Abstract
Purpose
Pancreatic ductal adenocarcinoma (PDA) is associated with an immunosuppressive microenvironment that supports the growth of the malignancy as well as immune system evasion. Here we examine markers of immunosuppression in PDA within the context of the glycolytic tumor microenvironment, their inter-relationship with tumor biology and association with overall survival.
Experimental design
We utilized tissue microarrays consisting of 223 PDA patients annotated for clinical stage, tumor size, lymph node involvement, and survival. Expression of CD163, FoxP3, PD-L1, and MCT4 was assessed by immunohistochemistry and statistical associations were evaluated by univariate and multivariate analysis. Multi-marker subtypes were defined by Random Forest analysis. Mechanistic interactions were evaluated using PDA cell lines and models for myeloid differentiation.
Results
PDA exhibits discrete expression of CD163, FoxP3 and PD-L1 with modest individual significance. However, combined low expression of these markers was associated with improved prognosis (P =0.02). PDA tumor cells altered macrophage phenotype and function, which supported enhanced invasiveness in cell-based models. Lactate efflux mediated by MCT4 was associated with, and required for, the selective conversion of myeloid cells. Correspondingly, MCT4 expression correlated with immune markers in PDA cases, and increased the significance of prognostic subtypes (P = 0.002).
Conclusions
There exists a complex interplay between PDA tumor cells and the host immune system wherein immunosuppression is associated with negative outcome. MCT4 expression, representative of the glycolytic state of PDA, contributes to the phenotypic conversion of myeloid cells. Thus, metabolic status of PDA tumors is an important determinant of the immunosuppressive environment.
Keywords: Pancreatic ductal adenocarcinoma, Immunosuppression, Tumor metabolism, Immune therapy, Tumor microenvironment
Introduction
Pancreatic ductal adenocarcinoma (PDA) is a highly aggressive malignancy and the fourth leading cause of cancer mortality in the United States. Most patients present with advanced disease and the prognosis of these patients is dismal, with 5-year overall survival less than 6% and a median survival of 4 to 6 months (1). Even when complete surgical resection is possible, recurrence is common and a majority of patients will die with distant metastasis within 2 years (2). As systemic treatments remain minimally effective, many studies have focused on the unique genetic characteristics of PDA to define new therapeutic targets (3). Meanwhile, recent studies have demonstrated unique aspects of metabolism and immune evasion in PDA that could offer rational targets for therapeutic intervention beyond genomic targets (1, 4–6).
PDA is characterized by the presence of desmoplastic stroma that can account for as much as 90% of the total tumor volume (7, 8). Interactions between the tumor cells and the surrounding stroma create an inflammatory microenvironment that is conducive to tumor growth and progression. This chronic inflammation recruits predominantly T-cells supported by additional leukocytes -- including dendritic cells, macrophages, neutrophils, and B-cells -- to the primary tumor (7, 9). The clinical relevance of this ongoing inflammatory program is reflected in poor outcomes correlated with infiltrating inflammatory cells and the contributions of specific cell types are being increasingly elucidated (10, 11). For instance, neutrophil-lymphocyte ratio may represent a prognostic marker in PDA patients with a lower ratio representing improved prognosis and, despite the lack of a significant increase in the total cell number, B-cells have now been implicated in PDA progression through their ability to impact myeloid cell function (12–14). Meanwhile, single-agent inhibitors of immune checkpoints have not demonstrated substantial efficacy in PDA (15). Thus, a better understanding of the mechanisms that regulate the PDA immunosuppressive environment could reveal specific disease subtypes with enhanced prognostic significance and allow for additional targeted treatment approaches.
Glycolysis plays an important role in inflammation and, specifically, lactate is a critical mediator of the macrophage inflammatory response (16–18). Alterations in glycolytic tumor metabolism also impact the contributions of other immune cells in PDA (19, 20). Since increased expression of the lactate exporter MCT4 (SLC16A3) also portends poor outcome in PDA (4), we hypothesized that the glycolytic imbalance in pancreatic cancer cells, represented by MCT4 expression, directly impacts the surrounding immune milieu and that these characteristics could be used to identify distinct disease subtypes. To this end, in this study we examine the tumor microenvironment of PDA patients for indicators of an immunosuppressive phenotype and utilize these elements to define prognostic subtypes of disease. Further, we demonstrate in vitro that the metabolic context of pancreatic cancer cells can influence the phenotype of associated immune cells and, by extension, expression of the lactate exporter MCT4 can further refine the disease-immune subtypes.
Materials and Methods
Tumor Microarray Construction and Population Study
Study cases were obtained from the surgical pathology files at Thomas Jefferson University with Institutional Review Board approval. The tissue microarray (TMA) contained tumor samples derived from 223 largely consecutive patients with PDA who had been treated at Thomas Jefferson University Hospitals between the years 2002 and 2010. Whole tissue section slides were constructed and TMAs were derived from them using a tissue arrayer (Veridiam) as previously described (4). Immunohistochemistry was performed as previously described (21) on 4μm TMA sections using a standard avidin-biotin immunoperoxidase method with antibodies specific for CD163 (1:100, clone 10D6, Leica), FOXP3 (1:50, clone 206D, Biolegend), PD-L1 (1:200, clone E1L3N, Cell Signaling Technologies), and MCT4 (1:250, developed and characterized by Dr. Nancy Philp (22)). Staining was performed on a Ventana Benchmark automated stainer.
TMA analysis
After staining, positive cells were counted and converted to a percentage of the total counted cells in a given field and expression of all examined markers was categorized as low or high based on either the median percentage of positive cells or 10% positivity (PD-L1), consistent with published reports (5, 23, 24). Correlation between markers was obtained using the spearman correlation method. Kaplan Meier (KM) curves for both independent as well as combined markers were obtained using the survival package in R statistical software (25). Statistical significance and hazard ratio along with 95% confidence intervals for the KM curves was established using the log rank p-value obtained from Cox proportional hazard regression analysis. An unsupervised random forest (RF) clustering method was used for clustering analysis of immune markers (26). RF dissimilarity measure between markers was obtained and classified into clusters using the partitioning around medoids method; both implemented using random forest and cluster packages in R. All heatmaps and 3D scatterplots were obtained using heatmap.2 and rgl packages in R.
