Abstract
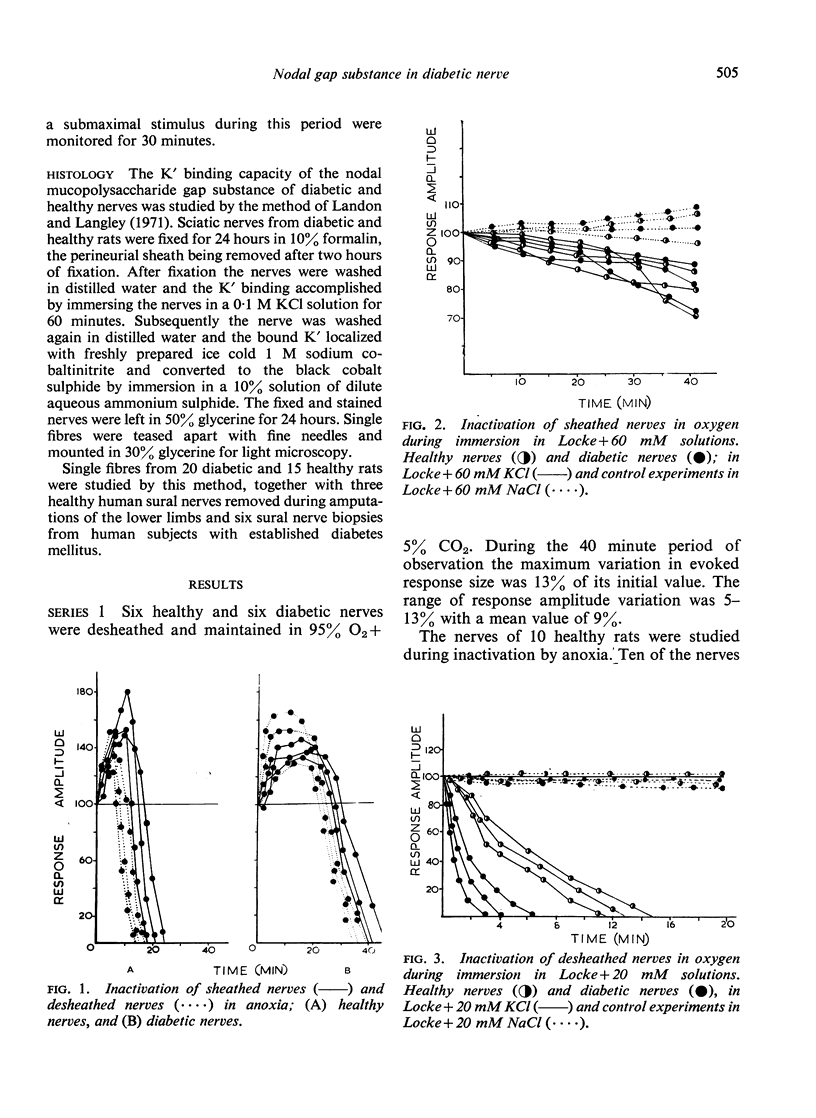
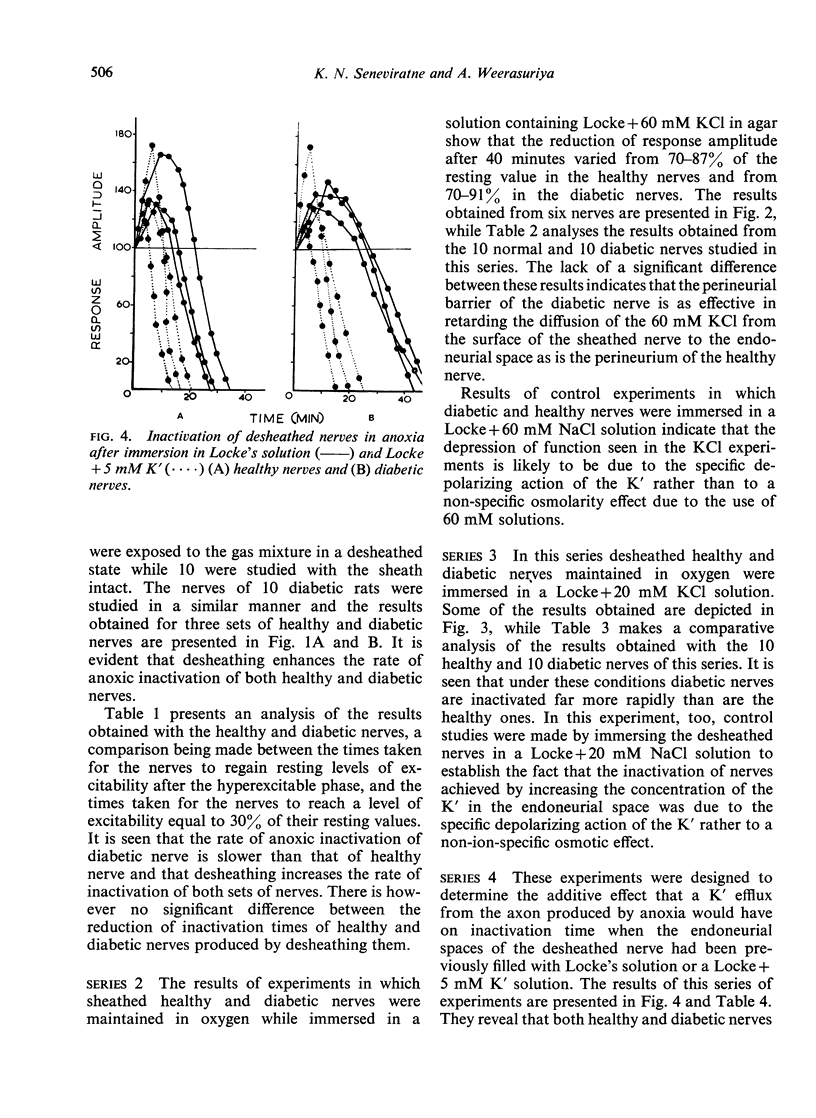
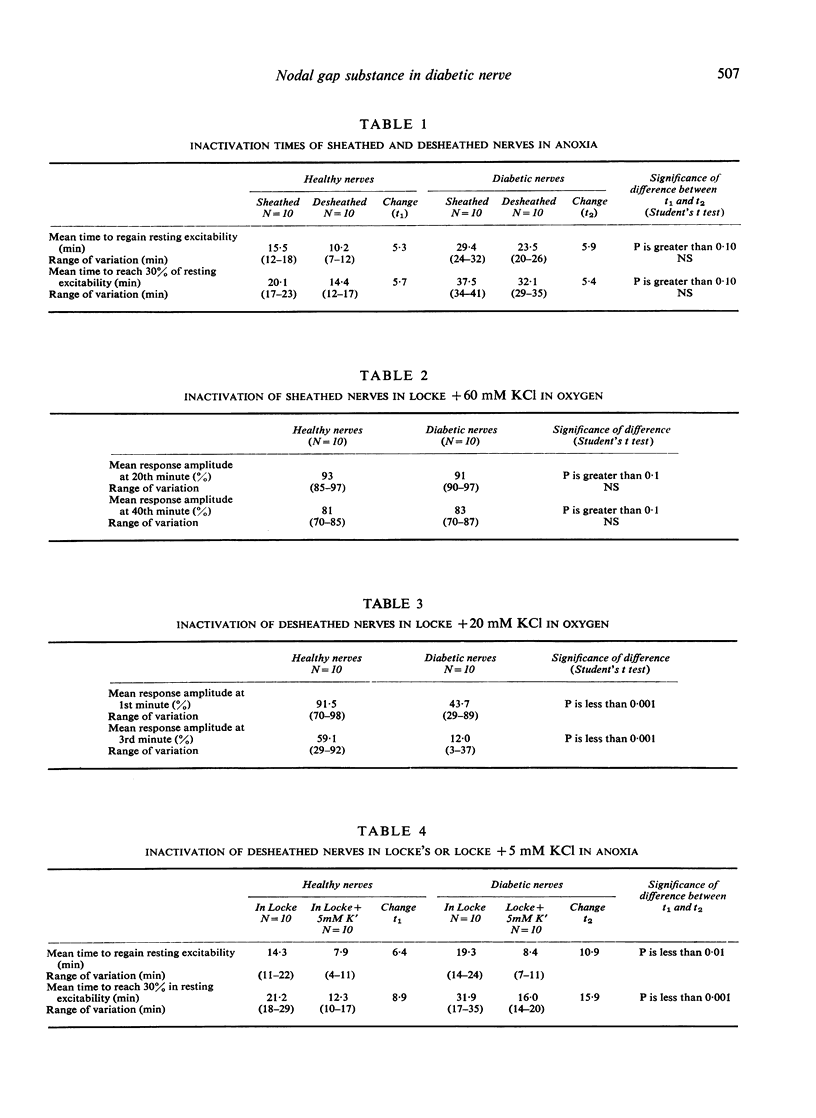
Anoxia and KC1 have been used to inactivate peripheral nerves by depolarization conduction block. Investigation of the inactivation patterns in isolated sciatic nerves of healthy and alloxan-diabetic rats suggests that the paranodal gap substance of healthy nerve behaves as an effective periaxonal diffusion barrier. In diabetic nerve the permeability of this barrier is significantly increased. A marked reduction in the K' binding capacity of the nodal gap substance has been demonstrated in myelinated nerves of human diabetics and alloxan diabetic rats.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisswenger P. G., Spiro R. G. Human glomerular basement membrane: chemical alteration in diabetes mellitus. Science. 1970 May 1;168(3931):596–598. doi: 10.1126/science.168.3931.596. [DOI] [PubMed] [Google Scholar]
- Burkel W. E. The histological fine structure of perineurium. Anat Rec. 1967 Jun;158(2):177–189. doi: 10.1002/ar.1091580207. [DOI] [PubMed] [Google Scholar]
- CRESCITELLI F. Nerve sheath as a barrier to the action of certain substances. Am J Physiol. 1951 Aug;166(2):229–240. doi: 10.1152/ajplegacy.1951.166.2.229. [DOI] [PubMed] [Google Scholar]
- Castaigne P., Cathala H. P., Dry J., Mastropaolo C. Les réponses der nerfs et des muscles à des stimulations électriques AU COURS D'une epreuve de garrot ischémique chez l'homme normal et chez le diabétique. Rev Neurol (Paris) 1966 Jul;115(1):61–66. [PubMed] [Google Scholar]
- Cravioto H. The perineurium as a diffusion barrier. Ultrastructural correlates. Bull Los Angeles Neurol Soc. 1966 Oct;31(4):196–208. [PubMed] [Google Scholar]
- EBASHI S. Calcium binding activity of vesicular relaxing factor. J Chir (Paris) 1961 Sep;82:236–244. doi: 10.1093/oxfordjournals.jbchem.a127439. [DOI] [PubMed] [Google Scholar]
- FENN W. O., GERSCHMAN R. The loss of potassium from frog nerves in anoxia and other conditions. J Gen Physiol. 1950 Jan 20;33(3):195–203. doi: 10.1085/jgp.33.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMBLE H. J. COMPARATIVE ELECTRON-MICROSCOPIC OBSERVATIONS ON THE CONNECTIVE TISSUES OF A PERIPHERAL NERVE AND A SPINAL NERVE ROOT IN THE RAT. J Anat. 1964 Jan;98:17–26. [PMC free article] [PubMed] [Google Scholar]
