In this article, we demonstrate robust modulation of beta activity in the human anterior temporal lobe during a name retrieval task. Increased beta power was consistently seen from both the left and right anterior temporal lobe for both proper and common name retrieval tasks. Measurement of these visual naming responses may provide the groundwork for future mapping modalities to localize eloquent cortex in the anterior temporal lobe.
Keywords: beta band, naming, anterior temporal lobe, eipliepsy, temporal pole
Abstract
Naming people, places, and things is a fundamental human ability that is often impaired in patients with language-dominant anterior temporal lobe (ATL) dysfunction or ATL resection as part of epilepsy treatment. Convergent lines of evidence point to the importance of the ATL in name retrieval. The physiologic mechanisms that mediate name retrieval in the ATL, however, are not well understood. The purpose of this study was to characterize the electrophysiologic responses of the human ATL during overt cued naming of famous people and objects. Eight neurosurgical patients with suspected temporal lobe epilepsy who underwent implantation of intracranial electrodes for seizure focus localization were the subjects of this study. Specialized coverage of the ATL was achieved in each subject. The subjects named pictures of U.S. presidents and images of common hand-held tools. Event-related band power was measured for each ATL recording site. Both the left and right ATL demonstrated robust and focal increases in beta-band (14–30 Hz) power during person and tool naming. The onset of this response typically occurred at 400 ms but sometimes as early as 200 ms. Visual naming of famous people and tools is associated with robust and localized modulation of the beta band in both the left and right ATL. Measurement of visual naming responses may provide the groundwork for future mapping modalities to localize eloquent cortex in the ATL.
NEW & NOTEWORTHY
In this article, we demonstrate robust modulation of beta activity in the human anterior temporal lobe during a name retrieval task. Increased beta power was consistently seen from both the left and right anterior temporal lobe for both proper and common name retrieval tasks. Measurement of these visual naming responses may provide the groundwork for future mapping modalities to localize eloquent cortex in the anterior temporal lobe.
the ability to name people, places, and things is unique to humans and fundamental to many daily activities. This ability is highly dependent on the anterior temporal lobes (ATL), especially the language-dominant ATL, where focal lesions (i.e., brain injury) result in a specific deficit for naming that is most pronounced for naming proper nouns (Damasio et al. 1996; Lambon Ralph 2014; Tranel 2009). ATL-related naming deficits are common after language-dominant anterior temporal lobectomy to treat seizures (Drane et al. 2008, 2013; Yucus and Tranel 2007) and also in patients with semantic dementia (Bozeat et al. 2000; Hurley et al. 2014; Lambon Ralph et al. 2001; Rogers et al. 2004). Evidence suggests that ATL-related naming deficits are heteromodal (e.g., anomia for faces and voices), meaning the ability to name both visual and auditory inputs is impaired (Belfi and Tranel 2013; Lambon Ralph 2014; Waldron et al. 2014). Naming impairment is far more pronounced for naming unique entities compared with common entities (Damasio et al. 1996; Semenza 2011).
Despite its clinical and scientific importance, the physiologic mechanisms underlying name retrieval in the ATL remain poorly understood, in large part because of technical limitations of noninvasive neuroimaging (e.g., signal dropout, limited spatiotemporal resolution) (Devlin et al. 2000; Grabowski et al. 2001; Visser et al. 2010). Invasive electrophysiological (Abel et al. 2015; Chan et al. 2011; Chen et al. 2016; Shimotake et al. 2014) and transcranial magnetic stimulation (Pobric et al. 2007) studies have provided important information regarding the time course and specificity of naming and recognition-related processes; however, the physiologic mechanisms of name retrieval in the ATL remain poorly understood. Previous work studying electrocorticographic (ECoG) responses of inferior frontal (i.e., Broca's area) or posterior temporal (i.e., Wernicke's area) language-dominant cortex during various language tasks (including naming) has focused on responses in the high- gamma band (70–150 Hz) (Canolty et al. 2007; Cervenka et al. 2013; Edwards et al. 2010; Miller et al. 2011; Sinai et al. 2005). Induced high-gamma power has also been observed at select ATL sites in response to visual and auditory naming (Cervenka et al. 2013). Studying the electrophysiology of the ATL in humans can be technically challenging due to limited cortical sampling by standard electrode configurations and the difficulty of identifying and rejecting prominent ocular muscle artifact (Kovach et al. 2011). With dense electrode coverage of ATL cortex, we have found robust and consistent responses at lower frequencies (beta; 14–30 Hz) during both visual and auditory proper naming (Abel et al. 2015).
Although classically thought to mediate sensorimotor function, the physiologic significance of the beta rhythm outside sensorimotor cortex remains poorly understood (Engel and Fries 2010). Recent findings demonstrate that augmentation of beta oscillations may be involved in mediating top-down feedback on sensory cortexes (Bastos et al. 2015; Brovelli et al. 2004), which means the beta rhythm may be an important candidate for the maintenance of cognitive states (Engel and Fries 2010). Additionally, in multisensory (heteromodal) cortex such as the ATL, augmentation of slower brain rhythms may play a role in the integration of multiple sensory inputs from distant cortexes (Engel and Fries 2010). Thus, given the evidence showing that the human ATL is crucial for name retrieval across input modalities, we hypothesized that name retrieval would be associated with robust and focal augmentation of low-frequency (i.e., beta band) responses in the human ATL. A previous study from our laboratory has described beta augmentation during both visual and auditory naming of U.S. presidents in a limited number of subjects (n = 3) with dense coverage of the language-dominant ATL (Abel et al. 2015). In the present study, we used an array specialized to provide dense coverage of ATL cortex (Abel et al. 2014), allowing us to identify and characterize proper and common naming-related modulation of the beta band in the human ATL using direct intracranial recordings in eight patients undergoing monitoring for seizure localization.
METHODS
Participants.
The study subjects were eight neurosurgical patients (all male, age range 21–49; Table 1) who each underwent chronic (1–3 wk) intracranial recordings with ECoG for epileptic network localization. The University of Iowa Human Subjects Review Board approved the research protocol and written informed consent was obtained from each subject before the study.
Table 1.
Clinical and demographic data
| Subject | Age | Sex | Education* | Handedness† | Language Dominance‡ | Age at Epilepsy Onset | Seizure Focus | Pathology |
|---|---|---|---|---|---|---|---|---|
| L206 | 48 | Male | 12 | +100 | Left | 33 | Left temporal | MTS |
| L222 | 33 | Male | 12 | +100 | Left | 19 | Left temporal | MTS |
| L242 | 49 | Male | 14 | −50 | Left | 16 | Left temporal | FCD |
| L258 | 38 | Male | 16 | +90 | Left | 29 | Multifocal: left frontal and temporal | N/A |
| L307 | 29 | Male | 12 | +100 | Left | 15 | Left insula | Cavernoma |
| R198 | 23 | Male | 13 | +100 | Left | 21 | Right temporal | Ganglioglioma, FCD |
| R212 | 41 | Male | 12 | +100 | Left | 26 | Right temporal | MTS, FCD |
| R288 | 21 | Male | 14 | +100 | Left | 16 | Right temporal | FCD |
MTS, mesial temporal sclerosis; FCD, focal cortical dysplasia.
