Abstract
Alterations in neuronal cytosolic calcium is a key mediator of the traumatic brain injury (TBI) pathobiology, but less is known of the role and source of calcium in shaping early changes in synaptic receptors and neural circuits after TBI. In this study, we examined the calcium source and potential phosphorylation events leading to insertion of calcium-permeable AMPARs (CP-AMPARs) after in vitro traumatic brain injury, a receptor subtype that influences neural circuit dynamics for hours to days following injury. We found that both synaptic and NR2B-containing NMDARs contribute significantly to the calcium influx following stretch injury. Moreover, an early and sustained phosphorylation of the S-831 site of the GluR1 subunit appeared after mechanical injury, and this phosphorylation was blocked with the inhibition of either synaptic NMDARs or NR2B-containing NMDARs. In comparison, mechanical injury led to no significant change in the S-845 phosphorylation of the GluR1 subunit. Although no change in S-845 phosphorylation appeared in injured cultures, we observed that inhibition of NR2B-containing NMDARs significantly increased S-845 phosphorylation one hour after injury while blockade of synaptic NMDARs did not change S-845 phosphorylation at any time point following injury. These findings show that a broad class of NMDARs are activated in parallel and that targeting either subpopulation will reverse some of the consequences of mechanical injury, providing distinct paths to treat the effects of mechanical injury on neural circuits after TBI.
INTRODUCTION
Phosphorylation of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptors (AMPARs) causes functional changes that are commonly implicated in synaptic plasticity, as well as pathological signaling occurring in several disease states. Several sites on the individual AMPAR subunits can be phosphorylated, with the resulting phosphorylation controlling the localization, insertion, channel conductance, and receptor kinetics of the AMPAR [Wang et al., 2005; Lee, 2006]. Two specific phosphorylation sites on the GluR1 subunit - serine 831 and serine 845 – are emerging as potentially important regulators defining the role AMPARs in specific neurological diseases [Fu et al., 2004; Rakhade et al., 2008]. The activation of upstream kinases and resultant phosphorylation of S-845 and S-831 affect trafficking and conductance of receptors containing the GluR1 subunit, and thus may play a critical role in the insertion of highly active CP-AMPARs in the form of GluR1 homomers [Derkach et al., 2007].
Although alterations in intracellular calcium levels are a proximal event for both S-831 and S-845 phosphorylation, each has a distinct pathway that suggests two independent control mechanisms. Phosphorylation of the GluR1 S-831 site by one of the most abundant, calcium sensitive proteins (CaMKIIα) [Sheng and Hoogenraad, 2007], increases single channel conductance [Derkach et al., 1999] and influences GluR1 trafficking [Boehm et al., 2006]. Moreover, several previous studies show that CaMKIIα activity alters AMPAR mediated currents [Hayashi et al., 2000; Goforth et al., 2004]. Given that CaMKIIα can achieve an autophosphorylation state to remain active well beyond the initial calcium transient [Hudmon and Schulman, 2002], this means that S-831 modification may play a more prominent and sustained role on AMPAR function than other kinases that are inactivated immediately after the removal of calcium.
Alternatively, phosphorylation of the S-845 residue of the GluR1 subunit is controlled by cGKII [Serulle et al., 2007], a kinase activated by an increase in nitric oxide (NO) levels within the cytosol, and PKA, activated by the calcium dependent production of cAMP [Willoughby and Cooper, 2007]. Largely independent of CaMKIIα signaling, increases in nitric oxide levels are linked to calcium mediated activation of neuronal nitric oxide synthase (nNOS) or activation of endothelial nitric oxide synthase (eNOS), whereas the late rise in NO is attributed to inducible nitric oxide synthase (iNOS) but may also include release from mitochondria (mNOS) [Wojda et al., 2008; Werner and Engelhard, 2007; Bayir et al., 2007]. For both S-845 phosphorylation mechanisms, delivery of GluR1 subunits to the cell surface increases and results in an increased AMPAR activity [Serulle et al, 2007]. Similar to CaMKIIα mediated phosphorylation of S-831 site, the increased delivery of GluR1 homomers may present a positive feedback loop to significantly potentiate calcium increases following synaptic activity, therefore leading to a rapid S-845 mediated increase in CP-AMPARs following the initial elevations in nitric oxide or cAMP.
Our past work using an in vitro model of traumatic brain injury shows that CP-AMPARs appear within four hours following mechanical injury, and the targeted inhibition of these receptors will lead to a reduction in neuronal death 24h following injury [Spaethling et al., 2008]. With this in vitro model of traumatic brain injury, several reports show that the initial mechanical injury is accompanied by an immediate increase in cytosolic calcium that is mediated largely by the NMDA receptor [Zhang et al., 1996; Geddes-Klein et al., 2006]. Activation of the NMDAR can represent a common initiation point for both NO, CaMKIIα, and cAMP signaling, in part due to the close physical association of CaMKIIα and nNOS with the NR2B subunit [Christopherson et al., 1999; Barria and Malinow, 2005], and the demonstrated link between NMDAR and cAMP activity [Chetkovich et al., 1993; Wang et al., 2007]. Moreover, there is evidence from in vivo models of ischemia and traumatic brain injury that activity of each of these key signaling molecules – NO, cAMP, and CaMKIIα – is associated with NMDAR activation in the acute phase following injury [Griesbach et al., 2004; Cherian et al., 2004; Folkerts et al., 2007; Atkins et al., 2007]. This has collectively led to our hypothesis that the regulation of CP-AMPAR appearance following mechanical injury to cortical neurons is primarily influenced by NMDAR activation. Recent reports show the relative importance of NMDAR subtype activation (e.g., NR1/NR2B, NR1/NR2A, NR1/NR2A/NR2B) in mediating signaling for both neuronal survival and different forms of neuronal death [DeRidder et al., 2006; Hardingham et al., 2002; Rameau et al., 2007], suggesting that the NMDA mediated regulation of CP-AMPAR insertion after mechanical injury may be controlled by the balance of synaptic and NR2B-containing NMDAR activation that occurs following mechanical injury in vitro.
We investigated the role of different NMDAR subpopulations in the enhanced expression of CP-AMPARs seen following in vitro mechanical injury. Moreover, we tested if these changes in either the phosphorylation of the GluR1 subunit or the appearance of CP-AMPARs were mediated by NR2B-NMDAR activation, a commonly cited mechanism associated with neuronal death. We used immunoblotting procedures to examine the timecourse of S-831 and S-845 phosphorylation following in vitro mechanical injury, and pharmacological manipulations to determine the relative role of synaptic vs. NR2B-containing NMDA receptors in controlling these phosphorylation changes. Finally, we tested if blocking either synaptic NMDARs or NR2B-containing NMDARs influenced the resulting appearance of CP-AMPARs following mechanical injury. Our results demonstrate important new regulatory mechanisms of CP-AMPAR appearance after in vitro TBI, and point to the utility of targeting NMDAR subpopulations for mitigating the early consequences of CP-AMPARs after traumatic mechanical injury.
