Abstract
Background
Heart failure (HF) is associated with excess morbidity and mortality for which noncardiac causes are increasingly recognized. We previously described an increased risk of cancer among HF patients compared with community controls.
Objectives
In the present study, we examined whether HF was associated with an increased risk of subsequent cancer among a homogenous population of first myocardial infarction (MI) survivors.
Methods
A prospective cohort study was conducted among Olmsted County, Minnesota residents with incident MI from 2002 to 2010. Patients with prior cancer or HF diagnoses were excluded.
Results
A total of 1,081 participants (mean age: 64 ± 15 years; 60% male) were followed for 5,327 person-years (mean: 4.9 ± 3.0 years). A total of 228 patients developed HF and 98 patients developed cancer (excluding nonmelanoma skin cancer). Incidence density rates for cancer diagnosis (per 1,000 person-years) were 33.7 for patients with HF and 15.6 for patients without HF (p = 0.002). The hazard ratio (HR) for cancer associated with HF was 2.16 (95% confidence interval [CI]: 1.39 to 3.35); adjusted for age, sex, and Charlson comorbidity index; HR: 1.71 (95% CI: 1.07 to 2.73). The HRs for mortality associated with cancer were 4.90 (3.10 to 7.74) for HF-free and 3.91 (1.88 to 8.12) for HF patients (p for interaction = 0.76).
Conclusions
Patients who develop HF after MI have an increased risk of cancer. This finding extends our previous report of an elevated cancer risk after HF compared with controls, and calls for a better understanding of shared risk factors and underlying mechanisms.
Keywords: epidemiology, follow-up studies, risk
Introduction
Heart failure (HF) is associated with excess morbidity and mortality (1). The morbidity in HF patients can be attributed to: direct cardiac causes, such as HF symptoms, ischemia, and arrhythmias; related conditions, such as anemia, infections, and depression; and other noncardiac causes (2). These noncardiac causes of morbidity in HF patients are increasingly recognized as their association with HF is unraveled.
A specific case is made for malignancy. Although usually considered a separate cause of morbidity, it may be that an association exists between heart disease and an increased risk of cancer. Our group has indeed shown a 70% increased risk of cancer among HF patients in Olmsted County, Minnesota compared with community controls (3). In that study, controls were matched to HF patients by age and sex, and efforts were made to comprehensively adjust for other recognized factors. However, unrecognized characteristics and treatment may have differed between groups to influence the occurrence and/or detection of incident cancer.
Patients surviving a first myocardial infarction (MI) might later present with HF (4). The comparison of those with and without HF after MI has advantages because they share a common disease mechanism (atherosclerosis), risk factor profile, treatment modalities, and follow-up routines. Although certain characteristics and risk factors may differ between those who develop HF and those who do not, such as older age, obesity, smoking, diabetes, larger anterior infarcts (5), and delayed reperfusion (6), these are largely known and can be controlled for in the analysis. Therefore, comparing these 2 groups may provide more information on the impact of heart failure on the occurrence of subsequent cancer.
In this study, we evaluated the association between HF and subsequent cancer risk among first MI survivors.
Methods
Study Setting
This study was conducted in Olmsted County, Minnesota under the auspices of the Rochester Epidemiology Project. Olmsted County is relatively isolated from other urban centers, and thus only a few providers deliver nearly all medical care to local residents (7). The medical records from these providers are indexed through the Rochester Epidemiology Project, resulting in the linkage of inpatient and outpatient medical records from all sources of care used by the population, thus providing a unique infrastructure to analyze disease determinants and outcomes (7).
Study Design
A prospective cohort study was conducted among Olmsted County, Minnesota residents with incident MI from November 2002 through December 2010. Those with a diagnosis of HF or cancer prior to the MI diagnosis were excluded from the cohort. Patients who developed HF after the MI were identified and the incidence of cancer, excluding nonmelanoma skin cancer, was compared to those without HF. The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
MI Cohort
Only patients with a first MI were included in this cohort, as previously described in detail (8,9). The diagnosis of MI was verified on the basis of the presence of 2 of the following: typical chest pain; elevated cardiac troponin T (cTnT); and electrocardiographic (ECG) changes. Cardiac troponin T was measured with a sandwich electrochemiluminescence immunoassay on the Elecsys 2010 (Roche Diagnostics Corp, Indianapolis, Indiana) in the laboratories of the Department of Medicine and Pathology at the Mayo Clinic.