Years of formal schooling.
Derived from the Geschwind-Oldfield Handedness Questionaire (Oldfield 1971), which runs from full right-handedness (+100) to full left-handedness (−100).
Determined by Wada test (Wada and Rasmussen 2007).
All subjects underwent neuropsychological evaluation before electrode implantation (Table 2). All of the subjects except one (subject L242) were right-handed as determined by neuropsychological measures (Oldfield 1971). Additionally, all subjects had left language dominance as determined by Wada testing (Wada and Rasmussen 2007). During the course of the intracranial electrode monitoring, seven subjects were determined to have epileptic foci in the temporal lobe, and one subject (L258) was found to have seizures arising from both the left frontal and left temporal lobe. Electrode sites with prominent interictal electrophysiologic abnormalities (e.g., interictal spikes) were removed from analysis to mitigate the influence of epileptiform abnormalities on data analysis.
Table 2.
Preoperative neuropsychological test scores for all subjects
| Subjects |
||||||||
|---|---|---|---|---|---|---|---|---|
| Test | L206 | L222 | L242 | L258 | L307 | R198 | R212 | R288 |
| Intellect/Premorbid | ||||||||
| WRAT-Reading | n/a | 74 | 96 | 82 | 88 | n/a | n/a | 112 |
| WAIS-FSIQ | 91 | 82 | 93 | 89 | 81 | 100 | 79 | 105 |
| VCI | 105 | 87 | 107 | 85 | 87 | 107 | 81 | 112 |
| Similarities | 11 | 9 | 11 | 9 | 6 | 14 | 8 | 13 |
| Information | 11 | 6 | 12 | 7 | 8 | 8 | 7 | 14 |
| Vocabulary | 11 | 8 | 11 | 6 | 9 | 12 | 6 | 10 |
| Attention/Working Memory | ||||||||
| WAIS-WMI | 97 | 86 | 89 | 100 | 86 | 95 | 92 | 86 |
| Digit Span | 9 | 4 | 7 | 7 | 7 | 8 | 10 | 6 |
| Arithmetic | 10 | 11 | 9 | 13 | 8 | 10 | 7 | 9 |
| WMS-Spatial Span | 8 | 9 | 7 | 13 | 1 | 13 | 10 | 8 |
| Anterograde Memory | ||||||||
| BVRT-Correct | 6 | 8 | 7 | 4 | 9 | 8 | 4 | 9 |
| AVLT-Trial 1 | 5 | 6 | 6 | 6 | 6 | 10 | 6 | 5 |
| AVLT-Trial 5 | 8 | 9 | 10 | 10 | 12 | 13 | 11 | 12 |
| AVLT-30′ Recall | 0 | 4 | 4 | 7 | 4 | 13 | 3 | 12 |
| CFT-30′ Recall | 3 | 16 | 8.5 | 17 | 14.5 | 11.5 | 11 | 21 |
| WMS-Logical Memory I | 8 | 5 | 11 | 9 | 11 | 15 | 8 | 14 |
| WMS-Logical Memory II | 8 | 6 | 8 | 9 | 9 | 13 | 8 | 14 |
| WMS-Faces I | 8 | 9 | 8 | 7 | 6 | 8 | 10 | 8 |
| WMS-Faces II | 7 | 10 | 12 | 5 | 8 | 9 | 8 | 9 |
| Perception/Construction | ||||||||
| WAIS-PRI | 81 | 88 | 88 | 81 | 88 | 104 | 89 | 111 |
| Block Design | 6 | 12 | 8 | 7 | 7 | 10 | 7 | 11 |
| Matrix Reasoning | 8 | 5 | 9 | 5 | 9 | 9 | 7 | 12 |
| Visual Puzzles | 6 | 7 | 7 | 8 | 8 | 13 | 8 | 13 |
| FRT | 45 | 43 | 49 | 41 | 47 | 43 | 41 | 49 |
| CFT-Copy | 22 | 33 | 33 | 24.5 | 31.5 | 30 | 30 | 30 |
| Motor/Processing Speed | ||||||||
| WAIS-PSI | 86 | 79 | 76 | 108 | 76 | 92 | 74 | 100 |
| Coding | 7 | 6 | 4 | 12 | 6 | 9 | 6 | 10 |
| Symbol Search | 8 | 6 | 7 | 11 | 5 | 8 | 4 | 10 |
| Trail-Making Test A | 33 | 48 | 24 | 20 | 19 | 39 | 55 | 33 |
| Trail-Making Test B | 106 | 90 | 102 | 56 | 82 | 61 | 106 | 59 |
| Pegboard-Dominant | 118 | 78 | 83 | 78 | 57 | 69 | 94 | 80 |
| Pegboard-NonDominant | 138 | 99 | 90 | 76 | 60 | 69 | 89 | 72 |
| Language | ||||||||
| Boston Naming Test | 53 | 36 | 50 | 41 | 34 | 56 | 41 | 53 |
| COWA | 25 | 23 | 25 | 36 | 23 | 23 | 26 | 32 |
| Experimental Naming Accuracy | ||||||||
| People, % | 99 | 98 | 93 | 99 | 98 | 77 | 100 | 100 |
| Tools, % | 93 | 100 | 87 | 96 | 100 | 98 | 99 | 100 |
| Overall, % | 98 | 98 | 92 | 98 | 99 | 80 | 99 | 100 |
Wide-Range Achieving Test (WRAT)-Reading, Wechsler Adult Intelligence Scale (WAIS)-Full Scale Intelligence Quotient (FSIQ), Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Working Memory Index (WMI), Processing Speed Index (PSI) (100 ± 15); WAIS-Similarities, Information, Vocabulary, Digit Span, Arithmetic, Block Design, Matrix Reasoning, Visual Puzzles, Coding, Symbol Search (10 ± 3); Weschler Memory Scale (WMS)-Spatial Span, Logical Memory, Faces (10 ± 3); Benton Visual Retention Test (BVRT)-Correct (#/10); Auditory Verbal Learning Test (AVLT; #/15); Complex Figure Test (CFT): #/36); Facial Recognition Test (FRT: #/54); Trail-Making Test (time to complete in seconds); Pegboard (time to complete in seconds); Boston Naming Test (#/60); Controlled Oral Word Association (COWA; total words over 3 60-s trials; age-education corrected score).
Electrode implantation.
