Abstract
α-Synuclein is the major component of Lewy bodies and Lewy neurites in Parkinson disease and dementia with Lewy bodies and of glial cytoplasmic inclusions in multiple system atrophy. It has been suggested that α-synuclein fibrils or intermediate protofibrils in the process of fibril formation may have a toxic effect on neuronal cells. In this study, we investigated the ability of soluble monomeric α-synuclein to promote microtubule assembly and the effects of conformational changes of α-synuclein on Tau-promoted microtubule assembly. In marked contrast to previous findings, monomeric α-synuclein had no effect on microtubule polymerization. However, both α-synuclein fibrils and protofibrils inhibited Tau-promoted microtubule assembly. The inhibitory effect of α-synuclein fibrils was greater than that of the protofibrils. Dot blot overlay assay and spin-down techniques revealed that α-synuclein fibrils bind to Tau and inhibit microtubule assembly by depleting the Tau available for microtubule polymerization. Using various deletion mutants of α-synuclein and Tau, the acidic C-terminal region of α-synuclein and the basic central region of Tau were identified as regions involved in the binding. Furthermore, introduction of α-synuclein fibrils into cultured cells overexpressing Tau protein induced Tau aggregation. These results raise the possibility that α-synuclein fibrils interact with Tau, inhibit its function to stabilize microtubules, and also promote Tau aggregation, leading to dysfunction of neuronal cells.
Keywords: fibril, microtubule, protein misfolding, synuclein, Tau protein (Tau)
Introduction
Parkinson disease (PD)2 is a progressive neurodegenerative disorder, clinically characterized by tremor, muscular rigidity, bradykinesia, and postural-reflex impairment. It is the second most common neurodegenerative disorder after Alzheimer disease (AD), affecting about 1–2% of the population over the age of 65 years. Neuropathological features of PD are the selective loss of dopaminergic neurons in substantia nigra and the appearance of intracellular inclusion bodies, referred to as Lewy bodies (LBs) and Lewy neurites. Ultrastructurally, the inclusions are composed of a dense core of filamentous and granular materials surrounded by radially oriented fibrils. In 1997, missense mutation in the α-synuclein gene SNCA, previously mapped to the PARK 1 locus, chromosome 4q21, was found to be associated with familial PD (1). Subsequent immunohistochemical studies using antibodies to synucleins demonstrated that α-synuclein is the major component of filaments or fibrils in LBs and also glial cytoplasmic inclusions in multiple system atrophy (2–4). To date, six missense mutations in SNCA (A30P, E46K, H50Q, G51D, A53T, and A53E) associated with familial forms of PD and dementia with Lewy bodies (DLB) have been reported (1, 5–9).
α-Synuclein is a 140-amino acid protein, which is enriched in presynaptic nerve terminals of the CNS (10). It belongs to the synuclein family, which currently consists of three members (α-, β-, and γ-synuclein) (11), and it consists of (i) an N-terminal amphipathic region, (ii) a central hydrophobic non-amyloid β component (NAC) region, and (iii) a highly acidic C-terminal region. Seven imperfect 11-mer tandem repeats, which display variations of a KTKEGV sequence, exist in the N-terminal half. α-Synuclein is natively unfolded (12), and therefore, it was thought not to have a well defined secondary structure. However, NMR studies suggest that monomeric α-synuclein may have a conformation that is stabilized by intramolecular long range interactions, fluctuating in the range of nanoseconds to microseconds, that appear to inhibit oligomerization and aggregation (13, 14). Although α-synuclein is found predominantly in the cytosolic fraction, it has also been assumed that the amphipathic N-terminal region of α-synuclein binds to lipid membranes, where it takes an α-helical conformation (15).
Although the precise physiological functions of α-synuclein remain to be fully elucidated, several putative functions have been proposed. α-Synuclein may be involved in the regulation of vertebrate synaptic plasticity, because the expression of synelfin, the avian homologue of α-synuclein, was found to be regulated at a critical period of song learning in zebra finch (16). α-Synuclein knock-out mice showed increased dopamine release at nigrostriatal terminals in response to paired electrical stimuli, suggesting that α-synuclein may act as a negative regulator of dopamine transmission (17). In addition, modulation of dopamine transporter, chaperone function, and microtubule polymerizing activity have been reported (18).
Recombinant α-synuclein has been shown to assemble into fibrils or protofibrils under some experimental conditions, and the morphologies of the fibrils are similar to those found in LBs (19). The fibrils show a cross-β fiber diffraction pattern similar to that of typical amyloid fibrils (20). Furthermore, it has been reported that A53T and E46K mutants form fibrils at an increased rate (21, 22), and A30P mutation promotes oligomerization (23) or formation of fragile fibrils (24). The C-terminally truncated form of α-synuclein shows an increased rate of fibrillization, suggesting that the C-terminal region inhibits oligomerization and aggregation to a certain extent (19).
α-Synuclein deposited in brains of patients with DLB as well as other α-synucleinopathies is highly phosphorylated at Ser-129 (25), suggesting that the extensive phosphorylation of α-synuclein is a pathological event. Phosphorylated α-synuclein deposited in brains with α-synucleinopathy is also partially ubiquitinated (26), and the ubiquitination sites have been identified (27, 28).
It was reported that α-synuclein promotes microtubule assembly and that the highly acidic C-terminal region of α-synuclein is critical for its function (29). However, many microtubule-associated proteins (MAPs) in neuronal cells, for example Tau and MAP2, contain a well conserved, positively charged microtubule-binding domain that mediates binding to microtubules. This raises the following question. Is α-synuclein truly a microtubule-associated protein? In this study, we analyzed the effect of α-synuclein on microtubule assembly, from the viewpoint of both its normal physiological function and its involvement in abnormal cytotoxic mechanisms.
Results
Monomeric α-Synuclein Has No Tubulin Polymerizing Activity
To investigate the physiological action of α-synuclein on microtubules, we carried out microtubule assembly assay using various concentrations of purified recombinant human α-synuclein. When 2.3 μm recombinant Tau (a microtubule-associated protein known to promote microtubule assembly) was incubated with 10 μm tubulin, microtubule assembly was detected as an increase in turbidity (Fig. 1A). In contrast, no microtubule polymerization was observed with α-synuclein in the concentration range from 6.9 to 276 μm, which is much higher than the concentrations used in the previous report (Fig. 1A).
FIGURE 1.
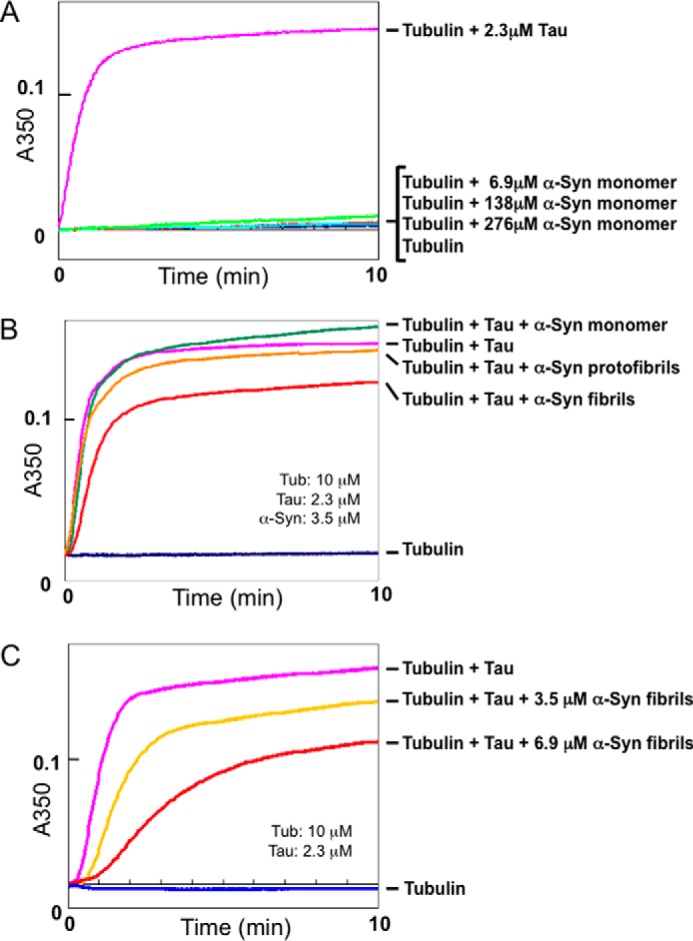
Effects of α-synuclein monomer, fibrils, or protofibirils on microtubule assembly. A, effect of α-synuclein monomer on microtubule assembly. Polymerization of tubulin with or without α-synuclein was monitored by measuring turbidity at 350 nm for 10 min. Blue, 10 μm tubulin only; pink, tubulin + 2.3 μm Tau; light blue, red, and green, tubulin + 69, 138, and 276 μm α-synuclein monomer. Mean values of three separate experiments are plotted. Similar results were obtained in all three experiments. B, effects of α-synuclein monomer, fibrils, or protofibrils on Tau-promoted microtubule assembly. Tubulin polymerization with Tau in the presence or absence of α-synuclein (monomer, fibrils, or protofibrils) was monitored in terms of turbidity. Blue, 10 μm tubulin only; pink, tubulin + 2.3 μm Tau; green, tubulin + Tau + 3.5 μm α-synuclein monomer; orange, tubulin + Tau + 3.5 μm α-synuclein protofibrils; red, tubulin + Tau + 3.5 μm α-synuclein fibrils. Mean values of three separate experiments were plotted on the graph. Similar results were obtained in all three experiments. C, dose-dependent inhibition of Tau-promoted microtubule assembly by α-synuclein fibrils. Tubulin polymerization with Tau in the presence of 3.5 or 6.9 μm α-synuclein fibrils was monitored. Blue, 10 μm tubulin only; pink, tubulin + 2.3 μm Tau; yellow, tubulin + Tau + 3.5 μm α-synuclein fibrils; red, tubulin + Tau + 6.9 μm α-synuclein fibrils. Mean values of three separate experiments are plotted. Similar results were obtained in all three experiments. α-Syn, α-synuclein.
