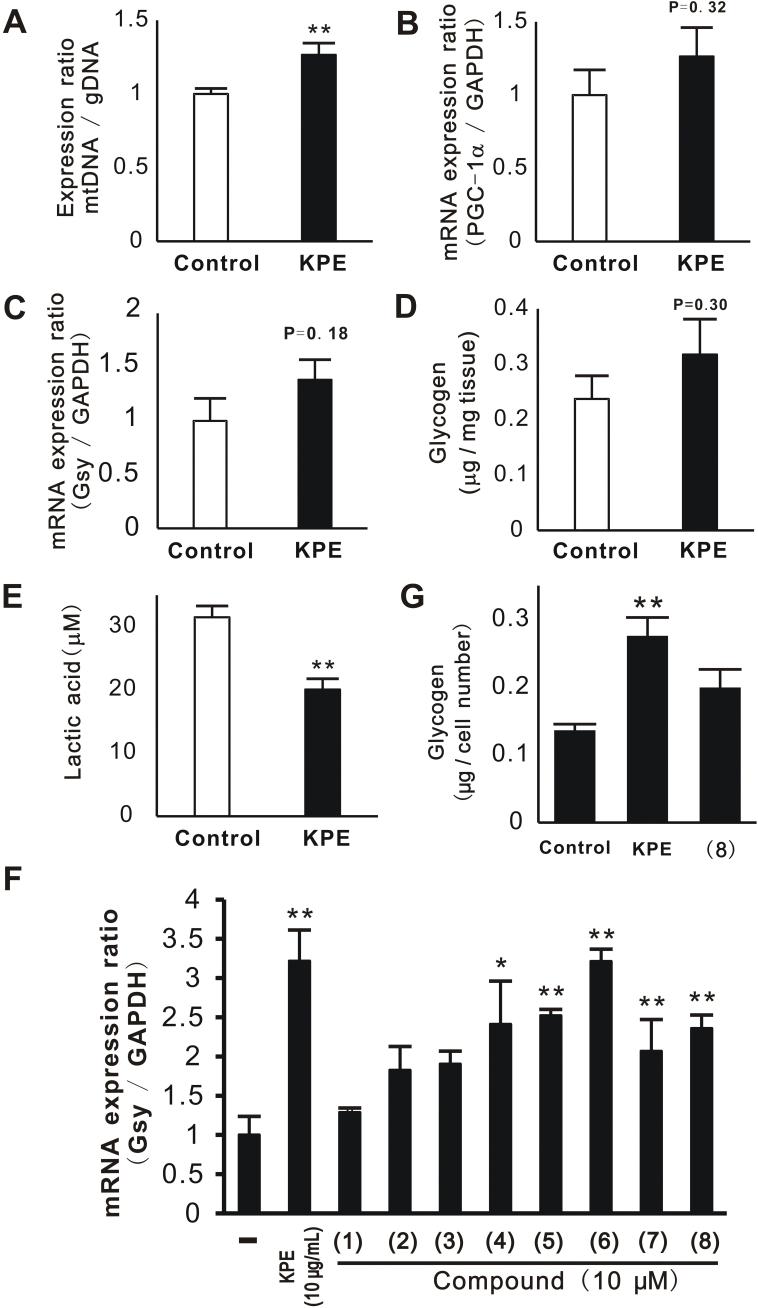
In order to investigate the contribution of mechanisms other than anti-oxidative activity to improvements in physical fitness performance and muscular endurance, the effects of KPE on metabolism were evaluated in relation to mitochondrial number, accumulation of glycogen, and blood LA level. Regarding mitochondrial number, the expression ratio of mitochondrial DNA to genomic DNA was measured. The results showed that KPE enhanced the number of mitochondria in the soleus muscle (Fig. 6A). The mRNA expression of PGC-1α, which is related to mitochondrial biosynthesis, tended to increase by KPE (Fig. 6B). The mRNA expression of Gsy and accumulation of glycogen were similarly evaluated. The results showed that KPE tended to enhance the mRNA expression of Gsy (Fig. 6C) and increase glycogen content (Fig. 6D). Therefore, KPE was suggested to increase mitochondrial numbers by enhancing the expression of PGC-1α and glycogen content by enhancing the expression of Gsy. Moreover, KPE decreased blood LA levels (Fig. 6E).
