Abstract
Objective
The objective of this study was to determine the contribution of lymphatic tissue to heterotopic ossification.
Background
Heterotopic ossification (HO) is the pathologic development of ectopic bone within soft tissues often following severe trauma. Characterization of the tissue niche supporting HO is critical to identifying therapies directed against this condition. Lymphangiogenesis is up-regulated during incidents of trauma, thereby co-incident with the niche supportive of HO. We hypothesized that lymphatic tissues play a critical role in HO formation.
Methods
Mice underwent hindlimb Achilles’ tendon transection and dorsal burn injury(burn/tenotomy) to induce HO. The popliteal and inguinal lymph nodes were excised ipsilateral to the tenotomy site. Flow cytometry and immunostaining were used to quantify and localize lymphoendothelium. MicroCT was used to quantify HO.
Results
Enrichment of mature lymphatic tissues was noted 2 weeks after injury at the tendon transection sites when compared with the contralateral, intact tendon based on LYVE1+ tubules (10.9% v. 0.8%, p<0.05). Excision of the inguinal and popliteal nodes with draining popliteal lymphatic vessel significantly decreased the presence of mature lymphoendothelium 2 weeks after injury (10.9% v. 3.3%, p<0.05). Bone-cartilage-stromal progenitor cells (CD105+/AlphaV+/Tie2−/CD45−/CD90−/BP1−) were also significantly decreased after lymph node excision (10.2% v. 0.5%, p<0.05). A significant decrease was noted in the volume of de novo HO present within the soft tissues (0.12 mm^3 v. 0.02 mm^3).
Conclusions
These findings suggest that lymphatic vessels are intimately linked with the formation de novo bone within soft tissues following trauma, and their presence may facilitate bone formation.
INTRODUCTION
Heterotopic ossification (HO) is a disease of pathologic ectopic bone formation in soft tissue occurring in the context of severe burns, trauma spinal cord injuries and chronic wounds. Growth and maturation of HO appears to occur as a consequence of aberrant wound healing resulting in a number of sequelae including poor wound healing, chronic pain, nerve entrapment, increased risk of infection, and diminished range-of-motion.1,2 Few non-surgical therapies exist and those, including high-dose NSAIDs and radiotherapy, are limited due to adverse effects and poor efficacy.3 Surgical excision, while beneficial, is limited by high rates of recurrence at the surgical site.4
The pathophysiology of HO has not been fully characterized. Mounting evidence suggests that degree of inflammation correlates with increased bone formation after trauma.5,6 However, it is unclear which cellular components of the inflammatory response may contribute to HO. Endothelial networks, specifically the lymphatic endothelium provides a logical link between inflammatory/immunologic signals and the local cellular populations at the site of HO development. Lymphangiogenesis and lymphatic trafficking after trauma is a necessary part in the initiation and regulation of the immunologic response.7 Furthermore, intact lymphatic drainage has been described in both adult fracture healing and in the normal development of bone, cartilage, and soft tissues.8–10
In this study, we evaluate the contribution of intact lymphatic drainage to the development of de novo ectopic bone within soft tissues using a model of trauma-induced HO.11 In particular, we identify the impact on a well-described population of bone-cartilage-stromal progenitor (BCSP) cells present within HO lesions, and overall bone volume.12 To disrupt lymphatic flow we relied on a model of combined superficial inguinal and popliteal lymphadenectomy.13 After combined lymphadenectomy the distal hindlimb remains lymphatically isolated for ~2 weeks. By combining these two models we tested whether lymphatic isolation has any effect on the cellular niche contributing to HO or on the development of ectopic bone itself.
METHODS
Animals
All animal procedures were carried out in accordance with the guidelines provided in the Guide for the Use and Care of Laboratory Animals from the Institute for Laboratory Animal Research (ILAR, 2011) and were approved by the Institutional Animal Care and Use Committee of the University of Michigan (PRO0005909). For our trauma model, young (6–8 weeks) C57BL/6J female mice from Charles River (Wilmington, MA) were used.
Animals were divided into two experimental groups, those receiving a burn plus Achilles tenotomy (n=11) and those receiving burn tenotomy (n=9) with additional regional lymphadenectomy. Five animals from each group were allocated to FACS analysis, 4 animals to uCT analysis, and two animals from the burn tenotomy group were sacrificed for histologic analysis.
Burn Trauma Model
All mice used for analysis received a partial thickness burn injury as previously described.11 Briefly, animals were anesthetized with 3–5% inhaled isoflurane. Hair was closely clipped on the left dorsum to expose the skin. Partial-thickness burn was achieved by placing a metal brand, heated to 60°C in a water bath, against the exposed skin for 18 seconds. Each mouse then received a concurrent Achilles tenotomy with sharp dissection at the midpoint in the left leg. Pain management was achieved with subcutaneous injections of buprenorphine every 12 hours for 3 days. Movement was not restricted after injury and mice were able to walk on injured limb as tolerated.