Next, we investigated the effect of α-synuclein fibrils on Tau-promoted microtubule assembly, because accumulation of filamentous α-synuclein is closely correlated with neuronal degeneration in PD and other α-synucleinopathies. We considered that abnormal α-synuclein (α-synuclein fibrils or protofibrils) might inhibit the normal physiological function of microtubules and lead to neuronal degeneration. Recombinant Tau (2.3 μm) was preincubated with 3.5 μm α-synuclein monomer, fibrils or protofibrils, and then incubated with tubulin, and the turbidity was monitored at 350 nm. As shown in Fig. 1B, both α-synuclein fibrils and protofibrils inhibited Tau-promoted microtubule assembly, whereas monomeric α-synuclein showed no effect at the same concentration. The inhibitory effect of α-synuclein fibrils was greater than that of protofibrils. To investigate whether this inhibitory effect of α-synuclein fibrils is dose-dependent, we carried out the assay with 3.5 or 6.9 μm α-synuclein fibrils. As shown in Fig. 1C, the Tau-promoted microtubule assembly was inhibited more effectively by 6.9 μm α-synuclein fibrils than by 3.5 μm α-synuclein fibrils, indicating that the fibrils inhibit Tau-promoted microtubule assembly in a dose-dependent manner (Fig. 1C).
Interaction of Tau with α-Synuclein Fibrils
To investigate the mechanisms that underlie the inhibitory properties of α-synuclein fibrils or protofibrils, we analyzed the interaction of Tau with α-synuclein monomer, fibrils, or protofibrils by means of dot blot overlay assay (Fig. 2). Tau immunoreactivities were hardly detectable on the spots of monomeric α-synuclein or the protofibrils, whereas strong immunoreactivity was detected on the α-synuclein fibrils, indicating that Tau bound to α-synuclein fibrils but not the monomer. Next, we investigated whether this interaction was in an ionic interaction or not by spin-down of α-synuclein fibrils (Fig. 3). In the absence of α-synuclein fibrils, most Tau was recovered in the supernatant after ultracentrifugation. In contrast, in the presence of α-synuclein fibrils, most Tau was recovered in the pellet with α-synuclein fibrils after ultracentrifugation under a low ionic condition (Fig. 3). However, under a high ionic condition (0.5 m NaCl), most Tau was recovered in the supernatant (Fig. 3). These data suggest that the Tau binds to α-synuclein fibrils via ionic interaction.
FIGURE 2.
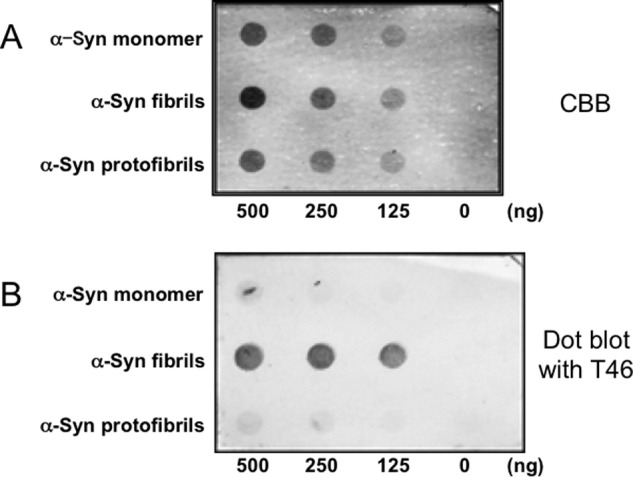
Interaction of Tau with α-synuclein monomer, fibrils and protofibrils. A, Coomassie Brilliant Blue (CBB) staining of α-synuclein monomer, fibrils, and protofibrils dotted on a nitrocellulose membrane. The amounts of α-synuclein proteins are shown in the figure. B, detection of Tau interacted with α-synuclein. α-Synuclein monomer, fibrils, and protofibrils dotted on a nitrocellulose membrane were incubated with Tau, and bound Tau was detected with anti-Tau antibody T46. α-Syn, α-synuclein.
FIGURE 3.
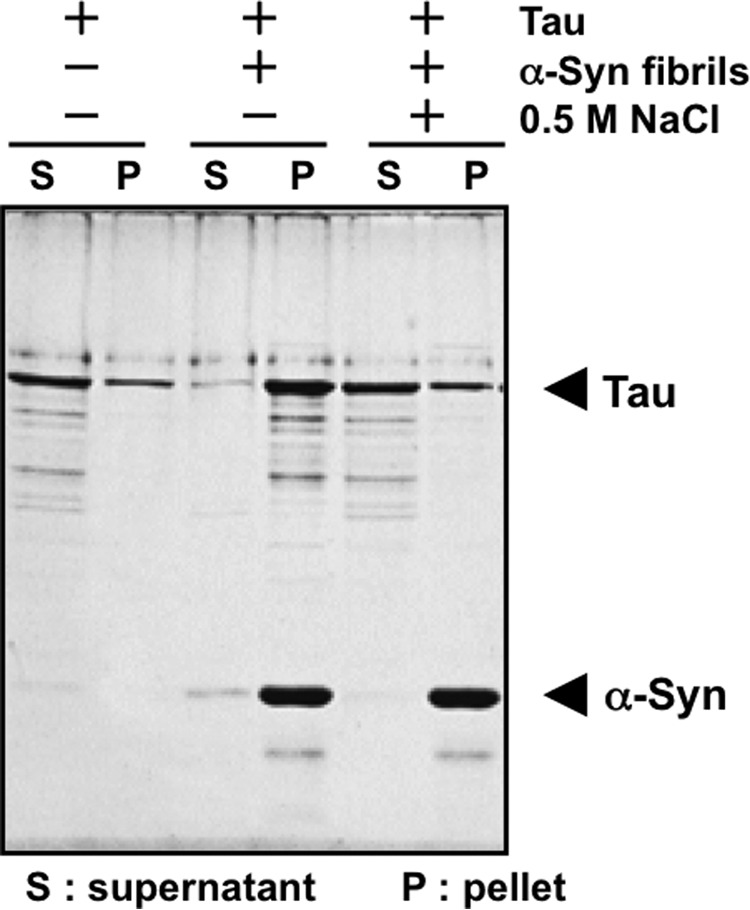
Evaluation of ionic interaction of Tau with α-synuclein fibrils by spin-down assay. Tau binding to α-synuclein fibrils was investigated by the spin-down technique, in the presence or absence of 0.5 m NaCl. The concentrations of α-synuclein and Tau used in this assay were 11.1 and 3.7 μm, respectively. α-Syn, α-synuclein.
Identification of Tau-binding Region in α-Synuclein Fibrils
Because Tau binds to tubulin or microtubules due to its positive charge, it is conceivable that the highly acidic C-terminal region of α-synuclein may be responsible for the binding to Tau. To determine which region of α-synuclein in the α-synuclein fibrils binds to Tau, we investigated the interaction of Tau with α-synuclein fibrils assembled with C-terminally truncated forms of α-synuclein (1–110, 1–120, and 1–130 α-synuclein), N-terminally truncated forms of α-synuclein (11–140), or full-length α-synuclein (WT). All truncated forms of α-synuclein assembled into fibrils similar to those formed by wild-type full-length α-synuclein (Fig. 4A). The binding of Tau to these fibrils was analyzed by spin-down assay (Fig. 4B). As shown in Fig. 4C, the ratios of fibril-bound Tau to Tau unbound to the fibrils was gradually reduced from 0.91 to 0.38 by successive deletion of 10 amino acid residues of the C-terminal region of α-synuclein (Fig. 4C), whereas no such difference was observed with N-terminally truncated α-synuclein fibrils 11–140 (data not shown). These results strongly suggest that Tau binds to α-synuclein fibrils at their highly acidic C-terminal region.
FIGURE 4.