Fig. 6.
Effect of KPE on mitochondria and glycogen in vitro and in vivo. Total DNA, mRNA, and glycogen were extracted from the mouse soleus muscle and C2C12 treated with KPE (10 μg/mL) or compounds 1–8 (10 μM). In vivo (A-E), total DNA was evaluated for the expression of mitochondrial DNA (mtDNA) using real-time PCR (A). mtDNA was normalized by the expression of genomic DNA (gDNA). mRNA expressions of PGC-1α (B) and glycogen synthase (Gsy, C) were evaluated using real-time PCR. Muscle glycogen was determined using the phenol-sulfuric acid method (D). The glycogen content was normalized by the tissue weight. Blood lactic acid (LA) was measured using a kit (E). In vitro (F, G), the mRNA expression of Gsy was evaluated using real-time PCR (F) and glycogen contents were determined (G). The glycogen contents in these cells were normalized by the cell numbers. Each column represents the mean with the S.E. (in vivo: control: n = 15, KPE: n = 14, in vitro: n = 4). Asterisks denote significant differences from the control at *: P < 0.05, **: P < 0.01, respectively.
3.5. PMFs promoted the accumulation of glycogen in vitro
We previously reported that PMFs in KPE increased the mRNA expression of glucose transporter type 4 (GLUT4) and PGC-1α. Therefore, we herein determined whether PMFs affect the accumulation of glycogen. The mRNA expression of Gsy was measured in C2C12 treated with KPE or PMFs. KPE and many of the PMFs (4, 5, 6, 7, and 8) significantly increased the expression of Gsy (Fig. 6F). The effects of KPE and compound 8 on the glycogen content in C2C12 were then evaluated. Compound 8 was selected because it is known to activate AMPK [28]. KPE significantly enhanced the glycogen content (Fig. 6G), while compound 8 slightly enhanced its contents. These results suggest that PMFs in KPE promote the accumulation of glycogen.
4. Discussion
The results of the present study indicate that KPE increases physical fitness performance (Fig. 4) and muscular endurance (Fig. 3 and Fig. 4). In the inclined plate test and wire hanging test after ST, endurance and performance were better in the KPE group than in the control group. Anti-inflammation and enhanced energy metabolism have been suggested as the mechanisms by which KPE improves performance. Hence, enhancements in mRNA expression related to inflammation and the accumulation of glycogen were evaluated. The results showed that KPE tended to down-regulate the mRNA expression of IL-6 and TNF-α in the soleus muscle (Fig. 5A, B) without significant differences being observed when compared with control mice. In the in vitro test, KPE suppressed mRNA expression of inflammatory factors induced by LPS. When PMFs (1–8) were examined using the same method, 1, 2, and 8 were found to significantly suppress the mRNA expression of IL-6 and TNF-α. KPE showed an inhibitory trend on the mRNA expression of IL-6 and TNF-α in the in vivo research. In order to clarify this, further study with more number of mice on the anti-inflammation effect of KPE in muscle is needed. Regarding the up-regulation of GLUT4 and PGC-1α, 2 and 8 exerted similar strong effects [27]. Therefore, the 3′- and 4′-methoxy groups may not be necessary for this activity.