Immune infiltrate scoring
Lymphocytes and neutrophils were assessed in haematoxylin and eosin stained whole tissue sections at 10x magnification in either the central area of the tumor (tumor cellular environment field) or at the tumor’s invasive margin (tumor stroma field). The presence of infiltrate was assessed using a four-degree scale in each area, where a score of 0 indicated no presence, 1 denoted a mild and patchy appearance of inflammatory infiltrate (rare), 2 signified a prominent inflammatory reaction (intermittent), and 3 represented dense infiltration (frequent).
Cells
Established pancreatic cancer cell lines (BXPC3, Capan-2, Hs766t, MIA PaCa-2, Panc-1, PL5, PL45) were cultured in DMEM +10%FBS. THP-1 cells were a generous gift from Dr. David Farrar and were maintained in RPMI-1640 supplemented with 10% FBS, L-glutamine (2mM), sodium pyruvate (1mM), non-essential amino acids, and beta-mercaptoethanol (75μM). To visualize wound healing, PL45 cells were stably transfected with mCherry fluorescent protein. For MCT4 knockdown experiments, a mixture of Lipofectamine RNAiMAX transfection reagent (Life Technologies, Grand Island, NY) and MCT4 siRNA (h2, sc-45892, Santa Cruz Biotechnology, Dallas, TX) was added to plates of 70% confluent pancreatic cancer cells as described by the manufacturer.
Macrophage differentiation and polarization
For macrophage differentiation assays, THP-1 cells were cultured for 72 hours in the presence of 50ng/ml phorbol 12-myristate 13-acetate (PMA) or 50% conditioned media, as indicated. For polarization assays, following incubation in the presence of PMA, adherent THP-1 cells were washed and differentiation media was replaced with fresh supplemented RPMI with or without 50% conditioned media. Conditioned media was isolated from ~80–90% confluent established pancreatic cancer cell lines 72 hours post-split, or post-transfection, centrifuged at 4000 RPM for 10 minutes, and filtered with a 0.45 micron syringe filter to remove contaminating cells and debris before use. For proteinase K and lactic acid experiments, following conditioned media collection, samples were treated with proteinase K (50μg/ml) for 30 minutes at 37°C followed by phenylmethylsulfonyl fluoride (PMSF, 5mM) for 1 hour at room temperature to inactivate proteinase K. Lactic acid (Sigma Aldrich, St. Louis, MO) was added at 8mM. Lactate readings were acquired on an automated electrochemical analyzer (Bioprofile Basic-4 Analyzer, NOVA Biomedical, Waltham, MA).
Flow cytometry
Adherent THP-1 cells were scraped from culture dishes, collected by centrifugation, and resuspended in flow wash buffer (PBS +1% FBS + 0.1% sodium azide). To stain for cell surface markers, cells were incubated in the presence of antibodies specific for galectin 3/Mac-2 (LGALS3, clone M3/38, Cedarlane Labs, Burlington, Ontario, Canada), CCR7/CD197 (clone 3D12), CD206 (clone 19.2), and HLA-DR (clone L243) (eBioscience, San Diego, CA). Cells were then washed and resuspended in 1% paraformaldehyde. Pancreatic cancer cells were collected by trypsinization and resuspended as described above. For MCT4 staining, intracellular staining was performed as previously described (27) using anti-MCT4 antibody (clone H-90, Santa Cruz Biotechnology) conjugated with Alexa fluor 555 (Z-25305, Life Technologies). Following incubation, cells were washed with 0.1% saponin and then resuspended in flow wash buffer. All samples were collected on an LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (FlowJo, Ashland, OR).
Drug sensitivity
Cells from established pancreatic cancer cell lines were plated for 72 hours in the presence or absence of PMA-differentiated macrophages, pancreatic cancer cell conditioned media-polarized macrophages, and gemcitabine (90nM). At the end of the time course, relative cell number was determined by CellTiter-Glo cell viability assay (Promega, Madison, WI) and luminescence was read on a Synergy 2 multi-mode plate reader (Biotek Instruments, Winooski, VT).
Cancer cell invasiveness
THP-1 cells were plated in 12-well plates, differentiated for 72 hours with PMA, and then polarized with conditioned media overnight. The following day, fresh media was added and Transwell membrane inserts with 0.4um pores (Corning, Corning, NY) were added onto each well. Pancreatic cancer cells (10,000 cells/membrane) were added to the top of each Transwell membrane and cultured for 24 hours. The top of each insert was carefully washed with PBS and the remaining cells on the bottom of the membrane were fixed in 10% formalin and stained with hematoxylin. Membranes were cut from the Transwell supports, placed on microscope slides, and imaged. The number of migrated cells was counted and the average number of cells was obtained from 10 high-powered fields. For wound healing assays, equivalent numbers of either PMA-differentiated or conditioned media-polarized THP-1 cells were added to wells of culture dishes confluent with pancreatic cancer cells. Each well was scored with a pipet tip and imaged at the indicated time points at 40x total magnification on an EVOS FL cell imaging system (Life Technologies).
Results
Differential recruitment of immune cells to tumor and stromal compartments is associated with survival differences in PDA patients
Pancreatic ductal adenocarcinoma often arises in the background of chronic inflammation suggesting that perturbations in immune function can have significant impact related to tumor biology, disease prognosis and therapeutic interventions. To elucidate the association of suppressive immune features in clinical cases a cohort of PDA cases (n=223) was employed (Table 1). Within this cohort, inflammation, as defined by the presence of lymphocytes and neutrophils, was widely distributed throughout the cases (Supplementary Figure S1A) and overall lymphocyte or neutrophil accumulation was not significantly associated with survival (Supplementary Figure S1B).
Table 1.