MATERIALS AND METHODS
Primary Cell Culture
Animal procedures were performed in accordance with the Institutional Animal Care and Use Committee at the University of Pennsylvania. Primary cortical neurons were isolated from embryonic day 18 (E18; Charles River, Wilmington, MA) rats and 250,000 cells were plated on a 1.227 in2 area of poly-D-lysine (PDL; Sigma, St. Louis, MO) and Laminin (BD Biosciences) coated silicone membrane. The culture surface (membrane) was attached to a stainless steel well. The silicone substrates used (Sylgard 184 + 186 mix, Dow Corning, Midland, MI) were transparent, flexible, and elastic, which allowed us to mimic the mechanical forces that occur during traumatic brain injury [Meaney et al., 1995]. Neurons were cultured in Neurobasal media (Invitrogen) with B27 and Glutamax (Gibco) in a humidified incubator at 37°C, 5% CO2 for 18-21 days in vitro (DIV). The length of time for culturing was used to allow for the full expression of glutamate receptors [Lin et al., 2002; Hall et al., 2007], and to avoid the spontaneous calcium oscillations that can appear in 11-14 DIV cultures. These spontaneous calcium oscillations are considered a characteristic of immature neurons and will render the neurons insensitive to mechanical injury [Geddes-Klein et al., 2006]. Moreover, published reports that show the expression profile of the AMPA subunits begins to plateau after 16DIV [Kumar et al., 2002; Pellegrini-Giampietro et al., 1991], suggesting neurons attain sufficient stable AMPAR profiles at DIV18-21.
Drug Treatments
All compounds were solubilized in neutral buffered saline solution (NBS; in mM: 51.3 NaCl, 5.4 KCl, 2 MgCl2, 1.8 CaCl2, 26 NaHCO3, 0.9 NaH2PO4, 10 HEPES, 0.001 TTX, pH 7.3), added to the cells 5 minutes prior to injury, and remained on the cultures for the duration of the experiment unless otherwise noted. Cultures were pre-incubated with Ro25-6981 (1μM; Sigma) to inhibit primarily NR1/NR2B NMDARs, applied 10 minutes before injury and remaining in the media for a total period of 30 minutes. To inhibit synaptic NMDARs, cells were treated following a modified protocol published by Bengtson et al [Bengtson et al., 2008]. Cortical neurons were incubated for 4 minutes at 37°C in 50μM bicuculline methobromide. Cells were then incubated for 10 minutes at 37°C in bicuculline and 10μM MK801 and rinsed in 1μM tetrodotoxin (TTX) for 1hr before the injury.
Injury
We used a previously described in vitro model of traumatic brain injury that reproduces the mechanical forces that occur during injury on a cultured monolayer of neurons [Lusardi et al., 2004]. Cultures at 18-21 days in vitro (DIV) were treated with drug or changed into NBS as desired, placed on a stainless steel plate, and covered with a top plate to form a sealed chamber. Increasing the chamber pressure to its peak level in 15 milliseconds caused the compliant silicone membrane to stretch, in turn applying a stretch to the cultured neurons. This pressurization phase was immediately followed by a release of the pressure, typically within 25 milliseconds of achieving the peak pressure. Although cells were cultured over a circular area (23 mm diameter), we designed the supporting stainless steel plate to expose only cells in a defined region (a 6mm × 18mm rectangular region; approximately 1/3 of the culture surface area) to a stretch, designing the system to provide a uniform membrane stretch over 95% of the surface within that region [Lusardi et al., 2004]. The membrane in the injured region was stretched uniaxially, where the width was extended 80% beyond its initial width before returning to its original dimension. After injury, cultures were returned to the incubator until the desired time point. We did not observe any obvious detachment of the cells from the membrane following stretch injury, nor did we observe any gross morphological changes in the cultures. This stretch level consistently causes a significant increase in cell death compared to unstretched controls [Spaethling et al., 2008]. Naïve unstretched neurons served as reference conditions for statistical comparison.
Calcium Imaging
A group of cultures were mechanically injured and then assessed for the response in cytosolic calcium to an application of AMPA (10 μM) to the media. Forty-five minutes prior to a scheduled observation time, neurons were loaded with the calcium sensitive fluorescent dye, Fura-2AM in NBS (Molecular Probes, Eugene, OR; 5μM), at 37°C. Immediately prior to imaging, neuronal cultures were rinsed and placed on a Nikon TE300 microscope (Optical Apparatus, Ardmore, PA) for calcium fluorescence imaging. Both prior to and following mechanical injury, cells were in NBS media. Cells were alternately excited at 340 and 380nm using an excitation shutter filter wheel (Sutter Instruments, Novato, CA) and the corresponding emission images (510 nm) were collected using a 14-bit Hamamatsu camera (model- c4742-98; Optical Apparatus, Ardmore, PA) at a rate of one ratio image approximately every three seconds. The fluorescence ratio from excitation at the two wavelengths (F340, F380) was used to calculate the Fura ratio (R = F340/F380).
To determine the response of neurons to AMPA stimulation, a baseline set of images was acquired for 30 seconds prior to the addition of 10μM AMPA, a concentration selected from pilot studies (1μM-100μM) to avoid saturating the receptor response [Glaum et al., 1990] and maintain fluorescence measurements within the linear range of the Fura-2 indicator. Immediately following AMPA treatment, the response was captured for 2.5 minutes in the presence of AMPA. In each cell, we normalized the response to AMPA stimulation to its baseline fluorescence ratio. Cells with abnormally high baseline ratios, defined as a fluorescence ratio greater than 1, were excluded from analysis. Similarly, cells with an abnormally low baseline ratio (< .3) were also excluded from analysis. The excluded cells were typically less than 5% of observed cells. All cells in the field of view, averaging approximately 75 cells per well, were imaged across multiple wells for each experimental condition studied. A nested analysis of variance, with the culture well for each cell as the nesting variable, was used to detect significant differences across experimental conditions. Tukey post-hoc analysis was used for planned multiple comparisons following the ANOVA analysis. For all statistical conditions, p-values of less than 0.05 were considered significant.