Follow-up
Participants were followed through their complete (inpatient and outpatient) medical records in the community, from the index MI date to death or the most recent clinical contact through March 2013.
Heart Failure
Clinical diagnoses of HF were reviewed and validated according to the Framingham criteria. These criteria require the presence of at least 2 major criteria, or 1 major criterion in addition to 2 minor criteria, to confirm HF (10). The type of HF was defined according to echocardiographically-measured ejection fraction (EF) as HF with reduced EF (EF <50%; HFrEF) or HF with preserved EF (EF ≥50%; HFpEF) (11). EF was measured as previously described (12). The EF measurement that was closest to the HF diagnosis (applying a predefined maximum period of 60 days) was recorded for each participant.
Cancer
Cancer types were classified by anatomic and system primary involvement (13); nonmelanoma skin cancers were excluded from the study. The date of first cancer diagnosis was used as the diagnosis date.
Death
Death was ascertained using multiple sources including autopsy reports, death certificates filed in Olmsted County, obituary notices, and electronic death certificates obtained from the Section of Vital Statistics, Minnesota Department of Health, as previously described (14).
Clinical Characteristics
Nurse abstractors collected clinical data from the medical record using the MI date as the index. Smoking was categorized as never, past, or current (at time of evaluation or within the previous 6 months). Clinical definitions were used to identify diabetes, hypertension, and dyslipidemia. Body mass index (BMI, kg/m2) was calculated using the current weight and earliest adult height. Overall comorbidity burden was assessed by the Charlson comorbidity index (15). MI presentation according to ST-segment elevation and anterior location was determined, as well as Killip class. The latter was assessed within 24 h of the index MI and analyzed as a categorical variable (class >1 vs. class 1). Peak cTnT was defined as the highest cTnT measurement after MI. Data on medications prescribed at discharge were collected. Reperfusion therapy or revascularization included coronary artery bypass grafting, percutaneous coronary intervention, or thrombolysis performed during the index hospitalization.
Statistical Analysis
Baseline characteristics, overall and by HF status during follow-up (regardless of the time of onset), are presented as mean and standard deviations for continuous variables, and as frequencies for categorical variables. Associations between baseline characteristics and development of HF were assessed using Cox proportional hazards regression models, using time from MI as the time scale. Cancer rates with person-time denominators were calculated for HF and HF-free categories and compared by Fisher exact test. Person-time at risk for the HF-free category was accumulated from the index MI until cancer, HF diagnosis, death, or end of follow-up, whichever came first. For the HF category, person-time at risk was accumulated from HF validation date until cancer, death, or end of follow-up, whichever came first. Cox proportional hazards regression models were constructed to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for risk of cancer associated with HF. HF was modeled as a time-dependent variable, allowing subjects to transfer from one exposure group to another during follow-up. Initial adjustment was made for age, sex, and Charlson comorbidity index. To assess the robustness of the main analysis, additional adjustment was made for hypertension, smoking, BMI, anterior MI, peak cTnT, Killip class, reperfusion/revascularization, and treatment with aspirin at hospital discharge. Stratified analyses were performed by sex and age groups; exploratory analyses stratified the analyses according to HFrEF and HFpEF. Because age is a strong determinant of cancer risk, models were repeated using age as the time scale in the Cox model. Data on EF were missing in 30% of the cases with HF. Multiple imputations were performed to account for missing EF values. Five datasets were created with missing values replaced by imputed values on the basis of a model that incorporated various demographic and clinical variables. The results of these datasets were then combined using Rubin’s rules (16).
The cumulative incidence of cancer after MI was estimated for up to 5 years and compared by HF status at 30 days following the index MI. In the customary Kaplan-Meier approach, patients who die are censored; however, this may overestimate the cumulative cancer incidence when the death rate is high. Therefore, death was treated as a competing event in this analysis (17). Finally, Cox models were constructed to assess the association between cancer and death, with cancer treated as a time-dependent variable. The proportional hazards assumption was tested and found to be valid. Analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc., Cary, North Carolina) and WINPEPI, version 11.23 (18).