Patient Characteristics
| Characteristics | No. of patients 223 (%) | |
|---|---|---|
|
| ||
| Median Age (range) | 65 (27–89) | |
|
| ||
| Gender | ||
| Male | 121 (54) | |
| Female | 102 (46) | |
|
| ||
| Tumor size (cm) | ||
| 0–2 | 42 (19) | |
| 2.1–4 | 123 (55) | |
| > 4 | 49 (22) | |
| Unknown | 9 (4) | |
|
| ||
| Node Involvement | ||
| Positive | 142 (64) | |
| Negative | 78 (35) | |
| Unknown | 3 (1) | |
|
| ||
| Grade | ||
| 1 | 20 (9) | |
| 2 | 138 (62) | |
| 3 | 57 (26) | |
| Unknown | 8 (3) | |
|
| ||
| Vital Status | ||
| Alive | 119 (53) | |
| Dead | 92 (42) | |
| Unknown | 12 (5) | |
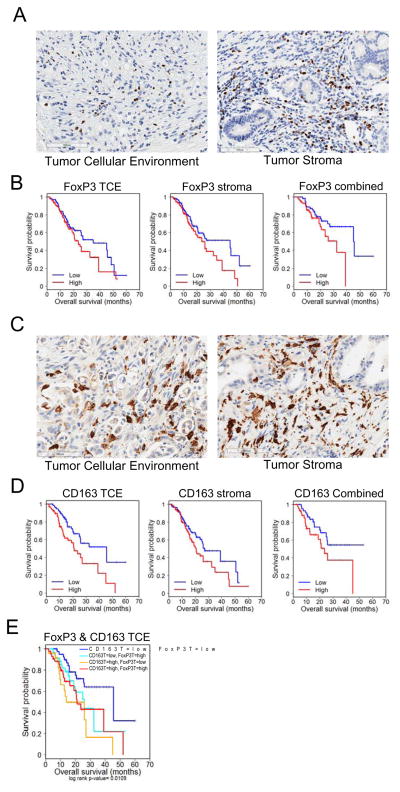
Proper T-cell function is necessary for efficient recognition and subsequent elimination of tumor cells by the immune system. As such, suppression of T-cell function is associated with poor outcome in multiple malignancies, including pancreatic cancer (28). Under normal circumstances, FoxP3+ regulatory T-cells play a critical role in blunting the T-cell response to prevent autoimmunity; however, the role of these cells is subverted in the context of tumorigenesis and disease progression (29, 30). Since PDA often contains abundant desmoplastic stroma, regulatory T-cells were scored in the tumor cellular environment and tumor stroma (stroma). Approximately 50% of PDAs harbored FoxP3 positive infiltrate scored as high in at least one compartment (Figure 1A). Higher levels of these cells only in the stroma were associated (HR=1.395, p<0.05) with worse survival and combined expression in both compartments was associated (HR=2.038, p<0.05) with decreased survival versus the absence of FoxP3 (Figure 1B). These data suggest that the immune milieu of the surrounding stroma is an important determinant of disease outcome in addition to that of the tumor itself (Supplementary Table S1).
Figure 1. Immunosuppressive cells are associated with poor outcome in PDA.
(A) Representative images of High FoxP3 staining in the tumor cellular environment (TCE, >0%) and stroma (>9.5%) of patient sections. (B) Kaplan-Meier plots indicating survival probability in human patients with High or Low accumulation of FoxP3+ cells in the tumor cellular environment and/or stroma. See supplementary Table S1,S2. (C) Representative images of High CD163 staining in the tumor cellular environment (>30%) and stroma (>33%) of patient sections. (D) Kaplan-Meier plots indicating survival probability in human patients with High or Low accumulation of CD163+ cells in the tumor cellular environment and/or stroma. See supplementary Table S3,S4. (E) Kaplan-Meier plots indicating survival probability in human patients with High or Low accumulation of CD163+ and FoxP3+ cells in the tumor stroma. See supplementary Table S5.
The presence of M2 (CD163+) macrophages was evaluated in both the tumor cellular environment and stroma (Figure 1C). Expression of CD163 corresponded (HR=2.058, p=0.005) to overall survival when present in the tumor cellular environment, as opposed to stromal expression that only trended (HR=1.481, p<0.08) toward poor survival (Figure 1D). Likewise, the combined absence of CD163 throughout both the tumor and stroma was suggestive (HR=1.852, p<0.08) of a favorable prognosis (Figure 1D, Supplementary Table S2). Patients with tumors expressing low levels of both FoxP3 and CD163 had a significantly improved outcome (median survival [MS] = 45.6mo) when compared against patients with high levels of both markers (HR=2.367, p>0.017, MS=21mo). Meanwhile, high CD163 expression in combination with low FoxP3 expression was significantly associated (HR=3.422, p<0.005, MS=13.8mo) with the shortest median survival (Figure 1E, Supplementary Table S3).
Pancreatic cancer cells influence macrophage differentiation in a cell line dependent manner
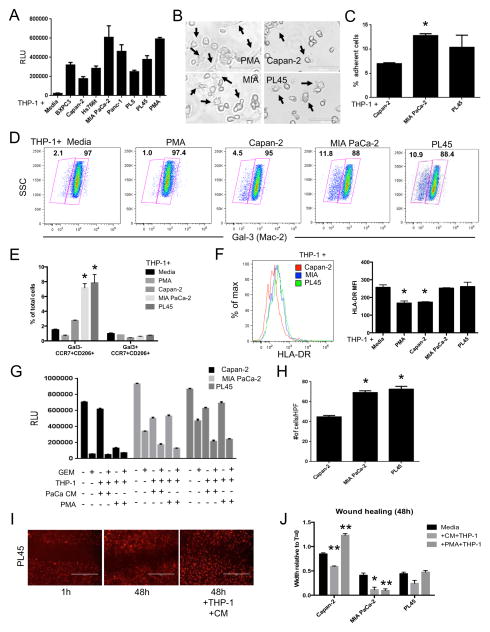
To further characterize the relationship between pancreatic cancer cells and macrophages, THP-1 cells were stimulated with conditioned media from various pancreatic cancer cell lines (Figure 2A). While the conditioned media demonstrated marked increases in macrophage differentiation over media alone, the response varied greatly between the different cell lines in terms of morphology (Figure 2B) as well as overall adherence (Figure 2C). Phenotypically, these differences were characterized by the increased prevalence of a galectin-3 (Mac-2)-negative population in MIA PaCa-2 or PL45 conditioned media treated THP-1 cells as compared to those cells treated with Capan-2 conditioned media (Figure 2D). Further, these galectin-3-negative cells expressed markers indicative of both M1 (CCR7, HLA-DR) and M2 (CD206) polarized macrophages (Figure 2E, 2F).
Figure 2. PDA cell products influence the phenotype and function of myeloid cells.