Western Blot
For injured samples, the marked injured region of membrane was excised from the rest of the culture. Neurons from that region were lysed in RIPA buffer (in mM: 10 HEPES, 200 NaCl, 30 EDTA, 0.5% Triton X-100, pH 7.4 with complete mini protease inhibitor (Roche Molecular Biochemicals), 0.5 NaF, 0.1 K4P2O7, 1 NaVO4). Cell lysates were cleared by centrifugation at 16,000 g for 30 min at +4°C. Ten (10) μg of protein per lysate was resolved on 4–12% Bis-Tris NuPage gels (Invitrogen) at 150 V for 1 h 30 min then transferred to a polyvinylidene difluoride (PVDF) membrane (Invitrogen) at 10V overnight in transfer buffer (Invitrogen) containing 20% methanol. The membranes were blocked in TBST (20 mM Tris-HCL, 1.5 M NaCl, 0.1% Tween-20, pH 7.4) with 5% dry milk for 1 hr at room temperature. The blots were then incubated with primary antibodies for p-845 GluR1 primary (Chemicon; 1:1000), p-831 GluR1 (1:500; Abcam), total GluR1 primary (Abcam; 1:1000), and GAPDH (Millipore; 1:10,000) in TBST with 5% dry milk overnight at 4°C. The blots were then rinsed in TBST and incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody (1:5000 for Rb; 1:20,000 for Ms; Amersham Biosciences, Piscataway NJ) for 2 h at room temperature. Blots were visualized using ECL detection (Pierce Biotechnology, Rockford IL). Protein bands were quantified with a computer assisted densitometric scanning (Kodak 1D Image Analysis Software, Eastman Kodak Company, Rochester, NY, USA). Averages of the intensity ratios were calculated for injured and uninjured groups. For comparisons between two conditions, a Student's t test was used (p<0.05). For comparisons of more than two groups, an analysis of variance (ANOVA) was used in combination with Tukey post-hoc analysis to determine significance (p<0.05).
RESULTS
AMPAR modification and mechanical injury
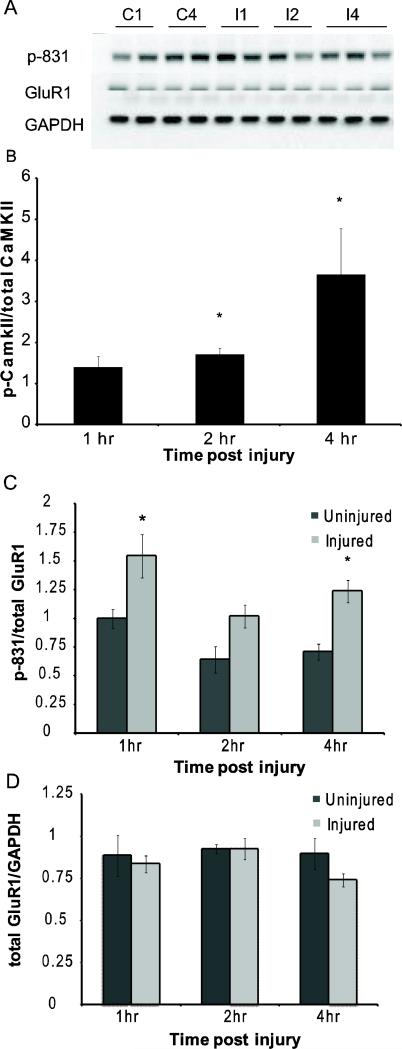
Our past work showed that mechanical injury causes a transient increase in cytosolic calcium, lasting for less than 30 minute after injury and showing no secondary elevation for at least six hours after the initial injury [Spaethling, et al., 2008]. In comparison to the short calcium transient, the phosphorylation of CaMKIIα lasts for a much longer period following injury [Spaethling et al., 2008]. Past work shows that the S-831 site on the GluR1 subunit is one of several CaMKIIα targets, and this phosphorylation step could be an early step leading to the appearance of CP-AMPARs. We observed a progressive increase in CaMKIIα phosphorylation after mechanical injury, showing a significant increase 2h and 4h after injury (Fig. 1B). In parallel, we found that p-831 levels significantly increased 1hr post-injury, and remained elevated 2 hours after injury (Fig. 1C). Four hours following injury, we again observed a significant elevation in S-831 phosphorylation. Total GluR1 levels remained constant across all time points, indicating that the observed increase in p-831 to total GluR1 ratio is due to the increase in phosphorylation instead of a change in total GluR1 (Fig. 1D).
Figure 1.
Sustained increase in phosphorylation of the S-831 site on the GluR1 subunit occurs following mechanical injury. (A) Representative blot of p-831 levels post-injury. (C1-1hr uninjured saline, C4-4hr uninjured saline, I1-1hr after injury, I2-2hrs after injury, 4I-4hrs after injury) (B) p-CaMKIIα levels increase 2 and 4 hr post-injury (n=4-8 per condition; * = p<0.05 compared to uninjured control) (C) In mechanically injured cultures, p-831 normalized to total GluR1 is increased 1hr after injury and has a second rise at 4hrs post-injury. (n = 4-8 per condition; * = p<0.05 compared to uninjured control) (D) No change in GluR1 as compared to GAPDH at any time measured post-injury (n=4-8 per condition)
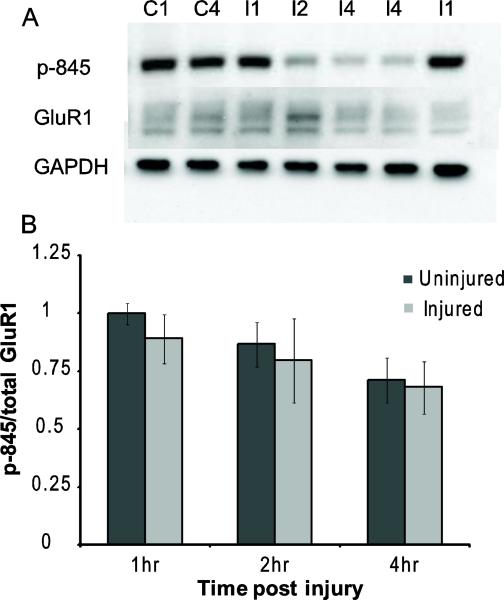
An alternative phosphorylation of the GluR1 at S-845 could also lead to the appearance of CP-AMPARs following mechanical injury [Serulle et al., 2007]. However, we observed no significant difference in p-845 levels (Fig. 2A, B) following injury relative to control, uninjured samples in saline solution. Although we observed a modest decrease in p-845 levels over time in uninjured, saline-treated cultures, these changes were not significantly different from naïve samples. Moreover, we did not observe a decrease in GluR1 subunit levels in these analyzed samples, suggesting that these changes were not confounded by alterations in receptor subunit levels from translation or proteolysis.
Figure 2.