Results
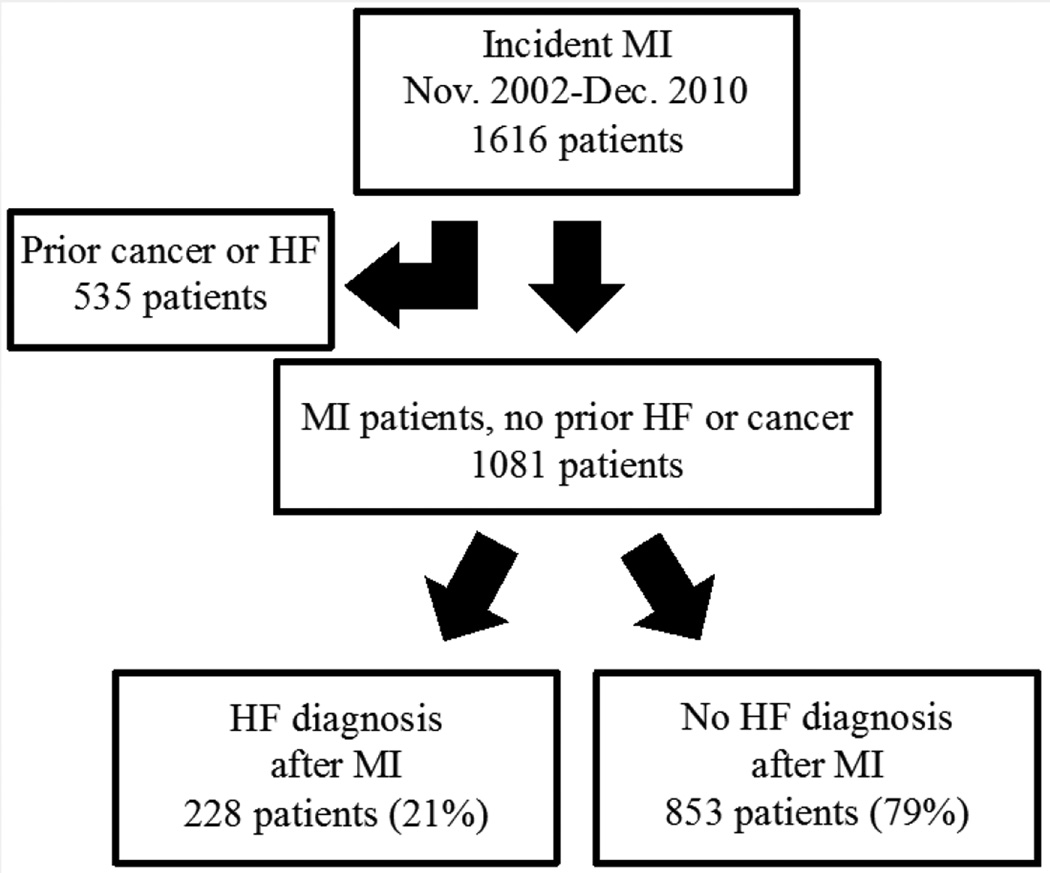
Between 2002 and 2010, 1,616 patients were identified with incident MI. Of those, 535 (33%) were excluded from the study because of a prior diagnosis of HF or cancer, resulting in a sample size of 1,081 (Figure 1). The mean age was 64 ± 15 years and 60% were men. ST-segment elevation MI occurred in 23% and anterior MI in 35% of the patients. Reperfusion or revascularization was performed in 61% of the patients during the index hospitalization. HF was diagnosed after MI in 228 patients (21%). Median time to HF diagnosis was 3 days (25th to 75th percentiles: 0 to 120 days). Patients with subsequent HF were, on average, 10 years older, more likely to be women, with adverse risk factors and larger infarctions. They also were less likely to receive reperfusion/revascularization. Treatment at discharge from MI with angiotensin blockade, beta-adrenergic blockade, and statins was similar between groups, whereas patients who developed HF were less often treated with aspirin (Table 1).
Figure 1. Study Flow Chart.
The study includes patients from Olmsted County, Minnesota after MI. Patients with and without post-MI heart failure were compared. HF = heart failure; MI = myocardial infarction.
Table 1.