(A) Relative level of THP-1 cell adherence induced by the indicated conditioned media (n=3). (B) Representative phase contrast images (20x) of THP-1 cells treated with PMA or the indicated conditioned media for 72h. Arrows denote cellular morphology indicative of activated macrophages. Scale bars represent 100μm. (C) The relative number of adherent THP-1 cells following treatment with the indicated conditioned media for 72h (n=3). (D) Representative pseudocolor dot plots depicting the differential expression of galectin-3 in THP-1 cells treated with the indicated conditioned media for 72h (n=3). (E) The relative number of Gal3-CCR7+CD206+ and Gal3+CCR7+CD206+ macrophage populations in THP-1 cells treated with PMA or the indicated conditioned media for 72h (n=3). (F) Representative histogram overlay (left) and quantitative analysis (right) of THP-1 cell surface expression of HLA-DR following 72h treatment with the indicated conditioned media. (G) Relative cell survival following treatment of PDA cell lines (n=3) with the indicated combination of gemcitabine (GEM) and/or THP-1 cells differentiated in PMA or PDA cell conditioned media (PaCa CM). (H) The number of PDA cells from the indicated cell lines (n=3) that migrated to the bottom of a transwell membrane in response to PDA-conditioned media activated THP-1 cells. Data were collected from ten randomly selected high-power fields (40x) per sample. (I) Representative images of wound healing in mCherry-expressing PL45 cells after 48h incubation with or without conditioned media (CM) differentiated THP-1 cells. (J) Wound healing in the indicated PDA cell lines over the course of 48h +/− THP-1 cells pre-differentiated for 72h with either PMA or matched PDA CM. For all panels, all data are representative of at least 3 experiments. Graphed data represent mean values ± SEM. * indicates p<0.05. ** indicates p<0.01.
Macrophages differentiated by pancreatic cancer cells enhance cancer cell invasiveness
Given that PDA cell-conditioned media differentially induced macrophage differentiation and activation, the impact these macrophages played on mechanisms of pancreatic cancer severity was gauged. Regardless of the initial stimuli, treated THP-1 cells had no discernable effect on gemcitabine response (Figure 2G). However, the addition of the macrophages resulted in some cell death in the co-cultures, as evidenced by decreased cellularity in THP-1-only groups. Conditioned media-differentiated macrophages promoted pancreatic cancer cell invasiveness, as measured by Boyden chamber (Figure 2H) and wound healing assays (Figure 2I, 2J). Of note, expedited wound healing is a cell line specific phenomenon, as evidenced by the differential impact PMA-differentiated macrophages have on Capan-2 cells as compared to the other cell types.
Combined expression of immunosuppressive markers defines a subset of PDA patients with improved prognosis
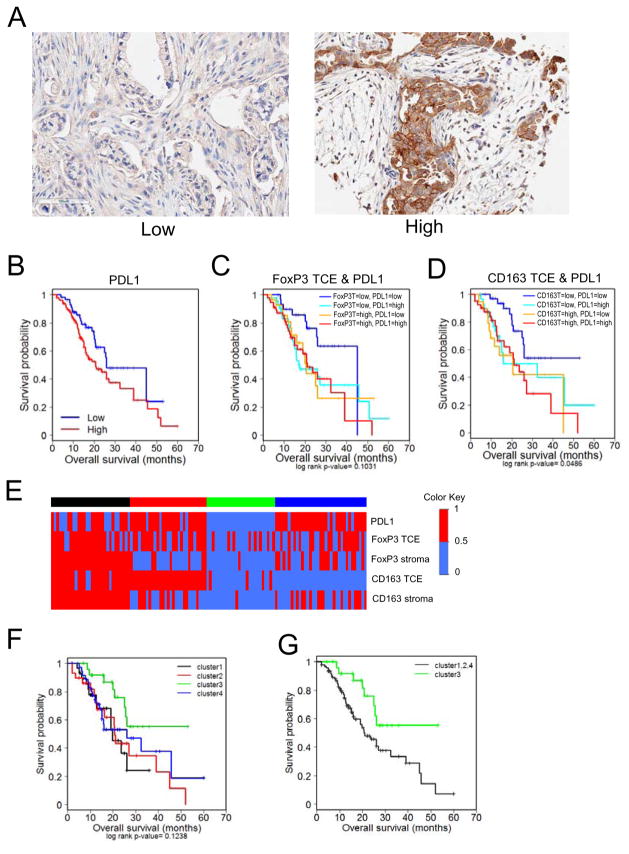
The presence of alternatively activated, M2 macrophages and regulatory T-cells in the PDA tumors suggested local immune evasion, whereby tumor cells escape immune surveillance. Therefore, the expression of PD-L1 (CD274), which is associated with suppression of local immune engagement, was evaluated in the PDA cohort. Tumor specific expression of PD-L1 was observed in the majority of PDA cases (Figure 3A) and was associated (HR=1.619, p=0.056) with overall survival (Figure 3B) suggesting that the presence of PD-L1 could be important for the maintenance of immune evasion.
Figure 3. Immune evasion and suppression signature correlates with poor prognosis in PDA.
(A) Representative images of Low (<10%) and High (≥10%) PD-L1 staining of patient PDA tumor sections. (B) Kaplan-Meier plots indicating survival probability in human patients with High or Low accumulation of PD-L1+ cells. See supplementary Table S6, S7. (C) Kaplan-Meier plots indicating survival probability in human patients with High or Low accumulation of FoxP3+ and PD-L1+ cells in the tumor cellular environment (TCE). See supplementary Table S8. (D) Kaplan-Meier plots indicating survival probability in human patients with High or Low accumulation of CD163+ and PD-L1+ cells in the tumor cellular environment. See supplementary Table S9. (E) Heat maps of unsupervised RF clustering of immune marker expression. Clusters were defined using the partitioning around medoids method. (F) Kaplan-Meier plots indicating survival probability in human patients based on the previously defined immune marker expression cluster. See supplementary Table S10. (G) Kaplan-Meier plots indicating survival probability in human patients from the cluster of lowest complex expression (cluster 3) with those from clusters with high expression of at least one marker (clusters 1,2,4). See supplementary Table S11.
Tumors that were dually absent of FoxP3 and PD-L1 (Figure 3C), or CD163 and PD-L1 (Figure 3D) demonstrated prolonged overall survival. In all patients, the median survival was 21 months, while these dual negative cases had a median survival of greater than 45 months (Supplementary Tables S5, S6). Importantly, this was not a feature of all combinations of these markers, as analysis of CD163- or FoxP3-negative stroma combined with the absence of PD-L1 expression had no such survival advantage. These data suggested that interactions between the immune markers could be clinically significant and define a subset of PDA patients with favorable prognosis.
Random Forest analysis was used to cluster cases based on the presence of CD163, PD-L1 and FoxP3 (Figure 3E). Using this approach, 4 clusters of cases that had distinct principle component features were defined (Figure 3F). In particular, cluster 3, consisting of approximately 25% of cases in the cohort, was largely devoid of CD163, FoxP3, and PD-L1, and had improved overall survival that was statistically significant vs. clusters 1 and 2 and trended for improved survival vs. cluster 4 (Supplementary Table S7). In comparison with all other clusters, the cases in cluster 3 were significantly associated with improved outcome exhibiting a median survival in excess of 50 months (Figure 3G, Supplementary Table S8).