Unchanged p-845 levels post-injury. (A) Representative blot of p-845 levels after injury (C1-1hr uninjured saline, C4-4hr uninjured saline, I1-1hr after injury, I2-2hrs after injury, 4I-4hrs after injury). (B) Following injury there is no significant change in p-845 levels compared to total GluR1 levels. (n = 4-10 per condition)
Source specificity of initiation of AMPAR modification
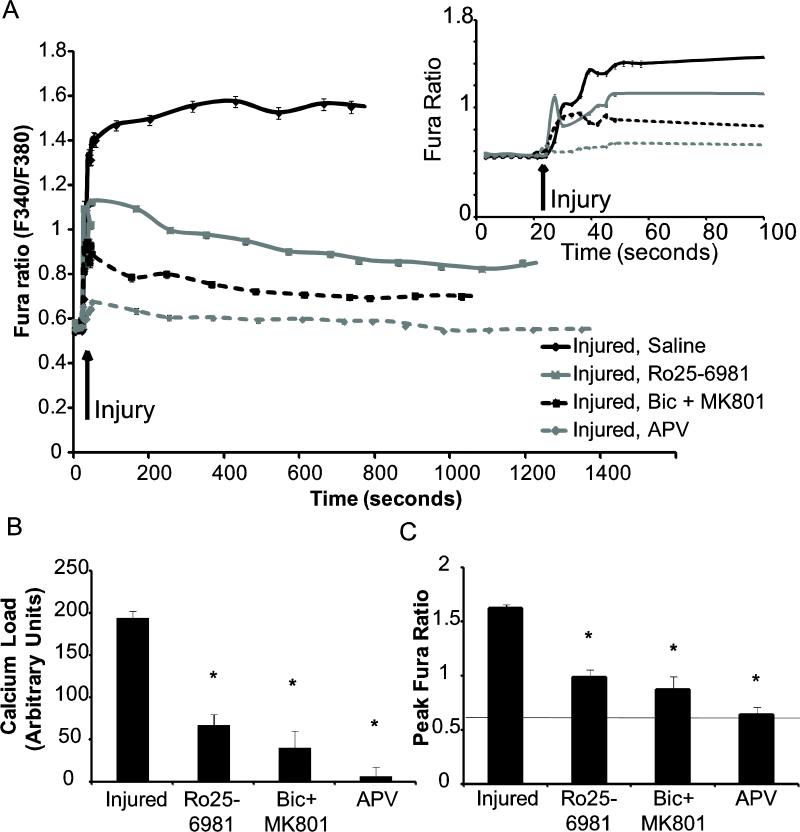
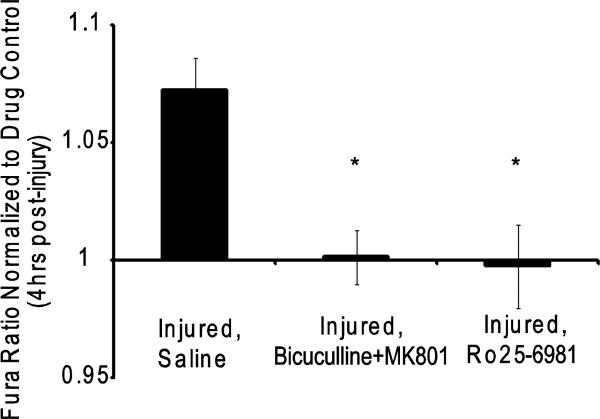
Given that changes in the different serine phosphorylation sites on GluR1 can regulate the appearance of CP-AMPARs, we next considered potential source-specific mechanisms regulating these changes. Several groups showed recently that calcium entering the cell through synaptic or extrasynaptic NMDARs lead to differential effects on neuronal survival or demise [Hardingham et al., 2002; Rameau et al., 2007]. We first considered if the mechanical injury caused a subsequent influx of calcium through either synaptic and/or NR2B-containing NMDA receptors, and if this pathway for calcium influx was a prominent contribution to the immediate changes in cytosolic calcium following injury. Past work from our group using this model of injury showed that moderate levels of injury (50% peak stretch) would lead to calcium influx that is primarily due to influx through synaptic NMDA receptors [Geddes-Klein et al., 2006]. At more severe injury levels necessary to cause neuronal death 24 hours following stretch, we found that 93% ± 14% of the initial calcium influx was due to influx through the NMDA receptors (Fig 3). Using a protocol for blocking synaptically localized NMDA receptors prior to mechanical injury, we observed a significant reduction in the measured calcium influx following mechanical injury (Fig. 3). Alternatively, treatment of cortical neurons with an antagonist directed towards the NR2B containing NMDARs (Ro25-6981) also reduced the initial calcium influx from untreated controls, although the response remained higher than in naïve neurons (Fig. 3). The relative levels of cytosolic calcium increases observed after mechanical injury by blocking synaptically localized or NR2B containing NMDARs was not significantly different. Collectively, these studies show that calcium entering the neuron at this level of mechanical injury is nearly exclusively through NMDARs, and is divided between synaptic and NR2B-containing NMDARs. Therefore, both pathways may contribute to the AMPAR subunit phosphorylation we measured following injury.
Figure 3.
Both synaptic and NR2B-containing NMDARs contribute to calcium increase after mechanical injury. Cultures were pretreated to inhibit synaptic (Bicuculline + MK-801), NR2B-containing (Ro25-6981), or all NMDARs (APV). (A) Cytosolic calcium levels were monitored prior to and following mechanical injury for up to 23 mins. Blocking NMDARs during the injury resulted in no calcium influx. Inhibiting either synaptic or NR2B-containing NMDAR NMDARs reduces the total injured mediated calcium load. (Shown is average taken every 2 minutes; n = 3-5 per condition). Inset: focus on first 100s of imaging. (B) The area under the curves shown in (A) represents the calcium load that enters the cell. All treatments resulted in a significant reduction in calcium load compared to the saline treated injured case (*=p<0.05). (C) The peak fura ratio was compared between the conditions and all treatments resulted in a significant decrease compared to the saline treated injured case (*=p<0.05)
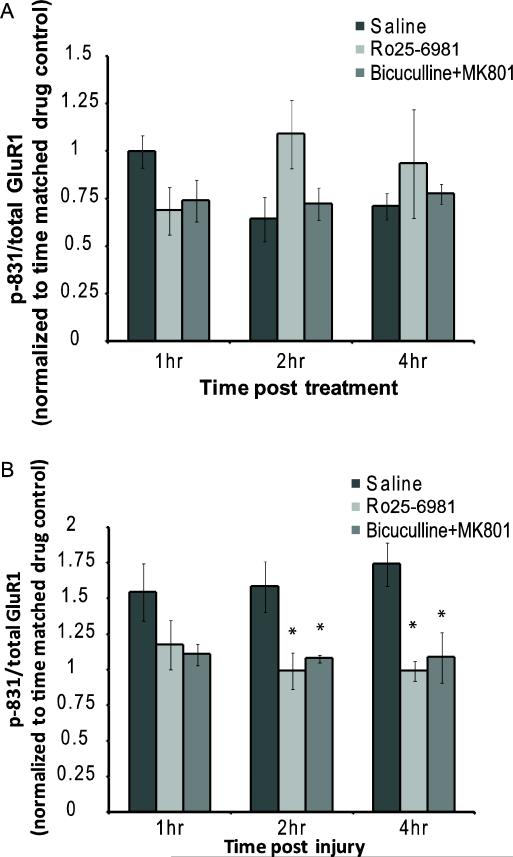
Despite this combined contribution of synaptic and NR2B-containing NMDARs to the resulting calcium influx following injury, several reports show that exact source specificity is critical for regulating the activation of specific synaptic signaling cascades, including those involved in CP-AMPAR insertion [Xu et al., 2009; Jin and Feig, 2010; Cheriyan et al., 2011]. To test the relative importance of specific calcium influx sources on AMPAR modification following mechanical injury, we measured the level of AMPAR phosphorylation following injury in either the presence of synaptic or NR2B-containing NMDAR blockade. In uninjured cultures, we observed some alteration in the relative phosphorylation of p-831 over time but none of these changes were significant (Fig. 4A). Inhibiting synaptic NMDARs during injury returned the p-831 level to baseline at 1hr (Fig. 4B) indicating that synaptically sourced calcium is responsible for phosphorylation at this site. However, our studies on the source of calcium influx also showed that influx through NR2B-containing NMDARs is also significant, and recent reports associate NR2B-containing, or extrasynaptic NMDAR activity, with adversely affecting neuronal fate [Papadia and Hardingham, 2007; Xu et al., 2009]. Injuring in the presence of Ro25-6981 also resulted in a significant decrease in p-831 levels not different from baseline (Fig. 4B) suggesting that calcium entry through the NR2B-containing NMDAR population also contributes to this AMPAR phosphorylation event. Together, these data indicate that more than one distinct subpopulation of NMDARs will lead to alterations in S-831 phosphorylation after injury.