We previously reported that PMFs including KPE activated AMPK [27]. AMPK activators such as AICAR have been shown to increase physical fitness performance and muscular endurance in vivo [27]. A chronic treatment with AICAR was found to increase the accumulation of glycogen and enhance fatty acid oxidation in skeletal muscles in insulin-deficient rats [31] while another activator, C24 decreased blood glucose and fatty acid levels in diabetic db/db mice [32]. AMPK has also been reported to play an important role in improvements in inflammation and insulin resistance [33]. These findings are consistent with the present results and our previous findings on black ginger. KPE and PMFs, particularly compound 8, promoted the uptake of glucose, accumulation of glycogen, activation of mitochondria, and increases in mitochondrial number (Fig. 6 and our previous findings [8]). Furthermore, KPE has been reported to suppress increases in body weight, visceral fat accumulation, dysfunctional lipid metabolism, hyperinsulinemia, insulin resistance, hypertension, and peripheral neuropathy in TSOD mice [1]. Therefore, the activation of AMPK by KPE may contribute to these activities including improvements in physical fitness performance and endurance. The activation of AMPK by food ingredients differs from that by medicines such as AICAR and C24. The application of black ginger as a new approach to prevent diseases is promising because of its safety and historical background as a food [34].
Clinical studies have been conducted on black ginger, with some reporting improved physical fitness performance in the elderly and athletes, [6, 7] and the enhanced antioxidant enzyme activities (increased activities of superoxide dismutase, glutathione peroxidase and catalase in serum) and decreased MDA level had been suggested to be one of the mechanisms for physical improvement effect of KPE [7]. The promotion of energy consumption through the activation of metabolism in brown adipose tissue has also been reported [35]. Therefore, black ginger may be a suitable ingredient for improving physical fitness performance, muscular endurance, fatigue, and metabolism. On the other hand, mild exercise is well-known to have similar effects as those of KPE on health [36, 37, 38, 39, 40]. We expect black ginger to become a useful ingredient in processed foods and dietary supplements that will have exercise- and health-promoting effects.
Declarations
Author contribution statement
Kazuya Toda: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shoketsu Hitoe: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Shogo Takeda: Performed the experiments.
Hiroshi Shimoda: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
All authors related to this study are employees of Oryza Oil and Fat Chemical Co., Ltd. (Aichi, Japan). The authors declare no conflict of interest associated with this manuscript.
Additional information
No additional information is available for this paper.
References
- 1.Akase T., Shimada T., Terabayashi S., Ikeya Y., Sanada H., Aburada M. Antiobesity effects of Kaempferia parviflora in spontaneously obese type II diabetic mice. J. Nat. Med. 2011;65:73–80. doi: 10.1007/s11418-010-0461-2. [DOI] [PubMed] [Google Scholar]
- 2.Shimada T., Horikawa T., Ikeya Y., Matsuo H., Kinoshita K., Taguchi T., Ichinose K., Takahashi K., Aburada M. Preventive effect of Kaempferia parviflora ethyl acetate extract and its major components polymethoxyflavonoid on metabolic diseases. Fitoterapia. 2011;82:1272–1278. doi: 10.1016/j.fitote.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 3.Rujjanawate C., Kanjanapothi D., Amornlerdpison D., Pojanagaroon S.J. Anti-gastric ulcer effect of Kaempferia parviflora. J. Ethnopharmacol. 2005;102:120–122. doi: 10.1016/j.jep.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Kusirisin W., Srichairatanakool S., Lerttrakarnnon P., Lailerd N., Suttajit M., Jaikang C., Chaiyasut C. Antioxidative activity: polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med. Chem. 2009;5:139–147. doi: 10.2174/157340609787582918. [DOI] [PubMed] [Google Scholar]