Pancreatic cancer cell-mediated changes in glycolytic metabolites influence macrophage differentiation independent of cytokines
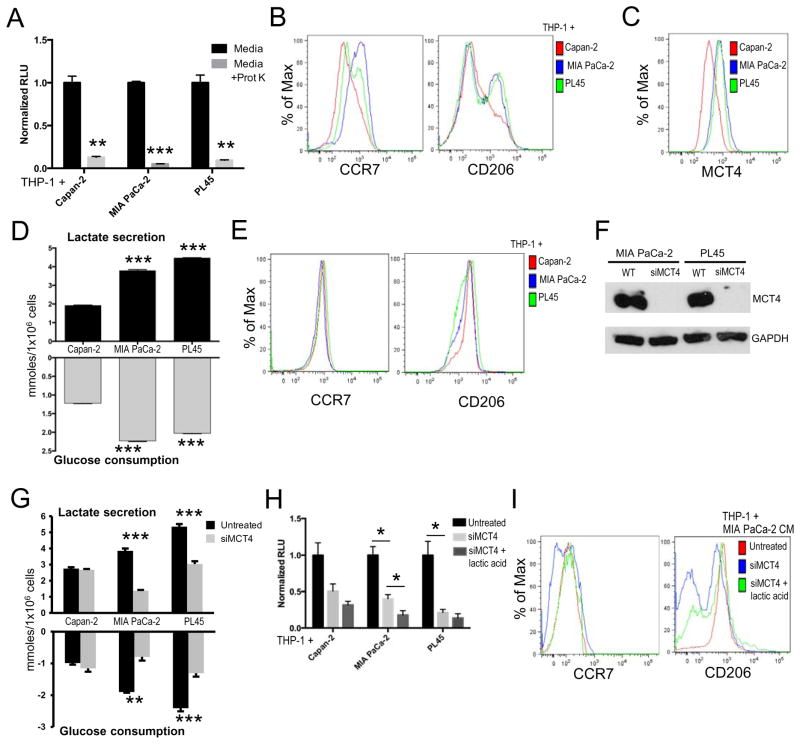
Local factors alter the function of immune cells in the tumor microenvironment. Established PDA cell lines secrete varying amounts of cytokines that would impact immune function, including high expression of CSF-1 by MIA PaCa-2 cells (31). To determine if the differences in macrophage activation were attributable to variations in cytokine secretion, THP-1 cells were cultured in protein depleted conditioned media. While protein depletion caused a significant reduction in macrophage adherence (Figure 4A), the phenotypic differences remained (Figure 4B). These data suggest that although secreted proteins (e.g. cytokines and chemokines) are important mediators of THP-1 adherence, additional factors are sufficient to skew macrophage polarization.
Figure 4. Pancreatic cancer cell metabolism influences myeloid cell differentiation and phenotype.
(A) Relative number of adherent THP-1 cells (n=3 per group) following 24h treatment with the indicated conditioned media +/− proteinase K. (B) Representative expression of CCR7 and CD206 on THP-1 cells (n=3 per group) following exposure to the indicated proteinase K pre-treated conditioned media. (C) Representative expression of MCT4 by the indicated pancreatic cancer cell lines. (D) Amount of lactate secreted and amount of glucose consumed per 1×106 cells by the indicated pancreatic cancer cell lines (n=3 per group). (E) Representative expression of CCR7 and CD206 on THP-1 cells (n=3 per group) following exposure to the indicated lactic acid pre-treated conditioned media. (F) Representative immunoblots of MCT4 expression in PDA cell lines ± siMCT4. (G) Amount of lactate secreted and amount of glucose consumed per 1×106 cells by the indicated pancreatic cancer cell lines ± siMCT4 knockdown (n=3 per group). (H) Relative level of THP-1 cell adherence induced by conditioned media collected from the indicated PDA cell lines ± siMCT4 knockdown and ± lactic acid (n=3). (I) Representative expression of CCR7 and CD206 on THP-1 cells (n=3 per group) following exposure to MIA PaCa-2 conditioned media under the indicated conditions. For all panels, all data are representative of at least 3 experiments. Graphed data represent mean values ± SEM. * indicates p<0.05, ** indicates p<0.01, *** indicates p<0.005.
Glycolysis has also been identified as a regulator of the inflammatory response (32). The PDA microenvironment is glycolytic in comparison to surrounding tissue and has been associated with poor outcome in PDA (4). As such, we investigated how the glycolytic environment impacts immune cell activation in PDA. Similar to our previous report (4), various established PDA cell lines differentially express monocarboxylate transporter 4 (MCT4, Figure 4C), which serves to export increased levels of lactate produced via glycolysis to the extracellular environment. Accordingly, the cell lines with increased MCT4 expression consume more glucose and secrete more lactate than those with lower MCT4 expression (Figure 4D). Meanwhile, the addition of exogenous lactic acid to conditioned media negated the phenotypic differences induced by the different PDA cell lines (Figure 4E). To determine if MCT4 expression on PDA cells influenced immune response, MCT4 was knocked down in PDA cell lines (Figure 4F) resulting in a significant decrease in lactate secretion and glucose consumption in PDA cell lines with higher basal MCT4 expression (Figure 4G). Conditioned media from these cells was added to undifferentiated THP-1 cells and in all cases, MCT4 knockdown resulted in decreased THP-1 cell differentiation, as measured by macrophage adherence, when compared with control cells (Figure 4H). While the addition of lactic acid did not increase THP-1 adherence in these cells following MCT4 knockdown (Figure 4H), this treatment was sufficient to rescue CCR7 and CD206 expression (Figure 4I). These data are consistent with our earlier findings (Figure 4A, 4B) in suggesting differential roles for glycolytic metabolites in adherence and polarization.
