In advanced HIV-1 disease, 24 weeks of ART-mediated recovery results in reversal of activated cell-specific monocyte subsets, with minimal recovery of soluble makers of tissue-associated myeloid activation.
Keywords: macrophage, activation resolution, pathogenesis, AIDS
Abstract
Reversal of monocyte and macrophage activation and the relationship to viral suppression and T cell activation are unknown in patients with advanced HIV-1 infection, initiating antiretroviral therapy. This study aimed to determine whether reduction in biomarkers of monocyte and macrophage activation would be reduced in conjunction with viral suppression and resolution of T cell activation. Furthermore, we hypothesized that the addition of CCR5 antagonism (by maraviroc) would mediate greater reduction of monocyte/macrophage activation markers than suppressive antiretroviral therapy alone. In the CCR5 antagonism to decrease the incidence of immune reconstitution inflammatory syndrome study, antiretroviral therapy-naïve patients received maraviroc or placebo in addition to standard antiretroviral therapy. PBMCs and plasma from 65 patients were assessed during 24 wk of antiretroviral therapy for biomarkers of monocyte and macrophage activation. Markers of monocyte and macrophage activation were reduced significantly by 24 wk, including CD14++CD16+ intermediate monocytes (P < 0.0001), surface CD163 (P = 0.0004), CD169 (P < 0.0001), tetherin (P = 0.0153), and soluble CD163 (P < 0.0001). A change in CD38+, HLA-DR+ CD8 T cells was associated with changes in CD169 and tetherin expression. Maraviroc did not affect biomarkers of monocyte/macrophage activation but resulted in greater percentages of CCR5-positive monocytes in PBMC. HIV-1 suppression after 24 wk of antiretroviral therapy, with or without maraviroc, demonstrates robust recovery in monocyte subset activation markers, whereas soluble markers of activation demonstrate minimal decrease, qualitatively differentiating markers of monocyte/macrophage activation in advanced disease.
Introduction
Persistent immune activation during HIV-1 infection is associated with disease progression and suboptimal immune recovery after ART [1–3]. Ongoing viremia has been noted to promote a state of innate immune activation inclusive of changes in NK [4] and monocytes/macrophages. Among innate effectors, activation of monocytes and macrophages has been noted as a potentially major source of inflammation attributed to mechanisms of activation that may not be fully reversed during ART [4–12].
Monocyte subset frequencies during viremia show that the CD14++CD16+ intermediate monocyte subset is expanded during HIV-1 [13, 14] or SIV infection [15] and is associated with HIV pathogenesis [16]. The significance of this subset as a potential indicator of long-term inflammation is supported by similar elevations described in HIV(−)-infected patients with acute coronary syndrome [17]. Soluble biomarkers of monocyte/macrophage activation are also well characterized in HIV disease, including sCD14 and sCD163. Elevated sCD14 has been described as a marker of HIV-associated microbial translocation and increases in plasma LPS [8, 18, 19]. Cell-associated CD163 expression and shedding (as sCD163) from monocytes and macrophages are also elevated during HIV/SIV infection [9, 20–22] and associated with both cardiac and CNS pathology [5, 6, 14, 20, 23]. The relationship between innate and adaptive activation during viremia and the expected decrease of both after ART raise the hypothesis that changes in T cell activation would be associated with changes in monocyte and macrophage activation.
ART-mediated resolution of monocyte/macrophage activation has become a growing area of focus because of the relationship with metabolic disease and retention of immune activation during HIV infection [6, 14, 16, 23]. A longitudinal study found that the CD14++CD16+ intermediate monocyte subset decreased in patients treated during early infection but not patients treated during chronic infection [9], and cross-sectional comparisons have demonstrated that suppressed patients reach levels comparable with HIV (−)-infected donors [17]. Likewise, sCD163 was also reduced in patients treated during early-onset of infection [9]; however, unlike the intermediate subset, cross-sectional studies suggest that sCD163 does not reach HIV(−) donor levels with viremic suppression [4–7]. On the other hand, sCD14 is less plastic. Longitudinal studies have found that elevated sCD14 levels persist despite viral suppression [8–10] and are resolved only partially after extended ART time points [10, 24]. In addition, cross-sectional comparisons have demonstrated that patients undergoing ART do not resolve sCD14 to the level of HIV(−) donors [4, 10–12].
The other major component of monocyte/macrophage activation during viremia is the up-regulation of ISG expression. Among these, CD169 (sialoadhesion, sialic acid-binding Ig-type lectin 1) is a surface-expressed ISG (IFN-α, IFN-γ inducible) on monocytes, macrophages, and DCs that is highly expressed during HIV-1 infection [25–27] and other inflammatory diseases [28, 29]. CD169 can also facilitate trans-infection of target cells by monocytes and DCs [25, 30, 31]. Its expression can be reversed with viral suppression after ART in SIV infection [32]. Furthermore, several HIV-1 restriction factors active in monocytes and macrophages are ISG induced and up-regulated with viremia [33, 34].
Although the addition of CCR5 antagonism (maraviroc) did not affect the degree of CD4 recovery in subjects, starting with nadir CD4 counts in the parent study [35], its impact on the resolution of monocyte and macrophage activation remains unclear. Some studies have suggested that CCR5 antagonism can improve resolution of T cell activation during ART initiation (temporary advantage) [36], yet data are not uniform across intensification studies to date [37–39]. Likewise, with regard to monocyte/macrophage activation, maraviroc-inclusive ART has yielded mixed results regarding sCD14 resolution [37, 39–41]. Some variability may be attributed to cohort and therapy differences, as intensification studies often target immunologic nonresponders [38, 39]. Taken together, the immunologic impact of a maraviroc-inclusive regimen in patients initiating ART from advanced disease has not been described.
Herein, we characterize the change in biomarkers of monocyte and macrophage activation and the impact of CCR5 antagonism in patients initiating ART from advanced disease (CD4 count <100 cells/mm3). Primary analysis aimed to determine the degree and kinetics of recovery of monocyte/macrophage activation with respect to T cell activation, and secondary analysis addressed any impact of CCR5 antagonism on monocyte/macrophage activation. With 24 wk of suppressive ART, we observed a differential recovery of biomarkers of monocyte/macrophage activation. Apart from increases in the percentage of peripheral blood monocytes expressing CCR5, no maraviroc-mediated effects on monocytes/macrophages with ART were noted.