- HASSELBACH W., MAKINOSE M. [The calcium pump of the "relaxing granules" of muscle and its dependence on ATP-splitting]. Biochem Z. 1961;333:518–528. [PubMed] [Google Scholar]
- HESS A., YOUNG J. Z. The nodes of Ranvier. Proc R Soc Lond B Biol Sci. 1952 Nov 20;140(900):301–320. doi: 10.1098/rspb.1952.0063. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F., STAMPFLI R. Effect of potassium and sodium on resting and action potentials of single myelinated nerve fibers. J Physiol. 1951 Feb;112(3-4):496–508. doi: 10.1113/jphysiol.1951.sp004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEBANOFF S. J., GREENBAUM A. L. The effect of pH on the diabetogenic action of alloxan. J Endocrinol. 1954 Nov;11(4):314–322. doi: 10.1677/joe.0.0110314. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K. Some observations on perfused frog sciatic nerves. J Physiol. 1954 Feb 26;123(2):338–356. doi: 10.1113/jphysiol.1954.sp005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Nicholls J. G., Orkand R. K. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966 Jul;29(4):768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., OGSTON A. G. THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. 4. THE OSMOTIC PRESSURE OF MIXTURES OF SERUM ALBUMIN AND HYALURONIC ACID. Biochem J. 1963 Nov;89:249–253. doi: 10.1042/bj0890249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENT T. C., PIETRUSZKIEWICZ A. The effect of hyaluronic acid on the sedimentation rate of other substances. Biochim Biophys Acta. 1961 May 13;49:258–264. doi: 10.1016/0006-3002(61)90125-1. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. Electrotonus in frog spinal roots and sciatic trunk. Acta Physiol Scand. 1951 Aug 25;23(2-3):234–262. doi: 10.1111/j.1748-1716.1951.tb00813.x. [DOI] [PubMed] [Google Scholar]
- Landon D. N., Langley O. K. Cationic binding at the node of Ranvier. J Anat. 1969 Jul;105(Pt 1):196–196. [PubMed] [Google Scholar]