Regional Lymphadenectomy
Regional lymphatic isolation was achieved through the combined popliteal and superficial inguinal lymph node excision ipsilateral to the site of injury. While anesthetized hair from the abdomen and posterior to the popliteal fossa was closely clipped. Lymph nodes were access via sharp dissection at the site of the popliteal fossa or along the lateral abdomen as previously described.13 Control animals received a skin incision comparable to that necessary to access the nodes without disruption of the popliteal fossa or inguinal fat pad.
Histologic Processing and Analyses
At 1- and 3-weeks animals (n=1 per time point) were euthanized for histology alongside uninjured controls. The distal hindlimb was removed by sharp dissection at the knee and skin was removed. Samples were fixed in buffered formalin solution for 24 hours at 4°C. Decalcification of the sample was completed with 19% ethylenediaminetetracetic acid (EDTA) solution for 28–42 days at 4°C. Decalcified tissues were dehydrated through graded ethanol, and paraffin embedded. Transverse sections from all sampled were completed with a width of 5 microns and mounted on Superfrost plus slides (Fisher Scientific, Pittsburg, PA). Paraffin samples were dried overnight at 37°C while cryo-sectioned slices were stored at −80°C.
Immunofluorescent staining was performed as previously described for LYVE1 (sc-19319, Santa Cruz), CDH5 (sc-28644, Santa Cruz), and Sox9 (sc-20095, Santa Cruz). Briefly, sections were deparaffinized and rehydrated in xylenes and graded ethanol. Antigen retrieval was performed with Citrate solution pH 6.0. Samples were then quenched for autofluorescence in 3% glycine before blocking and permeabilization. Primary antibodies were applied overnight at 4°C. Appropriate dilutions were determined prior to achieving final images. After washing, fluorescently conjugated secondary antibodies tagged with either AlexaFluor 488 or AlexaFluor 594 (Life Technologies) were applied. Nuclear counterstain was achieved using Hoechst 33342 (Life Technologies) and mounted with ProLong Gold Antifade Reagent (Life Technologies). Appropriate primary antibody, secondary antibody, and autofluorescent controls were run simultaneously with each tested sample.
Microscopy
All fluorescently stained images were taken using an Olympus BX-51 upright light microscope equipped with standard DAPI, 488, and TRITC reflector cubes attached to an Olympus DP-70 high resolution digital camera. Images were visualized in Adobe Photoshop to perform vessel counts and to overlay cells for co-staining.
Vessel Counts
Sections from the middle body an uninjured Achilles tendon and from areas of heterotopic ossification developing 3-weeks after burn tenotomy were analyzed for the presence of LYVE1+ ring structures. Counts were made across three section in 5 high-powered fields per section. LYVE1+ cells not forming clear endothelial ring structures were excluded from analysis.
Flow Cytometry
FACS analysis was performed on soft tissue collected from the site of injury in a defined area from the muscular attachment of the Achilles tendon superiorly to its insertion at the calcaneus inferiorly. A similar site was harvest from contralateral uninjured legs encompassing the entirety of the uninjured tendon and surrounding soft tissue.
Tissue was digested for 120 minutes in 0.75% Collagenase 2 (Sigma-Aldrich) in Hanks Balanced Salt Solution (HBSS) at 37°C under constant agitation. Samples were filtered using a 70-micron sterile strainer and centrifuged at 800 rpm for 5 minutes before removing the supernatant and washing in HBSS. This process was repeated three times before incubation with fluorescently labeled antibodies.
Antibodies used included: AlphaV-PE (12-0512-83, eBioscience), BP1-Biotin (13-5891-85, eBioscience), CD31-APC (551262, BD Pharmingen), CD34-eFluor450 (48-0341-82, eBioscience), CD45-APC (17-0451-83, eBioscience), CD90-PE-Cyanine7 (25-0902-82, eBioscience), CD105-eFluor450 (48-1057-42, eBioscience), CD133-AF488 (11-1331-82, eBioscience), Podoplanin-PE-Cyanine7 (25-5381-80, eBioscience), Tie2-AlexaFluor 488 (334208, BioLegend), Tie2-PE (12-5987-83, eBioscience), and VEGFR3-Biotin (13-5988-85, eBioscience) conjugated with Streptavidin-PerCP-Cyanine5.5 (45-4317-82, eBioscience).