Characteristics of Patients With Incident MI by HF Status During Follow-up
| Characteristics | All | Without HF | With HF |
|---|---|---|---|
| N | 1,081 | 853 | 228 |
| Age, mean (SD), yrs | 64 (15) | 62 (15) | 72 (14)* |
| Male, n (%) | 643 (60) | 539 (63) | 104 (46)* |
| Smoking, n (%) | |||
| Never | 443 (41) | 357 (42) | 86 (38) |
| Past | 257 (24) | 196 (23) | 61 (27) |
| Current | 381 (35) | 300 (35) | 81 (36) |
| BMI, mean (SD), kg/m2 | 28.9 (6.4) | 29.0 (6.4) | 28.5 (6.4) |
| Hypertension, % | 709 (66) | 524 (61) | 185 (81)* |
| Hyperlipidemia, % | 675 (62) | 524 (61) | 151 (66) |
| Diabetes, % | 201 (19) | 138 (16) | 63 (28)* |
| Charlson comorbidity index, mean (SD) | 1.14 (1.57) | 0.93 (1.38) | 1.93 (1.96)* |
| Anterior MI, % | 376 (35) | 265 (31) | 111 (49)* |
| ST elevation MI, % | 252 (23) | 207 (24) | 45 (20) |
| Peak cTnT, mean (SD), ng/ml | 1.96 (3.54) | 1.80 (3.04) | 2.58 (4.93)* |
| Killip >1, % | 218 (20) | 107 (13) | 111 (50)* |
| Reperfusion/revascularizaton, % | 661 (61) | 543 (64) | 118 (52)* |
| Discharged with ACEi or ARB†, % | 617 (60) | 477 (58) | 140 (65) |
| Discharge with beta-adrenergic blocker†, % |
867 (84) | 684 (84) | 183 (85) |
| Discharged with a statin†, % | 820 (80) | 646 (79) | 174 (81) |
| Discharged with aspirin†, % | 905 (88) | 727 (89) | 178 (82)* |
p < 0.05
Data for medications at discharge were available for 1,032 patients (48 died in hospital, 1 a day after discharge), of whom 216 had heart failure.
ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blocker; BMI = body mass index; cTnT = cardiac troponin T; HF = heart failure; MI = myocardial infarction.
Patients were followed for a total of 5,327 person-years (mean ± SD, 4.9 ± 3.0 years). During follow-up, 228 patients developed HF and 98 patients developed cancer (70 developed cancer with no HF diagnosed prior to the cancer [8.2% of patients without HF] and 28 developed cancer after HF diagnosis [12.3% of patients with HF]). Among patients who developed cancer, the median time from MI to cancer diagnosis was 2.8 years (25th to 75th percentiles: 1.5 to 4.7 years). Incidence density rates for cancer diagnosis (per 1,000 person-years) were 33.7 for patients with HF and 15.6 for patients without HF (p = 0.002) (Table 2).
Table 2.
Association of HF (Modeled as a Time-Dependent Variable) With Subsequent Cancer Risk
| Overall | Men | Women | Age<75 Yrs at Index MI |
Age ≥75 Yrs at Index MI |
||
|---|---|---|---|---|---|---|
| Cancer rate per 1,000 person-years |
HF | 33.7 | 41.0 | 26.4 | 35.9 | 30.3 |
| No HF | 15.6 | 15.7 | 15.4 | 15.0 | 17.9 | |
| p value | 0.002 | 0.003 | 0.20 | 0.004 | 0.28 | |
| HR (95% CI) | ||||||
| Time from MI as time scale |
Unadjusted | 2.16 (1.39–3.35) | 2.60 (1.49–4.54) | 1.71 (0.84–3.49) | 2.37 (1.39–4.04) | 1.65 (0.74–3.69) |
| Adjusted* | 1.71 (1.07–2.73) | 1.72 (0.95–3.14) | 1.61 (0.77–3.35) | 1.94 (1.11–3.41) | 1.55 (0.67–3.56) | |
| Adjusted† | 1.92 (1.11–3.31) | 1.39 (0.66–2.93) | 2.47 (1.08–5.66) | 1.97 (0.99–3.91) | 2.06 (0.74–5.73) | |
| Age as time scale | Unadjusted | 1.89 (1.21–2.97) | 2.03 (1.14–3.62) | 1.85 (0.89–3.84) | 1.95 (1.14–3.34) | 1.79 (0.80–4.01) |
| Adjusted‡ | 1.65 (1.04–2.64) | 1.72 (0.95–3.12) | 1.79 (0.84–3.80) | 1.64 (0.93–2.90) | 1.70 (0.75–3.90) | |
| Adjusted§ | 1.84 (1.06–3.19) | 1.35 (0.63–2.88) | 2.79 (1.18–6.63) | 1.54 (0.77–3.08) | 2.29 (0.86–6.09) |
Adjusted for age, sex, and Charlson comorbidity index (the sex-specific estimates are adjusted for age and comorbidity, whereas the age-specific estimates are adjusted for sex and comorbidity).