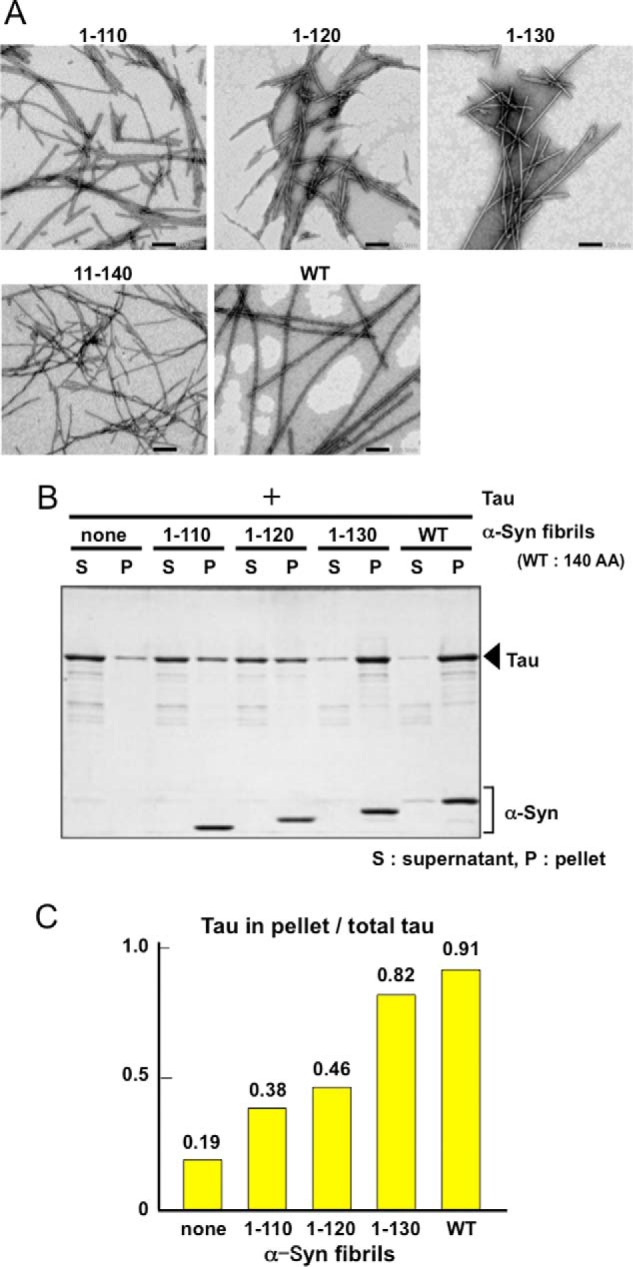
Interaction of Tau with full-length or C-terminally truncated α-synuclein fibrils. A, negative staining of electron microscopy of α-synuclein fibrils consisting of C-terminally truncated (1–110, 1–120, and 1–130), N-terminally truncated (11–140), and full-length wild-type α-synuclein (WT). B, α-synuclein (α-Syn) fibrils consisting of full-length wild-type or C-terminally truncated α-synuclein were incubated with Tau, and the binding was analyzed by means of spin-down assay. α-Synuclein fibrils of each type (22.1 μm) were mixed with an equal volume of 7.4 μm Tau and centrifuged. The fibril-bound Tau recovered in the pellet and the unbound Tau in the supernatant were analyzed by SDS-PAGE and Coomassie Brilliant Blue (CBB) staining. C, relative ratio of bound Tau in pellet to the total Tau is shown.
Interaction of Tau with α-Synuclein Fibrils Is Crucial for Inhibition of Tau-promoted Microtubule Assembly
To examine whether the interaction of Tau with α-synuclein fibrils actually inhibits Tau-promoted microtubule assembly, we examined microtubule assembly in the presence of α-synuclein fibrils assembled with C-terminally truncated or N-terminally truncated α-synuclein (Fig. 5). Although the N-terminally truncated form of α-synuclein(11–140) fibrils inhibited Tau-promoted microtubule assembly as effectively as wild-type α-synuclein fibrils, the C-terminally truncated form of α-synuclein(1–130) fibrils inhibited it only slightly (Fig. 5). These results indicated that the inhibition of Tau-promoted microtubule assembly was due to the interaction of Tau with the C-terminal region of α-synuclein fibrils, especially amino acid residues 131–140.
FIGURE 5.
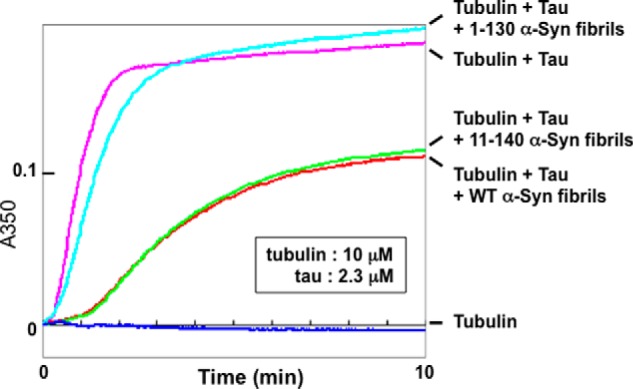
Effects of N- or C-terminally truncated α-synuclein fibrils on Tau-promoted microtubule assembly. Tubulin polymerization with Tau in the presence or absence of truncated α-synuclein fibrils was monitored in terms of turbidity. Blue, 10 μm tubulin only; pink, tubulin + 2.3 μm Tau; red, tubulin + Tau + 6.9 μm wild-type α-synuclein fibrils; light blue, tubulin + Tau + 6.9 μm 1–130 α-synuclein fibrils; green, tubulin + Tau + 6.9 μm 11–140 α-synuclein fibrils. Mean values of three separate experiments were plotted. Similar results were obtained in all three experiments.
Conformational Changes in α-Synuclein Fibrils and Protofibrils
To investigate the conformational difference between α-synuclein monomer and α-synuclein in the fibrils or protofibrils, we carried out dot blot assay using three different anti-α-synuclein antibodies (antibody 36, an antibody to the N terminus of α-synuclein; NAC1, an antibody to the mid-portion of α-synuclein; and Syn102, an antibody to the C terminus of α-synuclein) (Fig. 6A). Monomeric α-synuclein was hardly detectable with these three antibodies. In contrast, α-synuclein fibrils were strongly immunoreactive to antibodies to the N- or the C-terminal regions and were also positive to NAC1 (Fig. 6, C–E). These results are consistent with previous reports that the central half of the α-synuclein forms the core structure of the fibrils coated by the more accessible N- and C-terminal portions at the periphery (30, 31). The results are also in agreement with a previous report, in which monomeric α-synuclein assumes conformations stabilized by long range interactions between NAC (residues 61–95) and the C-terminal residues 110–130 and between the N-terminal region and nearby residue 120 (14). α-Synuclein protofibrils were equally well detected with all three antibodies, although the immunoreactivity to NAC1 was stronger than that of the fibrils (Fig. 6, C–E).
FIGURE 6.
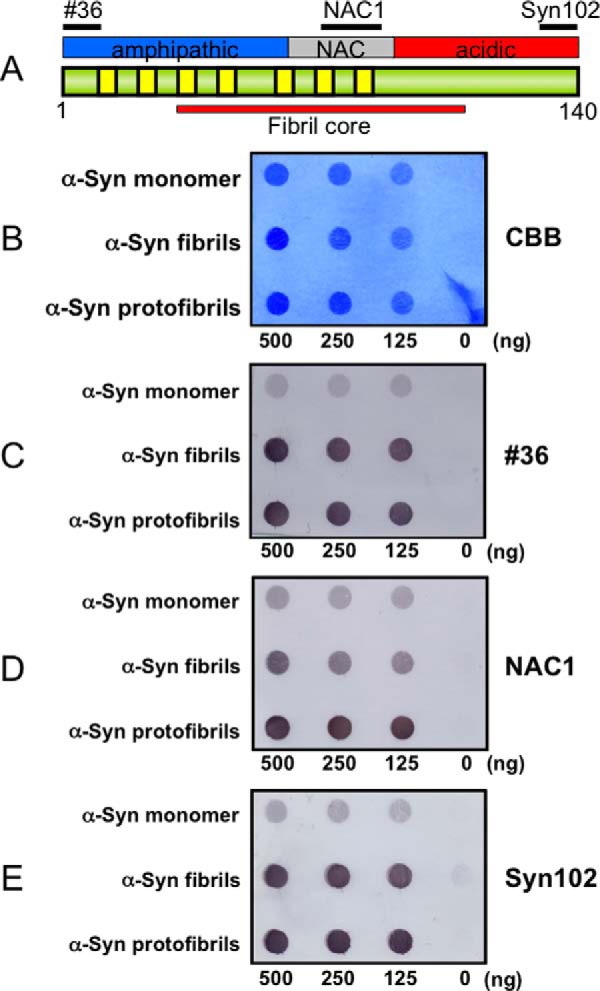
Dot blot assay of α-synuclein monomer, fibrils, and protofibirils with various anti-α-synuclein antibodies. A, schematic diagram of the domain structure of the α-synuclein (α-Syn) molecule. Epitopes of antibodies 36 (residues 1–10), NAC1 (residues 75–91), and Syn102 (residues 131–140) used in the dot blot assay are shown. B, Coomassie Brilliant Blue (CBB) staining of α-synuclein monomer, fibrils, and protofibrils dotted on a nitrocellulose membrane. C, immunodetection with an antibody to N terminus of α-synuclein (#36). D, immunodetection with an antibody to the mid-portion of α-synuclein (NAC1). E, immunodetection with an antibody to C terminus of α-synuclein (Syn102).