MATERIALS AND METHODS
Donor population
HIV-1-seropositive patients (n = 65; 56.9% women; median age = 38 yr) were recruited to participate in substudy B (South Africa study site) within the CADIRIS (maraviroc) cohort, as described in the parent study [35]. Inclusion criteria targeted ART-naïve individuals with a CD4 count <100 cells/mm3 (Table 1). Patients who failed to suppress viral load <400 RNA copies/ml by wk 24 were excluded from analysis. Patients who received placebo or maraviroc (600 mg), in addition to a background of efavirenz (600 mg), tenofovir (300 mg), and emtricitabine (200 mg), and plasma and PBMCs, were isolated from whole blood at 0 (pre-ART), 12, and 24 wk. Data showing less than the total cohort reflect unavailable patient material or limited assay capacity. Figures expressing paired, longitudinal data (see Figs. 1–4) include patients with only complete data (0, 12, and 24 wk). All participants produced informed consent in accordance with clinical trial NCT 00988780.
TABLE 1.
Baseline characteristics
| Variable | ART + placebo | ART + maraviroc | Total | Pa |
|---|---|---|---|---|
| n | 31 | 34 | 65 | |
| Age (yr) | 38 (34–45) | 37.5 (33.75–44) | 38 (34–44) | 0.712 |
| Gender, no. women (%) | 18 (58.1%) | 19 (55.9%) | 37 (56.9%) | 1.0000 |
| Log viral load (RNA copies/ml) | 5.201 (4.934–5.604) | 5.467 (5.000–5.728) | 5.407 (5.000–5.679) | 0.3703 |
| CD4 count (cells/mm3) | 46 (25–72) | 41 (23–53.5) | 41 (25–66.5) | 0.2483 |
| CD8 count (cells/mm3) | 585 (426–796) | 431 (339–706.5) | 531 (375.8–730.3) | 0.1544 |
| % HLA-DR+, CD38+ CD4 T cells | 14.5 (10.17–24.1) | 14.65 (8.990–21.10) | 14.5 (9.223–22.30) | 0.7561 |
| % HLA-DR+, CD38+ CD8 T cells | 23.65 (14.40–29.90) | 19.55 (12.73–24.95) | 20.55 (13.80–28.38) | 0.2112 |
| % CD14++CD16+ monocytes | 17.95 (11.53–23.50) | 15.70 (10.90–21.45) | 15.90 (11.00–21.90) | 0.4415 |
| % CD163 monocytes | 4.430 (0.735–11.40) | 3.870 (1.117–11.90) | 4.010 (0.8360–10.80) | 0.9102 |
| % CD169 monocytes | 81.95 (76.63–87.43) | 81.50 (68.70–88.95) | 81.50 (72.80–87.90) | 0.6544 |
| Tetherin (MFI) | 2195 (957.5–3407) | 1276 (943.0–2832) | 1688 (972.0–3298) | 0.6372 |
| sCD14 (ng/ml) | 2861 (2143–3712) | 2398 (1905–3223) | 2774 (2069–3464) | 0.1437 |
| sCD163 (ng/ml) | 1496 (1123–2408) | 1449 (1086–1712) | 1449 (1087–1926) | 0.4715 |
P values were calculated by Mann-Whitney U test for all comparisons, excluding gender, which was calculated by Fisher’s exact test.
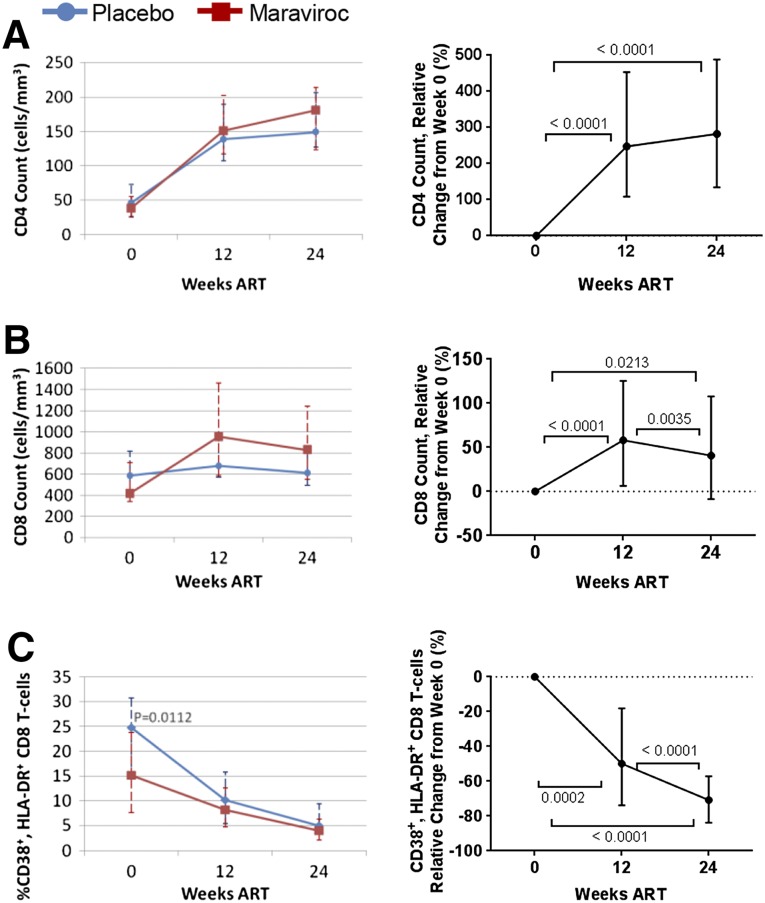
Figure 1. T cell count and CD38+, HLA-DR+ CD8 T cells during ART.
Shown are placebo (blue) versus maraviroc (red) group CD4 count (A), CD8 count (B), and percentage of CD38+, HLA-DR+, and CD8 T cells (C), absolute values and change relative to baseline. Median and interquartile range are displayed. Only patients with all (wk 0, 12, and 24) time points are shown. Mann-Whitney U comparison between groups was performed at each time point, with P < 0.05 considered significant and displayed (left). Longitudinally, Friedman test and post hoc Dunn test were performed, with P < 0.05 considered significant and displayed (right).