The inclusion of MCT4 expression enhances the prognostic value of immunosuppressive biomarkers
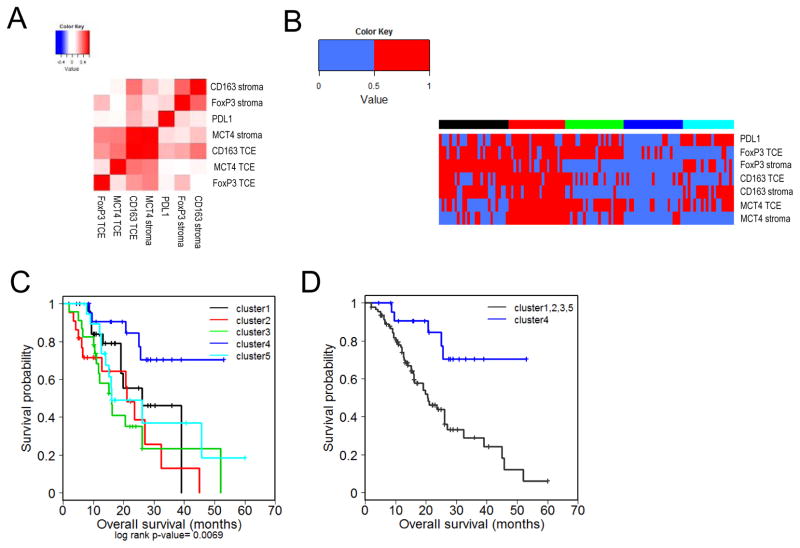
Given the role of MCT4 in regulating immune function, additional prognostic power could be added by incorporating immunological markers with MCT4 expression. Thus, we investigated the relationship of MCT4 expression in PDA with the immune related markers. These data showed that MCT4-status in both the tumor cellular environment and stroma were positively correlated with CD163 expression in the tumor cellular environment (Figure 5A). All other markers exhibited a tenuous or non-significant association with MCT4 in the tumor cellular environment, but interestingly stromal MCT4 correlated with veritably all immunological features (Figure 5A). These findings suggest that while there is a relationship between the glycolytic state of the tumor and the immunological features of disease, the dual absence of both is associated with increased overall survival. Random forest clustering revealed the presence of discrete clusters that were separated based on distinct principle component features (Figure 5B). Cluster 4 lacked almost all immune markers and the expression of MCT4 in the stroma. In Kaplan-Meier analysis this cluster harbored particularly favorable overall survival (Figure 5C, Supplementary Table S9). Importantly, this cluster was significant (HR=3.852, p<0.005, MS >53mo) vs. all other cases (MS=20.6mo) and remained significant in multivariate analysis including grade and lymph node status (Figure 5D, Supplementary Tables S10, S11).
Figure 5. Suppressive immune features and MCT4 expression define prognostic disease subtypes in PDA.
(A) Pearson correlation heat maps of immune markers and MCT4 expression. (B) Heat maps of unsupervised RF clustering of immune marker and MCT4 expression. (C) Kaplan-Meier plots indicating survival probability in human patients based on the previously defined immune marker and MCT4 expression clusters. See supplementary Table S12. (D) Kaplan-Meier plots indicating survival probability in human patients from the cluster of lowest complex expression (cluster 4) with those from clusters with high expression of at least one marker (clusters 1,2,3,5). See supplementary Tables S13, S14.
Discussion
Based on underlying tumor metabolism and mutation status, PDA actively promotes an inflammatory and immunosuppressive microenvironment by recruiting macrophages, and other immune cells. The composition of the cellular immune microenvironment in PDA is associated with variable patient outcome. We examined the tumor microenvironment of patients with PDA and identified tumor characteristics that were independently associated with prognosis. In addition, PDA can influence macrophages recruited to the tumor through alterations in the metabolic environment. MCT4 expression, a surrogate marker for the glycolytic tumor microenvironment, influenced macrophage differentiation in vitro and provided additional prognostic discrimination in patients.
The immune system is designed to recognize and remove pathologic insults, including malignancy, with the survival of cancer cells suggests a host immune deficiency or an active evasion by cancer cells. To this end, the makeup of the immune milieu in the PDA tumor microenvironment and the effects these cells have on pathogenesis and prognosis has garnered increased attention. Since cytotoxic (CD8+) T cells are abundant in PDA (14), the lack of tumor cell death would seem to be the result of an immunosuppressive microenvironment instead of an intrinsic defect of adaptive immunity. This is further supported by immunotherapy models where targeting the T cell checkpoint PD-L1 improved the efficacy of systemic therapy (23). However, the use of immune checkpoint blockade therapeutics as single agent treatments has resulted in only minimal success (15, 33, 34), suggesting that some additional aspects of PDA or the tumor microenvironment are promoting immunosuppression.
The combined blockade of both myeloid differentiation signaling (CSF1R) and T cell checkpoint signaling (PD-L1) has been shown to be more effective than either approach individually (35). While these data suggest that myeloid cell-mediated promotion of immunosuppression directly contributes to the general lack of efficacy of single agent immunotherapeutics in PDA, the mechanisms that promote this phenomenon remain unclear. Thus, although the emergence of this suppressive immune environment supporting PDA development provides new possibilities to distinguish disease subtypes and highlights new opportunities for therapeutic interventions, additional insights are necessary to guide these beneficial outcomes.
PDA is associated with a significant desmoplastic stroma that harbors infiltrating immune cells. Previously, increased tumor infiltrate of FoxP3-, CD163-, or PD-L1-expressing cells or M2 macrophages has been associated with tumor progression or poor outcome (5, 24, 36, 37). We expand on this finding by demonstrating the importance of the tumor stroma to the development of this suppressive phenotype. PDA stroma has been shown to support the recruitment and accumulation of immune cells, demonstrating a phenotype high in pro-inflammatory cytokine and chemokine expression (7). Random Forest Clustering of our suppressive immune phenotypes reflect the association between suppressive immune cells in the stroma and inferior outcomes (clusters 1 and 2). Although the exact mechanism is not clear, the stroma in PDA promotes primary tumor growth by creating an immunosuppressive microenvironment. Further examination of the relationship between the primary tumor and the stroma is important to better understand the biology of this aggressive cancer and to help devise novel therapies.
It has recently been demonstrated that macrophages are critical for PDA tumor growth and that KRAS status plays an important role in macrophage recruitment and activation (38–40). To explore this further, here we utilized a system by which cells from the monocytic THP-1 line were treated with conditioned media isolated from established PDA cell lines in vitro. Our data demonstrate that these PDA cell lines differentially influence macrophage development. For example, the phenotype induced by conditioned media isolated from MIA PaCa-2 and PL45 cells (CCR7+CD206+) strongly resembles that of myeloid-derived suppressor cells (MDSCs), which would further enhance the immunosuppressive environment (41). These cells also express increased HLA-DR expression as compared to cells differentiated by Capan-2 conditioned media. It has previously been shown that the expression of HLA-DR on MDSCs is associated with their ability to induce CD4+ T cell tolerance, further reducing the capability for an effective anti-tumor immune response (42). However, HLA-DR expression on these cells also provides a mechanism by which specifically designed CD4+ T cells can convert these MDSCs, decreasing their suppressive efficacy (42). This suggests that PDA influences the immune tumor microenvironment by modulating macrophage populations.