Identification of α-Synuclein Binding Region in Tau
Because the C-terminal region of α-synuclein is highly acidic, we considered that α-synuclein fibrils may bind to the basic region of Tau. To test this, we investigated the interaction of α-synuclein fibrils with various deletion mutants of Tau by means of a spin-down assay (Fig. 7A). The results are shown in Fig. 7, B and C. The reduction of binding of 4RTau(Δ2–163) was minimal compared with that of wild-type full-length 4RTau 412, suggesting very little contribution of the N-terminal region of Tau to the binding to α-synuclein fibrils. In contrast, the C-terminal deletion of Tau dramatically decreased the binding, as detected in particular with 4RTau 163 or 4RTau(Δ164–338). The binding affinities of 4RTau 163 and 4RTau(Δ164–338) were significantly lower than those of 4RTau 226 and Tau(Δ227–338), respectively, suggesting that residues 164–226 of Tau may play the greatest role in the binding of Tau to α-synuclein fibrils. Calculation of the ratio of basic amino acid residues to acidic residues in the deleted regions indicate that the 164–226-residue region is highly basic and support the idea that this region binds to the acidic C-terminal region of α-synuclein fibrils by ionic interaction (Fig. 7D).
FIGURE 7.
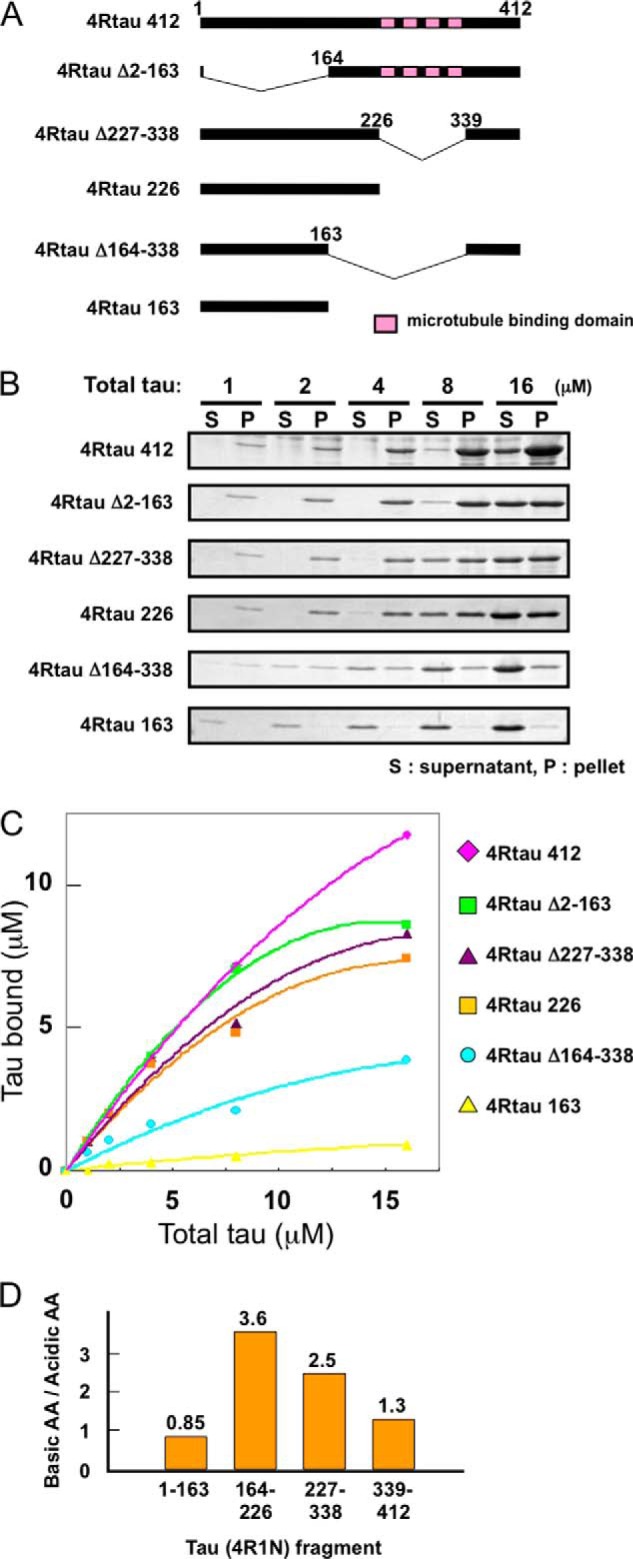
Analysis of the binding region of Tau to α-synuclein fibrils. A, schematic diagram of full-length 4-repeat Tau and the deletion constructs used in this study. Pink boxed regions represent microtubule binding repeats. B, Tau binding to α-synuclein fibrils was analyzed by the spin-down technique. 25 μm α-synuclein fibrils were mixed with various concentrations of wild-type or truncated Tau (final concentration of Tau is given in micromolars). After centrifugation, Tau bound to α-synuclein fibrils (recovered in the pellet) and Tau unbound to fibrils (recovered in supernatant) were analyzed by SDS-PAGE. C, amount of Tau bound to α-synuclein fibrils in each reaction was measured by densitometry and plotted versus total Tau used in each reaction. D, relative ratio of the number of basic amino acids (such as lysine) to that of acidic amino acids (such as glutamic acid) in Tau fragments (1–163, 164–226, 227–338, and 339–412) are shown.
Transduction of α-Synuclein Fibrils Induced Tau Aggregation Associated with α-Synuclein Aggregation in Cultured Cells
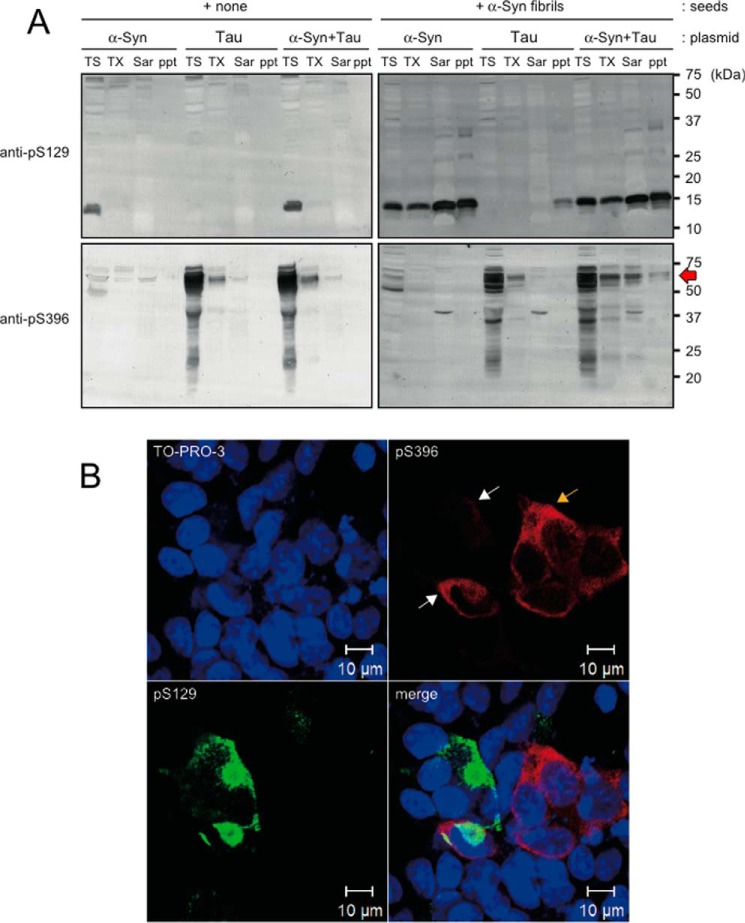
To investigate the effect of α-synuclein fibrils on Tau and microtubules in cultured cells, we transfected either Tau or α-synuclein or both into cultured cells and induced α-synuclein aggregation by treating with α-synuclein fibrils (32). First, we investigated whether there is any alteration in the free and polymerized tubulin in cells with or without α-synuclein aggregation by extracting with buffer containing taxol according to the protocol by Merrick et al. (33). However, there was no apparent difference in the ratio of free and polymerized tubulin (data not shown). We also investigated the co-localization of Tau, α-synuclein, and tubulin in cultured cells, with or without transduction of α-synuclein fibrils, by immunocytochemistry using antibodies to unphosphorylated Tau and α-synuclein. However, it was difficult to detect the differences in the localization of these proteins, because the antibody to unphosphorylated α-synuclein reacted with α-synuclein fibrils added extracellularly. In pathological conditions, aggregated Tau and α-synuclein are abnormally phosphorylated in cells, and phospho-specific antibodies are usually used to detect them sensitively in immunocytochemical or immunoblot analyses of diseased brains. Therefore, we investigated these cells with anti-phospho-α-synuclein antibody pS129 and anti-phospho-Tau antibody pS396 (Fig. 8). Immunoblot analysis of differentially extracted proteins with these antibodies revealed that phosphorylated Tau was accumulated in Sarkosyl-soluble and -insoluble fractions of cells expressing both Tau and α-synuclein after treatment with α-synuclein fibrils (Fig. 8A, indicated by red arrow). No such bands were detected in cells expressing only Tau or only α-synuclein even after treatment with α-synuclein fibrils (Fig. 8A). Immunocytochemical analysis also confirmed the partial co-localization of phospho-α-synuclein and phospho-Tau (Fig. 8B). These results suggest that α-synuclein aggregates also induce Tau aggregation if Tau is expressed in the cells sufficiently to permit self-aggregate formation. We also observed lower intensities of pS396 signals in cells with α-synuclein aggregation (white arrows), compared with those in cells without α-synuclein aggregation (yellow arrow), suggesting that bundling of Tau was inhibited by α-synuclein aggregates in cultured cells (Fig. 8B).