Further adjusted for hypertension, smoking, BMI, anterior MI, peak cTnt, Killip class, reperfusion/revascularization, and treatment with aspirin at hospital discharge.
Adjusted for sex and Charlson comorbidity index (the age-specific estimates are adjusted for comorbidity).
Further adjusted for hypertension, smoking, BMI, anterior MI, peak cTnT, Killip class, reperfusion/revascularization, and treatment with aspirin at hospital discharge.
CI = confidence interval; HR = hazard ratio. Other abbreviations as in Table 1.
Types of cancer were classified according to system involvement. Among patients with HF, the most common types of cancer were respiratory (n = 8; 29% of cancer diagnoses), digestive (n = 8; 29%), and hematologic (n = 4; 14%). The most common cancers among patients without HF were male reproductive (n = 15; 21%), respiratory (n =12; 17%), digestive (n = 10; 14%), and female breast (n = 8; 11%) (Table 3). The difference in cancer system involvement between groups was not statistically significant (Fisher exact test p = 0.087).
Table 3.
Type of Cancer by HF Status During Follow-up
| Without HF (N = 70) |
With HF (N = 28) |
|
|---|---|---|
| Respiratory | 12 (17.1) | 8 (28.6) |
| Digestive | 10 (14.3) | 8 (28.6) |
| Male reproductive | 15 (21.4) | 1 (3.6) |
| Skin | 8 (11.4) | 2 (7.1) |
| Other | 8 (11.4) | 2 (7.1) |
| Breast | 8 (11.4) | 1 (3.6) |
| Hematologic | 5 (7.1) | 4 (14.3) |
| Urinary | 4 (5.7) | 1 (3.6) |
| Female reproductive | 0 (0.0) | 1 (3.6) |
HF = heart failure.
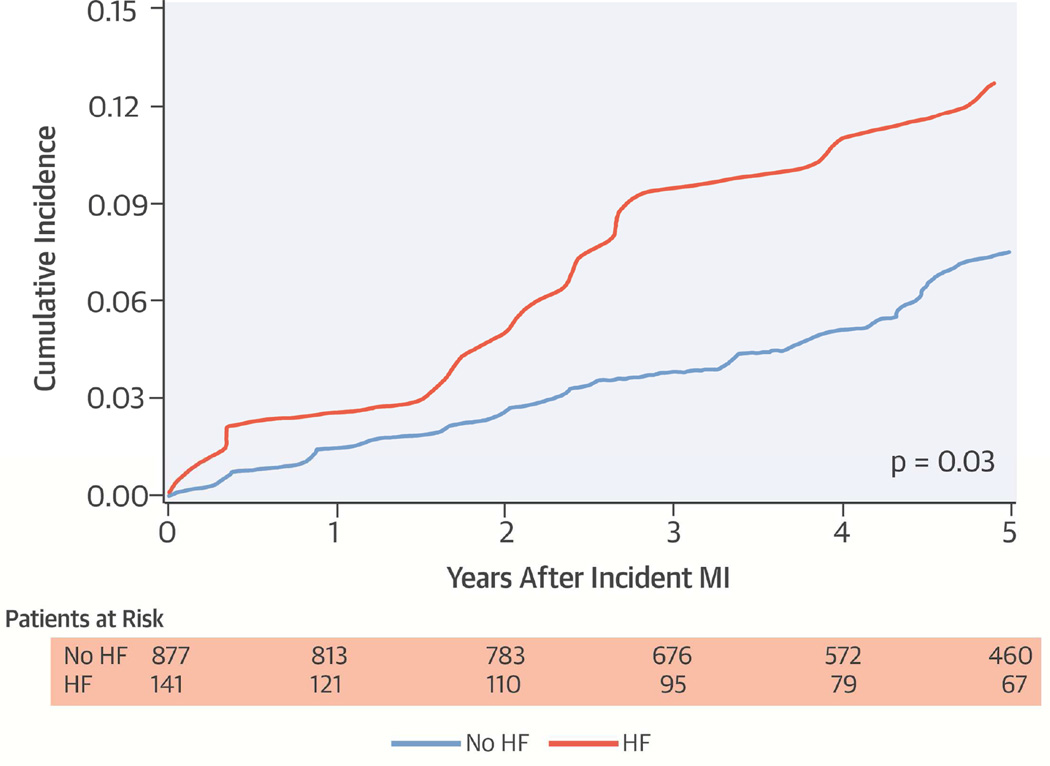
The cumulative incidence of cancer among patients with and without HF 30 days after MI is shown in the Central Illustration. The incidence of cancer between groups was similar initially, but diverged after 1.5 years of follow-up, with higher rates of cancer among the HF patients. Including all HF diagnoses after MI, the unadjusted and adjusted associations between HF and cancer in the entire cohort, and by sex and age subgroups are shown in Table 2. Patients who developed HF had more than a 2-fold increased risk of cancer (HR: 2.16; 95% CI: 1.39 to 3.35). After adjustment for age, sex, and Charlson comorbidity index, the HR was 1.71 (95% CI: 1.07 to 2.73). With further adjustment for hypertension, smoking, BMI, anterior MI, peak cTnT, Killip class, reperfusion/revascularization, and treatment with aspirin at hospital discharge, the HR was 1.92 (95% CI: 1.11 to 3.11). Similar associations were observed among men, women, and patients older or younger than 75 years of age. Modeling age as the time scale in the Cox model produced similar associations.