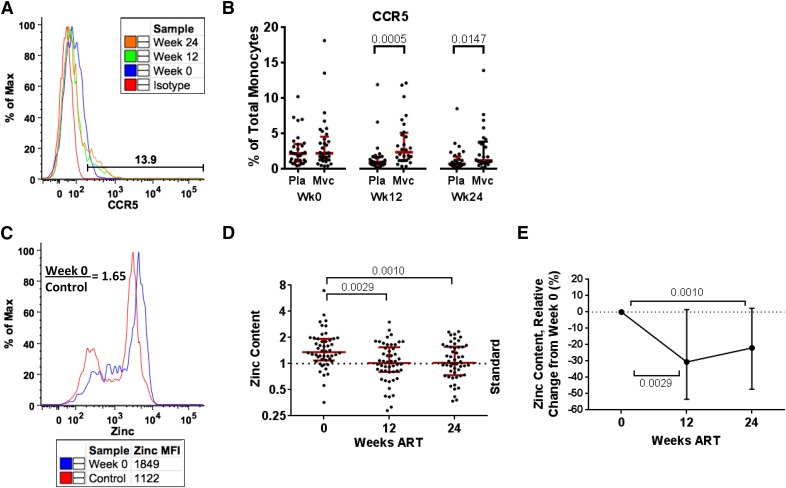
Figure 4. ART impact on monocyte apoptosis-resistance mechanisms.
CCR5 expression is displayed in a representative maraviroc (Mvc) patient (A) and the total cohort (B) during 24 wk of ART in placebo (Pla) versus maraviroc groups. Median and interquartile range are shown, and group comparison was performed using the Mann-Whitney U test, with P < 0.05 considered significant and displayed. Monocyte zinc content is shown [representative (C), relative value (D), relative change (E)] during 24 wk of ART. The dotted, horizontal lines (D and E) represent the MFI of the HIV(−) standard repeated during each experiment. Longitudinal comparisons display median and interquartile range using the Friedman test and Dunn post hoc test, with P < 0.05 considered significant and displayed.
T cell and monocyte flow cytometry
T cell activation was characterized in PBMC. Cells were thawed and stained with CD3-V450, CD38-APC, HLA-DR-APCH7, CD4-PerCPCy5.5, CCR5-PE, and CD8-FITC. Monocyte activation and ISG expression markers were assessed in PBMC with CD3-V450 (exclusion), CD14-APC, CD16-APCH7, CCR5-PerCPCy5.5, CD169-PE, and CD163-FITC, as well as with CD14-V450, CD16-APCH7, CD3-PerCPCy5.5 (exclusion), CD317-PE (tetherin), and CCR5-FITC. Cell type-matched gate (CD3+ for T cells; CD14, CD16 combined gate for monocytes) isotypes were used for negative/positive gating. Aqua Live/Dead (Invitrogen) was used for dead cell exclusion. All flow cytometry antibodies were purchased from BD Biosciences (San Jose, CA, USA), with the exception of CD169-PE (eBioscience, San Diego, CA, USA) and CD317-PE (BioLegend, San Diego, CA, USA), and used per the manufacturers’ instructions. T cells were gated on singlets by use of forward-scatter-height and -area, lymphoid-scatter profile, and CD3+ cells, followed by CD4+ and CD8+ T cells (Supplemental Fig. 1A). Monocytes were gated on singlets; monocyte-scatter profile; CD3 exclusion; total monocytes (CD14, CD16 combined gate); and the monocyte subsets, classic monocytes (CD14++CD16−), intermediate monocytes (CD14++CD16+), and nonclassic monocytes (CD14+CD16++; Supplemental Fig. 1B and C). All monocyte markers evaluated depict the total monocyte population (see Figs. 2–4 and Table 2). Activation biomarker data for total and all monocyte subsets have been provided in Supplemental Tables 1 and 2 and Supplemental Fig. 2.
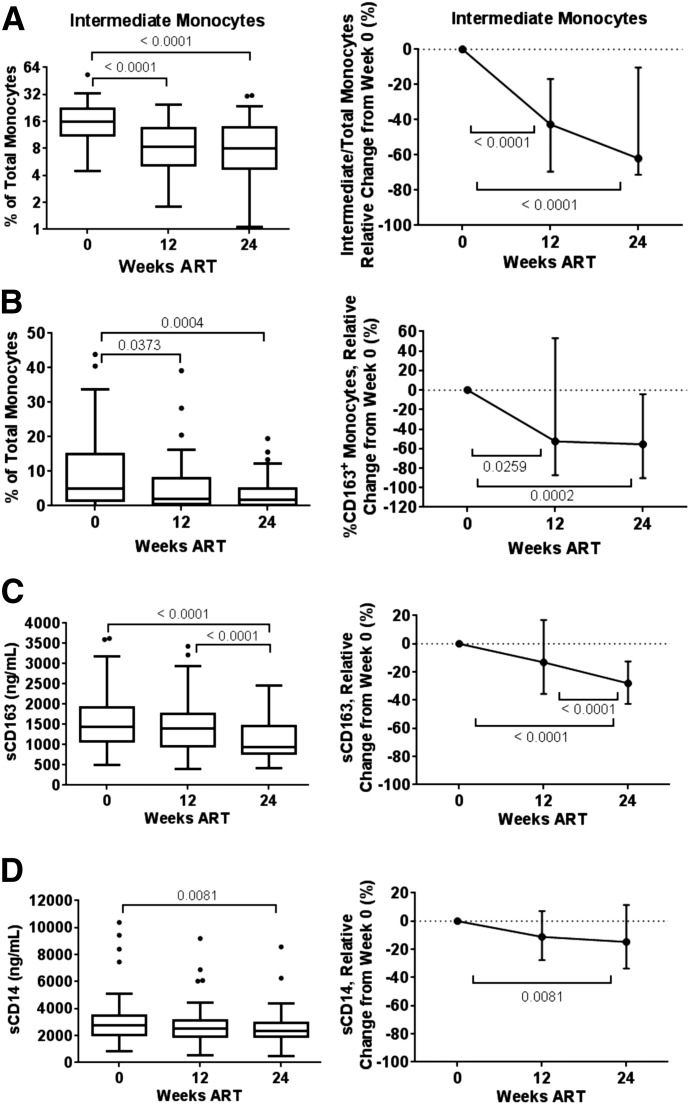
Figure 2. Monocyte and macrophage activation markers during ART.
Shown are percentage of CD14++CD16+ intermediate monocytes (A), CD163+ monocytes (B), plasma sCD163 (C), and plasma sCD14 (D), absolute and relative to baseline change. Longitudinal comparisons display median and interquartile range with Tukey’s method using the Friedman test and Dunn post hoc test, with P < 0.05 considered significant and displayed.
TABLE 2.
