Abstract
The red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) is a serious pest of the palm tree in tropical regions of the world. One strain of Metarhizium sp. ZJ-1, isolated from Chinese soils, was evaluated for growth characteristics, and screened for its virulence to R. ferrugineus larvae in laboratory conditions. An approximately 685-bp fragment was amplified by ITS (ITS1-5.8S-ITS2) PCR from strain ZJ-1, further phylogenetic analysis revealed that 93 % similarity to Metarhizium anisopliae. Inoculation of 1 × 108 conidia/mL caused 100 % mortality of R. ferrugineus, LT50 levels of ZJ-1 were 1.66 days (1 × 108 conidia/mL), indicating that the conidia of strain ZJ-1 were highly virulent. These results suggest that M. anisopliae ZJ-1 has potential as an effective and persistent biological control agent for R. ferrugineus.
Keywords: Metarhizium anisopliae, Red palm weevil, ITS, Mortality, Bioassay
Background
Invasive red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae), is one of the most severe pests of various palm species (Tagliavia et al. 2014). It was first observed in India, but now is widely distributed in Asia, Africa, Central America, South America, and the Caribbean, as well as in Curaçao, Netherlands Antilles (Fiaboe and Roda 2012; Kehat 1999).
In China, R. ferrugineusis was considered as quarantine pest and has 19 species of 15 palm genera (Kehat 1999; Dembilio et al. 2009). According to the international standards for pest measurements (ISPM), the results from an R. ferrugineus pest risk analysis model showed that its evaluation is close to that of a high-level quarantine pest. This indicates that R. ferrugineus can easily settle in China, where it potentially poses a great threat to palm trees (Wu et al. 2007).
Rhynchophorus ferrugineus can spread within tree trunks, destroy the vascular system, eventually, the trees soon died (Fetoh 2011). Female R. ferrugineus lay eggs in injured trunks of established palms, associated frond bases, tree crowns, and offshoots. R. ferrugineus larvae bore into palm crowns, trunks, and offshoots and are generally invisible until the infected palms are dying (Pinhas et al. 2008). According to integrated pest management strategy, several control methods have been used against R. ferrugineus invasion of palms. Some of these methods include cutting down and burning infected palms, trapping adult R. ferrugineus, chemical control, host plant resistance, and male sterile techniques (Faleiro 2006). Aside from these, numerous entomopathogenic fungi (Metarhizium anisopliae and Beauveria abassiana) isolated from R. ferrugineus in the Middle East have been evaluated for biocontrol of associated pest insects (Gindin et al. 2006). However, biocontrol has not been widely used, although it is friendly to the environment and harmless to humans. Despite the economic importance of investigations of infection patterns and histopathology of these entomopathogenic fungi in selected insects, no study has documented the histopathology of entomopathogenic fungi in R. ferrugineus (Toledo et al. 2010).
A new Chinese variety of M. anisopliae ZJ-1, characterized by high virulence and low repellency to R. ferrugineus, was obtained. In the present study, we sequenced the ITS1-5.8S-ITS2 region of M. anisopliae strain ZJ-1 and identified M. anisopliae within infected R. ferrugineus larvae.
Results
Fungal morphology of fungi
Sporulation color in Metarhizium can be light to very dark green (Bischoff et al. 2006), and while green sporulation is characteristic of Metarhizium spp., it cannot be considered as diagnostic, because other fungi also produce green spores. The morphology of strain ZJ-1 was typical of the genus M. anisopliae. It grew fast on SDAY and PDA plates. Round colonies on agar plates were white or cream, with villous and gossypine aerial hyphae on the surface. Punctiform olive green formed in the middle of the colony, which turned darker during growth and resulted in a tawny color at the back of the plates.