FIGURE 8.
Immunoblot and immunocytochemical analyses of Tau and α-synuclein in cultured cells treated with α-synuclein fibrils. A, immunoblot analysis of phospho-α-synuclein and Tau in cultured cells. Cells transfected with α-synuclein, Tau, or both were treated with or without α-synuclein fibrils, extracted sequentially with Tris saline (TS), 1% Triton-X (TX), and 1% Sarkosyl (Sar), fractionated to obtain the pellets (ppt), and immunoblotted with anti-phospho-α-synuclein (anti-pS129) and anti-phospho-Tau (anti-pS396). Tau accumulation was detected in the Sarkosyl-soluble and -insoluble fractions of cells expressing both Tau and α-synuclein after treatment with α-synuclein fibrils. B, immunocytochemistry of cells transfected with both Tau and α-synuclein after treatment with α-synuclein fibrils. Co-localization of phospho-α-synuclein and phospho-Tau in cultured cells is indicated by white arrows. Note that pS396 signal intensities were relatively low in cells having α-synuclein aggregates (white arrows), compared with those in cells without α-synuclein aggregation (yellow arrow).
Discussion
In this study, we have re-examined the microtubule polymerizing activity of α-synuclein, because it has been reported that the highly acidic C-terminal region of α-synuclein binds to tubulin and promotes microtubule assembly (29), which is in contrast to the cases of conventional microtubule-associated proteins such as Tau and MAP2 that bind to microtubules due to their positive charge. Contrary to the previous report, we found that α-synuclein did not promote microtubule assembly at concentrations ranging from 6.9 to 276 μm. It is clear that the tubulin and buffer conditions we used were appropriate for the assay, because recombinant Tau protein promoted microtubule assembly at a low concentration under our conditions. Because other experimental conditions, including preparation of recombinant protein from Escherichia coli, are also similar to those of the previous report, it is puzzling why their α-synuclein apparently showed activity. It is conceivable that some contaminants or aggregated α-synuclein present in their α-synuclein preparation may have promoted microtubule assembly or caused a turbidity change in the reaction mixture.
The cytotoxicity of α-synuclein fibrils and protofibrils remains controversial in relation to their roles in neurodegeneration. In this study, we have demonstrated that α-synuclein fibrils inhibited Tau-promoted microtubule assembly through an ionic interaction with Tau in vitro. Although α-synuclein protofibrils also inhibited Tau-promoted microtubule assembly, their inhibitory effect was weaker than that of α-synuclein fibrils. These data suggest that the fibrous or filamentous form of α-synuclein may be more toxic in neuronal cells, because they affected Tau-promoted microtubule assembly or stabilization more strongly than did protofibrils. It has been demonstrated that the N- and C-terminal regions of α-synuclein in fibrils are sensitive to protease and may be exposed outside the core structure of the fibrils (30). This idea is supported by our findings in dot blot assay, in which α-synuclein fibrils were highly immunoreactive to antibodies directed toward the N- and C-terminal regions of α-synuclein (Fig. 6). Because the C-terminal region of α-synuclein is highly acidic, it may have strong affinity for positively charged proteins or peptides and may contribute to pathogenic interactions with other molecules. Indeed, many proteins, such as proteasomal protein S6′, a subunit of the 19S cap, subunits of 20S proteasome particles, transcriptional regulator HMGB-1 (34), MAP1B (35), etc., have been reported to interact with α-synuclein fibrils in vitro. These abnormal interactions may cause dysfunction of these molecules, leading to impairment of neurons in PD or DLB brains. In this study, we found that α-synuclein fibrils bind to Tau through an ionic interaction and inhibit Tau-promoted microtubule assembly. It is possible that direct interaction of α-synuclein fibrils with tubulin may also have an inhibitory effect on microtubule assembly to some extent. α-Synuclein protofibrils showed high immunoreactivity to all three antibodies covering regions from the N to C terminus of α-synuclein in dot blot assay on the nitrocellulose membrane, which is similar but not identical to the case of the fibrils. In this study, although α-synuclein protofibrils slightly inhibited microtubule assembly, the inhibitory mechanism may be different from that of α-synuclein fibrils, based on the slight difference of reactivity with NAC1 antibody, which suggests the existence of a structural difference. The monomeric form of α-synuclein was less immunoreactive to all three antibodies than α-synuclein fibrils or protofibrils in the dot blot assay. This may be due to the specific structural character of monomeric α-synuclein, which has been shown to be compactly stabilized by intramolecular interactions, including the N- and C-terminal regions (14).
Tau is a major neuronal microtubule-associated protein localized mostly in axons. Tau directly binds to microtubules and regulates microtubule dynamics. The expression of Tau is regulated by alternative splicing, leading to the expression of either 3-repeat (3R) or 4-repeat (4R) Tau, and Tau function is regulated by phosphorylation. In this study, we showed that α-synuclein fibrils bind to Tau in vitro and inhibit microtubule assembly. It has been shown that Tau-deficient mice exhibit normal axonal elongation, except for a certain type of axon, suggesting compensation for at least some of the physiological functions of Tau by other MAPs. Many exonic and intronic mutations in the Tau gene MAPT are associated with frontotemporal dementia and parkinsonisms linked to chromosome 17 (FTDP-17), and the pathogenesis of FTDP-17 is linked to increased fibrillization, partial loss of function and alteration in the ratio of 3R Tau/4R Tau.
It is known that Tau pathologies in DLB and PD are often accompanied with α-synuclein pathologies (36, 37). Furthermore, co-localization of Tau and α-synuclein in the olfactory bulb neurons and neurites, where the severity of Tau pathology correlated with α-synuclein pathology, has been shown in AD with amygdala LBs (38), and in the restricted area that is vulnerable to both α-synuclein and Tau pathology in the brains of familial PD with A53T mutation (39). Because only a minority of LBs was recognized by Tau antibodies, co-aggregation of filamentous α-synuclein and Tau may not be crucial for the formation of LBs or for the pathogenesis of PD. However, it has been shown that α-synuclein induces fibrillization of Tau and that co-incubation of Tau and α-synuclein synergistically promotes mutual fibrillization in vitro (40, 41). We also showed in this study that α-synuclein fibrils induced pathological Tau aggregation in cultured cells expressing both Tau and α-synuclein, when α-synuclein fibrils were introduced into the cells with transfection reagent. Furthermore, dot-like phospho-Tau pathologies are induced in wild-type mouse brains inoculated with α-synuclein fibrils (42). Although further studies will be needed to define the interaction of Tau with α-synuclein fibrils in vivo, α-synuclein and Tau may interact with each other through an ionic interaction of immature α-synuclein or Tau fibrils at the initial phage or the early stage of fibrillization (Fig. 9).
FIGURE 9.
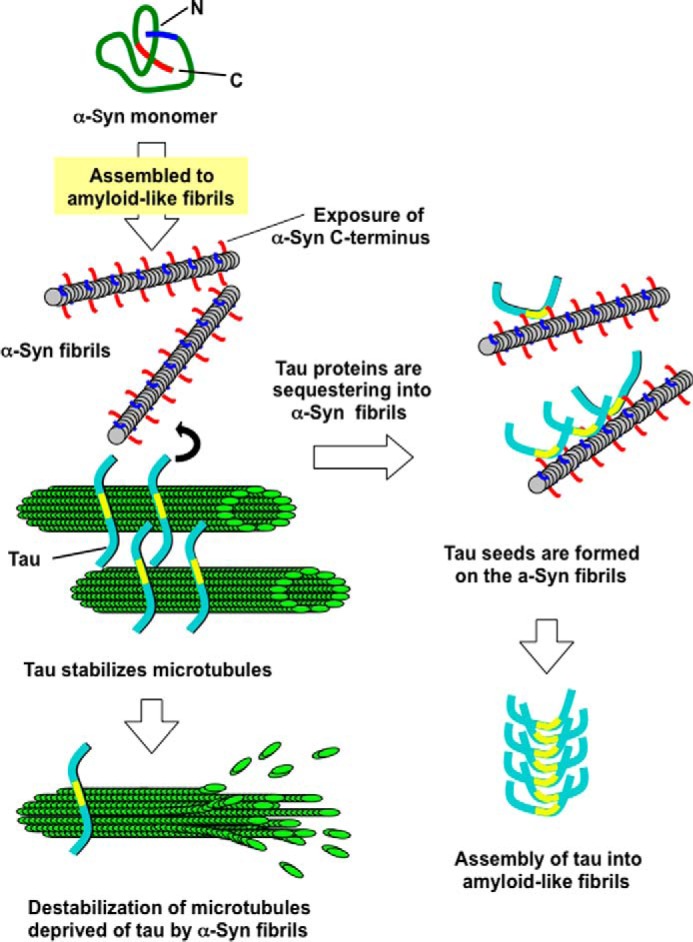
Schematic diagram of inhibition of Tau-promoted microtubule assembly by α-synuclein fibrils and Tau aggregation accompanying α-synuclein aggregation. α-Synuclein monomer assembles into fibrils, or intermediate oligomers, protofibrils, under certain conditions. α-Synuclein fibrils interact with Tau, which stabilizes microtubules, and deprive microtubules of Tau by binding to Tau. This causes destabilization or disruption of microtubules, resulting in neuronal dysfunction. Tau proteins sequestered into α-synuclein fibrils undergo conformational change and form seeds for assembly of Tau into amyloid-like fibrils.