Figure 4.
Influx through synaptic and NR2B-containing NMDARs after mechanical injury contribute to S-831 phosphorylation post-injury. (A) Synaptic (Bicuculline + MK-801) or NR2B-containing (Ro25-6981) NMDARs were blocked in control (uninjured) cultures and no changes were observed in p-831 levels. (B) These same populations were then blocked during the injury, isolating the effects of different NMDAR subpopulations. Using either the synaptic blockade protocol or Ro25-6981 eliminated the increase in p-831 levels observed 1hr post-injury. (n = 4-8 per condition; *= p<0.05 compared to time matched drug controls)
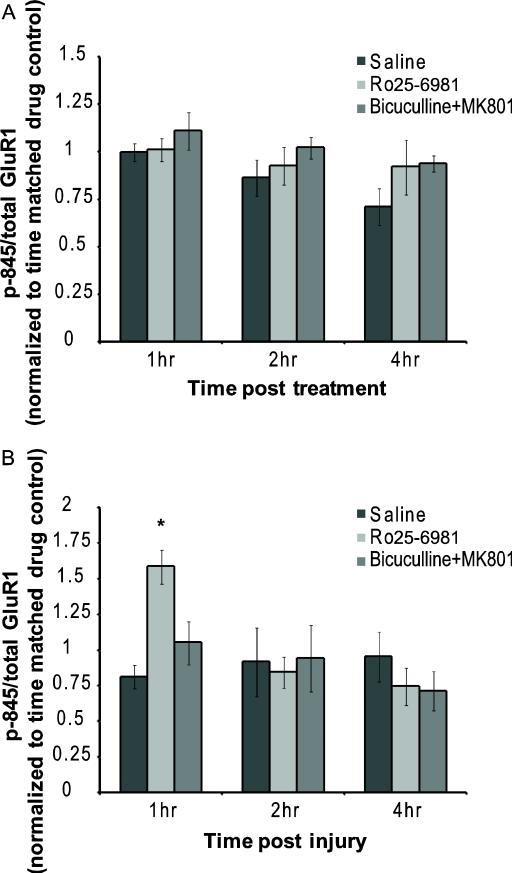
Although we did not observe significant differences in p-845 levels between injured and uninjured cortical neurons, it is possible that the parallel activation of the synaptic and NR2B-containing NMDARs during mechanical injury would produce competing signals for S-845 phosphorylation. To test this possibility, we examine if distinct NMDAR subgroups contributed to the regulation of p-845 after injury. Once again, drug treatments produced some change in the relative p-845 phosphorylation levels over time in control cultures, but these changes were not significant (Fig. 5A). An isolated synaptic block of NMDARs did not alter the course of S-845 phosphorylation after mechanical injury (Fig. 5B). Conversely, Ro25-6891 treatment increased the p-845 response to injury one hour after mechanical injury (Fig. 5B), returning to levels not significant from saline treated cultures at 2h and 4h after injury.
Figure 5.
Effects on p-845 levels dependent upon type of NMDA activation. (A) Synaptic or NR2B-containing NMDARs were blocked in control (uninjured) cultures and no changes were observed in p-845 levels. (B) Cultures treated with the synaptic block protocol (Bicuculline + MK-801) had less maintenance of p-845 levels than saline treated cultures at all observed times post-injury. (n = 3-8 per condition). Cultures that were treated with Ro25-6981, a NR2B antagonist, before injury had higher levels of p-845 normalized to total GluR1 than their saline treated injured group 1 hr post-injury. (n = 3-6 per condition ; * = p<0.05 compared to time matched drug controls)
NMDAR subpopulation responsible for AMPAR modification
These GluR1 phosphorylation events affect subunit localization and receptor kinetics; however, it was still unclear how the combination of effector kinases works together to result in the changes we have observed. As mentioned previously, S-831 phosphorylation is linked to CaMKIIα activity, and our evidence suggested that controlling CaMKIIα activation would lead to changes that could limit the appearance of CP-AMPARs via S-831 phosphorylation. Consistent with our expectations, inhibiting CaMKIIα significantly reduced, but did not eliminate, the observed enhanced AMPA-mediated calcium influx 4 hours post-injury [Spaethling et al., 2008]. Given the role of both synaptic and NR2B-containing NMDARs in the initial calcium transient and subsequent S-831 phosphorylation of the GluR1 subunit after injury, we next tested if these NMDAR populations differentially controlled the appearance of calcium-permeable AMPARs after stretch injury. Treating mechanically injured cultures with AMPA four hours after injury led to an enhanced calcium influx relative to uninjured cultures, similar to results from a previous study [Spaethling et al., 2008]. Pretreatment with Joro spider toxin eliminated the enhanced calcium influx in injured neurons, showing that the injury led to an enhanced contribution of calcium-permeable AMPARs after injury. Blocking either synaptic or NR2B-containing NMDARs led to a significant attenuation of the AMPA-mediated calcium influx response (Fig. 6). Similar to reversing changes in S-831 phosphorylation, these results show that targeting subpopulations of NMDARs is effective in in altering early changes in AMPARs after mechanical injury.
Figure 6.