The morphology of hyphae and conidial of M. anisopliae ZJ-1 from R. ferrugineus cadavers were showed. The mycelium was smooth and hyaline, well-branched and separated, with major hyphae up to 2.0–3.2 μm wide. Conidiophores were podgy, simple, or highly branched, with 2–5 sterigma at the branch top. Conidia were colorless, elliptical, and cylindrical, with obtuse ends. Most conidia were 4.8–5.5 μm × 2.0–2.5 μmin length. Growth temperatures were between 10 and 38 °C, with an optimum of 28 °C.
Molecular identification
An approximately 685 bp fragment was amplified by ITS (ITS1-5.8S-ITS2) PCR from strain ZJ-1. The ITS sequence was submitted to GenBank (accession No. JN377427). The ZJ-1 sequence bore 93 % similarity to that of M. anisopliae (FJ545294) according to an NCBI Blast search. This result indicated that strain ZJ-1 could represented a new species and possibly a member of a new genus.
The dendrogram bifurcated into two distinct clades (1 and 2) with a dissimilarity of 0.02 (Fig. 1). Clade 1 consists of four additional clusters, in which isolates ZJ-1, M. anisopliae, M. pinghaense, M. album, and M. robertsii are identical (cluster I); M. guizhouense, M. majus, M. acridum, and M. minus show a high similarity (cluster II, cluster IV); and cluster IV separates from the other clusters. Clade 2 is represented by two large clusters (V and VI); these sequences show the lowest similarity to the ITS sequences of the isolate ZJ-1 used in this investigation. The origin of 17 kinds of Metarhizium ITS sequences are shown in Table 1.
Fig. 1.
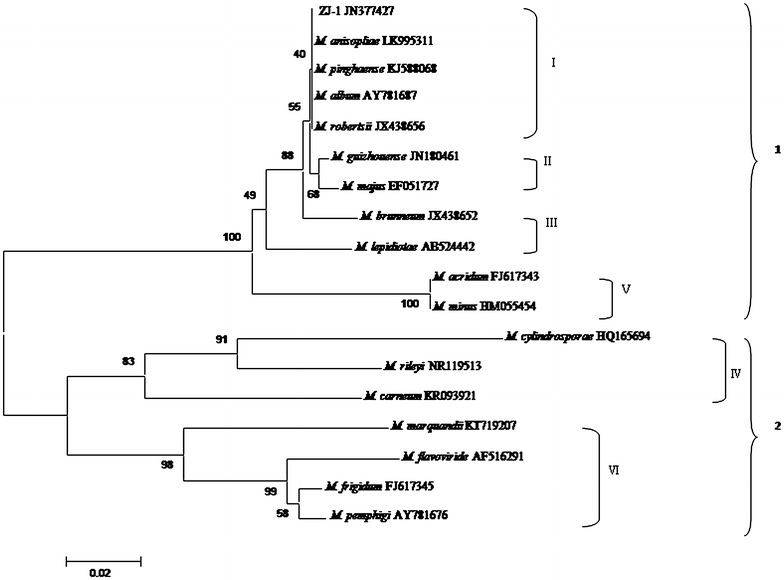
The clustering analysis tree of sample strains and 17 kinds of Metarhizium ITS sequences
Table 1.
Origin of 17 kinds of Metarhizium ITS sequences
| Name | Location | Bankit | Name | Location | Bankit |
|---|---|---|---|---|---|
| Metarhizium pinghaense | Italy | KJ588068 | Metarhizium anisopliae | Algeria | LK995311 |
| Metarhizium album | Brazil | AY781687 | Metarhizium robertsii | Poland | JX438656 |
| Metarhizium guizhouense | China | JN180461 | Metarhizium majus | Brasil | EF051727 |
| Metarhizium brunneum | Poland | JX438652 | Metarhizium pemphigi | Brazil | AY781676 |
| Metarhizium lepidiotae | Japan | AB524442 | Metarhizium acridum | USA | FJ617343 |
| Metarhizium frigidum | USA | FJ617345 | Metarhizium minus | China | HM055454 |
| Metarhizium cylindrosporae | Thailand | HQ165694 | Metarhizium rileyi | USA | NR119513 |
| Metarhizium carneum | Brazil | KR093921 | Metarhizium marquandii | China | KT719207 |
| Metarhizium flavoviride | Greece | AF516291 |
Laboratory bioassays
Results showed that conidia of M. anisopliae ZJ-1 killed 60 % of test specimens at the lowest concentration (1 × 106 conidia/mL) 10 days post-inoculation. The mortality rate increased with conidial concentration (P < 0.05). Conidia were found to be highly virulent to R. ferrugineus and caused approximately 100 % mortality 8 days post-inoculation of 1 × 108 conidia/mL conidia. In contrast, the control group showed significantly lower mortality rates (3.33 ± 2.87 %) (Fig. 2). LT50 values for M. anisopliae ZJ-1 were 1.66 day (1 × 108 conidia/mL) (Table 2). Probit analysis showed that LD50 values were 2175.29 conidia/larvae 3-day post-treatment.