Correlation of change in T cell and total monocyte/macrophage activation biomarkers
| Cell type marker | T cell activation |
Monocyte and macrophage activation |
Clinical |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 |
CD8 |
Total monocytes |
Plasma |
||||||||
| CD38 | CD38, HLA-DR | CD38 | CD38, HLA-DR | % Intermediate | CD163 | CD169 | Tetherin | sCD14 | sCD163 | CD4 count | |
| CD38/CD4 (−16.90%) | — | 0.7296a | 0.5721a | 0.4975a | 0.0129 | 0.0703 | 0.1755 | 0.4299c | 0.2246 | −0.0493 | −0.0703 |
| CD38, HLA-DR/CD4 (−9.66%) | — | 0.4657a | 0.5194a | −0.1036 | −0.0872 | 0.0871 | 0.3834c | 0.2083 | −0.0104 | −0.0971 | |
| CD38/CD8 (−36.73%) | — | 0.7158a | 0.1226 | 0.3155b | 0.3962c | 0.5263a | 0.3443b | 0.2073 | −0.4692a | ||
| CD38, HLA-DR/CD8 (−12.80%) | — | 0 | 0.1291 | 0.3815c | 0.5315a | 0.2464 | 0.2886b | −0.0587 | |||
| Intermediate monocytes (−8.20%) | — | −0.0633 | 0.1400 | −0.2373 | −0.0020 | −0.0450 | 0.0397 | ||||
| CD163 (−1.91%) | — | 0.2140 | 0.2239 | 0.3315b | 0.1151 | −0.4593a | |||||
| CD169 (−78.02%) | — | 0.4333c | 0.2968b | 0.2191 | −0.1662 | ||||||
| Tetherin (−546.0 MFI units) | — | 0.2027 | 0.0623 | −0.1458 | |||||||
| sCD14 (−313.3 ng/ml) | — | 0.1484 | −0.2973b | ||||||||
| sCD163 (−394.5 ng/ml) | — | −0.1095 | |||||||||
| CD count (+116.0 cells/mm3) | — | ||||||||||
Median 24-wk change is shown in first column within parentheses. Spearman rho is displayed with statistical significance as follows: aP < 0.001, bP < 0.05, and cP < 0.01.
Monocyte zinc content
Monocyte zinc content was determined in PBMC by use of CD11b-V450, CD14-APC, CD16-APCH7, CD3-PerCPCy5.5 (exclusion), and 5 μM Newport Green DCF diacetate cell permeate zinc indicator (Invitrogen). Archived PBMC from the same HIV-seronegative donor was used as the internal control for the entire study. Control PBMC was thawed each time that the assay was performed and stained in parallel as a standard for each study subject analyzed. The MFI of each sample was divided by the MFI of the control donor to calculate the relative zinc content (see Fig. 4C).
Plasma activation markers
Plasma samples were assayed for sCD14 (R&D Systems, Minneapolis, MN, USA) and sCD163 (Trillium Diagnostics, Brewer, ME, USA) using 1:500 dilutions, according to the manufacturers’ instructions. Plates were read on a BioTek Synergy HT microplate reader at 450 nm with the appropriate wavelength corrections.
Flow cytometry analysis
Flow cytometry data analysis and images were generated with FlowJo software (TreeStar, Ashland, OR, USA). All flow data reported display percentage of positive cells relative to the parent population, with the exception of tetherin (MFI; see Fig. 3B) and zinc content (see Fig. 4C–E).
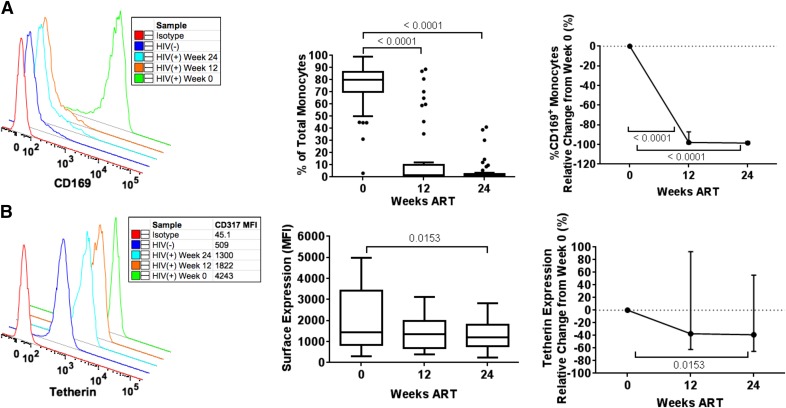
Figure 3. ART reduction of monocyte ISG markers.
Shown are the percentage of CD169+ monocytes (A) and tetherin expression (B) with representative donors (left), absolute values (middle), and change relative to baseline (right) during 24 wk of ART. Longitudinal comparisons display median and interquartile range with Tukey’s method using the Friedman test and Dunn post hoc test, with P < 0.05 considered significant and displayed.
Statistical analysis
All dot and Box-and-Whisker Plots were generated and tested statistically with Prism 6 (GraphPad Software, La Jolla, CA, USA), and 2-group longitudinal graphs (see Fig. 1) were generated using Microsoft Excel. All graphical plots show median and interquartile ranges, and Box-and-Whisker Plots are displayed using Tukey’s method. Relative change (see Figs. 1–4) was calculated as percentage change relative to wk 0 (pre-ART). Group comparisons at the same time point (see Figs. 1 and 4) were performed using the Mann-Whitney U test (2-tailed). Longitudinal comparisons were performed using the Friedman test, with the Dunn post hoc test between time points (Table 2 and Supplemental Fig. 2.
RESULTS AND DISCUSSION
Patients were recruited from a parent study examining the impact of CCR5 antagonism on the incidence of IRIS in patients with low CD4-count HIV-1, initiating ART [35] among 5 study sites in Mexico and 1 study site in South Africa. Archived plasma and PBMCs were analyzed from 65 patients from the South Africa site who were virologically suppressed at wk 24 (defined as viral load <400 RNA copies/ml). Inclusion criteria of the parent study required a baseline CD4 count of ≤100 CD4 T cells/mm3, allowing us to investigate the impact of ART-mediated suppression on monocyte/macrophage activation biomarkers in advanced HIV-1 disease. Consistent with the parent study, no difference was detected between placebo and maraviroc groups in the substudy for gender, age, and baseline clinical metrics (viral load, CD4 count, CD8 count; Table 1). Baseline CD8 T cell and monocyte/macrophage activation markers were also not different between groups.
The change in T cell count and CD38+, HLA-DR+ CD8 T cells was first analyzed. Patients increased median CD4 count from 46 and 41 (placebo and maraviroc group, respectively) to 149 and 177 cells/mm3 (P < 0.0001 vs. wk 0, pre-ART for both groups; P = 0.5710 between groups, wk 24; Fig. 1A). The parent study and similar studies [39] have observed higher CD8 counts in maraviroc groups during ART. The substudy observed a similar trend (Fig. 1B) but did not reach significance (P = 0.0579 at wk 24). Because these patients initiated therapy from advanced disease, we did not expect uniform decreases in T cell activation markers [2, 3] by wk 24. However, CD8 T cell activation, as measured by the percentage of HLA-DR+ and CD38+ cells, was uniformly reduced in both groups by wk 24 (P < 0.0001 vs. week 0, pre-ART; Fig. 1C). Independent of the group, the percentage of CD8 T cells expressing CD38 and HLA-DR was reduced by 70.76% from baseline.