Following thirty minutes of incubation at 4°C, sample were washed and filtered through a 45 micron mesh filter before being run on a FACSAria II (BD Biosciences) Cell Sorter at the University of Michigan Flow Cytometry Core in the Biomedical Science Research Center. Values were normalized to total viable cells to control for possible differences in the amount of tissue harvested. Data was then analyzed using the FlowJo software
uCT Analysis
Mouse hind limbs were imaged with μCT at 9-weeks post-injury (μCT: Siemens Inveon, using 80kVp, 80mA and 1100 ms exposure). Images were reconstructed and ectopic bone volume formation was calculated. All scans were analyzed with threshold Hounsfield units (HU) of 800 (total), 1250 (intermediate-density), or 1800 (high-density) to determine the volume of tissues.
Samples were then further analyzed to look at the arrangement of ectopic tissue with abnormal bone in close proximity, adjacent to, or contiguous with native bone being defined as bone associated HO. Ectopic tissue present in muscle or soft tissue areas and not immediately adjacent to native bone was defined as soft tissue HO
Statistical analysis
Data were analyzed using SPSS software (v21, IBM). Means and standard deviations were calculated from numerical data and statistical analysis was performed using Welch’s t-test. Kurtosis values were calculated to confirm normality of data. In figures, bar graphs represent means, whereas error bars represent one standard deviation. For all assays, significance was defined as a p < 0.05.
RESULTS
Lymphatic endothelium is enriched at the tendon transection site, and contributes tissue to the developing HO anlagen
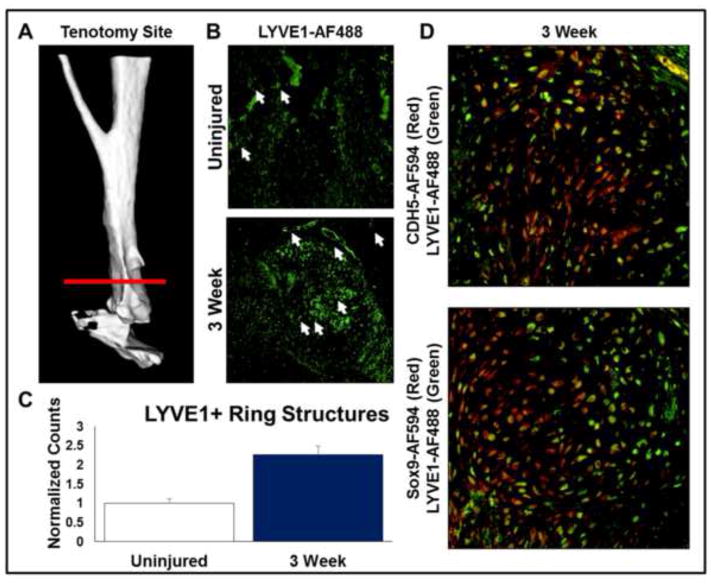
First we sought to demonstrate the presence and enrichment of lymphatic vessels at the tendon transection site (Fig. 1A). Based on histologic analysis, LYVE1+ cells in a tubule formation were significantly increased at the tenotomy site 3 weeks after injury by 2.3-fold (p<0.05) when compared with the contralateral, uninjured hindlimb (Fig. 1B,C). To further characterize the location of these LYVE1+ cells, histologic evaluation was performed; LYVE1+ cells were evident in fibroproliferative and chondrogenic centers of the tenotomy site (Fig 1D). Furthermore, these tissues co-stained with both vascular (VeCadherin) and cartilaginous (Sox9) markers in the anlagen suggesting a contribution to the developing HO anlagen (Fig 1D).
Figure 1. Endothelial infiltration is increased during development of the developing HO anlagen after trauma.
A. 3D Micro-CT of distal hindlimb demonstrating site of tenotomy and subsequent HO formation (white arrows – LYVE1+ ring structures). B. LYVE1 stain (green) demonstrating enrichment of lymphatic vessels in the early HO anlagen. C. Quantification of lymphatic endothelial vessels counted from the site of HO formation and contralateral uninjured controls. D. Immunofluorescent imaging demonstrating co-localization of LYVE1+ (green) cells with SOX9 and CDH5 (red) in the developing HO anlagen (white arrows – LYVE1+ ring structures).