Intracranial electrodes were implanted using a standard frontotemporal craniotomy in all subjects. The decision to perform chronic intracranial monitoring and the position of intracranial electrodes was based solely on clinical criteria. Dense coverage of the ATL was achieved in six subjects by combining a standard anteromedial strip electrode (Cohen-Gadol and Spencer 2003) with a specialized array tapered to fit over the convexity of the ATL (Abel et al. 2014). In two subjects (R212 and R288), dense coverage of the ATL was obtained using multiple anterior temporal strip electrodes. Five subjects had predominantly left-sided coverage and three patients had predominantly right-sided coverage (however, 4 of the subjects had bilateral ATL coverage). Additionally, all subjects had coverage of the posterior fusiform gyrus (pFG), and one subject (L242) had coverage of the lateral occipital cortex. In all subjects, a subgaleal contact was used as a reference.
All subjects underwent whole brain MR and CT scanning before electrode implantation. To localize electrodes on each subject's three-dimensional-rendered brain MR, high-resolution T1-weighted structural MRIs (in-plane resolution 0.78 × 0.78 × 1.0 mm) were obtained both before and after electrode implantation. Pre- and postimplantation MR and CT volumes were nonlinearly coregistered using a three-dimensional thin-plate spline morphing. Calculated transformation is applied to the coordinates for each electrode contact and plotted onto the cortical surface created from preimplantation images.
Task.
Subjects named pictures of U.S. presidents or hand-held tools that were intermixed in the same blocks (Fig. 1). The clinical naming deficit seen in patients after resection of the language dominant ATL is most pronounced for unique concrete entities (e.g., famous people); thus U.S. presidents were chosen as the stimuli to represent this category (Tranel 2009). For the U.S. president category, stimuli consisted of at least 150 pictures of Barack Obama, William Clinton, and George W. Bush (50 unique pictures for each president). In some cases, subjects also named John F. Kennedy (50 unique pictures) and Abraham Lincoln (50 unique pictures). We also studied tool naming. For the tool category, 50 unique pictures of 5–10 different hand held tools (e.g., hammer, saw) were presented. All pictures were obtained from the Internet and did not contain any text. Stimuli were chosen based on the results of preoperative naming performance on a screening test that included pictures of U.S. presidents. Since many patients with temporal lobe epilepsy have comorbid naming deficits, specific U.S. presidents (e.g., Barack Obama) were only included in the experiment if the subject was able to name them during preoperative screening. This preoperative screening was not performed for tool naming since object-naming deficits are less robust for patients with ATL dysfunction.
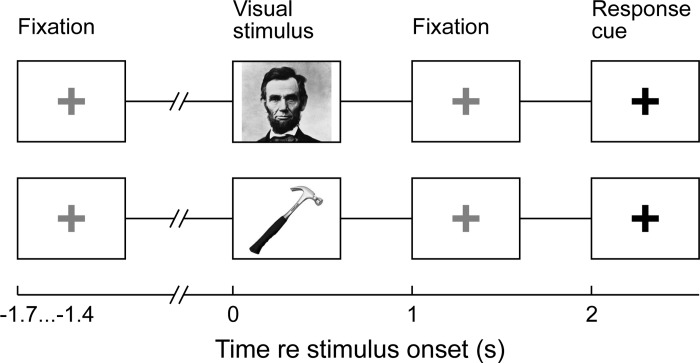
Fig. 1.
Schematic representation of person- and tool-naming tasks. Picture stimuli are displayed for 1 s, followed by a red fixation cross. This red fixation cross changes to a green fixation cross after 1 s signaling the subject to name the picture.
Stimulus randomization and presentation were performed with Presentation Software (version 14.9, www.neurobs.com). Picture stimuli were presented on a computer monitor placed ∼1 m from the subject's face. For the task, each picture was presented for 1 s. After showing the picture for 1 s, the picture was replaced by a red cross that turned green after 1 s (2 s after the beginning of the trial) prompting the subject to verbally respond with the name of the item or person in the picture. A microphone located at the subject's bedside recorded verbal responses. In subjects L258, R288, and R307, the experimenter (T. J. Abel) manually triggered the onset of the next trial after the subject's response, also resulting in a variable intertrial interval (not <1 s). In all other subjects, the verbal response triggered the next trial, resulting in a variable intertrial interval (not <1 s).
Data analysis.
Epicortical ECoG signals were recorded continuously, amplified, filtered (1- to 1,000-Hz bandpass, 12 dB/octave rolloff), digitized at a sampling rate of 2,034 Hz, and stored for subsequent offline analysis. Data were downsampled to 1,000 Hz, filtered to remove 60-Hz line noise and its harmonics, and were segmented into 4-s trial epochs, from −1 to 3 s relative to stimulus onset.
Time-frequency analysis was implemented using two methods. A modified demodulated band transform-based algorithm (Clochon et al. 1996; Hao et al. 1992; Kovach and Gander 2016) was used for spectral analysis. For visualization, time-frequency analysis employed a computationally efficient form of frequency-domain complex demodulation (Bingham et al. 1967). For a detailed description of our technique, we refer the reader to Kovach and Gander (2016). The primary advantage of this approach, in addition to its efficiency, is its minimal susceptibility to spectral leakage artifacts. In brief, the technique is closely analogous to the standard short-time Fourier transform, except the signal is segmented and windowed in the frequency domain rather than the time domain. This is done by computing the discrete Fourier transform over the entire duration of the recording, segmenting the discrete Fourier transform over positive frequencies into overlapping windows of 1-, 2-, 4-, 10-, and 20-Hz bandwidth for theta (4–7 Hz), alpha (7–14 Hz), beta (14–30 Hz), gamma (30–70 Hz), and high gamma (70–150 Hz), respectively. Each segment was multiplied with a cosine window and separately applying the inverse FFT to each to obtain a bandpass-filtered and demodulated analytic signal, which is downsampled to an optimal rate. The result is equivalent to a bank of Nth order finite impulse response bandpass filters, where N is the number of samples in the recording. For a given center frequency, ωc, the impulse response of the filter is for h defined as:
where Δω is the half-width of the frequency window and Ωs its sampling rate, with all frequencies in radian angular units. The time resolution of the filter is therefore on the order of , where Δf is the full window bandwidth in Hz. The minor (less than −20 dB total) additional time leakage of energy resulting from the oscillatory side lobes of this window is not expected to have any material effect on time resolution.