- Landon D. N., Langley O. K. The local chemical environment of nodes of Ranvier: a study of cation binding. J Anat. 1971 Apr;108(Pt 3):419–432. [PMC free article] [PubMed] [Google Scholar]
- Langley O. K. Ion-exchange at the node of Ranvier. Histochem J. 1969 May;1(4):295–301. doi: 10.1007/BF01003276. [DOI] [PubMed] [Google Scholar]
- Lieberman A. R. The connective tissue elements of the mammalian nodose ganglion. An electron microscope study. Z Zellforsch Mikrosk Anat. 1968;89(1):95–111. doi: 10.1007/BF00332655. [DOI] [PubMed] [Google Scholar]
- MAGLADERY J. W., McDOUGAL D. B., Jr, STOLL J. Electrophysiological studies of nerve and reflex activity in normal man. II. The effects of peripheral ischemia. Bull Johns Hopkins Hosp. 1950 May;86(5):291–312. [PubMed] [Google Scholar]
- NATHAN P. W. Ischaemic and post-ischaemic numbness and paraesthesiae. J Neurol Neurosurg Psychiatry. 1958 Feb;21(1):12–23. doi: 10.1136/jnnp.21.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGSTON A. G., PHELPS C. F. The partition of solutes between buffer solutions and solutions containing hyaluronic acid. Biochem J. 1961 Apr;78:827–833. doi: 10.1042/bj0780827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson Y., Reese T. S. Permeability of vasa nervorum and perineurium in mouse sciatic nerve studied by fluorescence and electron microscopy. J Neuropathol Exp Neurol. 1971 Jan;30(1):105–119. doi: 10.1097/00005072-197101000-00011. [DOI] [PubMed] [Google Scholar]
- POOLE E. W. Ischaemic and post-ischaemic paraesthesiae; normal responses in the upper limb with special reference to the effect of age. J Neurol Neurosurg Psychiatry. 1956 May;19(2):148–154. doi: 10.1136/jnnp.19.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott C. W., Goldstein M. A. Sarcoplasmic reticulum of striated muscle: localization of potential calcium binding sites. Science. 1967 Feb 24;155(3765):1019–1021. doi: 10.1126/science.155.3765.1019. [DOI] [PubMed] [Google Scholar]
- SHANTHAVEERAPPA T. R., BOURNE G. H. The 'perineural epithelium', a metabolically active, continuous, protoplasmic cell barrier surrounding peripheral nerve fasciculi. J Anat. 1962 Oct;96:527–537. [PMC free article] [PubMed] [Google Scholar]