Lymphatic enrichment after trauma is abrogated by lymph node excision
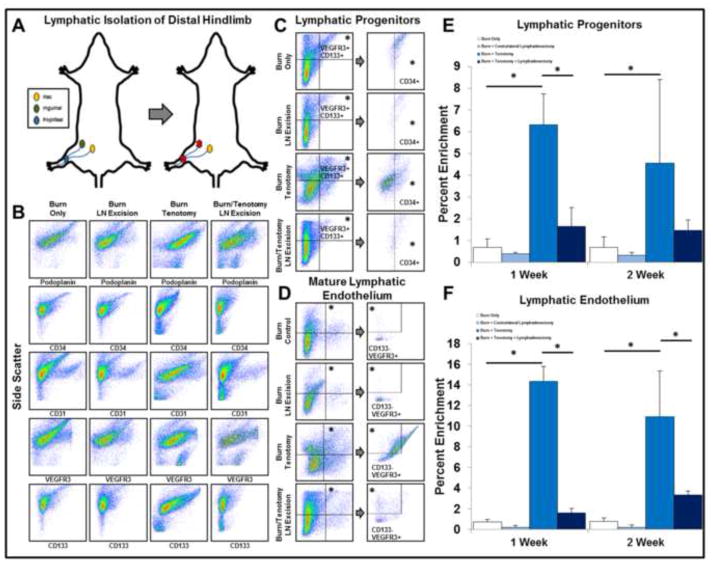
Given the presence of lymphatic tissues in the developing anlagen, we sought to examine whether this lymphatic enrichment could be manipulated. Therefore, we used a previously validated model of lymph node excision to evaluate its impact on tenotomy site lymphatic tissue (Fig. 2A).13 Mice in this model exhibit delayed lymphatic drainage and immunologic recognition without evidence of significant lymphedema in the hindlimb (S.Fig 1A).13
Figure 2. Lymphatic isolation of the distal hindlimb results in abrogation of lymphagiogenic markers at the site of tenotomy.
A. Model of lymphatic isolation of the distal hindlimb via combined popliteal and inguinal lymph node excision. B. Lymphatic isolation reduces the enrichment of lymphatic endothelial and progenitor markers at the site of tenotomy. C. Flow panel identifying lymphatic progenitor cells (LPCs: CD34+/VEGFR3+/CD133+). D. Flow panel identifying mature lymphatic endothelium (MLE: CD31/+Podoplanin+/VEGFR3+/CD133−). E. Quantitative analysis of lymphatic progenitors in the distal hindlimb in the presence or absence of injury models. F. Quantitative analysis of lymphatic endothelium in the distal hindlimb in the presence or absence of injury models. All error bars represent standard deviation. Black asterisks on graph represent p<0.05.
Samples were obtained at each time point from the site of tendon transection in mice either with or without regional lymph node excision (burn/tenotomy and burn/tenotomy/LN excision). Contralateral uninjured Achilles tendons were collected as internal controls (burn only and burn/LN wxcision). At 1 and 2 weeks following burn/tenotomy, there was enrichment of immature (CD133) and mature (CD31) endothelial markers at the site of tendon transection when compared with the burn/tenotomy/LN excision group (CD133: 1 Week: 8.2-fold increase; 2 Weeks: 9.0-fold increase; p<0.05) and mature (CD31: 1 Week: 3.9-fold increase; 2 Weeks: 3.7-fold increase; p<0.05) (Fig. 2B).
Given this endothelial cell profile, we assessed our tissues for the presence of lymphatic progenitor cells (LPC: CD34+/VEGFR3+/CD133+)14 and mature lymphatic endothelium (MLE: CD31+/Podoplanin+/VEGFR3+/CD133−)14–16 in injured and contralateral limbs in the presence or absence of regional lymphadenectomy (Fig 2C,D). We found that LPCs and MLE were both significantly enriched in the burn/tenotomy when compared with the burn only limbs (LPC: 1-Week: 6.3% v. 0.7%, 2-Week: 4.6% v. 0.7%; MLE: 1-Week: 14.3% v. 0.7%; 2-Week: 10.9% v. 0.7%) (Fig 2E,F). Strikingly, we found that LPCs and MLE were both significantly decreased in the burn/tenotomy/LN excision group versus burn/tenotomy (LPC: 1-Week: 6.3% v. 1.6%, 2-Week: 4.6% v. 1.3%; MLE: 1-Week: 14.3% v. 1.6%; 2-Week: 10.9% v. 3.3%) (Fig 2E,F). Taken together, these findings suggest that lymph node excision actually modulates the growth of lymphatic vessels within trauma sites.