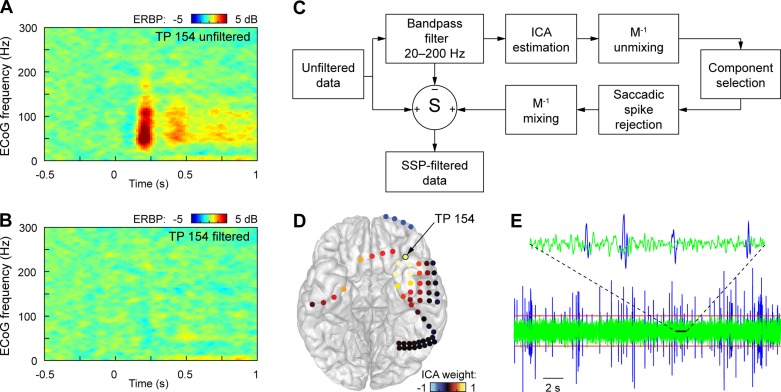
For statistical analysis comparing conditions of interest, event-related band power (ERBP) in the beta band (14–30 Hz, obtained from the ECoG signal using 100th order FIR filter) was also calculated relative to the average ERBP of the entire 4-s epoch. This frequency range was selected based on spectral profile of recording sites within the ATL (Abel et al. 2015). Contamination by ocular EMG artifact, which can be particularly prominent when recording from the ATL, was removed as previously described (Kovach et al. 2011) (Fig. 2).
Fig. 2.
Removal of eye-muscle contamination. The presence of the saccade-related EMG response from extraocular muscles, the saccadic spike potential (SSP), may create the appearance of an induced cortical high-gamma responses following trial onset (A), which is eliminated when the EMG contamination is suppressed (B) (exemplary data from a temporal pole contact TP 154). The procedure to remove the SSP is summarized in C. Independent component analysis (ICA) is performed on data filtered in the spectral the range of the SSP. Components related to oculomotor EMG are identified by their spatial loadings, which are characteristically large near the orbits (D), and by the presence of visually obvious SSPs in the component activation (E, blue line). To minimize the suppression of cortical responses at the temporal pole that may result from a simple ICA spatial filter, SSPs identified by thresholding (E, red line) are removed from the selected ICA component activations through windowing (E, green line). Decontaminated data are reconstructed by applying the inverse mixing transformation to the modified ICA activations, subtracting the untreated frequency-filtered data from the original data and adding the ICA-filtered response.
To determine the spatial distribution of beta band modulation, average beta ERBP increase in a 1-s window (300-1,300 poststimulus onset) from each ATL site was compared with prestimulus baseline activity with a one-tailed t-test, corrected for multiple comparisons using false discovery rate criterion (Benjamini and Hochberg 1995), with significance threshold set to q = 0.01.
RESULTS
Behavioral performance.
The accuracy of person and tool naming during ECoG recording was determined for each subject. Incorrect responses were not used for subsequent ECoG analyses. Across all subjects, the overall accuracy for picture naming was 95.3%. Stratified by category, the accuracy of person naming was 95.6% and the accuracy of tool naming was 96.5%. Specific naming accuracies for each subject are listed in Table 2. Most naming errors consisted of either the response “don't know” or a response omission (typically filler words such as “uh” or “um”). Substitution errors were more common for person naming (e.g., Bill Clinton for George Bush and vice versa) than tools.
Responses to person and tool naming.
Visual person and tool naming tasks were associated with localized modulation of low-frequency power in the ATL (Figs. 3 and 4). Low-frequency power alterations in the ATL were most consistent with what would classically be considered the beta band (i.e., 14–30 Hz). Beta power modulation occurred in both the left and right ATL as seen in different subjects (see Fig. 3 for left ATL and Fig. 4 for right ATL). Localized ATL beta modulation occurred regardless of whether a person or tool was being named. In most cases, increases in beta power were observed at responsive ATL sites; however, some ATL sites showed decreased beta power during name retrieval. For example, electrode 218 in L222 and electrode 194 in R212 both exhibited attenuated beta power during name retrieval tasks. Overall, attenuated beta power was a less common response during name retrieval than augmented beta power; therefore, we focused our quantitative analysis on increases in the beta band.
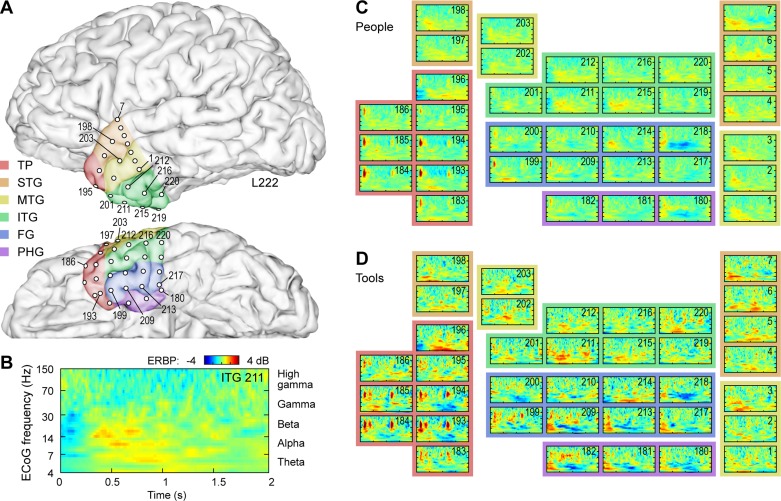
Fig. 3.
Electrocorticographic (ECoG) activity within left anterior temporal lobe (ATL) in a representative subject (L222) during naming tasks. A: anatomical reconstruction of recording site locations within the left ATL. Colors represent the 6 regions of interest [temporal pole (TP); superior temporal gyrus (STG); middle temporal gyrus (MTG); inferior temporal gyrus (ITG); fusiform gyrus (FG); and parahippocampal gyrus (PG)]. B: time-frequency analysis of ECoG activity during naming of U.S. presidents recorded from a representative site (contact no. 211, ITG). C and D: responses from all ATL sites during people and tool naming task, respectively. Each event-related band power (ERBP) time-frequency plot is depicted using the axes as those shown in B.
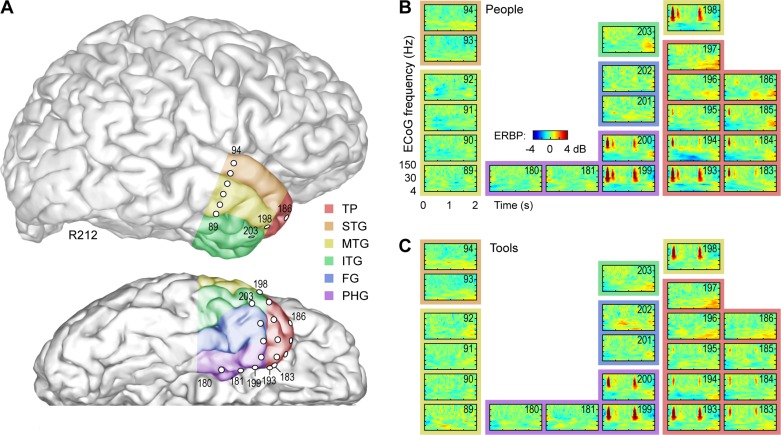
Fig. 4.
ECoG activity within right ATL in a representative subject (R212; anatomical reconstruction shown in A) during naming of U.S. presidents and tools (B and C, respectively). See legend of Fig. 3 for details.