- SPIRO R. G. Glycoproteins and diabetes. Diabetes. 1963 May-Jun;12:223–230. doi: 10.2337/diab.12.3.223. [DOI] [PubMed] [Google Scholar]
- STEINESS I. Vibratory perception in diabetics during arrested blood flow to the limb. Acta Med Scand. 1959 Mar 4;163(3):195–205. doi: 10.1111/j.0954-6820.1959.tb10400.x. [DOI] [PubMed] [Google Scholar]
- Seneviratne K. N., Peiris O. A. The effect of ischaemia on the excitability of human sensory nerve. J Neurol Neurosurg Psychiatry. 1968 Aug;31(4):338–347. doi: 10.1136/jnnp.31.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne K. N., Peiris O. A. The effect of ischaemia on the excitability of sensory nerves in diabetes mellitus. J Neurol Neurosurg Psychiatry. 1968 Aug;31(4):348–353. doi: 10.1136/jnnp.31.4.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne K. N., Peiris O. A. The effects of hypoxia on the excitability of the isolated peripheral nerves of alloxan-diabetic rats. J Neurol Neurosurg Psychiatry. 1969 Oct;32(5):462–469. doi: 10.1136/jnnp.32.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne K. N., Peiris O. A. The role of diffusion barriers in determining the excitability of peripheral nerve. J Neurol Neurosurg Psychiatry. 1970 Jun;33(3):310–318. doi: 10.1136/jnnp.33.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne K. N., Peiris O. A., Weerasuriya A. Effects of hyperkalaemia on the excitability of peripheral nerve. J Neurol Neurosurg Psychiatry. 1972 Apr;35(2):149–155. doi: 10.1136/jnnp.35.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne K. N. Permeability of blood nerve barriers in the diabetic rat. J Neurol Neurosurg Psychiatry. 1972 Apr;35(2):156–162. doi: 10.1136/jnnp.35.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S. R., Berenson G. S., Radhakrishnamurthy B. Glycoprotein changes in diabetic kidneys. Diabetes. 1970 Mar;19(3):171–175. doi: 10.2337/diab.19.3.171. [DOI] [PubMed] [Google Scholar]
- THOMAS P. K. The connective tissue of peripheral nerve: an electron microscope study. J Anat. 1963 Jan;97:35–44. [PMC free article] [PubMed] [Google Scholar]