Enrichment of osteogenic precursors in developing HO requires intact lymphatics
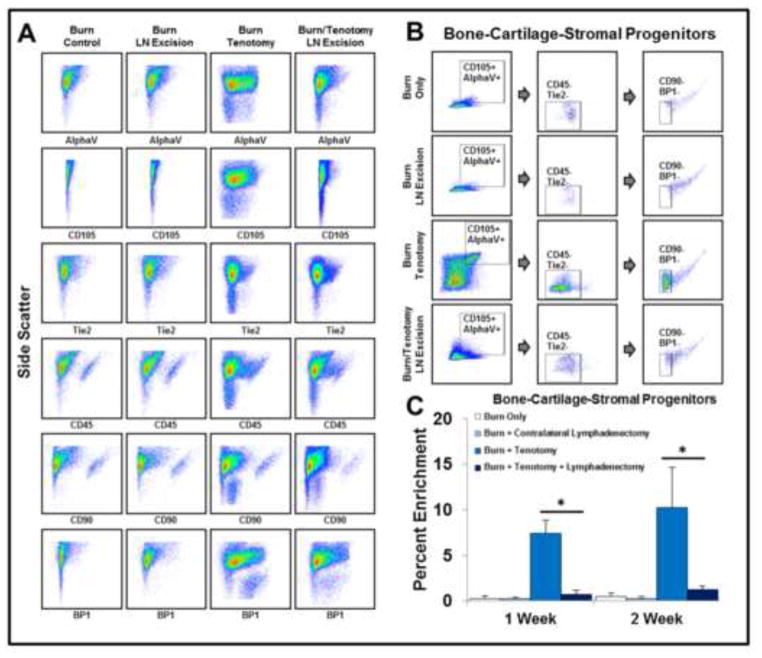
Given the finding that lymph node excision modulates the growth of lymphatic tissue within trauma sites, we next sought to understand the impact on pathologic extra-skeletal bone formation. Therefore, we first investigated the impact of lymph node excision on a population of bone-cartilage-stromal progenitors (BCSP: CD105+/AlphaV+/CD45−/Tie2−/CD90−/BP1−). These cells have previously been shown to develop into bone through endochondral ossification similar to the development of HO.12 Flow cytometry demonstrated enrichment of CD105+ (1-Week: 14.7 v. 5.3%; 2-Week: 17.4 v. 4.8%) and AlphaV+ (1-Week: 41.9 v. 12.5%; 2-Week: 47.1% v. 13.9%) cells in mice with burn/tenotomy when compared to the burn/tenotomy/LN excision group (Fig. 3A). Interestingly, CD45+ cells were significantly diminished in injured hindlimbs receiving lymph node excision when compared with burn/tenotomy alone after 1 week (33.2% v. 27.2%). However, after 2 weeks, the levels of CD45+ cells at the tenotomy site in burn/tenotomy and burn/tenotomy with lymph node excision mice were similar (Fig. 3A).
Figure 3. Enrichment of bone-cartilage-stromal progenitors in early HO requires intact regional lymph nodes.
A. Lymphatic isolation reduces the enrichment of CD105+ and AlphaV+ at the site of tenotomy. B. Flow panel identifying BCSPs in the distal hindlimb (BCSP: CD105+/AlphaV+/CD45−/Tie2−/CD90−/BP1−). C. Quantitative analysis of BCSPs in the distal hindlimb in the presence or absence of injury models. All error bars represent standard deviation. Black asterisks on graph represent p<0.05.
We next assessed the presence and enrichment of BCSPs in our samples using the complete panel (CD105+/AlphaV+/CD45−/Tie2−/CD90−/BP1−) (Fig 3B). BCSPs were significantly enriched at both 1 and 2 weeks after injury when compared with contralateral controls (1-Week: 7.4% v. 0.3%; 2-Week: 10.2% v. 0.5%) (Fig. 3C). This effect was significantly decreased at 1-week in mice receiving lymph node excision (1-Week: 7.4% v. 0.7%; 2-Week: 10.2% v. 1.2%) (Fig 3C). Taken together, these findings suggest that manipulation of lymph nodes alters the enrichment of BCSPs at the tendon injury site.
De novo ectopic bone formation within soft tissue is significantly decreased with lymph node excision
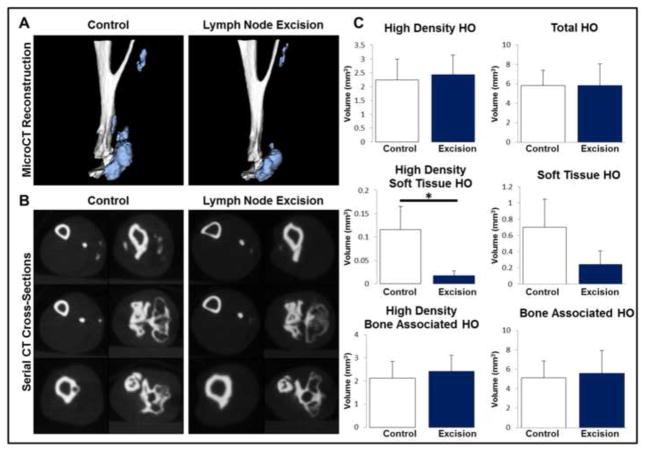
We next determined the effect of lymph node excision on HO volume in burn/tenotomy mice. Mice were euthanized 9 weeks after injury and microCT performed to quantify HO and native tibial volume (Fig. 4A; S.Fig 1B,C). Lymph node excision did not significantly alter tibial volume, however, tissue mineral density analysis demonstrated a significant increase when compared with mice receiving burn/tenotomy only (992.52 mg/cc vs. 1014.02 mg/cc; p<0.05; S.Fig 1B,C). Sites of de novo heterotopic bone within soft tissue were significantly diminished in volume in mice with lymph node excision, when compared with mice which did not have lymph node excision (0.12 mm^3 v. 0.02 mm^3, p<0.05) using a threshold of 1250 Hounsfield Units (Fig 4A–C). The decrease in total soft tissue HO volume approached statistical significance (0.70 mm^3 v. 0.24 mm^3, p=0.11) (Fig 4C). Finally, total, combined HO volume quantified as a combination of ectopic bone growing within soft tissues and ectopic bone growing in continuity with the calcaneus were similar between groups (5.8 mm^3 v. 5.8 mm^3) (Fig 4C).