Fig. 2.
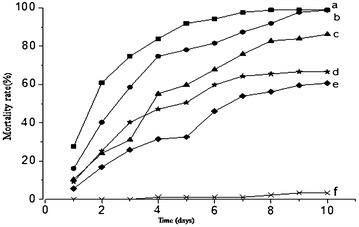
Mortality rates of R. ferrugineus larvae treated with different doses of M. anisopliae. Dose of Conidia/mL: cross symbol control; filled square 1 × 108; filled circle 1 × 107; filled triangle 1 × 106; filled star 1 × 105; filled diamond 1 × 104. a–e Different letters indicate statistically significant differences (P < 0.05)
Table 2.
LT50 values of R. ferrugienus infected by M. anisopliae isolates (95 % CL)
| Spores concentration | LT50 (day) | Correlation coefficient R | 95 % CL | |
|---|---|---|---|---|
| Lower | Upper | |||
| 1 × 108 | 1.66 | 0.9967 | 1.46 | 1.87 |
| 1 × 107 | 2.44 | 0.9969 | 2.20 | 2.70 |
| 1 × 106 | 3.78 | 0.9764 | 3.46 | 4.12 |
| 1 × 105 | 4.73 | 0.9923 | 4.23 | 5.31 |
| 1 × 104 | 7.14 | 0.9810 | 6.37 | 8.01 |
LT50, lethal time for 50 % mortality
Discussion
This study showed that entomopathogenic fungus M. anisopliae ZJ-1 was highly and stably virulent to R. ferrugineus LT50, ZJ-1 killing the treated larvae rather quickly (LT50:2.44d) compared with M. anisopliae isolate (LT50:3.5) (Gindin et al. 2006). It was a serious pest of various palm species. biological control with pathogenic fungi can offer long-terminsect control without destroying environment and non-target organisms(Rossetti et al. 2015). The pathogenic fungi seem (easy) to survive and distribute with R. ferrugineus in dark and humid surroundings. It is generally known that the studies in China describing the biological control of R. ferrugienus with entomopathogenic fungi have not yet been reported.
Driver et al. (2000) analyzed ITS1 and ITS2 sequences of 100 representative M. anisopliae strains and found ITS to be a good basis for the classification of M. anisopliae. Islam et al. (2014) sequenced the internal transcribed spacer (ITS) 1-5.8-ITS4 region of various M. anisopliae strains for the detection and identification of M. anisopliae. In the present study, we determined rDNA-ITS sequences of M. anisopliae ZJ-1 and analyzed associated sequences of relevant varieties. Results indicated isolate ZJ-1 to be a novel variety of M. anisopliae, with 93 % sequence identity to M. anisopliae, which was consistent with results from morphological characterization. M. anisopliae is applied as conidia or mycelia in various formulations. The new conidia and viable cells can be spread to the healthy insects and thus achieve the control effect through infecting the insects and the induction of a fungal epizootic (Genthner et al. 1997). After the series of adhesion, prepenetration growth, penetration into the host, and establishment of the pathogen in the host, the insects would be infected by M. anisopliae.