Tumor associated macrophages have been shown to promote tumor growth through various mechanisms including TLR4/IL-10 mediated epithelial-mesenchymal transition, pro-migratory factor secretion, and enhanced drug resistance (37, 43–46). Here, we show that macrophages increase invasiveness in established PDA cell lines, as demonstrated by increased recruitment of PDA cells (Figure 2H) and expedited wound healing response (Figure 2I, J) in the presence of macrophages differentiated by PDA-conditioned media. MIA PaCa-2 and PL45 cells were able to overcome the effect of PMA-differentiated macrophages on wound healing while Capan-2 cells were not. Thus, our data suggest a PDA cell-specific mechanism that facilitates tumor growth and prevents anti-tumor response, in line with previous reports (38, 39, 47). Taken together, these data highlight the complex regulation of the immune environment in PDA.
While it has been shown that tumor-derived cytokines such as GM-CSF can directly influence the immune milieu in and around the tumor (38, 39), our data indicate that a cytokine-independent mechanism also contributes to skew immune cell differentiation (Figure 4B). PDA can directly influence macrophages, myeloid cells and the tumor microenvironment by altering the local metabolic conditions. It has become increasingly evident that glycolysis plays an important role in regulating immune response, particularly where increased glycolysis produces glycolytic byproducts like lactic acid (32). In macrophages, high levels of intracellular lactate result in decreased responsiveness to LPS, and thus macrophage MCT4 expression is associated with more effective inflammatory response (16). Conversely, tumor-derived lactic acid is capable of skewing macrophages towards the anti-inflammatory, tumor-promoting M2 phenotype (48). Here we show decreased macrophage differentiation in THP-1 cells treated with conditioned media from cell lines where MCT4 has been knocked down. These data provided sufficient rationale for examining how PDA expression of MCT4 is associated with immune markers of disease.
Extensive profiling of established PDA cell lines has revealed distinct stratification of these lines on the basis of their metabolic characteristics. These findings demonstrate that various cell lines are fundamentally predisposed towards different metabolic programs, including a subset of cells with an elevated reliance on glycolysis (49). Along these lines, we have recently shown that enhanced glycolysis in PDA, represented by the expression of MCT4, is associated with prognosis. Our findings demonstrated that glycolysis was largely suppressed in PDA cell lines with higher endogenous levels of MCT4 (e.g. PL45 and MIA PaCa-2) while cell lines with lower endogenous MCT4 expression were generally unaffected (4). Here we have demonstrated that MCT4 knockdown is sufficient to attenuate lactate efflux and reduce glucose uptake (Figure 4G), consistent with our previous findings, which also indicated that MCT4 is necessary to maintain proper lactate production (4). Strikingly, patients that had low expression of MCT4 in conjunction with diminished markers of suppressive immune cells demonstrated significantly improved survival as compared to other clusters. These data suggest that a combination of immune and metabolic features in PDA define subsets of disease that sufficiently correspond to prognosis. Taken together, these data suggest that the individual metabolic state of PDA malignancies may portend the efficacy of potential immunotherapies and that, ultimately, a combined approach comprised of immunotherapy and a targeted metabolic therapy may increase treatment efficacy.
Supplementary Material
Translational Relevance.
Recent findings in pancreatic ductal adenocarcinoma (PDA) have shown that an influx of immunosuppressive inflammatory cells around the tumor is associated with poor outcome. However, additional insights are necessary to determine whether these prognostic findings can be utilized as predictive markers. We used a cohort of PDA patients to characterize the immunosuppressive tumor microenvironment and demonstrated the association of multiple markers with clinical outcome. Utilizing established PDA cell lines, we showed that byproducts of glycolysis are capable of skewing immune cell phenotypes. Based on these findings we refined our immune expression panel by incorporating the lactate exporter MCT4. Patients with low expression of immune markers and MCT4 exhibited a significantly improved overall survival. Thus, metabolic features of PDA can impact the local immune environment which is likely relevant to prognosis and treatment of disease.
Acknowledgments
Financial Support: NIH
Footnotes
Disclosures: The authors have nothing to disclose.
References
- 1.Fokas E, O’Neill E, Gordon-Weeks A, Mukherjee S, McKenna WG, Muschel RJ. Pancreatic ductal adenocarcinoma: From genetics to biology to radiobiology to oncoimmunology and all the way back to the clinic. Biochimica et biophysica acta. 2014;1855:61–82. doi: 10.1016/j.bbcan.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Buchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. The British journal of surgery. 2004;91:586–94. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 3.Franco J, Witkiewicz AK, Knudsen ES. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget. 2014;5:6512–25. doi: 10.18632/oncotarget.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek G, Tse YF, Hu Z, Cox D, Buboltz N, McCue P, et al. MCT4 Defines a Glycolytic Subtype of Pancreatic Cancer with Poor Prognosis and Unique Metabolic Dependencies. Cell reports. 2014;9:2233–49. doi: 10.1016/j.celrep.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 5.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–23. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamieson NB, Mohamed M, Oien KA, Foulis AK, Dickson EJ, Imrie CW, et al. The relationship between tumor inflammatory cell infiltrate and outcome in patients with pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2012;19:3581–90. doi: 10.1245/s10434-012-2370-y. [DOI] [PubMed] [Google Scholar]
- 7.Tjomsland V, Niklasson L, Sandstrom P, Borch K, Druid H, Bratthall C, et al. The desmoplastic stroma plays an essential role in the accumulation and modulation of infiltrated immune cells in pancreatic adenocarcinoma. Clinical & developmental immunology. 2011;2011:212810. doi: 10.1155/2011/212810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartel M, Di Mola FF, Gardini A, Zimmermann A, Di Sebastiano P, Guweidhi A, et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World journal of surgery. 2004;28:818–25. doi: 10.1007/s00268-004-7147-4. [DOI] [PubMed] [Google Scholar]