Central Illustration. Incidence of Cancer Among HF and HF-Free Patients 30 Days After MI.
Cumulative incidence of cancer according to HF status 30 days after incident MI, with death considered a competing event. HF = heart failure; MI = myocardial infarction.
We conducted an exploratory analysis according to EF. Ejection fraction data were available for 160 (70%) HF patients. After multiple imputations for those missing EF data, of the 228 patients with HF, 120 (53%) had HFrEF. Cancer was diagnosed in 16 (13%) patients with HFrEF and in 12 (11%) with HFpEF. Patients with HFrEF had an increased risk of cancer (unadjusted HR: 2.53; 95% CI: 1.47 to 4.35). The association remained with adjustment for age and sex (HR: 2.28; 95% CI: 1.31 to 3.95). In patients with HFpEF, a trend for increased risk of cancer was observed (unadjusted HR: 1.80; 95% CI: 0.97 to 3.51). Adjusting for age and sex weakened the association (HR: 1.65; 95% CI: 0.88 to 3.10).
The age- and sex-adjusted HR (95% CI) for all-cause mortality associated with post-MI cancer (modeled as a time-dependent variable) was 4.83 (3.35 to 6.97). Stratified by HF status at 30 days post-MI, the HRs were 4.90 (3.10 to 7.74) for HF-free and 3.91 (1.88 to 8.12) for HF patients (p for interaction = 0.76). In a complementary analysis, HF and cancer were both treated as time-dependent variables. Although both variables were strongly associated with mortality (age- and sex-adjusted HR: 4.49 [3.11 to 6.49] for cancer; 2.67 [2.08 to 3.42] for HF), the interaction between the 2 terms was nonsignificant (p = 0.20).
Discussion
The present study compared the incidence of cancer (excluding nonmelanoma skin cancer) among survivors of a first MI with and without subsequent HF. Patients with HF were 71% more likely to have subsequent cancer, adjusted for age, sex, and the Charlson comorbidity index. This trend was seemingly more apparent among patients with reduced EF. Cancer involved multiple organ systems, without significant differences between the groups regarding system involvement. The increased incidence in cancer began approximately 1.5 years after HF diagnosis. Both HF and cancer were independently associated with increased mortality after MI.