Overlapping pathways contribute to enhanced AMPA signaling post-injury. Either synaptic or NR2B NMDAR blockade leads to the elimination of the enhanced calcium response due to CP-AMPARs (n=4-8 per condition; *=p<0.05 compared to saline, injured cultures)
DISCUSSION
In this study, we examined potential changes in phosphorylation of the GluR1 subunit of the AMPA receptor following traumatic mechanical injury, testing if the mechanisms that would lead to these phosphorylation events also contributed to the appearance of calcium permeable AMPA receptors (CP-AMPARs) after injury. We showed that our model of traumatic mechanical injury activates both synaptic and NR2B-containing NMDARs at the moment of injury, but this activation is largely transient. Our data illustrated that stretch injury induces alterations at two critical GluR1 phosphorylation sites, S-831 and S-845. We found a significant increase in S-831 phosphorylation 1 hr following injury that was significantly sustained four hours after injury. In contrast, p-845 levels remain unchanged over time after injury. Moreover, both synaptic and NR2B-containing NMDAR populations were synergistic for S-831 phosphorylation, while p-845 regulation was more complex. Finally, pharmacological manipulations showed that both synaptic and NR2B-NMDARs contribute to the enhanced AMPA-mediated calcium influx following injury, suggesting a role for either receptor subpopulation in mediating this specific injury response. Together our findings provide insight into the mechanisms of how AMPAR function can be disrupted by injury and give multiple targets for therapeutic intervention.
Past reports highlight an emerging importance of AMPAR mediated changes following traumatic mechanical injury. Goforth et al. was the first to observe alterations in the AMPAR functions using a system similar to the one used in the current study [Goforth et al., 1999]. Observing an immediate loss in the normal desensitization property of AMPARs following stretch injury, a subsequent study showed that the loss of desensitization was mediated by the NMDAR and CaMKIIα phosphorylation, similar to the role of the NMDAR and CaMKIIα we observe in the current study. In addition, a recent set of studies by Bell et al using a combined chemical and mechanical insult show that the appearance of CP-AMPARs in cerebellar neurons is also influenced by NMDAR stimulation [Bell et al., 2009]. Although examining a different regulating mechanism of AMPAR function – the PICK-1 mediated retention of the GluR2 subunit – these data underscore the complex mechanisms that can alter AMPAR function and composition after either a single mechanical or combined chemical/mechanical injury. Although these past studies point to the importance of the NMDAR in controlling AMPAR function and trafficking following mechanical injury, our work is the first to start discriminating among different NMDA receptor subpopulations in this process. Our data showing that mechanical injury can activate both synaptic and NR2B-containing NMDARs should be considered with past work showing that less severe stretch levels activate only synaptic NMDARs [Geddes-Klein et al., 2006]. Collectively, these data suggest a continuum of NMDAR activation, progressing from synaptic to include both synaptic and extrasynaptic NMDARs as the level of mechanical injury gradually shifts from mild to more severe. Therefore, we should be careful in not extending our current results across all levels of mechanical injury, as it is likely that the measured phosphorylation changes will differ with the degree of synaptic/NR2B-NMDAR activation.
Certainly the most direct link in the literature between subunit phosphorylation and subsequent insertion and/or stabilization of the CP-AMPAR isoforms appears for S-845 site. Past work shows a relatively high fidelity of this phosphorylation step leading to the stability of GluR1 homomers [He et al., 2009]. NMDAR activation can lead to the production of nitric oxide and cGKII, which in turn can phosphorylate the S-845 site on GluR1 [Serulle et al., 2007]. Our data also highlights an additional interplay between NMDAR receptor subpopulation stimulation and GluR1 phosphorylation. Although mechanical injury does not lead to a significant increase in S-845 phosphorylation, our data suggests a possible complex interplay between NR1/NR2A and NR1/NR2B NMDARs in regulating this phosphorylation after stretch injury. Given the synaptic blockade and Ro25-6981 blockade produced only modestly different changes in the acute calcium influx, these data suggest that a significant fraction of NR1/NR2B receptors appear at synaptic locations. Therefore, in mechanically injured neurons pretreated with Ro25-6981, the NMDARs activated include the smaller fraction of NR1/NR2A and NR1/NR2A/NR2B NMDARs at the synapse. The significant increase in p-845 phosphorylation with Ro25-6981 treatment, when compared with no significant increase when all synaptic NMDARs were blocked, suggests that activation of NR2A-containing receptors is a strong stimulus to enhance phosphorylation at the S-845 site, although the pathways responsible for this phosphorylation is not clear. Alternatively, when only the smaller fraction of NR1/NR2B extrasynaptic receptors is activated after injury with the synaptic blockade protocol, there is a modest decrease in S-845 that is not significant. Future studies with more specific NR2A-containing NMDAR antagonists would help clarify if the stimulation of only NR1/NR2B NMDARs at both synaptic and extrasynaptic locations would provide a counterbalance to the phosphorylation that appears to be triggered by activation of NR2A-containing NMDARs.
Given the prior evidence linking AMPAR changes to NMDAR activation and CaMKIIα phosphorylation, our changes in S-831 phosphorylation shows that the sustained activation of CaMKIIα beyond the initial calcium transient observed [Spaethling et al., 2008] may be important in regulating AMAPR subunit changes following injury. Past studies point to the role of activated CaMKIIα in phosphorylating specific serine residues on the GluR1 subunit of the AMPA receptor, its role in delivery of AMPARs to the synapse following activation, and its central role in NMDA receptor mediated LTP [Barria et al, 1997; Hayashi et al., 2000; Lisman and Zhabotinsky, 2001]. The presence and extent of CaMKIIα activation following traumatic brain injury in vivo was the subject of several recent investigations, with many showing that CaMKIIα is phosphorylated within 15-30 minutes following injury and is followed by a longer term reduction [Atkins et al., 2006; Griesbach et al., 2009] in the kinase. Interestingly, one report shows that during the acute period of activation, the autophosphorylated form of CaMKIIα appears in some, but not all, of the pyramidal neurons in the hippocampus [Folkerts et al., 2007]. These in vivo data suggest that there is an important early activation of CaMKIIα that could precede the AMPAR modifications that we studied in vitro in this report, and that this activation of the kinase may not be universal across all neurons within specific hippocampal subregions. Moreover, although our data shows a clear increase in CaMKIIα phosphorylation, we measured these changes only at stretch levels that would cause neuronal death after injury. The S-831 site of the GliuR1 subunit is not a high affinity substrate for CaMKIIα, and it has been reported that phosphorylation at this site requires a high level of CaMKIIα activation [Barria et al., 1997]. Thus, it is possible that a significant overall calcium overload, possibly caused by the activation of both NMDAR populations, is necessary for this injury-mediated phosphorylation. We observe a wide range of calcium increases across the cortical neuron population following stretch injury, raising the additional possibility that CaMKIIα activation and AMPAR subunit phosphorylation after injury may vary among individual cells and, at a finer scale, individual synapses. Defining the threshold for consistent CaMKIIα activation, its spatial variation within individual neurons, as well as the downstream receptor subunit phosphorylation changes, would provide more detailed information on specific, injury severity-dependent approaches for addressing the consequences of this kinase activation cascade across a length scale that would span single synapses, dendritic branches, and neuronal ensembles.