- 9.Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Lohr JM, et al. Pancreatic cancer microenvironment. International journal of cancer Journal international du cancer. 2007;121:699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 10.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–34. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 11.Benson DD, Meng X, Fullerton DA, Moore EE, Lee JH, Ao L, et al. Activation state of stromal inflammatory cells in murine metastatic pancreatic adenocarcinoma. American journal of physiology Regulatory, integrative and comparative physiology. 2012;302:R1067–75. doi: 10.1152/ajpregu.00320.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asari S, Matsumoto I, Toyama H, Shinzeki M, Goto T, Ishida J, et al. Preoperative independent prognostic factors in patients with borderline resectable pancreatic ductal adenocarcinoma following curative resection: the neutrophil-lymphocyte and platelet-lymphocyte ratios. Surg Today. 2015 doi: 10.1007/s00595-015-1206-3. [DOI] [PubMed] [Google Scholar]
- 13.Gunderson AJ, Kaneda MM, Tsujikawa T, Nguyen AV, Affara NI, Ruffell B, et al. Bruton’s Tyrosine Kinase (BTK)-dependent immune cell crosstalk drives pancreas cancer. Cancer discovery. 2015 doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu YF, Lu Y, Cheng H, Shi S, Xu J, Long J, et al. Abnormal distribution of peripheral lymphocyte subsets induced by PDAC modulates overall survival. Pancreatology. 2014;14:295–301. doi: 10.1016/j.pan.2014.05.797. [DOI] [PubMed] [Google Scholar]
- 15.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan Z, Xie N, Banerjee S, Cui H, Fu M, Thannickal VJ, et al. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J Biol Chem. 2015;290:46–55. doi: 10.1074/jbc.M114.603589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei L, Zhou Y, Yao J, Qiao C, Ni T, Guo R, et al. Lactate promotes PGE2 synthesis and gluconeogenesis in monocytes to benefit the growth of inflammation-associated colorectal tumor. Oncotarget. 2015;6:16198–214. doi: 10.18632/oncotarget.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peter K, Rehli M, Singer K, Renner-Sattler K, Kreutz M. Lactic acid delays the inflammatory response of human monocytes. Biochem Biophys Res Commun. 2015;457:412–8. doi: 10.1016/j.bbrc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, et al. Hif1alpha deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia. Cancer discovery. 2015 doi: 10.1158/2159-8290.CD-15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller BW, Morton JP, Pinese M, Saturno G, Jamieson NB, McGhee E, et al. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol Med. 2015;7:1063–76. doi: 10.15252/emmm.201404827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witkiewicz AK, Whitaker-Menezes D, Dasgupta A, Philp NJ, Lin Z, Gandara R, et al. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11:1108–17. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67:4182–9. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- 23.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 24.Geng L, Huang D, Liu J, Qian Y, Deng J, Li D, et al. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. Journal of cancer research and clinical oncology. 2008;134:1021–7. doi: 10.1007/s00432-008-0364-8. [DOI] [PubMed] [Google Scholar]
- 25.R_Core_Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2014. [Google Scholar]
- 26.Shi T, Seligson D, Belldegrun AS, Palotie A, Horvath S. Tumor classification by tissue microarray profiling: random forest clustering applied to renal cell carcinoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18:547–57. doi: 10.1038/modpathol.3800322. [DOI] [PubMed] [Google Scholar]
- 27.Hutcheson J, Perlman H. Loss of Bim results in abnormal accumulation of mature CD4-CD8-CD44-CD25- thymocytes. Immunobiology. 2007;212:629–36. doi: 10.1016/j.imbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 29.Linehan DC, Goedegebuure PS. CD25+ CD4+ regulatory T-cells in cancer. Immunologic research. 2005;32:155–68. doi: 10.1385/IR:32:1-3:155. [DOI] [PubMed] [Google Scholar]
- 30.Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–6. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shieh JH, Cini JK, Wu MC, Yunis AA. Purification and characterization of human colony-stimulating factor 1 from human pancreatic carcinoma (MIA PaCa-2) cells. Archives of biochemistry and biophysics. 1987;253:205–13. doi: 10.1016/0003-9861(87)90653-9. [DOI] [PubMed] [Google Scholar]
- 32.Ghesquiere B, Wong BW, Kuchnio A, Carmeliet P. Metabolism of stromal and immune cells in health and disease. Nature. 2014;511:167–76. doi: 10.1038/nature13312. [DOI] [PubMed] [Google Scholar]
- 33.Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–69. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang Y, Du Z, Yang F, Di Y, Li J, Zhou Z, et al. FOXP3+ lymphocyte density in pancreatic cancer correlates with lymph node metastasis. PLoS One. 2014;9:e106741. doi: 10.1371/journal.pone.0106741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshikawa K, Mitsunaga S, Kinoshita T, Konishi M, Takahashi S, Gotohda N, et al. Impact of tumor-associated macrophages on invasive ductal carcinoma of the pancreas head. Cancer science. 2012;103:2012–20. doi: 10.1111/j.1349-7006.2012.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–35. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–47. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liou GY, Doppler H, Necela B, Edenfield B, Zhang L, Dawson DW, et al. Mutant KRAS-induced expression of ICAM-1 in pancreatic acinar cells causes attraction of macrophages to expedite the formation of precancerous lesions. Cancer discovery. 2015;5:52–63. doi: 10.1158/2159-8290.CD-14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–94. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagaraj S, Nelson A, Youn JI, Cheng P, Quiceno D, Gabrilovich DI. Antigen-specific CD4(+) T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling. Cancer Res. 2012;72:928–38. doi: 10.1158/0008-5472.CAN-11-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng F, Li C, Li W, Gao Z, Guo K, Song S. Interaction between pancreatic cancer cells and tumor-associated macrophages promotes the invasion of pancreatic cancer cells and the differentiation and migration of macrophages. IUBMB life. 2014 doi: 10.1002/iub.1336. [DOI] [PubMed] [Google Scholar]
- 44.Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, et al. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93:844–54. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 45.Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, et al. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–52. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 46.Weizman N, Krelin Y, Shabtay-Orbach A, Amit M, Binenbaum Y, Wong RJ, et al. Macrophages mediate gemcitabine resistance of pancreatic adenocarcinoma by upregulating cytidine deaminase. Oncogene. 2014;33:3812–9. doi: 10.1038/onc.2013.357. [DOI] [PubMed] [Google Scholar]
- 47.Panni RZ, Sanford DE, Belt BA, Mitchem JB, Worley LA, Goetz BD, et al. Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer. Cancer immunology, immunotherapy : CII. 2014;63:513–28. doi: 10.1007/s00262-014-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Daemen A, Peterson D, Sahu N, McCord R, Du X, Liu B, et al. Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc Natl Acad Sci U S A. 2015;112:E4410–7. doi: 10.1073/pnas.1501605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.