Figure 4. Proximal but not total ectopic bone volume 9-weeks after tenotomy is diminished by regional lymphatic isolation.
A. Representative 3D micro-CT reconstructions of the distal hindlimb demonstrating the pattern of HO formation after trauma in the presence or absence of regional lymphadenectomy. B. Serial cross-sections of the distal hindlimb demonstrating the localization of ectopic bone. C. Quantification of total, proximal, and bone-associated HO volumes in the distal hindlimb. All error bars represent standard deviation. Black asterisks on graph represent p<0.05.
DISCUSSION
In this study, we focus on the relationship between lymphatic vessels and de novo ectopic bone, as forms during trauma-induced heterotopic ossification. Because trauma is followed by inflammation, and lymphangiogenesis contributes to inflammation, we sought to determine whether lymphangiogenesis can be modulated to alter ectopic bone formation. Evidence from clinical and animal models suggests that degree of inflammation correlates positively with ectopic bone volume.5,6
Regional lymph node excision within the hindlimb is a simple surgical technique to temporarily disrupt lymphatic flow, as has been previously described.13 However, our findings uniquely indicate that lymph node excision actually modulates the presence of lymphatic progenitor cells and mature lymphatic endothelium in response to soft tissue injury (e.g. tendon transection). It is particularly interesting to note that accumulation of mature (CD31) and immature (CD133) endothelial markers was abrogated by regional lymphadenectomy suggesting some role of either regional nodes or intact lymphatic drainage in the generation of the lymphangiogenic response to trauma. This finding is striking, and may represent modulation of the inflammatory response.17–22 Therefore, it is possible that excision of the hindlimb lymph nodes may alter lymphatic vessel formation by mediating inflammation.
Importantly, we found that a population of bone-cartilage-stromal progenitor cells (BCSP: CD105+/AlphaV+/CD45−/Tie2−/CD90−/BP1−) are diminished in the tenotomy site in the setting of lymph node excision even 2 weeks after injury. Again, this may represent a sequela of diminished inflammation at the trauma site. However, that CD45+ cells are similar between injury sites of mice with and without lymph node excision suggests relatively similar levels of overall inflammatory components. It should be noted that BCSPs have recently been shown to undergo de novo endochondral ossification, and were the only population of cells capable of this in a developing bone fractions.12,22. Despite similar levels of CD45+ cells, it is likely that specific components of the inflammatory infiltrate are depleted with lymph node excision. Pro-osteogenic monocyte and lymphocyte populations could thus be altered in a way that is not reflected by CD45+ alone.17–22,23–26 Inhibition of monocyte/macrophage homing/antigen presentation could potentially also lead to the increased in tibial TMD secondary to perturbations in osteoclast homeostasis and is a logical next step for future studies.26
In addition to our demonstration of decreased BCSP populations within the tenotomy site, we also noted a decrease in de novo soft tissue ectopic bone in mice which received lymph node excision. These findings were significant when assessing higher density HO (HU: 1,250 units) and approached significance when assessing both low and high density HO (p=0.11). These findings suggest that modulation of lymphatic vasculature may decrease de novo ectopic bone formation. This effect may be mediated through a decrease in BCSP populations, or in general through its effect on inflammation. However, we cannot discount the fact that overall HO when including calcaneal HO, was not altered with lymph node excision. It is possible that the cellular niche which supports de novo HO within soft tissues is distinct from that which forms along the calcaneus. In particular, HO which forms along the calcaneus may rely more on continuous growth from nearby skeletal structures such as the periosteum, whereas soft tissue HO must form in the absence of these nearby contributors. In the case of soft tissue HO then, the presence or absence of lymphatic vessels and/or BCSP populations may have a more marked impact on HO volume.