Although CaMKIIα activity can lead to the insertion of CP-AMPARs, we are careful not to suggest that phosphorylation at S-831 of the GluR1 subunit alone directly leads to CP-AMPAR surface expression. Studies show that CaMKIIα activity leads to enhanced GluR1 surface expression, possibly through the lateral translocation of the GluR1 subunit [Oh et al., 2006; Hayashi et al., 2000], but CaMKIIα has other non-direct pathways which lead to insertion of CP-AMPARs. CaMKIIα phosphorylation decreases the interaction between GluR1 and its PDZ-domain binding proteins [Hayashi et al., 2000] and phosphorylates a scaffolding protein, SAP-97, both leading to synaptic insertion of the GluR1 subunit. In addition, we should also consider other consequences of S-831 phosphorylation, including a decreased regulation of GluR1 by a binding partner, AKAP79, resulting in an increase in AMPA currents [Mauceri et al., 2004; Nikandrova et al., 2009]. Additionally, the activity of phosphatases that may dephosphorylate AMPAR subunits or their interacting proteins is an area that has been neglected and could clearly alter the injury-mediated effects on AMPA signaling. As more studies detail the exact steps that can regulate the function and subtype of AMPARs following S-831 phosphorylation, these will likely lead to more points of intervention to control the consequences of mechanical injury to neuronal networks.
Given the available knowledge on steps leading to GluR1 phosphorylation, we evaluated if stretch-induced changes in S-831 phosphorylation were uniquely linked to activation of NMDAR subpopulations during mechanical injury. With an increasing number of therapeutic options to target specific NMDAR subtypes, this analysis would also provide insight into possible therapeutic directions for TBI treatment. Our analysis is complicated by the developmental expression of NMDAR subtypes in dissociated neurons, where the expression of the NR2B subunit dominates and NR1/NR2B heteromeric NMDARs are found in both synaptic and extrasynaptic locations in cultures less than two weeks after initial isolation and plating. As the NR2A expression increases in maturing hippocampal and cortical neurons, there are two new types of NMDARs – the NR1/NR2A and NR1/NR2A/NR2B – that appear in synaptic locations. Preliminary testing of our cultures showed that extrasynaptic NMDARs were almost exclusively the NR1/NR2B subtype, but it was also evident that a proportion of NR1/NR2B NMDARs also appeared in the synaptic region. Thus, our pharmacological blockade of synaptic NMDARs and, separately, NR2B-NMDARs likely produces inhibition of some overlapping NMDAR subpopulations and could explain why these two separate strategies produce a significant reduction in the initial calcium influx whose sum is greater than the initial calcium transient observed in uninjured cultures. Given that both strategies show equal effectiveness in reversing the effects of both S-831 phosphorylation and appearance of calcium permeable AMPARs, the most appealing option is targeting NR2B-containing NMDARs. Past work shows that inhibiting synaptic NMDARs will limit the pro-survival signaling through this subpopulation, and therefore the blockade of synaptic NMDARs may produce a net negative effect on neuronal outcome. Alternatively, inhibiting NR1/NR2B NMDARs with an NR2B-specific antagonist will target largely extrasynaptic NMDARs and therefore limit the impact on synaptically-based neuronal signaling. One factor that would need consideration in this approach is the possible emergence of NR1/NR2B NMDARs at the synapse in the traumatically injured brain [Giza, 2006; Osteen, 2004], which would lead to unintended adverse effects NR2B antagonisms if the drug was delivered in the delayed postacute phase of injury. We observed excellent protection against neuronal death using ifenprodil, an alternative NR2B-NMDAR antagonist, if administered 2hr after mechanical injury in organotypic slice cultures. Future work could examine if this therapeutic window would also extend to inhibit the appearance of CP-AMPARs, an important first step in establishing the therapeutic value of this subtype specific approach for treating TBI.
Taken together, our data show that important early changes in the phosphorylation of the GluR1 subunit are mediated by both synaptic and NR2B-containing NMDARs, and the potential mediators of these phosphorylation steps appear as central elements in the appearance of CP-AMPARs following mechanical injury in vitro. Determining exactly how these pathways lead to the expression of CP-AMPARs and the mechanisms that regulate the long-term expression of these receptors will lead to a more complete approach for regulating their appearance in the days to weeks following injury. We have shown that AMPAR modification and kinase activation through NMDAR dependent initiation leads to enhanced AMPAR signaling, which provides several new targets for potential therapies and further investigation.
Highlights.
-Mechanical injury of cortical neurons increases calcium permeable AMPARs (CP-AMPARs)
-Synaptic and NR2B-containing NMDARs contribute to acute calcium influx after injury
-Stretch induced NMDAR activation contributes to AMPAR phosphorylation after injury
-Synaptic and NR2B-containing NMDARs contribute to CP-AMPARs after injury
Acknowledgments
Funding was provided by: NICHD 41699, NS 35712, and from the Commonwealth of Pennsylvania
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkins CM, Chen S, Alonso OF, Dietrich WD, Hu BR. Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:1507–1518. doi: 10.1038/sj.jcbfm.9600301. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Oliva AA, Jr., Alonso OF, Pearse DD, Bramlett HM, Dietrich WD. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007;208:145–158. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKIIα. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during longterm potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Bayir H, Kagan VE, Clark RS, Janesko-Feldman K, Rafikov R, Huang Z, Zhang X, Vagni V, Billiar TR, Kochanek PM. Neuronal NOS-mediated nitration and inactivation of manganese superoxide dismutase in brain after experimental and human brain injury. J Neurochem. 2007;101:168–181. doi: 10.1111/j.1471-4159.2006.04353.x. [DOI] [PubMed] [Google Scholar]
- Bell JD, Park E, Ai J, Baker AJ. PICK1-mediated GluR2 endocytosis contributes to cellular injury after neuronal trauma. Cell Death Differ. 2009:1665–80. doi: 10.1038/cdd.2009.106. [DOI] [PubMed] [Google Scholar]
- Bengtson CP, Dick O, Bading H. A quantitative method to assess extrasynaptic NMDA receptor function in the protective effect of synaptic activity against neurotoxicity. BMC Neurosci. 2008;9:11. doi: 10.1186/1471-2202-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Bredt DS. Targeting nitric oxide to its targets. Proc Soc Exp Biol Med. 1996;211:41–48. doi: 10.3181/00379727-211-43950f. [DOI] [PubMed] [Google Scholar]
- Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J, Kumar P, Mayadevi M, Surolia A, Omkumar RV. Calcium/calmodulin dependent protein kinase II bound to NMDA receptor 2B subunit exhibits increased ATP affinity and attenuated dephosphorylation. PLoS One. 2011;6(3):e16495. doi: 10.1371/journal.pone.0016495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetkovich DM, Sweatt JD. nMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. J Neurochem. 1993;61:1933–1942. doi: 10.1111/j.1471-4159.1993.tb09836.x. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- DeRidder MN, Simon MJ, Siman R, Auberson YP, Raghupathi R, Meaney DF. Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition. Neurobiol Dis. 2006;22:165–176. doi: 10.1016/j.nbd.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007 Feb;8(2):101–13. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Folkerts MM, Parks EA, Dedman JR, Kaetzel MA, Lyeth BG, Berman RF. Phosphorylation of calcium calmodulin-dependent protein kinase II following lateral fluid percussion brain injury in rats. J Neurotrauma. 2007;24:638–650. doi: 10.1089/neu.2006.0188. [DOI] [PubMed] [Google Scholar]
- Fu XZ, Zhang QG, Meng FJ, Zhang GY. NMDA receptor-mediated immediate Ser831 phosphorylation of GluR1 through CaMKIIalpha in rat hippocampus during early global ischemia. Neurosci Res. 2004;48:85–91. doi: 10.1016/j.neures.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Geddes-Klein DM, Serbest G, Mesfin MN, Cohen AS, Meaney DF. Pharmacologically induced calcium oscillations protect neurons from increases in cytosolic calcium after trauma. J Neurochem. 2006;97:462–474. doi: 10.1111/j.1471-4159.2006.03761.x. [DOI] [PubMed] [Google Scholar]
- Giza CC, Maria NS, Hovda DA. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J Neurotrauma. 2006 Jun;23(6):950–61. doi: 10.1089/neu.2006.23.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Holzwarth JA, Miller RJ. Glutamate receptors activate Ca2+ mobilization and Ca2+ influx into astrocytes. Proc Natl Acad Sci U S A. 1990;87(9):3454–8. doi: 10.1073/pnas.87.9.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth PB, Ellis EF, Satin LS. Enhancement of AMPA-mediated current after traumatic injury in cortical neurons. J Neurosci. 1999;19:7367–7374. doi: 10.1523/JNEUROSCI.19-17-07367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goforth PB, Ellis EF, Satin LS. Mechanical injury modulates AMPA receptor kinetics via an NMDA receptor-dependent pathway. J Neurotrauma. 2004;6:719–32. doi: 10.1089/0897715041269704. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016:154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BJ, Ripley B, Ghosh A. NR2B signaling regulates the development of synaptic AMPA receptor current. J Neurosci. 27:13446–13456. doi: 10.1523/JNEUROSCI.3793-07.2007. 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Harris AZ, Pettit DL. Extrasynaptic and synaptic NMDA receptors form stable and uniform pools in rat hippocampal slices. J Physiol. 2007;584:509–519. doi: 10.1113/jphysiol.2007.137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKIIα: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Feig LA. Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling. PLoS One. 2010;5(7):e11732. doi: 10.1371/journal.pone.0011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Takeuchi K, Rodenas-Ruano A, Takayasu Y, Murphy J, Bennett MV, Zukin RS. Regulation of NMDA receptor Ca2+ signalling and synaptic plasticity. Biochem Soc Trans. 2009;37:1369–1374. doi: 10.1042/BST0371369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-C, Huang Z-H, Jan I-S, Yeh C-C, Wu H-J, Chou Y-C, Chang YC. Development of excitatory synapses in cultured neurons dissociated from the cortices of rat embryos and rat pups at birth. J. Neurosci. Res. 2002;67:484–493. doi: 10.1002/jnr.10077. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Zhabotinsky AM. A model of synaptic memory: a CaMKIIα/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron. 2001;31:191–201. doi: 10.1016/s0896-6273(01)00364-6. [DOI] [PubMed] [Google Scholar]
- Lusardi TA, Wolf JA, Putt ME, Smith DH, Meaney DF. Effect of acute calcium influx after mechanical stretch injury in vitro on the viability of hippocampal neurons. J Neurotrauma. 2004;21:61–72. doi: 10.1089/089771504772695959. [DOI] [PubMed] [Google Scholar]
- Mauceri D, Cattabeni F, Di Luca M, Gardoni F. Calcium/calmodulin-dependent protein kinase II phosphorylation drives synapse-associated protein 97 into spines. J Biol Chem. 2004;279:23813–23821. doi: 10.1074/jbc.M402796200. [DOI] [PubMed] [Google Scholar]
- Meaney DF, Smith DH, Shreiber DI, Bain AC, Miller RT, Ross DT, Gennarelli TA. Biomechanical analysis of experimental diffuse axonal injury. J Neurotrauma. 1995;12:689–694. doi: 10.1089/neu.1995.12.689. [DOI] [PubMed] [Google Scholar]
- Nikandrova YA, Jiao Y, Baucum AJ, Tavalin SJ, Colbran RJ. Ca2+/calmodulin dependent protein kinase II binds to and phosphorylates a specific SAP97 splice variant to disrupt association with AKAP79/150 and modulate alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor (AMPAR) activity. J Biol Chem. 2009;285:923–934. doi: 10.1074/jbc.M109.033985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Osteen CL, Giza CC, Hovda DA. Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience. 2004;128(2):305–22. doi: 10.1016/j.neuroscience.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–579. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Bennett MV, Zukin RS. Differential expression of three glutamate receptor genes in developing rat brain: an in situ hybridization study. Proc Natl Acad Sci U S A. 1991;88:4157–4161. doi: 10.1073/pnas.88.10.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhade SN, Zhou C, Aujla PK, Fishman R, Sucher NJ, Jensen FE. Early alterations of AMPA receptors mediate synaptic potentiation induced by neonatal seizures. J Neurosci. 2008;28:7979–7990. doi: 10.1523/JNEUROSCI.1734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau GA, Tukey DS, Garcin-Hosfield ED, Titcombe RF, Misra C, Khatri L, Getzoff ED, Ziff EB. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J Neurosci. 2007;27:3445–3455. doi: 10.1523/JNEUROSCI.4799-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- Spaethling JM, Klein DM, Singh P, Meaney DF. Calcium-permeable AMPA receptors appear in cortical neurons after traumatic mechanical injury and contribute to neuronal fate. J Neurotrauma. 2008;25:1207–1216. doi: 10.1089/neu.2008.0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SE, Wenthold RJ, Soderling TR. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J Neurosci. 1994;14:1123–1129. doi: 10.1523/JNEUROSCI.14-03-01123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gong B, Vadakkan KI, Toyoda H, Kaang BK, Zhuo M. Genetic evidence for adenylyl cyclase 1 as a target for preventing neuronal excitotoxicity mediated by N-methyl-D-aspartate receptors. J Biol Chem. 2007;282(2):1507–17. doi: 10.1074/jbc.M607291200. [DOI] [PubMed] [Google Scholar]
- Wang HG, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, Walter U, Lohmann SM, Hawkins RD, Antonova I. Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45:389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- Wojda U, Salinska E, Kuznicki J. Calcium ions in neuronal degeneration. IUBMB Life. 2008;60:575–590. doi: 10.1002/iub.91. [DOI] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rzigalinski BA, Ellis EF, Satin LS. Reduction of voltage-dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science. 1996;274:1921–1923. doi: 10.1126/science.274.5294.1921. [DOI] [PubMed] [Google Scholar]