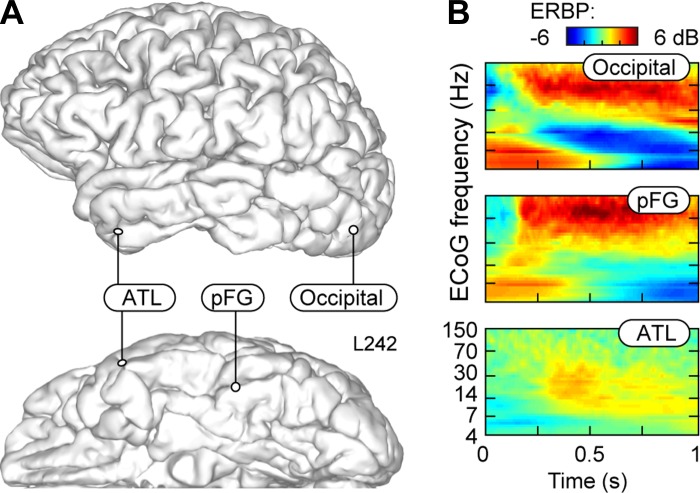
In contrast, pFG recordings during person and tool naming demonstrated robust modulation of high-frequency power coinciding best with the high-gamma band (70–150 Hz). In the single subject with recordings from the occipital lobe (L242), time-frequency analysis also revealed prominent increases in high-gamma power (Fig. 5). Time-frequency analyses of pFG and occipital lobe were typified by this increased high-gamma power and also decreased beta power, as is described in numerous reports of visual (Kawasaki et al. 2012; Tsuchiya et al. 2008), auditory (Nourski and Howard 2015), and sensorimotor cortex (Crone et al. 1998; Miller et al. 2007). Based on time frequency analyses, ATL beta band responses typically began at ∼400 ms, although some ATL responses were seen as early as 200 ms. In contrast, all high-gamma pFG responses began at ∼200 ms or earlier (Fig. 5). High-gamma responses from occipital cortex were seen earlier than 200 ms.
Fig. 5.
Timing of naming-related responses in the lateral occipital lobe, posterior FG, and ATL in subject L242. A: anatomical reconstruction of locations of 3 representative recording sites. B: ERBP time-frequency plots showing ECoG activity recorded from the 3 sites during naming of U.S. presidents.
Spatial distribution of ATL responses.
To evaluate which cortical sites elicited U.S. president naming responses, increases in beta power were calculated 300-1,300 ms from visual stimulus onset were assessed for statistical significance. This time window was selected for statistical analysis based on the time-frequency characteristics of the ATL beta-band responses (Abel et al. 2015). Thus ATL cortexes that exhibited significant (q < 0.001) for person and tool naming are depicted in Fig. 6.
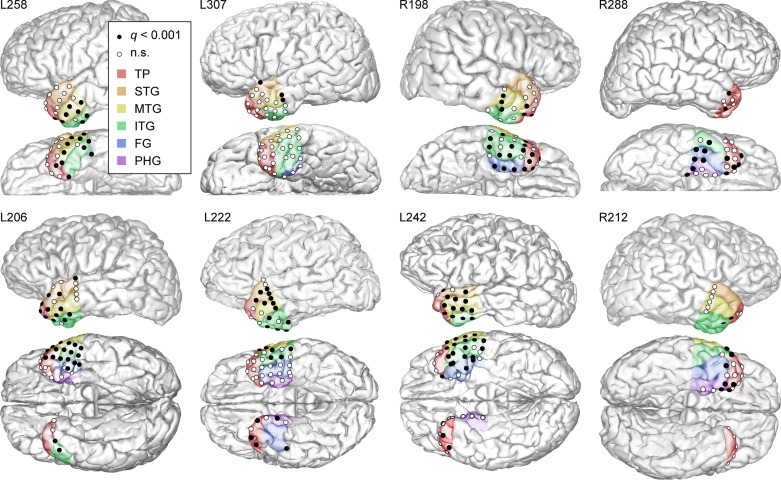
Fig. 6.
Spatial distribution of significant beta-band responses during the U.S. president naming task at all ATL recording sites in all subjects. Recording sites within anatomical regions of interest that exhibited significant (q < 0.001) responses are depicted by black dots. White circles represent recording sites within regions of interest that did not exhibit significant responses. The 6 regions of interest are color coded using the same convention as in Figs. 2 and 3.
Of the anterior temporal regions of interest depicted in Fig. 3, visual naming responses were most common (>60% cortical sites responsive) in the right FG (8/10 responsive sites; 80%), left middle temporal gyrus (22/32 responsive sites; 67%), right inferior temporal gyrus (12/18 responsive sites; 67%), and left inferior temporal gyrus (22/37 sites; 60%). Other responsive regions (25–60%) of cortical sites responsive were the right temporal pole (19/45 responsive sites; 42%), left superior temporal gyrus (11/29 responsive sites; 38%), right middle temporal gyrus (3/9 responsive sites; 30%), right parahippocampal gyrus (5/16 responsive sites; 30%), and left fusiform gyrus (1/7 responsive sites; 14%). The left parahippocampal gyrus, right superior temporal gyrus, and left temporal pole had a considerably lower proportion of responsive sites.
The right FG showed robust and consistent responses, a finding that was present in all subjects with right FG coverage. However, this was not found in the left FG. The middle temporal gyrus was the most reliably responsive region in the left ATL and was also responsive in all subjects with left ATL recordings. The inferior temporal gyri were also highly responsive on both the left and right side. Interestingly, the right temporal pole exhibited more responsive sites than the left temporal pole (42 vs. 11%).
DISCUSSION
Using high-density direct electrophysiologic recordings, we found robust modulation of beta oscillations in the language-dominant and nondominant ATL during visual famous person and tool naming tasks. The ATLs are known to play a crucial role in visual processing (Perrett et al. 1992; Tsao et al. 2003), recognition (Drane et al. 2008; Tranel et al. 1997), and language-dominant ATL name retrieval (Damasio et al. 1996, 2004). One of the most common complaints after neurosurgical resection of the language-dominant ATL, a common procedure for medically intractable temporal lobe epilepsy, is a severe and disabling impairment in naming (Drane et al. 2013; Lambon Ralph 2014; Tranel 2006). Naming impairment resulting from language-dominant ATL damage is most pronounced for unique concrete entities, such as famous people (Damasio et al. 2004) or landmarks (Tranel 2006) but can also affect other categories (Griffin and Tranel 2007). However, deficits for common nonunique entities are less pronounced and anomia is often not detected by the commonly used Boston Naming Test (Drane et al. 2008).