Conclusions
In summary, this study showed high susceptibility of R. ferrugienus larva to certain concentrations of local M. anisopliae ZJ-1. Future application of M. anisopliae ZJ-1 in the field is recommended for in situ protection of local palm trees. However, the new delivery methods along with more persistent formulations for this fungus are necessary to improve its efficiency and persistence under field conditions in the future.
Methods
Entomopathogenic fungus
Naturally dead R. ferrugineus cadavers were collected from Wenchang, Hainan Island, China (19_32N, 110_47E). The samples were soaked in 70 % alcohol for 1 min and rinsed using sterile distilled water. The cadavers were subsequently surface-sterilized using 0.1 % mercury chloride, followed by three rinses in sterile distilled water. Parts of the tissues were cut and inoculated on Sabouraud Dextrose Agar with Yeast Extract (SDAY) containing 40 g/L dextrose, 10 g/L peptone, 10 g/L yeast, 20 g/L agar and 500 µg/mL streptomycin. These tissues were separately placed on sterile petri dishes sealed with preservative film at 28 ± 1 °C, 75 ± 5 % RH for 6 days. Purification was achieved using a monospore culture, named ZJ-1. Scanning electron microscopy (Hitachi S-3000N) was performed to study morphologic characteristics of ZJ-1 (Driver et al. 2000; Tulloch 1976; Su 2006).
Experimental insects
A laboratory population of R. ferrugineus was established by collecting larvae from infected palm trees in the Wenchang suburb (19_32N, 110_47E), Hainan, China (Li et al. 2010). Larvae were reared on sugarcane stem tissues at 28 ± 1 °C, 75 ± 5 % RH. After adults emerged, they were placed in jars and supplied with cotton wicks saturated with 8–10 % honey for feeding. Subsequently, eggs were transferred to a moist sterile filter paper within an unsealed petri dish (12 cm in diameter). Upon hatching, neonate larvae were individually transferred to 50 mL vials containing 10 g weevil’s artificial diet (Martín and Cabello 2006). Approximately 7 days later, laboratory-reared larvae were obtained for further analysis.
DNA extraction and sequencing
Genomic DNA (gDNA) was extracted following the method described by Lee and Taylor (Lee and Taylor 1990). A fragment of the ITS spacer region was amplified using universal primer sets ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) and ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′). PCR reactions (50 µL) contained 50 ng of template gDNA, 1 µL of each 10 pM oligonucleotide, 1 µL of 10 mM dNTPs, 1 µL of 2 U/µL Taq DNA polymerase (Sino Geno Max Co., Ltd, Beijing), 5 µL of 10× PCR buffer. The PCR protocol for amplification of ITS regions included initial denaturation at 94 °C for 5 min, 35 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, followed by a final elongation at 72 °C for 10 min. PCR products were kept at 4 °C. The size and quality of PCR products were determined by gel electrophoresis using 1.25 % agarose gel, which was stained with ethidium bromide (0.5 mg/mL) and visualized under UV light. The ITS1-5.8S-ITS2 amplified products were purified using a Fungus Genomic DNA Extraction Kit (Omega) and sequenced in an automated system (Sino Geno Max Co., Ltd, Beijing).
DNA sequences obtained from this work were compared with existing 17 Metarhizium species sequences data in GenBank using the Basic Local Alignment Search Tool (NCBI BLAST) (Destéfano 2004); a summary of these comparisons is shown in Table 1. Phylogenetic relationships among DNA sequences of ZJ-1 were determined using multiple sequence alignment MAGA6.0 via the maximum likelihood method (ML). Confidence levels for generated groups were determined via 1000-repetition bootstrap analysis.