We did not detect a difference in any marker of monocyte/macrophage activation examined between the placebo and maraviroc groups (data not shown). As evidence of therapy exposure, we did observe a higher percentage of CCR5-expressing monocytes in peripheral blood during maraviroc-inclusive ART (P = 0.0005 wk 12; P = 0.0147 wk 24; see Fig. 4B). As maraviroc treatment had no impact on monocyte/macrophage biomarkers of activation, we addressed ART-mediated changes, independent of study group.
We focused on the change in multiple biomarkers of monocyte and macrophage activation, because their recovery is not well described during ART in advanced HIV-1 infection. Within total monocytes, the percentage of CD14++CD16+ intermediate monocytes and CD163+ monocytes was reduced significantly by wk 24 (P < 0.0001 and 0.0004, respectively), with a relative reduction of 61.96 and 55.54% from baseline (Fig. 2A and B and Supplemental Table 1). (Subset CD163 expression is reported in Supplemental Table 2.) In plasma, sCD163 was also reduced significantly at wk 24, with a relative reduction of 28.04% from baseline (P < 0.0001; Fig. 2C). sCD14, a surrogate of microbial translocation, was reduced significantly by wk 24 (P = 0.0081) but with only a 14.70% change from baseline (Fig. 2D). These data indicate that markers, such as the CD14++CD16+ subset and CD163 expression, are more likely to show greater change after viral suppression in subjects with advanced disease than plasma variables sCD14 or sCD163.
We also investigated the change in ISGs associated with the pathogenesis (CD169) and control of HIV-1 replication (tetherin). CD169 was highly expressed on total monocytes pre-ART and was reduced to marginal expression after 12 wk ART (P < 0.0001; Fig. 3A). Tetherin expression was also reduced by 24 wk (P = 0.0153, 38.94%; Fig. 3B). The expression of both CD169 and tetherin was reduced significantly in all monocyte subsets at wk 12 and 24 (Supplemental Table 2). The rapid and near complete reduction of monocyte CD169 expression stands out as a very early and reversible marker of HIV-1 viremia, supporting that control of viral replication removes a major stimulus for monocyte ISG induction.
As previous findings have demonstrated that signaling through CCR5 [42, 43] and elevated intracellular monocyte zinc content [44] are mechanisms associated with apoptosis resistance in monocytes from patients with viremic HIV-1, we investigated monocyte CCR5 expression and zinc content (Fig. 4). As noted earlier, the percentage of monocytes expressing CCR5 was higher in the maraviroc group during therapy (Fig. 4B); however, no difference in zinc content was noted between therapy groups (data not shown). Zinc content was decreased by 12 wk in total monocytes (Fig. 4D) and the classic subset but was unchanged in the intermediate and nonclassic subset (Supplemental Table 2). We interpret that ART may reduce the antiapoptotic impact of monocyte zinc content by reduction within classic monocytes or reduction of the size of the intermediate subset.
We last examined the correlation of the change in T cell activation with that of monocyte and macrophage activation markers measured over 24 wk (Table 2 and Supplemental Fig. 2). We found that the change in CD8 activation (CD38+, HLA-DR+) was closely associated with changes in CD169 and tetherin expression on total monocytes. It is noteworthy that CD4 or CD8 T cell activation (CD38+, HLA-DR+) change was not associated with changes in the CD14++CD16+ intermediate subset, CD163 expression, or plasma sCD14. These data suggest that decreases in markers of T cell activation, observed after viremic suppression, are mirrored by monocyte markers of ISG expression (CD169, tetherin) as opposed to those associated with inflammatory cascades.
Taken together, we show that changes in markers of macrophage activation and monocyte subsets after ART in patients with low CD4-count result in differential changes relative to decreases in T cell activation. Furthermore, CCR5 antagonism (maraviroc) did not have an impact on markers of monocyte and macrophage activation beyond the impact of ART alone. The latter indicates that suppression of viral replication is dominant over CCR5 antagonism in determining change in innate immune activation. However, because we do not examine tissue activation, as done elsewhere [39], we do not exclude a long-term impact of CCR5 antagonism on tissue damage, repair, and reconstitution, as should be a focus of future studies. Our data strongly indicate that monocyte/macrophage biomarkers of activation, often interpreted jointly, may reflect different mechanisms of activation that are not efficiently reversed with ART in advanced disease. Although other studies have addressed retention of activation in monocyte subsets after viral suppression, our study reports for the first time the differential changes observed when ART is initiated in advanced disease.
Although we generally observed decreases across biomarkers of monocyte and macrophage activation with viral suppression, different degrees of change were observed between markers. At one end, monocyte CD169 expression was greatly reduced by 12 wk (median 97.94% relative reduction), representing the marker with the closest alignment to control of viral replication. Although others have described a correlation of CD169 expression and viral load [25], we did not detect this association, possibly because of the narrow viral load distribution of our cohort. CD169 expression is with ART in the SIV macaque model [32] and, as shown here, during HIV-1 ART in advanced disease. The level of intermediate monocytes and cell-associated CD163 was also reduced significantly by >50% by wk 24 (Fig. 2). Previous studies have observed ART-mediated recovery that reaches HIV(−) levels for intermediate monocytes [17] but not sCD163 [4, 5, 7, 9], reinforcing the qualitative differences in the recovery of these biomarkers. As this cohort was characterized by favorable CD4 gains, it remains to be determined what, if any, changes occur in cell-associated monocyte activation markers in immunologic nonresponders.
Unexpectedly, CD163 expression in monocytes did not associate with sCD163 levels, and as follows, resolution of CD163 surface expression with ART may represent independent immunological pressures than those mediating receptor shedding. It is noteworthy that our measurement of CD163 expression may under-represent in vivo expression levels, as others have described lower values on PBMC compared with whole-blood staining (CD163 antibody clone GHI/61 used both here and referenced studies [22, 45]). Our prior work has described the impact of viral replication on monocyte ISG expression and apoptotic regulation [42–44, 46]. Data from this study reinforce the strong relationship between viral suppression and reduced ISG expression compared with markers of TLR-mediated activation that may reflect more gradual recovery from microbial translocation and associated retained activation.