Despite numerous lesion studies and functional neuroimaging studies demonstrating a crucial role for the nondominant ATL in recognition (Tranel et al. 1997) and the dominant ATL in name retrieval (Gorno-Tempini et al. 1998; Grabowski et al. 2001), the physiologic mechanisms responsible for these processes are poorly understood. This is due in part to the limited spatial and temporal resolution of noninvasive functional neuroimaging techniques (Visser et al. 2010). The spatial resolution of functional (f)MRI is specifically limited at the ATL (and other brain structures abutting the skull base) due to MR susceptibility artifact (Devlin et al. 2000; Ojemann et al. 1997), which can significantly influence responses as seen in the difference between positron emission tomography (PET) and fMRI responses in the ATL with the same task (Devlin et al. 2000). Secondly, visual processing and name retrieval, both key components of clinically relevant naming, occur on millisecond timescales that cannot be resolved by blood-flow-dependent response measures. Finally, ECoG-blood oxygen level-dependent (BOLD) correlation studies demonstrate that the BOLD signal is best correlated with high-frequency brain oscillations suggesting that lower frequency brain oscillations (e.g., theta or beta) may not be represented by the BOLD signal (Conner et al. 2011; Hermes et al. 2014; Lachaux et al. 2007). Newer fMRI analysis techniques (e.g., spatially corrected spin EPI and dual echo EPI) have mitigated the effects of fMRI signal dropout in anterior temporal cortex and when these techniques are adopted peak cortical activation reportedly occurs in the same region for both fMRI and grid recordings (Shimotake et al. 2014); however, it remains unclear what electrical activity may be missed by the limitations of the BOLD signal (Abel et al. 2015). Taken together, these limitations represent substantial barriers to characterizing the neurophysiology of perception and language processes in the human ATL via noninvasive functional neuroimaging.
Several studies have investigated the electrophysiologic properties of language processes in the ATL using intracranial recordings. The inherent limitation of this work, as with all intracranial recording research, is limited sampling of cortex by electrodes brought about by the clinical necessity of recording specific brain regions and also technical difficulties in attaining coverage of particular brain structures. We recently developed an electrode array specialized to provide dense coverage of the ATL for clinical localization of anterior temporal epileptic networks (Abel et al. 2014). Using this array in three patients with left ATL recordings, we showed that visual and auditory naming responses in the beta band occur at overlapping recording sites on ATL cortex (Abel et al. 2015). Others have also studied language processes in the ATL using ECoG, and some studies have found alterations in high-frequency oscillations (i.e., the gamma band) as opposed to low-frequency oscillations (Cervenka et al. 2013; Chan et al. 2011). Cervenka et al. (2013) reported high-gamma (50–150 Hz) modulation in response to visual and auditory naming tasks in the ATL, with some of the responsive cortical sites responsive to both visual and auditory naming tasks. However, the authors did not evaluate specifically for significant increases in lower frequencies. Chan et al. (2011) performed intracerebral recordings from the posterior aspect of the ATL during a semantic categorization task and also found high-gamma modulation. We have also found high-gamma responses from the ATL during visual and auditory naming; however, these responses are relatively sparse compared with the beta band response (Abel et al. 2015). Also, unlike the beta-band response, we did not find any ATL recording sites to exhibit high-gamma responses to both visual and auditory naming tasks (unlike Cervenka et al.). It is possible that the discrepancies in findings regarding high-gamma or beta-band responses in the ATL are due to relatively limited or more posterior coverage in other studies, but this remains to be clarified. An additional challenge of interpreting intracranial recordings from anterior temporal and temporopolar cortex is the prominence of ocular EMG artifact, which generates spikes of broad-band power increase that must be considered during analysis (Jerbi et al. 2009; Kovach et al. 2011).
Our main finding is that both the dominant and nondominant ATL demonstrate a robust, consistent, and localized beta-modulation response during retrieval of names for visually presented famous people and tools. It is important to note that because our subject population is all male, it is possible that our results would not generalize across both males and females. These responses were most consistently found in the right FG and left middle temporal gyrus but also commonly in the bilateral inferior temporal gyri. Interestingly, we found a relatively high proportion of responses from the right temporal pole compared with the left temporal pole, despite an equal number of recording contacts in each group (n = 45). At first glance, this may be unexpected given numerous lesion studies demonstrating the importance of the left temporal pole in name retrieval (Damasio et al. 1996; Drane et al. 2008). However, the right temporal pole is thought to play a crucial role in the recognition of unique concrete entities, and this process is thought to precede name retrieval (Drane et al. 2008; Tranel et al. 1997). For example, after seeing a stimulus, the right anterior temporal lobes may become engaged to process the meaning of the stimulus, while the left anterior temporal lobes are engaged to retrieve the name of the stimulus. Another interpretation is that the right and left ATL are both involved in semantic processing, which would provide a reason for the right ATL to be just as responsive as the left, but that the left side is associated with language deficits due to its more robust connectivity with left-side (language dominant) brain structures (Lambon Ralph et al. 2001). Secondly, another reason why responses are not seen from the temporal pole itself may represent a limitation of lesion studies. Most of the patients examined in the lesion studies have undergone anterior temporal lobectomies and have concomitant resection of both the middle temporal gyrus and temporal pole. Therefore, it is possible that damage to the anterior middle temporal gyrus is responsible for the naming deficit seen in anterior temporal lobectomy patients, despite that the most concentrated lesion overlap is typically the temporal pole itself. Ultimately, however, the causal significance of increased beta power in the right temporal pole remains unknown. Future studies (e.g., electrical stimulation) may be able to isolate the effects of the right temporal pole, which would improve our understanding of the role of beta augmentation in right temporopolar cortex.
Interestingly, although language-dominant ATL damage is associated with more pronounced declines in naming unique concrete entities than common nouns, we observed similar activation patterns for both U.S. presidents and tools in both the language-dominant and nondominant ATLs. One possible interpretation of this is that the ATL is involved in category-general semantic processing (Lambon Ralph 2014).
It is important to note that the physiologic significance and role of beta oscillations outside of sensorimotor cortexes is a topic of debate (Engel and Fries 2010). One hypothesis is that beta oscillations mediate top-down maintenance of cognitive states (Engel and Fries 2010). As reviewed by Engel and Fries (2010), evidence for this hypothesis from the motor system comes from the observation that beta oscillations are attenuated during voluntary movements (Miller et al. 2007) but increased following during motor holding periods (Brovelli et al. 2004; Chakarov et al. 2009). In support of this view, recent findings from conditional Granger causality analysis suggest that beta oscillations mediate top-down feedback interactions in the primate visual system (Bastos et al. 2015). In contrast, this Granger causality study found that theta-gamma oscillations mediate feed-forward interactions in the visual system. Lower frequency oscillations such as beta may also play a role in the integration of neuronal inputs over large distances, for example, between fusiform gyrus and the ATL. Support for this comes from modeling studies that show that the increased cycle length of beta oscillations can support synchrony of distance inputs in the setting of increased conduction delay (Kopell et al. 2000). A combination of slow and fast oscillations may support cross-modal interactions of distant neuronal inputs (Schroeder et al. 2008), which may be relevant to low-frequency oscillations in the ATL. Recently, evidence from nonhuman primates has shown that phase resetting of low-frequency oscillations in ATL by face stimuli increases the probability of voice cell firing (Perrodin et al. 2015). Both the integration of distant neural inputs and integration of cross-modal inputs are feasible roles for low-frequency oscillations in the ATL.