Laboratory bioassays
We selected R. ferrugineus larvae of similar size during the feeding period. Conidia of divided purified ZJ-1 were placed in a sterile 10 mL centrifuge tube containing aqueous 0.05 % Tween-80 and the mixture was vortexed to attain homogenization. Conidial concentration was determined using a hemocy to meter. A dilution series of aqueous conidial suspension (1.0 × 108, 1.0 × 107, 1.0 × 106, 2.0 × 105, 1 × 104 conidia/mL) was prepared thorough mixing, then sprayed on larvae. Treated larvae were separately transferred to 50 mL vials containing 10 g weevil’s artificial diet under controlled conditions (28 ± 1 °C, 80 ± 5 % RH). Each trial was performed in triplicate with a total of 30 insects per process. After the treatment, insects were scored as dead at 24 h intervals for 10 days. Meanwhile, dead larvae were selected and tested for potential pathological changes (Anggraeni and Putra 2011).
Authors’ contributions
XS and WQ conceived this study. WY, JZ and XN collected the reference data, FL and GM corrected the manuscript. XS wrote the manuscript. XS mainly and WQ partly performed the experiments. All authors read and approved the final manuscript.
Acknowledgements
This study was supported by Special Provincial science projects of HaiNan (Grant No. ZDXM2015024); Major Provincial Science and Technology Projects of HaiNan (Grant No. ZDZX2013008-02-02); National Nonprofit Institute Research Grant of CATAS-ITBB (High Virulence of Metarhizium against red palm weevil and the application Research of new Formulations); 211 Project for Northeast Agricultural University.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- M. anisopliae
Metarhizium anisopliae
- B. abassiana
Beauveri abassiana
- RPW
red palm weevil
- R. ferrugineus
Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae)
- ITS
internal transcribed spacer
- SDAY
Sabouraud dextrose agar with yeast extract
Footnotes
Xiaodong Sun and Weiquan Qin have equal contribution to the manuscript
Contributor Information
Xiaodong Sun, Phone: 0086-898-63330001, Email: sxd1949@163.com, Email: sxd1949@126.com.
Wei Yan, Email: 184848439@qq.com.
Weiquan Qin, Email: qwq268@163.com.
Jing Zhang, Email: 178109409@qq.com.
Xiaoqing Niu, Email: 287826866@qq.com.
Guangchang Ma, Email: 283251861@qq.com.
Fuheng Li, Email: lifuheng1963@126.com.
References
- Anggraeni T, Putra RE. Cellular and humoral immune defenses of Oxya japonica (Orthoptera: Acrididae) to entomopathogenic fungi Metarhizium anisopliae. Entomol Res. 2011;41:1–6. doi: 10.1111/j.1748-5967.2010.00311.x. [DOI] [Google Scholar]
- Bischoff JF, Rehner SA, Humber RA. Metarhizium frigidum sp. nov.: a cryptic species of M. anisopliae and a member of the M. flavoviride complex. Mycologia. 2006;98:737–745. doi: 10.3852/mycologia.98.5.737. [DOI] [PubMed] [Google Scholar]
- Dembilio Ó, Jacas JA, Llácer E. Are the palms Washingtonia filifera and Chamaerops humilis suitable hosts for the red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae) Appl Entomol. 2009;133:565–567. doi: 10.1111/j.1439-0418.2009.01385.x. [DOI] [Google Scholar]
- Destéfano RHR. Detection of Metarhizium anisopliae var. anisopliae within infected sugarcane borer Diatraea saccharalis (Lepidoptera, Pyralidae) using specific primers. Genet Mol Biol. 2004;27:245–252. doi: 10.1590/S1415-47572004000200020. [DOI] [Google Scholar]
- Driver F, Milner RJ, Trueman WH. A taxonomic revision of Metarhizium based on a phylogenetic analysis of rDNA sequence data. Mycol Res. 2000;104:134–150. doi: 10.1017/S0953756299001756. [DOI] [Google Scholar]
- Faleiro JR. A review of the issues and management of the red palm weevil Rhynchophoru s ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int J Trop Insect Sci. 2006;26:135–154. [Google Scholar]