The monocyte/macrophage activation metric that changed the least over 24 wk was sCD14, one of the least plastic biomarkers of activation examined [4, 8–10, 24]. Indeed, sCD14 showed no change in our cohort upon removal of the Mycobacterium tuberculosis IRIS cases, which generally fell in the upper region of sCD14 distribution (data not shown). (The relationship of baseline sCD14 and incidence of M. tuberculosis IRIS is beyond the scope of this study.) Our data clearly suggest that sCD14 is stable in the majority of advanced patients during 24 wk of ART.
Our study contrasts findings of positive impacts of maraviroc-supplemented ART on sCD14 resolution [40], potentially as a result of cohort differences, as other studies had higher baseline CD4 counts. The capacity of sCD14 and gut epithelial barrier damage to predict poor clinical outcome [12, 47] necessitates the evaluation of additional interventions to improve the reduction of this activation marker after ART in low CD4-count subjects [48]. It also remains to be determined whether the sCD14 and sCD163 are more clinically significant biomarkers of residual immune cross-talk and microbial translocation-associated ligands, such as LPS, than ISG-associated changes. However, our data also highlight the benefits of viral suppression in advanced disease, despite sub-200 CD4 reconstitution at 24 wk, and may provide monocyte immune reconstitution benchmarks that can be monitored to inform CD4 recovery.
Within the limitations of this study, the absence of age, gender, and geographically matched HIV-seronegative subjects limits our ability to interpret resolution to uninfected levels. However, this does not affect our ability to interpret what variables best reflect changes in T cell activation and viral suppression. The absence of functional responses limits assessment regarding retention of plasma sCD14 and altered TLR responses. Data analysis on patients with viral suppression by wk 24 and complete datasets (wk 0, 12, and 24) may introduce selection bias, limiting our interpretation to subjects with favorable clinical outcomes. Finally, as a result of the use of cryopreserved PBMC and the absence of gene expression data, we are unable to measure monocyte apoptotic outcome directly and therefore are limited to describing markers of previously described apoptotic resistance mechanisms (Fig. 4) [42–44].
In summary, we describe a spectrum of reduction of indicators of monocyte/macrophage activation in patients with advanced HIV-1 during 24 wk of ART. We also differentiate activation of variables closely associated with control of viral replication (CD14++CD16+ monocyte subset, CD163, CD169, and tetherin) and those (CD169 and tetherin) showing a strong relationship with decreasing CD8 T cell activation. The lack of greater change in sCD14 and sCD163 after ART in advanced disease supports that microbial translocation and associated activation may be of greater concern in advanced disease subjects, independent of viral suppression. The impact of this report will be in the differentiation of soluble and cell-associated markers of monocyte/macrophage activation when addressing activation recovery after ART in this population.
AUTHORSHIP
S.C.P., L.A., and L.J.M. designed experiments and prepared the first draft of manuscript. S.C.P., J.J., and M.G.F. prepared samples and performed assays. J.G.S-M., M.S.R., and I.S. contributed to design and execution of the parent study, cohort recruitment and monitoring, and manuscript preparation. L.J.M. supervised team operations.
Acknowledgments
The CADIRIS trial was funded as investigator-initiated research by Pfizer (New York, NY, USA). This work was supported by U.S. National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) (Grant R01 AI073219); the Philadelphia Foundation (Robert I. Jacobs Fund); Henry S. Miller, Jr., and J. Kenneth Nimblett; the Commonwealth Universal Research Enhancement Program; the Pennsylvania Department of Health, the Penn Center for AIDS Research, NIH NIAID (Grant P30AI045008); and the NIH-T32 HIV Pathogenesis Training Grant through the University of Pennsylvania. Support for shared resources used in this study was provided by NIH National Cancer Institute to The Wistar Institute (Grant P30 CA010815). The CADIRIS Study Team investigators include the following: J. Andrade-Villanueva, L. Azzoni, P. F. Belaunzarán-Zamudio, S. Ellenberg, M. M. Lederman, L. J. Montaner, M. Magaña-Aquino, J. L. Mosqueda-Gómez, A. Piñeirúa-Menéndez, C. Rivera-Benítez, B. Rodriguez, I. Sanne, I. Sereti, J. G. Sierra-Madero, and Ann Tierney. The authors thank Jeffery Faust (Wistar Flow Cytometry) for help with experiments, Wistar phlebotomist Deborah Davis for blood donor recruitment, and the CADIRIS study team.
Glossary
- APC
allophycocyanin
- ART
antiretroviral therapy
- CADIRIS
CCR5 antagonism to decrease the incidence of immune reconstitution inflammatory syndrome
- DC
dendritic cell
- IRIS
immune reconstitution inflammatory syndrome
- ISG
IFN-stimulated gene
- MFI
mean fluorescence intensity
- s
soluble
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institutes of Health.