The potential significance of beta oscillations for the cognitive task examined in our study is not currently known, but the integration of visual and auditory input and maintenance of cognitive states is relevant to name retrieval by the ATL. Convergent lines of evidence demonstrate that the ATL is a “convergence zone” that links visual and auditory input for retrieval of names and conceptual knowledge (Belfi and Tranel 2013; Binney et al. 2012; Lambon Ralph 2014). For example, anatomical studies have shown that the ATL receives convergent input from visual, auditory, and olfactory cortexes (Moran et al. 1987). More recently, MR diffusion tensor imaging analyses have shown graded convergence of visual and auditory inputs in the human ATL, also consistent with the idea that the ATL mediates retrieval of information in response to both visual and auditory inputs (Binney et al. 2012). Neuropsychologically, damage to the left ATL is known to cause more severe impairment to naming, specifically for unique concrete entities (Tranel 2006), and this may be explained by unique connectivity of the language dominant ATL to the language system, which is distinct from the nondominant ATL (Hurley et al. 2014). We have previously demonstrated that 50% of ATL sites showing beta-band responses overlap for both visual and auditory naming of U.S. presidents, consistent with the idea of graded convergence in the ATL. Thus the necessity of multisensory integration from distant cortical inputs may explain why the ATL exhibits prominent beta-band responses. The integration of distant cortical inputs is also an important feature of the “hub and spoke” model of semantic representation (Lambon Ralph 2014; Rogers et al. 2004), of which the ATL is an important hub. Thus another possible interpretation is that beta modulation in the ATL represents this integration across distant cortical areas.
The ATL may also utilize beta oscillations to maintain cognitive states necessary for the retrieval of information across sensory modalities. Primate electrophysiologic studies have reported beta-mediated feedback within the visual system hierarchy (Bastos et al. 2015). Previous theories of information retrieval by heteromodal convergence zones (e.g., the ATL) postulate that retroactivation of sensory cortexes may mediate the retrieval of modality-specific information (Damasio 1989). Thus one possible interpretation is that ATL beta oscillations represent top-down interactions between the ATL and sensory-specific cortexes to mediate information retrieval. ATL-mediated feedback to visual cortex has been previously reported in fMRI studies (Pehrs et al. 2015).
In conclusion, we demonstrate robust and consistent beta modulation in the human ATL in response to visual naming of famous people and tools. The presence of induced beta oscillations in the ATL during a visual naming task is consistent with the idea that ATL is a convergence zone for the retrieval of visually presented entities. The cognitive and clinical significance of these induced low-frequency rhythms should be further evaluated.
GRANTS
This work was supported by National Institutes of Health Grants F32-NS-087664 and RO1-DC-004290 and James S. McDonnell Foundation Grant 220020387.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
T.J.A., H.K., M.A.H., and D.T. conception and design of research; T.J.A. and A.E.R. performed experiments; T.J.A. and D.T. interpreted results of experiments; T.J.A., K.V.N., and C.K.K. prepared figures; T.J.A. drafted manuscript; T.J.A., A.E.R., K.V.N., H.K., and D.T. edited and revised manuscript; T.J.A., A.E.R., K.V.N., T.K.A., H.O., C.K.K., H.K., M.A.H., and D.T. approved final version of manuscript; A.E.R., T.K.A., H.O., and C.K.K. analyzed data.
ACKNOWLEDGMENTS
We thank Ken Manzel for assistance in data collection.
REFERENCES
- Abel TJ, Rhone AE, Nourski KV, Granner MA, Oya H, Griffiths TD, Tranel DT, Kawasaki H, Howard MA 3rd. Mapping the temporal pole with a specialized electrode array: technique and preliminary results. Physiol Meas 35: 323–337, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel TJ, Rhone AE, Nourski KV, Kawasaki H, Oya H, Griffiths TD, Howard MA, Tranel D. Direct physiologic evidence of a heteromodal convergence region for proper naming in human left anterior temporal lobe. J Neurosci 35: 1513–1520, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85: 390–401, 2015. [DOI] [PubMed] [Google Scholar]
- Belfi AM, Tranel D. Impaired naming of famous musical melodies is associated with left temporal polar damage. Neuropsychology 28: 429–435, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate-a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 57: 289–300, 1995. [Google Scholar]
- Bingham C, Godfrey M, Turkey JW. Modern techniques of power spectrum estimation. IEEE Trans Audio Electroacoustics 15, 56–66, 1967. [Google Scholar]
- Binney RJ, Parker GJ, Lambon Ralph MA. Convergent connectivity and graded specialization in the rostral human temporal lobe as revealed by diffusion-weighted imaging probabilistic tractography. J Cogn Neurosci 24: 1998–2014, 2012. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia 38: 1207–1215, 2000. [DOI] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL. Beta oscillations in a large-scale sensorimotor cortical network: directional influences revealed by Granger causality. Proc Natl Acad Sci USA 101: 9849–9854, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Soltani M, Dalal SS, Edwards E, Dronkers NF, Nagarajan SS, Kirsch HE, Barbaro NM, Knight RT. Spatiotemporal dynamics of word processing in the human brain. Front Neurosci 1: 185–196, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka MC, Corines J, Boatman-Reich DF, Eloyan A, Sheng X, Franaszczuk PJ, Crone NE. Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. Neuroimage 69: 267–276, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakarov V, Naranjo JR, Schulte-Monting J, Omlor W, Huethe F, Kristeva R. Beta-range EEG-EMG coherence with isometric compensation for increasing modulated low-level forces. J Neurophysiol 102: 1115–1120, 2009. [DOI] [PubMed] [Google Scholar]
- Chan AM, Baker JM, Eskandar E, Schomer D, Ulbert I, Marinkovic K, Cash SS, Halgren E. First-pass selectivity for semantic categories in human anteroventral temporal lobe. J Neurosci 31: 18119–18128., 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Shimotake A, Matsumoto R, Kunieda T, Kikuchi T, Miyamoto S, Fukuyama H, Takahashi R, Ikeda A, Lambon Ralph MA. The “when” and “where” of semantic coding in the anterior temporal lobe: temporal representational similarity analysis of electrocorticogram data. Cortex 79: 1–13, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clochon P, Fontbonne JM, Lebrun N, Etévenon P. A new method for quantifying EEG event-related desynchronization: amplitude envelope analysis. Electroencephalogr Clin Neurophysiol 98: 126–129, 1996. [DOI] [PubMed] [Google Scholar]
- Cohen-Gadol AA, Spencer DD. Use of an anteromedial subdural strip electrode in the evaluation of medial temporal lobe epilepsy. Technical note. J Neurosurg 99: 921–923, 2003. [DOI] [PubMed] [Google Scholar]