The presence of LYVE1+ cells in the HO anlagen highlights a potentially interesting relationship between developing HO and lymphatic endothelium. Heterotopic ossification is characterized by the aggregation of mesenchymal stem cells (MSCs) prior to chondrification.27 MSCs have also previously been shown to support the proliferation of lymphatic endothelium, suggesting that HO may drive its own lymphatic invasion.26 This is supported by the significant upregulation of AlphaV+ and CD105+ cells at 1- and 2- weeks after injury. Enrichment of both AlphaV+ and CD105+ populations have been linked to lymphangiogenic invasion and metastasis in tumor models.28–32 It is also important to consider the timing of CD45 enrichment. Previously, lymphatic isolation of murine tissue was reported as being a temporary process with reconstitution of lymphatic drainage occurring some time 14-days post-surgery. This is consistent with the normalization of CD45+ between treatment groups by 2-weeks after injury and may suggest that surgical lymphatic isolation is too temporary a process to affect long term development of ectopic bone.13
The results of this study present a novel link between lymphatic endothelium and trauma-induced HO. Ectopic bone forms in several other conditions such as fibrodysplasia ossificans progressiva (FOP) and ossification of the posterior longitudinal ligament (OPLL). Patients with FOP have a hyperactivating mutation of the type I bone morphogenetic protein (BMP) receptor ACVR1 (ACVR1 R206H) and develop ectopic bone lesions within soft tissues after even minor trauma33. The pathogenesis of OPLL remains poorly understood although genetic risk factors have been identified34. In both of these clinical scenarios of ectopic bone however, the role of lymphatic drainage remains unexplored. In these conditions and trauma-induced HO, it is critical to determine whether lymphatic isolation may alter HO formation, and what the impact of lymphedema or lymphatic injury may be on the pathogenesis of ectopic bone. Thus while surgical lymph node excision in our study has provided an interesting link between bone formation and lymphatic drainage, further evaluation is required to determine whether this presents a promising area of therapeutic intervention.
CONCLUSION
Here we identify a previously unpublished link between the lymphatic system and the development of ectopic bone after trauma. Our data highlight a potential avenue for exploration of immunologic recognition and priming in the study of HO and suggests that aberrations in the normal endothelial response to injury may play a more complicated role in the pathogenesis of ectopic bone formation than previously known.
Supplementary Material
A. Photographs of mouse hindlimbs 9 weeks after burn/tenotomy in the presence or absence of regional lymph node excision demonstrating no appreciable lymphedema. B. Representative 3D micro-CT reconstructions of the distal tibia demonstrating normal morphology. C. Quantitative evaluation of the volume and total mineral density of native tibia at the site of HO formation in the presence or absence of lymph node excision.
Acknowledgments
Funding: BL funded by 1K08GM109105-01, Plastic Surgery Foundation National Endowment Award, American Association of Plastic Surgery Award. SJL funded by a Howard Hughes Medical Institute research fellowship. SA funded by the NIH LRP, Coller Society, and Plastic Surgery Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
S.L and S.A. researched the data, wrote the manuscript, and edited figures. M.S and C.B. researched the data and edited figures. J.L., J.P., and H.H.S. researched the data. S.W. and B.M. reviewed/edited the manuscript. B.L. researched the data, developed the idea, analyzed the data, reviewed/edited manuscript.
References
- 1.Perosky JE, Peterson JR, Eboda ON, et al. Early detection of heterotopic ossification using near-infrared optical imaging reveals dynamic turnover and progression of mineralization following Achilles tenotomy and burn injury. J Orthop Res. 2014;32:1416–1423. doi: 10.1002/jor.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polfer EM, Forsberg JA, Fleming ME, et al. Neurovascular entrapment due to combat-related heterotopic ossification in the lower extremity. J Bone Joint Surg Am. 2013;95:e195(1)–e195(6). doi: 10.2106/JBJS.M.00212. [DOI] [PubMed] [Google Scholar]
- 3.Nauth A, Giles E, Potter BK, et al. Heterotopic ossification in orthopaedic trauma. J Orthop Trauma. 2012;26:684–688. doi: 10.1097/BOT.0b013e3182724624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee EK, Namdari S, Hosalkar HS, et al. Clinical results of the excision of heterotopic bone around the elbow: a systematic review. J Shoulder Elbow Surg. 2013;22:716–722. doi: 10.1016/j.jse.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Forsberg JA, Potter BK, Polfer EM, et al. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res. 2014;472:2845–2854. doi: 10.1007/s11999-014-3694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavey GJ, Qureshi AT, Hope DN, et al. Bioburden Increases Heterotopic Ossification Formation in an Established Rat Model. Clin Orthop Relat Res. 2015;473:2840–2847. doi: 10.1007/s11999-015-4272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aller MA, Arias JL, Sánchez-Patán F, et al. The inflammatory response: an efficient way of life. Med Sci Monit. 2006;12:RA225–234. [PubMed] [Google Scholar]