REFERENCES
- 1.Liu Z., Cumberland W. G., Hultin L. E., Prince H. E., Detels R., Giorgi J. V. (1997) Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16, 83–92. [DOI] [PubMed] [Google Scholar]
- 2.Hunt P. W., Martin J. N., Sinclair E., Bredt B., Hagos E., Lampiris H., Deeks S. G. (2003) T Cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 187, 1534–1543. [DOI] [PubMed] [Google Scholar]
- 3.Benito J. M., López M., Lozano S., Ballesteros C., Martinez P., González-Lahoz J., Soriano V. (2005) Differential upregulation of CD38 on different T-cell subsets may influence the ability to reconstitute CD4+ T cells under successful highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 38, 373–381. [DOI] [PubMed] [Google Scholar]
- 4.Lichtfuss G. F., Cheng W. J., Farsakoglu Y., Paukovics G., Rajasuriar R., Velayudham P., Kramski M., Hearps A. C., Cameron P. U., Lewin S. R., Crowe S. M., Jaworowski A. (2012) Virologically suppressed HIV patients show activation of NK cells and persistent innate immune activation. J. Immunol. 189, 1491–1499. [DOI] [PubMed] [Google Scholar]
- 5.Burdo T. H., Weiffenbach A., Woods S. P., Letendre S., Ellis R. J., Williams K. C. (2013) Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 27, 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burdo T. H., Lo J., Abbara S., Wei J., DeLelys M. E., Preffer F., Rosenberg E. S., Williams K. C., Grinspoon S. (2011) Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J. Infect. Dis. 204, 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hearps A. C., Maisa A., Cheng W. J., Angelovich T. A., Lichtfuss G. F., Palmer C. S., Landay A. L., Jaworowski A., Crowe S. M. (2012) HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS 26, 843–853. [DOI] [PubMed] [Google Scholar]
- 8.Brenchley J. M., Price D. A., Schacker T. W., Asher T. E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., Blazar B. R., Rodriguez B., Teixeira-Johnson L., Landay A., Martin J. N., Hecht F. M., Picker L. J., Lederman M. M., Deeks S. G., Douek D. C. (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371. [DOI] [PubMed] [Google Scholar]
- 9.Burdo T. H., Lentz M. R., Autissier P., Krishnan A., Halpern E., Letendre S., Rosenberg E. S., Ellis R. J., Williams K. C. (2011) Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J. Infect. Dis. 204, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallet M. A., Rodriguez C. A., Yin L., Saporta S., Chinratanapisit S., Hou W., Sleasman J. W., Goodenow M. M. (2010) Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS 24, 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lederman M. M., Calabrese L., Funderburg N. T., Clagett B., Medvik K., Bonilla H., Gripshover B., Salata R. A., Taege A., Lisgaris M., McComsey G. A., Kirchner E., Baum J., Shive C., Asaad R., Kalayjian R. C., Sieg S. F., Rodriguez B. (2011) Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J. Infect. Dis. 204, 1217–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandler N. G., Wand H., Roque A., Law M., Nason M. C., Nixon D. E., Pedersen C., Ruxrungtham K., Lewin S. R., Emery S., Neaton J. D., Brenchley J. M., Deeks S. G., Sereti I., Douek D. C.; INSIGHT SMART Study Group (2011) Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 203, 780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pulliam L., Gascon R., Stubblebine M., McGuire D., McGrath M. S. (1997) Unique monocyte subset in patients with AIDS dementia. Lancet 349, 692–695. [DOI] [PubMed] [Google Scholar]
- 14.Liang H., Duan Z., Li D., Li D., Wang Z., Ren L., Shen T., Shao Y. (2015) Higher levels of circulating monocyte-platelet aggregates are correlated with viremia and increased sCD163 levels in HIV-1 infection. Cell. Mol. Immunol. 12, 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim W. K., Sun Y., Do H., Autissier P., Halpern E. F., Piatak M. Jr, Lifson J. D., Burdo T. H., McGrath M. S., Williams K. (2010) Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. J. Leukoc. Biol. 87, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer-Smith T., Croul S., Sverstiuk A. E., Capini C., L’Heureux D., Régulier E. G., Richardson M. W., Amini S., Morgello S., Khalili K., Rappaport J. (2001) CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J. Neurovirol. 7, 528–541. [DOI] [PubMed] [Google Scholar]
- 17.Funderburg N. T., Zidar D. A., Shive C., Lioi A., Mudd J., Musselwhite L. W., Simon D. I., Costa M. A., Rodriguez B., Sieg S. F., Lederman M. M. (2012) Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 120, 4599–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Sánchez M., González-Serna A., Pacheco Y. M., Ferrando-Martínez S., Machmach K., García-García M., Alvarez-Ríos A. I., Vidal F., Leal M., Ruiz-Mateos E. (2012) Different biological significance of sCD14 and LPS in HIV-infection: importance of the immunovirology stage and association with HIV-disease progression markers. J. Infect. 65, 431–438. [DOI] [PubMed] [Google Scholar]
- 19.Noel N., Boufassa F., Lécuroux C., Saez-Cirion A., Bourgeois C., Dunyach-Remy C., Goujard C., Rouzioux C., Meyer L., Pancino G., Venet A., Lambotte O.; ANRS C021 CODEX Study Group (2014) Elevated IP10 levels are associated with immune activation and low CD4⁺ T-cell counts in HIV controller patients. AIDS 28, 467–476. [DOI] [PubMed] [Google Scholar]
- 20.Burdo T. H., Soulas C., Orzechowski K., Button J., Krishnan A., Sugimoto C., Alvarez X., Kuroda M. J., Williams K. C. (2010) Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 6, e1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim W. K., Alvarez X., Fisher J., Bronfin B., Westmoreland S., McLaurin J., Williams K. (2006) CD163 identifies perivascular macrophages in normal and viral encephalitic brains and potential precursors to perivascular macrophages in blood. Am. J. Pathol. 168, 822–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tippett E., Cheng W. J., Westhorpe C., Cameron P. U., Brew B. J., Lewin S. R., Jaworowski A., Crowe S. M. (2011) Differential expression of CD163 on monocyte subsets in healthy and HIV-1 infected individuals. PLoS One 6, e19968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker J. A., Sulciner M. L., Nowicki K. D., Miller A. D., Burdo T. H., Williams K. C. (2014) Elevated numbers of CD163+ macrophages in hearts of simian immunodeficiency virus-infected monkeys correlate with cardiac pathology and fibrosis. AIDS Res. Hum. Retroviruses 30, 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajasuriar R., Booth D., Solomon A., Chua K., Spelman T., Gouillou M., Schlub T. E., Davenport M., Crowe S., Elliott J., Hoy J., Fairley C., Stewart G., Cameron P., Lewin S. R. (2010) Biological determinants of immune reconstitution in HIV-infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor α and microbial translocation. J. Infect. Dis. 202, 1254–1264. [DOI] [PubMed] [Google Scholar]
- 25.Pino M., Erkizia I., Benet S., Erikson E., Fernández-Figueras M. T., Guerrero D., Dalmau J., Ouchi D., Rausell A., Ciuffi A., Keppler O. T., Telenti A., Kräusslich H. G., Martinez-Picado J., Izquierdo-Useros N. (2015) HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulliam L., Sun B., Rempel H. (2004) Invasive chronic inflammatory monocyte phenotype in subjects with high HIV-1 viral load. J. Neuroimmunol. 157, 93–98. [DOI] [PubMed] [Google Scholar]