We previously reported an increased risk of cancer among HF patients as compared with community controls (3). In the present, study, the HF and non-HF patients shared many of the risk factors, diagnostic procedures, and medications because all patients were survivors of MI. Therefore, the present study supports the previous finding that HF is associated with an increased risk of cancer.
Increased physician encounters and diagnostic procedures that occur in patients with HF may enhance or facilitate cancer diagnosis (detection bias). Similar to the previous study, a lag time of 1.5 years was observed between the HF diagnosis (occurring in the majority of cases within days of the MI) and cancer diagnosis. Most of the increased health demands of HF patients occur early (during the first year of diagnosis) (19). Therefore, the timing of cancer diagnosis observed herein argues against detection bias as a major cause of cancer diagnosis in the HF group.
The association between HF and cancer raises concerns regarding the effects of specific cardiovascular medications, including angiotensin-receptor blockers, cardiac glycosides, diuretic agents, statins, and prasugrel. Data on a possible association have been inconclusive in most cases (20), and studies disputing the association with glycosides (21,22), and with diuretics (23) were recently published. In the current study, patients who developed HF did not differ by statins or angiotensin antagonists prescribed at MI hospital discharge compared to patients who did not develop HF. Although data on prasugrel are lacking, patients with subsequent HF were less likely to undergo reperfusion/revascularization and less likely to be discharged on aspirin; therefore, it is unlikely that treatment with prasugrel was more frequent in this group. Although the study design limits a conclusive statement, there was no indication that treatment was associated with an increased risk of cancer.
In an exploratory analysis, we observed a seemingly more prominent risk of cancer in patients with reduced EF. This observation should be interpreted as hypothesis-generating, rather than hypothesis-testing. The pathways leading to altered healing and reduced EF after MI are complex, and HF after MI was reported to be associated with altered immunity, highlighting the role of the monocyte system (24). These or similar mechanisms for repair of damaged tissue might also be associated with an increased risk of cancer.
Cancer constitutes an enormous burden to society (25), and both cancer and HF are well-known causes of increased mortality (26). Although these 2 chronic conditions were previously viewed as unlinked (with the occasional patient suffering HF as a consequence of cancer treatment), the data presented herein suggest there may be a connection between them. Although HF patients have an increased risk of mortality, cancer also increases that risk. By better understanding the mechanism involved, we can hope to decrease the risk of mortality. In the meantime, physicians taking care of HF patients should be aware of the increased risk of cancer and endorse the current guidelines for proper cancer surveillance for early detection.
Strengths and Limitations
The observational nature of the analysis entails the obvious limitations. Data were not available for ongoing medical treatment and laboratory results. Echocardiographic data were available for only 70% of the HF patients, necessitating the use of multiple imputations. Although findings were statistically significant, the small sample size and number of events are a potential limitation, and confirmation with a larger cohort may be warranted. Conversely, the utilization of a well-defined contemporary cohort of first MI patients, the availability of baseline characteristics including discharge medications, the long-term follow-up with comprehensive validated data on HF, cancer, and mortality, and the utilization of advanced statistical methods are all important strengths of the present study.
Conclusions
Patients who develop HF after MI have an increased risk of cancer. The risk is increased irrespective of age and sex, and confers an additional risk of death. The current findings extend our previous report of an elevated cancer risk after HF compared with controls, and calls for a better understanding of shared risk factors and underlying mechanisms.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
Patients developing HF after MI exhibit an increased risk of developing cancer, underscoring the importance of multimorbidity as a determinant of outcomes among patients with cardiovascular disease.
TRANSLATIONAL OUTLOOKS
Future research is needed to explore the mechanisms linking cardiovascular disease and cancer, and to identify common risk factors
Acknowledgments
The authors thank Ellen Koepsell, RN and Deborah S. Strain for their study support.