In this study, we have demonstrated that α-synuclein fibrils bind to Tau in vitro. It is reasonable to speculate that α-synuclein fibrils may also bind to other MAPs, because conventional microtubule-associated proteins in neuronal cells have similar microtubule-binding domains and bind to tubulin or microtubules through their positive charge. Indeed, it has been reported that MAP2 exists in neuronal nuclei and LBs in substantia nigra in PD, and MAP1B exists in cortical and brainstem-type LBs. Considering these observations, levels of normally functioning MAPs may be reduced in neurons of affected regions in the presence of filamentous α-synuclein. This may lead to disruption of the microtubule network and neuronal death. Because the specialized function and morphology of neuronal cells is maintained by their cytoskeletons, especially the microtubules, and these cells are not renewed, it is not surprising that neuronal cells are highly vulnerable to disruption of the microtubule network. Microtubules also play an important role in transport of mitochondria, vesicles, organelles, or molecules, acting as rails on which motor proteins move. Therefore, disruption of microtubules in axons may lead to axonal impairment followed by neurodegeneration. Indeed, several reviews support the idea that impairment of axonal transport causes various neurodegenerative diseases (43–46). Furthermore, it has been shown that dopaminergic neurons are selectively vulnerable to microtubule depolymerization (47). Overall, it seems plausible that α-synuclein fibrils bind to MAPs and inhibit their function to assemble and stabilize microtubules, leading to disruption of microtubules (Fig. 9). This putative pathogenic mechanism may be related to neurodegeneration in PD, which exhibits selective dopaminergic neuronal loss.
Protein aggregation and the appearance of inclusion bodies in neurons or glial cells are common neuropathological features of many neurodegenerative diseases, including PD, AD, amyotrophic lateral sclerosis, and frontotemporal lobar degeneration. Although it is still a matter of debate whether these aggregations or inclusion bodies are cytotoxic, cytoprotective, or an incidental event, recent studies have suggested that the major abnormal protein components of these intracytoplasmic inclusion bodies, such as α-synuclein, Tau, and TDP-43, have prion-like properties and can propagate from cell to cell, resulting in neuronal dysfunctions and progressive neurodegeneration. In fact, the distributions and spreading of these intracellular abnormal proteins have been shown to closely correlate with disease progression (48–51). Abnormal interaction of Tau with α-synuclein resulting from conformational change of α-synuclein may induce not only dysfunctions of both Tau and α-synuclein but also production of abnormal Tau protein and its propagation. These synergistic pathological pathways may accelerate neuronal dysfunction.
In this study, we showed that α-synuclein fibrils interact strongly with Tau through the C-terminal acidic regions and affect microtubule assembly and stabilization. No such abnormal activity was seen with the monomeric form of α-synuclein. This gain of toxic function is considered to be due to the structural features of α-synuclein fibrils. By dot blot assay using three different site-specific antibodies to α-synuclein, we have shown that α-synuclein fibrils have a distinct conformation in which the N- and C-terminal regions are exposed. Therefore, we consider that the conversion of the monomeric form to the filamentous form is the central event in the appearance of abnormal function of α-synuclein, which may be responsible for the cytotoxicity. Thus, inhibitors of fibril formation could be potential candidates for therapeutic intervention, for example in PD. It has been shown that compounds belonging to several chemical classes inhibit filament formation of α-synuclein, as well as Tau or Aβ, and dimers or oligomers formed in the presence of these inhibitory compounds were non-toxic in SH-SY5Y cells. In addition, it has been shown that oligomeric intermediates of α-synuclein are degraded via the lysosomal degradation pathway in differentiated SH-SY5Y cells and primary culture of rat cortical neurons. Moreover, several compounds have been shown to bind to the C-terminal region of α-synuclein and inhibit filament formation (52), raising the possibility that the reactivity of the C-terminal region is attenuated by binding to these compounds. Such inhibitory compounds may contribute to both inhibition of filament formation and suppression of cytotoxicity of α-synuclein fibrils (53). Further studies of this approach may open up new possibilities for the treatment of PD.
Experimental Procedures
Expression and Purification of Recombinant Wild-type, C-terminally Truncated, and N-terminally Truncated Human α-Synuclein
Wild-type, C-terminally truncated (residues 1–130, 1–120, and 1–110), and N-terminally truncated (residues 11–140) α-synuclein were subcloned into pRK172 and expressed in E. coli BL21 (DE3) as described (54). For purification of wild-type, 1–130, and 11–140 α-synuclein, bacterial pellets were sonicated for 45 s twice in extraction buffer 1 (50 mm Tris-HCl, pH 7.5, 1 mm EGTA, 1 mm DTT) on ice. For 1–120 and 1–110 α-synuclein, bacterial pellets were sonicated for 45 s twice in extraction buffer 2 (50 mm MES, pH 6.25, 1 mm EGTA, 1 mm DTT) on ice. The homogenates were centrifuged at 16,000 × g for 15 min, and the supernatants were boiled for 5 min. Then, the supernatants (heat-stable fraction) of a 15-min centrifugation at 16,000 × g were subjected to anion-exchange (for wild-type, 1–130, and 11–140) or cation-exchange (for 1–120 and 1–110) chromatography on a column (Q-Sepharose or SP-Sepharose fast flow, respectively; Amersham Biosciences) equilibrated with extraction buffer. The columns were washed with extraction buffer and eluted with extraction buffer containing 0.35 m NaCl. The eluates were concentrated by precipitation with 50% saturated ammonium sulfate. The pellets were resuspended in and dialyzed against 30 mm Tris-HCl, pH 7.5. The protein concentrations were determined by comparing the absorbance at 215 nm of samples with that of α-synuclein of known concentration on reverse phase HPLC, using an Aquapore RP300 column.
Expression and Purification of Recombinant Wild-type and Deletion Mutants of Human Tau
Wild-type human 4RTau (412 amino acid residues) and the deletion mutants (1–163, 1–226, Δ227–338, and Δ164–338 Tau; numbering based on the 441 Tau isoform) were subcloned into pRK172 and expressed in E. coli BL21 (DE3). For purification of wild-type and Δ2–163 Tau, bacterial pellets were sonicated for 45 s twice in extraction buffer C (50 mm PIPES, pH 6.8, 5 mm EGTA, 1 mm DTT, 0.5 mm PMSF) on ice. For purification of 1–163, 1–226, Δ227–338, and Δ164–338 Tau, bacterial pellets were sonicated for 45 s twice in extraction buffer A on ice. The homogenates were centrifuged at 16,000 × g for 15 min, and the supernatants were boiled for 5 min. The supernatants (heat-stable fraction) of a 15-min centrifugation at 16,000 × g were subjected to cation-exchange (for wild type and Δ2–163 Tau) or anion-exchange (for 1–163, 1–226, Δ227–338, and Δ164–338) chromatography on columns equilibrated with extraction buffer. The columns were washed with extraction buffer and eluted with extraction buffer containing 0.35 m NaCl. The eluates were concentrated by precipitation with 50% saturated ammonium sulfate. The pellets were resuspended in and dialyzed against 30 mm Tris-HCl, pH 7.5.
Preparation of α-Synuclein Fibrils and Protofibrils
α-Synuclein fibrils were prepared as follows. Recombinant α-synuclein was incubated in 30 mm Tris-HCl, pH 7.5, containing 0.1% NaN3, with shaking at 37 °C for 2 or 3 days. The assembled α-synuclein was ultracentrifuged at 255,000 × g for 20 min at 25 °C. The resultant pellet was resuspended and sonicated in 30 mm Tris-HCl, pH 7.5, on ice and ultracentrifuged again. Then the pellet was resuspended and sonicated in 30 mm Tris-HCl, pH 7.5, on ice, and used as the α-synuclein fibril preparation. For electron microscopy, the fibrils were placed on collodion-coated 300-mesh copper grids and stained with 2% (v/v) phosphotungstate. Micrographs were recorded on a JEOL 1200EX electron microscope.
α-Synuclein protofibrils were prepared as described with slight modifications (55). Briefly, 4 mg of recombinant α-synuclein was suspended in 20 mm NH4HCO3. The solution was lyophilized and dissolved in 200 μl of 30 mm Tris-HCl, pH 7.5. The solution was cleared by centrifugation at 16,000 × g, for 5 min at 4 °C and then subjected to a Superdex 200 gel-filtration chromatography (Amersham Biosciences) on a column equilibrated in 30 mm Tris-HCl, pH 7.5, at a flow rate of 0.4 ml/min. The separated proteins were monitored at 215 nm, and the void volume fraction was collected. The fraction was analyzed by SDS-PAGE and used as α-synuclein protofibrils. The concentrations of α-synuclein fibrils and protofibrils were determined as described above after solubilization in 6 m guanidine hydrochloride.