- Fetoh BEA. Latent effects of gamma radiation on certain biological aspects of the red palm weevil (Rhynchophorus ferrugineus Olivier) as a new control technology. J Agric Technol. 2011;7:1169–1175. [Google Scholar]
- Fiaboe KKM, Roda AL. Predicting the potential worldwide distribution of the red palm weevil Rhynchophorusferrugineus (Olivier) (Coleoptera: Curculionidae) using ecological niche modeling. Fla Entomol. 2012;95:659–673. doi: 10.1653/024.095.0317. [DOI] [Google Scholar]
- Genthner FJ, Foss SS, Glast PS. Viruence of Metarhizium anisopliae to embryo s of the grass shrimp Palaemonetes pugio. J Invertebr Pathol. 1997;69:157–164. doi: 10.1006/jipa.1996.4653. [DOI] [PubMed] [Google Scholar]
- Gindin G, Levski S, Glazer I, Soroker V. Evaluation of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana against the red palm weevil Rhynchophorus ferrugineus. Phytoparasitica. 2006;34:370–379. doi: 10.1007/BF02981024. [DOI] [Google Scholar]
- Islam MT, Omar D, Shabanimofrad M. Molecular identification and virulence of six isolates of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) to Bemisia tabaci Q biotype. J Asia-Pac Entomol. 2014;17:237–241. doi: 10.1016/j.aspen.2014.01.008. [DOI] [Google Scholar]
- Kehat M. Threat to date palms in Israel, Jordan and the Palestinian authority by the red palm weevil, Rhynchophorus ferrugineus. Phytoparasitica. 1999;27:241–242. doi: 10.1007/BF02981465. [DOI] [Google Scholar]
- Lee SB, Taylor JW. Isolation of DNA from fungal mycelia and single spores. In: Innis M, Gelfand D, Sninsky J, Thomas JW, editors. PCR protocols: a guide to methods and applications. Orlando: Academic Press; 1990. pp. 282–287. [Google Scholar]
- Li L, Qin WQ, Ma ZL. Effect of temperature on the population growth of Rhynchophorus ferrugineus (Coleoptera: Curculionidae) on sugarcane. Environ Entomol. 2010;39:999–1003. doi: 10.1603/EN09316. [DOI] [PubMed] [Google Scholar]
- Martín MM, Cabello T. Manejo de la cría del picudo rojo de la palmera, Rhynchophorus ferrugineus (Olivier, 1790) (Coleoptera, Dryophthoridae), en dieta artificial y efectos en su biometría y biología. Bol San Veg Plagas. 2006;32:631–641. [Google Scholar]
- Pinhas J, Soroker V, Hetzroni A. Automatic acoustic detection of the red palm weevil. Comput Electron Agric. 2008;63:131–139. doi: 10.1016/j.compag.2008.02.004. [DOI] [Google Scholar]
- Rossetti SB, Mastore M, Protasoni M, Brivio MF. Effects of an entomopathogen nematode on the immune response of the insect pest red palm weevil: focus on the host antimicrobial response. J Invertebr Pathol. 2015;133:110–119. doi: 10.1016/j.jip.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Su XQ. A new species of Pythium isolated from mosquito larvae and its ITS region of rDNA. Mycosystema. 2006;25:523–528. [Google Scholar]
- Tagliavia M, Messina E, Manachini B, Cappello S, Quatrini P. The gut microbiota of larvae of Rhynchophorus ferrugineus Oliver (Coleoptera: Curculionidae) BMC Microbiol. 2014;14:136. doi: 10.1186/1471-2180-14-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo AV, de Remes Lenicov AMM, LópezLastra CC. Histopathology caused by the entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae, in the adult planthopper, Peregrinus maidis, a maize virus vector. J Insect Sci. 2010;10:35. doi: 10.1673/031.010.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch M. The genus Metarhizium. Trans Br Mycol Soc. 1976;66:407–411. doi: 10.1016/S0007-1536(76)80209-4. [DOI] [Google Scholar]
- Wu GC, Luo XY, Heng H, Dong YX, Ye LH. Risk analysis of alien invasive pest Rhyncnophorus ferrugineus (Olivier) Chin For Sci Technol. 2007;21:44–46. [Google Scholar]