- 27.van der Kuyl A. C., van den Burg R., Zorgdrager F., Groot F., Berkhout B., Cornelissen M. (2007) Sialoadhesin (CD169) expression in CD14+ cells is upregulated early after HIV-1 infection and increases during disease progression. PLoS One 2, e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.York M. R., Nagai T., Mangini A. J., Lemaire R., van Seventer J. M., Lafyatis R. (2007) A macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and Toll-like receptor agonists. Arthritis Rheum. 56, 1010–1020. [DOI] [PubMed] [Google Scholar]
- 29.Biesen R., Demir C., Barkhudarova F., Grün J. R., Steinbrich-Zöllner M., Backhaus M., Häupl T., Rudwaleit M., Riemekasten G., Radbruch A., Hiepe F., Burmester G. R., Grützkau A. (2008) Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis Rheum. 58, 1136–1145. [DOI] [PubMed] [Google Scholar]
- 30.Rempel H., Calosing C., Sun B., Pulliam L. (2008) Sialoadhesin expressed on IFN-induced monocytes binds HIV-1 and enhances infectivity. PLoS One 3, e1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puryear W. B., Akiyama H., Geer S. D., Ramirez N. P., Yu X., Reinhard B. M., Gummuluru S. (2013) Interferon-inducible mechanism of dendritic cell-mediated HIV-1 dissemination is dependent on Siglec-1/CD169. PLoS Pathog. 9, e1003291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim W. K., McGary C. M., Holder G. E., Filipowicz A. R., Kim M. M., Beydoun H. A., Cai Y., Liu X., Sugimoto C., Kuroda M. J. (2015) Increased expression of CD169 on blood monocytes and its regulation by virus and CD8 T cells in macaque models of HIV infection and AIDS. AIDS Res. Hum. Retroviruses 31, 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strebel K. (2013) HIV accessory proteins versus host restriction factors. Curr. Opin. Virol. 3, 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergamaschi A., Pancino G. (2010) Host hindrance to HIV-1 replication in monocytes and macrophages. Retrovirology 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sierra-Madero J. G., Ellenberg S. S., Rassool M. S., Tierney A., Belaunzarán-Zamudio P. F., López-Martínez A., Piñeirúa-Menéndez A., Montaner L. J., Azzoni L., Benítez C. R., Sereti I., Andrade-Villanueva J., Mosqueda-Gómez J. L., Rodriguez B., Sanne I., Lederman M. M.; CADIRIS Study Team (2014) Effect of the CCR5 antagonist maraviroc on the occurrence of immune reconstitution inflammatory syndrome in HIV (CADIRIS): a double-blind, randomised, placebo-controlled trial. Lancet HIV 1, e60–e67. [DOI] [PubMed] [Google Scholar]
- 36.Funderburg N., Kalinowska M., Eason J., Goodrich J., Heera J., Mayer H., Rajicic N., Valdez H., Lederman M. M. (2010) Effects of maraviroc and efavirenz on markers of immune activation and inflammation and associations with CD4+ cell rises in HIV-infected patients. PLoS One 5, e13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutiérrez C., Díaz L., Vallejo A., Hernández-Novoa B., Abad M., Madrid N., Dahl V., Rubio R., Moreno A. M., Dronda F., Casado J. L., Navas E., Pérez-Elías M. J., Zamora J., Palmer S., Muñoz E., Muñoz-Fernández M. A., Moreno S. (2011) Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected. PLoS One 6, e27864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkin T. J., Lalama C. M., McKinnon J., Gandhi R. T., Lin N., Landay A., Ribaudo H., Fox L., Currier J. S., Mellors J. W., Gulick R., Tenorio A. R. (2012) A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4⁺ T-cell recovery despite sustained virologic suppression: ACTG A5256. J. Infect. Dis. 206, 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hunt P. W., Shulman N. S., Hayes T. L., Dahl V., Somsouk M., Funderburg N. T., McLaughlin B., Landay A. L., Adeyemi O., Gilman L. E., Clagett B., Rodriguez B., Martin J. N., Schacker T. W., Shacklett B. L., Palmer S., Lederman M. M., Deeks S. G. (2013) The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood 121, 4635–4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero-Sánchez M. C., Alvarez-Ríos A. I., Bernal-Morell E., Genebat M., Vera F., Benhnia M. R., Bravo-Urbieta J., Galera-Peñaranda C., de Pablo-Bernal R. S., Abad-Carrillo M. A., Leal M., Ruiz-Mateos E.; VIH-GEMUVIH Región de Murcia Study Group (2014) Maintenance of virologic efficacy and decrease in levels of β2-microglobulin, soluble CD40L and soluble CD14 after switching previously treated HIV-infected patients to an NRTI-sparing dual therapy. Antiviral Res. 111, 26–32. [DOI] [PubMed] [Google Scholar]
- 41.Puertas M. C., Massanella M., Llibre J. M., Ballestero M., Buzon M. J., Ouchi D., Esteve A., Boix J., Manzardo C., Miró J. M., Gatell J. M., Clotet B., Blanco J., Martinez-Picado J.; MaraviBoost Collaborative Group (2014) Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. AIDS 28, 325–334. [DOI] [PubMed] [Google Scholar]
- 42.Giri M. S., Nebozyhn M., Raymond A., Gekonge B., Hancock A., Creer S., Nicols C., Yousef M., Foulkes A. S., Mounzer K., Shull J., Silvestri G., Kostman J., Collman R. G., Showe L., Montaner L. J. (2009) Circulating monocytes in HIV-1-infected viremic subjects exhibit an antiapoptosis gene signature and virus- and host-mediated apoptosis resistance. J. Immunol. 182, 4459–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gekonge B., Raymond A. D., Yin X., Kostman J., Mounzer K., Collman R. G., Showe L., Montaner L. J. (2012) Retinoblastoma protein induction by HIV viremia or CCR5 in monocytes exposed to HIV-1 mediates protection from activation-induced apoptosis: ex vivo and in vitro study. J. Leukoc. Biol. 92, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raymond A. D., Gekonge B., Giri M. S., Hancock A., Papasavvas E., Chehimi J., Kossenkov A. V., Nicols C., Yousef M., Mounzer K., Shull J., Kostman J., Showe L., Montaner L. J. (2010) Increased metallothionein gene expression, zinc, and zinc-dependent resistance to apoptosis in circulating monocytes during HIV viremia. J. Leukoc. Biol. 88, 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moniuszko M., Kowal K., Rusak M., Pietruczuk M., Dabrowska M., Bodzenta-Lukaszyk A. (2006) Monocyte CD163 and CD36 expression in human whole blood and isolated mononuclear cell samples: influence of different anticoagulants. Clin. Vaccine Immunol. 13, 704–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patro S. C., Pal S., Bi Y., Lynn K., Mounzer K. C., Kostman J. R., Davuluri R. V., Montaner L. J. (2015) Shift in monocyte apoptosis with increasing viral load and change in apoptosis-related ISG/Bcl2 family gene expression in chronically HIV-1-infected subjects. J. Virol. 89, 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunt P. W., Sinclair E., Rodriguez B., Shive C., Clagett B., Funderburg N., Robinson J., Huang Y., Epling L., Martin J. N., Deeks S. G., Meinert C. L., Van Natta M. L., Jabs D. A., Lederman M. M. (2014) Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J. Infect. Dis. 210, 1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Funderburg N. T., Jiang Y., Debanne S. M., Labbato D., Juchnowski S., Ferrari B., Clagett B., Robinson J., Lederman M. M., McComsey G. A. (2015) Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 68, 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]