In Vitro Microtubule Assembly Assay
Tubulin was purified from porcine brains by three cycles of polymerization-depolymerization followed by removal of co-purified proteins on an SP-Sepharose column as described, with some modifications (56). Tubulin concentration was determined by measuring the absorbance at 280 nm. To investigate the effect of α-synuclein on microtubule assembly, recombinant α-synuclein at various concentrations was incubated with tubulin (10 μm) in assembly buffer (80 mm PIPES, pH 6.9, 1 mm EGTA, 0.2 mm MgCl2, 1 mm DTT, 1 mm GTP) at 37 °C. As a positive control, recombinant Tau (4-repeat, 412-amino acid isoform of human Tau, 2.3 μm) was incubated with tubulin at 37 °C as described (57). Polymerization of tubulin was monitored continuously by measuring the turbidity at 350 nm with a spectrophotometer (UV-1600 PC, Shimadzu Co). To investigate the effects of α-synuclein (monomer, fibrils, or protofibrils) on Tau-promoted microtubule assembly, tubulin was incubated with Tau in the presence or absence of α-synuclein (monomer, fibrils, or protofibrils). α-Synuclein was pre-incubated with Tau before incubation with tubulin. The assembly reaction was carried out by incubating tubulin with a mixture of recombinant Tau and α-synuclein at 37 °C.
Dot Blot and Overlay Assay
α-Synuclein monomer, fibrils, and protofibrils were spotted onto nitrocellulose membrane. To detect the total proteins spotted, the membrane was stained with Coomassie Brilliant Blue. To investigate the conformational changes in α-synuclein fibrils or protofibrils, the degrees of immunoreactivity to three different anti-α-synuclein antibodies (antibody 36, an antibody raised against the N-terminal residues 1–10 of α-synuclein (30); NAC1, an antibody raised against residues 75–91 of α-synuclein (31); and Syn102, an antibody that recognizes the C-terminal residues 131–140 of α-synuclein) were analyzed as described (24, 31). Immunoreactivity was visualized using the avidin-biotin detection system (Vector Laboratories). For Tau overlay assay, the membrane was blocked with PBS containing 3% gelatin and overlaid with 0.6 μm Tau in PBS containing 10% fetal bovine serum (FBS) for 1.5 h and then washed twice with PBS. The Tau bound to α-synuclein was detected with a monoclonal anti-Tau antibody (T46) using the avidin-biotin detection system.
Analysis of Tau Bound to α-Synuclein Fibrils by Spin-down Technique
α-Synuclein fibrils were incubated with an equal volume of various concentrations of Tau in 30 mm Tris-HCl, pH 7.5, for 10 min at room temperature, and the mixtures were ultracentrifuged at 453,000 × g for 20 min at 25 °C. The resultant pellets containing α-synuclein fibrils were resuspended in the buffer (equal volume to that of the supernatant). The pellets and the supernatants (containing the fibril-bound Tau and the fibril-free Tau, respectively) were subjected to SDS-PAGE and stained with Coomassie Brilliant Blue. The stained areas were measured with ImageJ software (National Institutes of Health).
Effect of α-Synuclein Fibrils on Tau Aggregation in Cultured Cells
Wild-type human 4R1N-Tau and/or wild-type human α-synuclein were transiently overexpressed in SH-SY5Y cells by transfection of the plasmid with X-tremeGENE 9 (Roche Applied Science), followed by culture for 14 h. α-Synuclein fibrils or the monomer were introduced into the cells with Multifectam (Promega) as described previously (36). After incubation for 6 h, the medium was changed, and culture was continued for 2–3 days. Cells were harvested, and the cellular proteins were differentially extracted and immunoblotted with the indicated antibodies, as described (32).
Author Contributions
M. H. and T. O. designed the research and wrote the manuscript. T. O. performed most of the biochemical experiments. T. N. provided key reagents and conducted the experiments of cellular models. M. T. performed negative staining electron microscopy. T. O., S. H., T. N., A. T., and M. H. analyzed the data.
Acknowledgment
We thank Dr. Michel Goedert for the plasmids encoding Tau and α-synuclein.
This work was supported by Ministry of Education, Culture, Sports, Science and Technology KAKENHI Grants 26117005 and 15H02356 (to M. H.) and 26111730 (to T. N.), Japan Society for the Promotion of Science KAKENHI Grant 23228004 (to M. H.), Ministry of Health, Labor, and Welfare of Japan Grant 13800916 (to M. H.), grant-in-aid for research on rare and intractable diseases, the Research Committee on Establishment of Novel Treatments for Amyotrophic Lateral Sclerosis, and the Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and Development, AMED (to M. H.). The authors declare that they have no conflicts of interest with the contents of this article.
- PD
- Parkinson disease
- ALS
- amyotrophic lateral sclerosis
- AD
- Alzheimer disease
- LB
- Lewy body
- DLB
- dementia with LB
- NAC
- non-amyloid β component
- MAP
- microtubule-associated protein.
References
- 1. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., et al. (1997) Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 2. Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., and Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 3. Spillantini M. G., Crowther R. A., Jakes R., Cairns N. J., Lantos P. L., and Goedert M. (1998) Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson's disease and dementia with Lewy bodies. Neurosci. Lett. 251, 205–208 [DOI] [PubMed] [Google Scholar]
- 4. Wakabayashi K., Hayashi S., Kakita A., Yamada M., Toyoshima Y., Yoshimoto M., and Takahashi H. (1998) Accumulation of α-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol. 96, 445–452 [DOI] [PubMed] [Google Scholar]
- 5. Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J. T., Schöls L., and Riess O. (1998) Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat. Genet. 18, 106–108 [DOI] [PubMed] [Google Scholar]
- 6. Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D. G., and de Yebenes J. G. (2004) The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 7. Appel-Cresswell S., Vilarino-Guell C., Encarnacion M., Sherman H., Yu I., Shah B., Weir D., Thompson C., Szu-Tu C., Trinh J., Aasly J. O., Rajput A., Rajput A. H., Jon Stoessl A., and Farrer M. J. (2013) α-Synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Mov. Disord. 28, 811–813 [DOI] [PubMed] [Google Scholar]
- 8. Lesage S., Anheim M., Letournel F., Bousset L., Honoré A., Rozas N., Pieri L., Madiona K., Dürr A., Melki R., Verny C., Brice A., and French Parkinson's Disease Genetics Study Group. (2013) G51D α-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann. Neurol. 73, 459–471 [DOI] [PubMed] [Google Scholar]
- 9. Pasanen P., Myllykangas L., Siitonen M., Raunio A., Kaakkola S., Lyytinen J., Tienari P. J., Pöyhönen M., and Paetau A. (2014) Novel α-synuclein mutation A53E associated with atypical multiple system atrophy and Parkinson's disease-type pathology. Neurobiol. Aging 35, 2180. [DOI] [PubMed] [Google Scholar]
- 10. Maroteaux L., Campanelli J. T., and Scheller R. H. (1988) Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 8, 2804–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Goedert M. (2001) α-Synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2, 492–501 [DOI] [PubMed] [Google Scholar]
- 12. Weinreb P. H., Zhen W., Poon A. W., Conway K. A., and Lansbury P. T. Jr. (1996) NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry 35, 13709–13715 [DOI] [PubMed] [Google Scholar]
- 13. Bertoncini C. W., Fernandez C. O., Griesinger C., Jovin T. M., and Zweckstetter M. (2005) Familial mutants of α-synuclein with increased neurotoxicity have a destabilized conformation. J. Biol. Chem. 280, 30649–30652 [DOI] [PubMed] [Google Scholar]
- 14. Bertoncini C. W., Jung Y. S., Fernandez C. O., Hoyer W., Griesinger C., Jovin T. M., and Zweckstetter M. (2005) Release of long-range tertiary interactions potentiates aggregation of natively unstructured α-synuclein. Proc. Natl. Acad. Sci. U.S.A. 102, 1430–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davidson W. S., Jonas A., Clayton D. F., and George J. M. (1998) Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273, 9443–9449 [DOI] [PubMed] [Google Scholar]
- 16. George J. M., Jin H., Woods W. S., and Clayton D. F. (1995) Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 15, 361–372 [DOI] [PubMed] [Google Scholar]
- 17. Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W. H., Castillo P. E., Shinsky N., Verdugo J. M., Armanini M., Ryan A., Hynes M., Phillips H., Sulzer D., and Rosenthal A. (2000) Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252 [DOI] [PubMed] [Google Scholar]
- 18. Lee F. J., Liu F., Pristupa Z. B., and Niznik H. B. (2001) Direct binding and functional coupling of α-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J. 15, 916–926 [DOI] [PubMed] [Google Scholar]