- Conner CR, Ellmore TM, Pieters TA, DiSano MA, Tandon N. Variability of the relationship between electrophysiology and BOLD-fMRI across cortical regions in humans. J Neurosci 31: 12855–12865, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis-II. Event-related synchronization in the gamma band. Brain 121: 2301–2315, 1998. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition 33: 25–62, 1989. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature 380: 499–505, 1996. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition 92: 179–229, 2004. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, Matthews PM, Tyler LK. Susceptibility-induced loss of signal: Comparing PET and fMRI on a semantic task. Neuroimage 11: 589–600, 2000. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, Miller JW, Tranel D. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia 46: 1242–1255, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann JG, Phatak V, Loring DW, Gross RE, Hebb AO, Silbergeld DL, Miller JW, Voets NL, Saindane AM, Barsalou L, Meador KJ, Ojemann GA, Tranel D. Famous face identification in temporal lobe epilepsy: support for a multimodal integration model of semantic memory. Cortex 49: 1648–1667, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E, Nagarajan SS, Dalal SS, Canolty RT, Kirsch HE, Barbaro NM, Knight RT. Spatiotemporal imaging of cortical activation during verb generation and picture naming. Neuroimage 50: 291–301, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations–signalling the status quo? Curr Opin Neurobiol 20: 156–165, 2010. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS. The neural systems sustaining face and proper-name processing. Brain 121: 2103–2118, 1998. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp 13: 199–212, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin S, Tranel D. Age of seizure onset, functional reorganization, and neuropsychological outcome in temporal lobectomy. J Clin Exp Neuropsychol 29: 13–24, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao YL, Ueda Y, Ishii N. Improved procedure of complex demodulation and an application to frequency analysis of sleep spindles in EEG. Med Biol Eng Comput 30: 406–412, 1992. [DOI] [PubMed] [Google Scholar]
- Hermes D, Miller KJ, Vansteensel MJ, Edwards E, Ferrier CH, Bleichner MG, van Rijen PC, Aarnoutse EJ, Ramsey NF. Cortical theta wanes for language. Neuroimage 85: 738–748, 2014. [DOI] [PubMed] [Google Scholar]
- Hurley RS, Bonakdarpour B, Wang X, Mesulam MM. Asymmetric connectivity between the anterior temporal lobe and the language network. J Cogn Neurosci 27: 464–473, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Dalal S, Kahane P, Bertrand O, Berthoz A, Lachaux JP. Saccade related gamma-band activity in intracerebral EEG: dissociating neural from ocular muscle activity. Brain Topogr 22: 18–23, 2009. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Tsuchiya N, Kovach CK, Nourski KV, Oya H, Howard MA, Adolphs R. Processing of facial emotion in the human fusiform gyrus. J Cogn Neurosci 24: 1358–1370, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA 97: 1867–1872, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CK, Gander PE. The demodulated band transform. J Neurosci Methods 261: 135–154, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CK, Tsuchiya N, Kawasaki H, Oya H, Howard MA 3rd, Adolphs R. Manifestation of ocular-muscle EMG contamination in human intracranial recordings. Neuroimage 54: 213–233, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, Baciu M. Relationship between task-related gamma oscillations and BOLD signal: new insights from combined fMRI and intracranial EEG. Hum Brain Mapp 28: 1368–1375, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA. Neurocognitive insights on conceptual knowledge and its breakdown. Philos Trans R Soc Lond B Biol Sci 369: 20120392, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. J Cogn Neurosci 13: 341–356, 2001. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Abel TJ, Hebb AO, Ojemann JG. Rapid online language mapping with electrocorticography. J Neurosurg Pediatr 7: 482–490, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG. Spectral changes in cortical surface potentials during motor movement. J Neurosci 27: 2424–2432, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. J Comp Neurol 256: 88–103, 1987. [DOI] [PubMed] [Google Scholar]
- Nourski KV, Howard MA 3rd. Invasive recordings in the human auditory cortex. Handb Clin Neurol 129: 225–244, 2015. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage 6: 156–167, 1997. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Pehrs C, Zaki J, Schlochtermeier LH, Jacobs AM, Kuchinke L, Koelsch S. The temporal pole top-down modulates the ventral visual stream during social cognition. Cereb Cortex pii: bhv226, 2015. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Hietanen J, Oram M, Benson P, Rolls E. Organization and functions of cells responsive to faces in the temporal cortex [and discussion]. Philos Trans R Soc Lond B Biol Sci 335: 23–30, 1992. [DOI] [PubMed] [Google Scholar]
- Perrodin C, Kayser C, Logothetis NK, Petkov CI. Natural asynchronies in audiovisual communication signals regulate neuronal multisensory interactions in voice-sensitive cortex. Proc Natl Acad Sci USA 112: 273–278, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Ralph MA. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci USA 104: 20137–20141, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev 111: 205–235, 2004. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P, Kajikawa Y, Partan S, Puce A. Neuronal oscillations and visual amplification of speech. Trends Cogn Sci 12: 106–113, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza C. Naming with proper names: the left temporal pole theory. Behav Neurol 24: 277–284, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotake A, Matsumoto R, Ueno T, Kunieda T, Saito S, Hoffman P, Kikuchi T, Fukuyama H, Miyamoto S, Takahashi R, Ikeda A, Lambon Ralph MA. Direct exploration of the role of the ventral anterior temporal lobe in semantic memory: cortical stimulation and local field potential evidence from subdural grid electrodes. Cereb Cortex 25: 3802–3817, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, Lenz FA, Crone NE. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain 128: 1556–1570, 2005. [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology 20: 1–10, 2006. [DOI] [PubMed] [Google Scholar]
- Tranel D. The left temporal pole is important for retrieving words for unique concrete entities. Aphasiology 23: 867–884, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia 35: 1319–1327, 1997. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci 6: 989–995, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N, Kawasaki H, Oya H, Howard MA 3rd, Adolphs R. Decoding face information in time, frequency and space from direct intracranial recordings of the human brain. PLoS One 3: e3892, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci 22: 1083–1094, 2010. [DOI] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. 1960. J Neurosurg 106: 1117–1133, 2007. [DOI] [PubMed] [Google Scholar]
- Waldron EJ, Manzel K, Tranel D. The left temporal pole is a heteromodal hub for retrieving proper names. Front Biosci 6: 50–57, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucus CJ, Tranel D. Preserved proper naming following left anterior temporal lobectomy is associated with early age of seizure onset. Epilepsia 48: 2241–2252, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]