- 8.Melrose J, Little CB. Immunolocalization of lymphatic vessels in human fetal knee joint tissues. Connect Tissue Res. 2010;51:289–305. doi: 10.3109/03008200903318287. [DOI] [PubMed] [Google Scholar]
- 9.Szczesny G, Olszewski WL, Gewartowska M, et al. The healing of tibial fracture and response of the local lymphatic system. J Trauma. 2007;63:849–854. doi: 10.1097/01.ta.0000236641.51515.8f. [DOI] [PubMed] [Google Scholar]
- 10.Szczesny G, Olszewski WL, Gorecki A. Lymphoscintigraphic monitoring of the lower limb lymphatic system response to bone fracture and healing. Lymphat Res Biol. 2005;3:137–145. doi: 10.1089/lrb.2005.3.137. [DOI] [PubMed] [Google Scholar]
- 11.Peterson JR, De La Rosa S, Sun H, et al. Burn injury enhances bone formation in heterotopic ossification model. Ann Surg. 2014;259:993–998. doi: 10.1097/SLA.0b013e318291da85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan CK, Lindau P, Jiang W, et al. Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proc Natl Acad Sci U S A. 2013;110:12643–12648. doi: 10.1073/pnas.1310212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal S, Loder S, Wood S, et al. Engendering allograft ignorance in a mouse model of allogeneic skin transplantation to the distal hind limb. Ann Surg. 2015;261:611–618. doi: 10.1097/SLA.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salven P, Mustjoki S, Alitalo R, et al. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 15.Al-Rawi MA, Mansel RE, Jiang WG. Molecular and cellular mechanisms of lymphangiogenesis. Eur J Surg Oncol. 2005;31:117–121. doi: 10.1016/j.ejso.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Kato S, Shimoda H, Ji RC, et al. Lymphangiogenesis and expression of specific molecules as lymphatic endothelial cell markers. Anat Sci Int. 2006;81:71–83. doi: 10.1111/j.1447-073X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 17.Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005;115:2316–2319s. doi: 10.1172/JCI26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 19.Kataru RP, Jung K, Jang C, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama K, Li M, Cursiefen C, et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji RC. Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell Mol Life Sci. 2012;69:897–914. doi: 10.1007/s00018-011-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J Leukoc Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- 23.Chan CK, Seo EY, Chen JY, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smyth DC, Takenaka S, Yeung C, et al. Oncostatin M regulates osteogenic differentiation of murine adipose-derived mesenchymal progenitor cells through a PKCdelta-dependent mechanism. Cell Tissue Res. 2015;360:309–319. doi: 10.1007/s00441-014-2099-y. [DOI] [PubMed] [Google Scholar]
- 25.Guihard P, Danger Y, Brounais B, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells. 2012;30:762–772. doi: 10.1002/stem.1040. [DOI] [PubMed] [Google Scholar]
- 26.Hienz SA, Paliwal S, Ivanovski S. Mechanisms of Bone Resorption in Periodontitis. J Immunol Res. 2015:615486. doi: 10.1155/2015/615486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staines KA, Pollard AS, McGonnell IM, et al. Cartilage to bone transitions in health and disease. J Endocrinol. 2013;219:R1–R12. doi: 10.1530/JOE-13-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maertens L, Erpicum C, Detry B, et al. Bone marrow-derived mesenchymal stem cells drive lymphangiogenesis. PLoS One. 2014;9:e106976. doi: 10.1371/journal.pone.0106976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felding-Habermann B, Fransvea E, O’Toole TE, et al. Involvement of tumor cell integrin alpha v beta 3 in hematogenous metastasis of human melanoma cells. Clin Exp Metastasis. 2002;19:427–436. doi: 10.1023/a:1016377114119. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann UB, Westphal JR, Van Kraats AA, et al. Expression of integrin alpha(v)beta(3) correlates with activation of membrane-type matrix metalloproteinase-1 (MT1-MMP) and matrix metalloproteinase-2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer. 2000;87:12–19. doi: 10.1002/1097-0215(20000701)87:1<12::aid-ijc3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Sato H, Takino T. Coordinate action of membrane-type matrix metalloproteinase-1 (MT1-MMP) and MMP-2 enhances pericellular proteolysis and invasion. Cancer Sci. 2010;101:843–847. doi: 10.1111/j.1349-7006.2010.01498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seon BK, Haba A, Matsuno F, et al. Endoglin-targeted cancer therapy. Curr Drug Deliv. 2011;8:135–143. doi: 10.2174/156720111793663570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatsell SJ, Idone V, Wolken DM, et al. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to Activin A. Sci Transl Med. 2015;7:303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsunaga S, Sakou T. Ossification of the posterior longitudinal ligament of the cervical spine: etiology and natural history. Spine. 2012;37:E309–E314. doi: 10.1097/BRS.0b013e318241ad33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Photographs of mouse hindlimbs 9 weeks after burn/tenotomy in the presence or absence of regional lymph node excision demonstrating no appreciable lymphedema. B. Representative 3D micro-CT reconstructions of the distal tibia demonstrating normal morphology. C. Quantitative evaluation of the volume and total mineral density of native tibia at the site of HO formation in the presence or absence of lymph node excision.