- 19. Crowther R. A., Jakes R., Spillantini M. G., and Goedert M. (1998) Synthetic filaments assembled from C-terminally truncated α-synuclein. FEBS Lett. 436, 309–312 [DOI] [PubMed] [Google Scholar]
- 20. Serpell L. C., Berriman J., Jakes R., Goedert M., and Crowther R. A. (2000) Fiber diffraction of synthetic α-synuclein filaments shows amyloid-like cross-β conformation. Proc. Natl. Acad. Sci. U.S.A. 97, 4897–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Conway K. A., Harper J. D., and Lansbury P. T. (1998) Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat. Med. 4, 1318–1320 [DOI] [PubMed] [Google Scholar]
- 22. Choi W., Zibaee S., Jakes R., Serpell L. C., Davletov B., Crowther R. A., and Goedert M. (2004) Mutation E46K increases phospholipid binding and assembly into filaments of human α-synuclein. FEBS Lett. 576, 363–368 [DOI] [PubMed] [Google Scholar]
- 23. Conway K. A., Lee S. J., Rochet J. C., Ding T. T., Harper J. D., Williamson R. E., and Lansbury P. T. Jr. (2000) Accelerated oligomerization by Parkinson's disease linked α-synuclein mutants. Ann. N.Y. Acad. Sci. 920, 42–45 [DOI] [PubMed] [Google Scholar]
- 24. Yonetani M., Nonaka T., Masuda M., Inukai Y., Oikawa T., Hisanaga S., and Hasegawa M. (2009) Conversion of wild-type α-synuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. J. Biol. Chem. 284, 7940–7950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M. S., Shen J., Takio K., and Iwatsubo T. (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 [DOI] [PubMed] [Google Scholar]
- 26. Hasegawa M., Fujiwara H., Nonaka T., Wakabayashi K., Takahashi H., Lee V. M., Trojanowski J. Q., Mann D., and Iwatsubo T. (2002) Phosphorylated α-synuclein is ubiquitinated in α-synucleinopathy lesions. J. Biol. Chem. 277, 49071–49076 [DOI] [PubMed] [Google Scholar]
- 27. Nonaka T., Iwatsubo T., and Hasegawa M. (2005) Ubiquitination of α-synuclein. Biochemistry 44, 361–368 [DOI] [PubMed] [Google Scholar]
- 28. Anderson J. P., Walker D. E., Goldstein J. M., de Laat R., Banducci K., Caccavello R. J., Barbour R., Huang J., Kling K., Lee M., Diep L., Keim P. S., Shen X., Chataway T., Schlossmacher M. G., et al. (2006) Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic Lewy body disease. J. Biol. Chem. 281, 29739–29752 [DOI] [PubMed] [Google Scholar]
- 29. Alim M. A., Ma Q. L., Takeda K., Aizawa T., Matsubara M., Nakamura M., Asada A., Saito T., Kaji H., Yoshii M., Hisanaga S., and Uéda K. (2004) Demonstration of a role for α-synuclein as a functional microtubule-associated protein. J. Alzheimers Dis. 6, 435–442 [DOI] [PubMed] [Google Scholar]
- 30. Miake H., Mizusawa H., Iwatsubo T., and Hasegawa M. (2002) Biochemical characterization of the core structure of α-synuclein filaments. J. Biol. Chem. 277, 19213–19219 [DOI] [PubMed] [Google Scholar]
- 31. Masuda M., Hasegawa M., Nonaka T., Oikawa T., Yonetani M., Yamaguchi Y., Kato K., Hisanaga S., and Goedert M. (2009) Inhibition of α-synuclein fibril assembly by small molecules: analysis using epitope-specific antibodies. FEBS Lett. 583, 787–791 [DOI] [PubMed] [Google Scholar]
- 32. Nonaka T., Watanabe S. T., Iwatsubo T., and Hasegawa M. (2010) Seeded aggregation and toxicity of α-synuclein and τ: cellular models of neurodegenerative diseases. J. Biol. Chem. 285, 34885–34898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merrick S. E., Trojanowski J. Q., and Lee V. M. (1997) Selective destruction of stable microtubules and axons by inhibitors of protein serine/threonine phosphatases in cultured human neurons. J. Neurosci. 17, 5726–5737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lindersson E. K., Højrup P., Gai W. P., Locker D., Martin D., and Jensen P. H. (2004) α-Synuclein filaments bind the transcriptional regulator HMGB-1. Neuroreport 15, 2735–2739 [PubMed] [Google Scholar]
- 35. Jensen P. H., Islam K., Kenney J., Nielsen M. S., Power J., and Gai W. P. (2000) Microtubule-associated protein 1B is a component of cortical Lewy bodies and binds α-synuclein filaments. J. Biol. Chem. 275, 21500–21507 [DOI] [PubMed] [Google Scholar]
- 36. Lee V. M., Giasson B. I., and Trojanowski J. Q. (2004) More than just two peas in a pod: common amyloidogenic properties of tau and α-synuclein in neurodegenerative diseases. Trends Neurosci. 27, 129–134 [DOI] [PubMed] [Google Scholar]
- 37. Colom-Cadena M., Gelpi E., Charif S., Belbin O., Blesa R., Martí M. J., Clarimón J., and Lleó A. (2013) Confluence of α-synuclein, τ, and β-amyloid pathologies in dementia with Lewy bodies. J. Neuropathol. Exp. Neurol. 72, 1203–1212 [DOI] [PubMed] [Google Scholar]
- 38. Fujishiro H., Tsuboi Y., Lin W. L., Uchikado H., and Dickson D. W. (2008) Co-localization of tau and α-synuclein in the olfactory bulb in Alzheimer's disease with amygdala Lewy bodies. Acta Neuropathol. 116, 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duda J. E., Giasson B. I., Mabon M. E., Miller D. C., Golbe L. I., Lee V. M., and Trojanowski J. Q. (2002) Concurrence of α-synuclein and τ brain pathology in the Contursi kindred. Acta Neuropathol. 104, 7–11 [DOI] [PubMed] [Google Scholar]
- 40. Giasson B. I., Forman M. S., Higuchi M., Golbe L. I., Graves C. L., Kotzbauer P. T., Trojanowski J. Q., and Lee V. M. (2003) Initiation and synergistic fibrillization of τ and α-synuclein. Science 300, 636–640 [DOI] [PubMed] [Google Scholar]
- 41. Waxman E. A., and Giasson B. I. (2011) Induction of intracellular τ aggregation is promoted by α-synuclein seeds and provides novel insights into the hyperphosphorylation of τ. J. Neurosci. 31, 7604–7618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Masuda-Suzukake M., Nonaka T., Hosokawa M., Kubo M., Shimozawa A., Akiyama H., and Hasegawa M. (2014) Pathological α-synuclein propagates through neural networks. Acta Neuropathol. Commun. 2, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Vos K. J., Grierson A. J., Ackerley S., and Miller C. C. (2008) Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 31, 151–173 [DOI] [PubMed] [Google Scholar]
- 44. Feany M. B., and La Spada A. R. (2003) Polyglutamines stop traffic: axonal transport as a common target in neurodegenerative diseases. Neuron 40, 1–2 [DOI] [PubMed] [Google Scholar]
- 45. Hinckelmann M. V., Zala D., and Saudou F. (2013) Releasing the brake: restoring fast axonal transport in neurodegenerative disorders. Trends Cell Biol. 23, 634–643 [DOI] [PubMed] [Google Scholar]
- 46. Millecamps S., and Julien J. P. (2013) Axonal transport deficits and neurodegenerative diseases. Nat. Rev. Neurosci. 14, 161–176 [DOI] [PubMed] [Google Scholar]
- 47. Ren Y., Liu W., Jiang H., Jiang Q., and Feng J. (2005) Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J. Biol. Chem. 280, 34105–34112 [DOI] [PubMed] [Google Scholar]
- 48. Braak H., and Braak E. (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 [DOI] [PubMed] [Google Scholar]
- 49. Del Tredici K., and Braak H. (2016) Review: sporadic Parkinson's disease: development and distribution of α-synuclein pathology. Neuropathol. Appl. Neurobiol. 42, 33–50 [DOI] [PubMed] [Google Scholar]
- 50. Saito Y., Kawashima A., Ruberu N. N., Fujiwara H., Koyama S., Sawabe M., Arai T., Nagura H., Yamanouchi H., Hasegawa M., Iwatsubo T., and Murayama S. (2003) Accumulation of phosphorylated α-synuclein in aging human brain. J. Neuropathol. Exp. Neurol. 62, 644–654 [DOI] [PubMed] [Google Scholar]
- 51. Saito Y., Ruberu N. N., Sawabe M., Arai T., Tanaka N., Kakuta Y., Yamanouchi H., and Murayama S. (2004) Staging of argyrophilic grains: an age-associated tauopathy. J. Neuropathol. Exp. Neurol. 63, 911–918 [DOI] [PubMed] [Google Scholar]
- 52. Masuda M., Suzuki N., Taniguchi S., Oikawa T., Nonaka T., Iwatsubo T., Hisanaga S., Goedert M., and Hasegawa M. (2006) Small molecule inhibitors of α-synuclein filament assembly. Biochemistry 45, 6085–6094 [DOI] [PubMed] [Google Scholar]
- 53. Lee H. J., Khoshaghideh F., Patel S., and Lee S. J. (2004) Clearance of α-synuclein oligomeric intermediates via the lysosomal degradation pathway. J. Neurosci. 24, 1888–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Masuda M., Dohmae N., Nonaka T., Oikawa T., Hisanaga S., Goedert M., and Hasegawa M. (2006) Cysteine misincorporation in bacterially expressed human α-synuclein. FEBS Lett. 580, 1775–1779 [DOI] [PubMed] [Google Scholar]
- 55. Ding T. T., Lee S. J., Rochet J. C., and Lansbury P. T. Jr. (2002) Annular α-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry 41, 10209–10217 [DOI] [PubMed] [Google Scholar]
- 56. Weisenberg R. C., and Cianci C. (1984) ATP-induced gelation–contraction of microtubules assembled in vitro. J. Cell Biol. 99, 1527–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hasegawa M., Smith M. J., and Goedert M. (1998) Tau proteins with FTDP-17 mutations have a reduced ability to promote microtubule assembly. FEBS Lett. 437, 207–210 [DOI] [PubMed] [Google Scholar]