- 5.Chaturapanich G., Chaiyakul S., Verawatnapakul V., Yimlamai T., Pholpramool C. Enhancement of aphrodisiac activity in male rats by ethanol extract of Kaempferia parviflora and exercise training. Andrologia. 2012;44:323–328. doi: 10.1111/j.1439-0272.2011.01184.x. [DOI] [PubMed] [Google Scholar]
- 6.Promthep K., Eungpinichpong W., Sripanidkulchai B., Chatchawan U. Effect of Kaempferia parviflora extract on physical fitness of soccer players: A randomized double-blind placebo-controlled trial. Med. Sci. Monit. Basic Res. 2015;21:100–108. doi: 10.12659/MSMBR.894301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wattanathorn J., Muchimapura S., Tong-Un T., Saenghong N., Thukhum-Mee W., Sripanidkulchai B. Positive modulation effect of 8-week consumption of Kaempferia parviflora on health-related physical fitness and oxidative status in healthy elderly volunteers. Evid. Based Complement. Alternat. Med. 2012;2012:732816. doi: 10.1155/2012/732816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toda K., Takeda S., Hitoe S., Nakamura S., Matsuda H., Shimoda H. Enhancement of energy production by black ginger extract containing polymethoxy flavonoids in myocytes through improving glucose, lactic acid and lipid metabolism. J. Nat. Med. 2015;70:163–172. doi: 10.1007/s11418-015-0948-y. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher W.M. Lactic acid in amphibian muscle. J. Physiol. 1907;35:247–309. doi: 10.1113/jphysiol.1907.sp001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westerblad H., Bruton J.D., Lännergren J. The effect of intracellular pH on contractile function of intact: single fibres of mouse muscle declines with increasing temperature. J. Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stary C.M., Hogan M.C. Intracellular pH during sequential: fatiguing contractile periods in isolated single Xenopus skeletal muscle fibers. J. Appl. Physiol. 2005;99:308–312. doi: 10.1152/japplphysiol.01361.2004. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen O.B., de Paoli F., Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J. Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedersen T.H., Nielsen O.B., Lamb G.D., Stephenson D.G. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- 14.Favero T.G. Sarcoplasmic reticulum Ca2+ release and muscle fatigue. J. Appl. Physiol. 1999;87:471–483. doi: 10.1152/jappl.1999.87.2.471. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen O.B., Clausen T. The Na+/K+-pump protects muscle excitability and contractility during exercise. Exerc. Sport Sci. Rev. 2000;28:159–164. [PubMed] [Google Scholar]
- 16.Allen D.G., Kabbara A.A., Westerblad Hk. Muscle fatigue: the role of intracellular calcium stores. Can. J. Appl. Physiol. 2002;27:83–96. doi: 10.1139/h02-006. [DOI] [PubMed] [Google Scholar]
- 17.Vandenboom R. The myofibrillar complex and fatigue: a review. Can. J. Appl. Physiol. 2004;29:330–356. doi: 10.1139/h04-022. [DOI] [PubMed] [Google Scholar]
- 18.Powers S.K., Ji L.L., Kavazis A.N., Jackson M.J. Reactive oxygen species: impact on skeletal muscle. Compr. Physiol. 2011;1:941–969. doi: 10.1002/cphy.c100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosker H.R., Schols A.M. Fatigued muscles in COPD but no finishing line in sight. Eur. Respir. J. 2008;31:693–694. doi: 10.1183/09031936.00015308. [DOI] [PubMed] [Google Scholar]
- 20.Finsterer J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2012;13:218. doi: 10.1186/1471-2474-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X., Wu G., Shi D., Zhu R., Zeng H., Cao B., Huang M., Liao H. Effects of nitric oxide on notexin-induced muscle inflammatory responses. Int. J. Biol. Sci. 2015;11:156–167. doi: 10.7150/ijbs.10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn B.B., Alquier T., Carling D., Hardie D.G. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Hardie D.G., Schaffer B.E., Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Astratenkova I.V., Rogozkin V.A. Participation AMPK in the regulation of skeletal muscles metabolism. Ross Fiziol Zh Im I M Sechenova. 2013;99:657–673. [PubMed] [Google Scholar]