Sources of Funding: Research reported in this publication was supported by the National Institutes of Health under National Heart, Lung and Blood Institute Award Numbers R01HL59205 and R01HL72435 and the National Institute on Aging Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations and Acronyms
- BMI
body mass index
- CI
confidence interval
- cTnT
cardiac troponin T
- ECG
electrocardiographic
- EF
ejection fraction
- HF
heart failure
- HR
hazard ratio
- MI
myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Chen J, Hsieh AF, Dharmarajan K, et al. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998–2010. Circulation. 2013;128:2577–2584. doi: 10.1161/CIRCULATIONAHA.113.003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamberlain AM, St Sauver JL, Gerber Y, et al. Multimorbidity in heart failure: a community perspective. Am J Med. 2015;128:38–45. doi: 10.1016/j.amjmed.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasin T, Gerber Y, McNallan SM, et al. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62:881–886. doi: 10.1016/j.jacc.2013.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerber Y, Weston SA, Berardi C, et al. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am J Epidemiol. 2013;178:1272–1280. doi: 10.1093/aje/kwt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoni ML, Hoogslag GE, Boden H, et al. Cardiovascular mortality and heart failure risk score for patients after ST-segment elevation acute myocardial infarction treated with primary percutaneous coronary intervention (Data from the Leiden MISSION! Infarct Registry) Am J Cardiol. 2012;109:187–194. doi: 10.1016/j.amjcard.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Maeng M, Nielsen PH, Busk M, et al. DANAMI-2 Investigators. Time to treatment and three-year mortality after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction-a DANish Trial in Acute Myocardial Infarction-2 (DANAMI-2) substudy. Am J Cardiol. 2010;105:1528–1534. doi: 10.1016/j.amjcard.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 8.Roger VL, Killian JM, Weston SA, et al. Redefinition of myocardial infarction: prospective evaluation in the community. Circulation. 2006;114:790–797. doi: 10.1161/CIRCULATIONAHA.106.627505. [DOI] [PubMed] [Google Scholar]
- 9.Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62:1495–1539. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Dunlay SM, Roger VL, Weston SA, et al. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circ Heart Fail. 2012;5:720–726. doi: 10.1161/CIRCHEARTFAILURE.111.966366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pahor M, Guralnik JM, Ferrucci L, et al. Calcium-channel blockade and incidence of cancer in aged populations. Lancet. 1996;348:493–497. doi: 10.1016/S0140-6736(96)04277-8. [DOI] [PubMed] [Google Scholar]
- 14.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J. Wiley & Sons; 1987. [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Abramson JH. WINPEPI (PEPI-for-Windows): computer programs for epidemiologists. Epidemiol Perspect Innov. 2004;1:6. doi: 10.1186/1742-5573-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamberlain AM, Gerber Y, Dunlay SM, et al. Abstract 16807: Burden and timing of hospitalizations in heart failure: a community study. Circulation. 2014;130:A16807. doi: 10.1016/j.mayocp.2016.11.009. (abstr) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alameddine AK, Visintainer P, Normand SL, et al. Cancer rates in adults after cardiac interventions: a preliminary observational report. Am J Clin Oncol. 2014 Sep 5; doi: 10.1097/COC.0000000000000120. [E-pub ahead of print], http://dx.doi.org/10.1097/COC.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 21.Couraud S, Dell'Aniello S, Bouganim N, et al. Cardiac glycosides and the risk of breast cancer in women with chronic heart failure and supraventricular arrhythmia. Breast Cancer Res Treat. 2014;146:619–626. doi: 10.1007/s10549-014-3058-8. [DOI] [PubMed] [Google Scholar]
- 22.Biggar RJ, Wohlfahrt J, Oudin A, et al. Digoxin use and the risk of breast cancer in women. J Clin Oncol. 2011;29:2165–2170. doi: 10.1200/JCO.2010.32.8146. [DOI] [PubMed] [Google Scholar]
- 23.Mackenzie IS, Macdonald TM, Thompson A, et al. Spironolactone and risk of incident breast cancer in women older than 55 years: retrospective, matched cohort study. BMJ. 2012;345:e4447. doi: 10.1136/bmj.e4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Laan AM, Ter Horst EN, Delewi R, et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2014;35:376–385. doi: 10.1093/eurheartj/eht331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 26.Stewart S, Ekman I, Ekman T, et al. Population impact of heart failure and the most common forms of cancer: a study of 1 162 309 hospital cases in Sweden (1988 to 2004) Circ Cardiovasc Qual Outcomes. 2010;3:573–580. doi: 10.1161/CIRCOUTCOMES.110.957571. [DOI] [PubMed] [Google Scholar]