- 25.Nasri H., Rafieian-Kopaei M. Metformin: Current knowledge. J. Res. Med. Sci. 2014;19:658–664. [PMC free article] [PubMed] [Google Scholar]
- 26.Malin S.K., Kashyap S.R. Effects of metformin on weight loss: potential mechanisms. Curr. Opin. Endocrinol. Diabetes Obes. 2014;21:323–329. doi: 10.1097/MED.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 27.Narkar V.A., Downes M., Yu R.T., Embler E., Wang Y.X., Banayo E., Mihaylova M.M., Nelson M.C., Zou Y., Juguilon H., Kang H., Shaw R.J., Evans R.M. AMPK and PPARδ agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henagan T.M., Cefalu W.T., Ribnicky D.M., Noland R.C., Dunville K., Campbell W.W., Stewart L.K., Forney L.A., Gettys T.W., Chang J.S., Morrison C.D. In vivo effects of dietary quercetin and quercetin-rich red onion extract on skeletal muscle mitochondria, metabolism, and insulin sensitivity. Genes Nutr. 2015;10:2. doi: 10.1007/s12263-014-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lo S., Russell J.C., Taylor A.W. Determination of glycogen in small tissue samples. J. Appl. Physiol. 1970;28:234–236. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- 30.Frost R.A., Nystrom G.J., Lang C.H. Lipopolysaccharide and proinflammatory cytokines stimulate interleukin-6 expression in C2C12 myoblasts: role of the Jun NH2-terminal kinase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1153–1164. doi: 10.1152/ajpregu.00164.2003. [DOI] [PubMed] [Google Scholar]
- 31.Vitzel K.F., Bikopoulos G., Hung S., Pistor K.E., Patterson J.D., Curi R., Ceddia R.B. Chronic treatment with the AMP-kinase activator AICAR increases glycogen storage and fatty acid oxidation in skeletal muscles but does not reduce hyperglucagonemia and hyperglycemia in insulin deficient rats. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y.Y., Yu L.F., Zhang L.N., Qiu B.Y., Su M.B., Wu F., Chen D.K., Pang T., Gu M., Zhang W., Ma W.P., Jiang H.W., Li J.Y., Nan F.J., Li J. Novel small-molecule AMPK activator orally exerts beneficial effects on diabetic db/db mice. Toxicol. Appl. Pharmacol. 2013;273:325–334. doi: 10.1016/j.taap.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Liong S., Lappas M. Activation of AMPK improves inflammation and insulin resistance in adipose tissue and skeletal muscle from pregnant women. J. Physiol. Biochem. 2015;71:703–717. doi: 10.1007/s13105-015-0435-7. [DOI] [PubMed] [Google Scholar]
- 34.Sudwan P., Saenphet K., Saenphet S., Suwansirikul S. Effect of Kaempferia parviflora Wall. ex: Baker on sexual activity of male rats and its toxicity. Southeast Asian J. Trop. Med. Public Health. 2006;37:210–215. [PubMed] [Google Scholar]
- 35.Matsushita M., Yoneshiro T., Aita S., Kamiya T., Kusaba N., Yamaguchi K., Takagaki K., Kameya T., Sugie H., Saito M. Kaempferia parviflora extract increases whole-body energy expenditure in humans: roles of brown adipose tissue. J. Nutr. Sci. Vitaminol. (Tokyo) 2015;61:79–83. doi: 10.3177/jnsv.61.79. [DOI] [PubMed] [Google Scholar]
- 36.Pingitore A., Lima G.P., Mastorci F., Quinones A., Iervasi G., Vassalle C. Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Nutrition. 2015;31:916–922. doi: 10.1016/j.nut.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Horowitz J.F., Klein S. Lipid metabolism during endurance exercise. Am. J. Clin. Nutr. 2000;72:558S–563S. doi: 10.1093/ajcn/72.2.558S. [DOI] [PubMed] [Google Scholar]
- 38.Johnson N.A., Stannard S.R., Thompson M.W. Muscle triglyceride and glycogen in endurance exercise: implications for performance. Sports Med. 2004;34:151–164. doi: 10.2165/00007256-200434030-00002. [DOI] [PubMed] [Google Scholar]
- 39.Stephens F.B., Galloway S.D. Carnitine and fat oxidation. Nestlé Nutr. Inst. Workshop Ser. 2013;76:13–23. doi: 10.1159/000350224. [DOI] [PubMed] [Google Scholar]
- 40.Pedersen B.K., Saltin B. Exercise as medicine − evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015;25(Suppl. 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]