Abstract
Human cells are exposed to exogenous insults and continuous production of different metabolites. These insults and unwanted metabolic products might interfere with the stability of genomic DNA. Recently many studies demonstrated that different psychiatric disorders show substantially high level of oxidative DNA damage in the brain accompanied with morphological and functional alterations. It reveals that damaged genomic DNA may contribute to the pathophysiology of these mental illnesses. Here we review the role of oxidative damage and reduced antioxidant ability in some vastly studied psychiatric disorders and emphasize the inclusion of treatment options involving DNA repair. In addition, while most currently used antidepressants are based on the manipulation of the neurotransmitter regulation in managing different mental abnormalities, they are able to prevent or reverse neurotoxin-induced DNA damage. Therefore, it may be plausible to target on genomic DNA alterations for psychiatric therapies, which is of pivotal importance for future anti-psychiatric drug development.
Keywords: Major depression, bipolar disorder, schizophrenia, oxidative stress, DNA damage, DNA repair
1. Introduction
Psychiatric illnesses such as mood disorders and schizophrenia are common, chronic mental disorders involving alterations in neuronal structures and functions. These diseases affect the lives of millions of individual worldwide and account for a significant proportion of the global disease burden. Despite considerable progress in research on these diseases in past decades, many fundamental challenges related to their etiologies and pathophysiology are far from being fully elucidated. For example, as one of the most prevalent and costly psychiatric disorders worldwide, the major depression disorder (MDD) has been hypothesized to be etiologically related to alterations in brain monoamine (Schildkraut 1965), corticosteroid receptors (Holsboer 2000), neurotrophic factors (Duman & Li 2012), and cellular plasticity (Kempermann & Kronenberg 2003), as well as associated to inflammation (Muller & Schwarz 2007, Krishnadas & Cavanagh 2012) and oxidative stress (Manji et al. 2012, Smaga et al. 2015). While these hypotheses have triggered extensive studies and have resulted in an accumulated evidence to make pathogenic mechanisms underlying these diseases gradually being disclosed, none of them has been widely accepted. Nevertheless, it may be promising that there is a possibly common pathogenic feature among these psychiatric diseases: DNA damage and impaired DNA damage repair (Shiwaku & Okazawa 2015). Human cells are constantly exposed to cytotoxic and genotoxic agents coming from endogenous and exogenous sources. As one of the consequences of such exposure, accumulation of unrepaired damaged DNA leads to ultimate neuronal loss and associated neural deterioration (Harman 1981). Brain shows a high rate of metabolic activity and a high ratio of membrane surface area to cytoplasmic volume. Especially, to cope with greater energy requirement for functional maintenance, the brain consumes about 20% of the body’s total oxygen (Zecca et al. 2004). Continuously facing so active oxidative and metabolic reactions, brain neurons are more vulnerable to oxidative damage than other cells, which leads to pronounced neuropathology, like mutagenesis, cellular dysfunction and aberrant phenotypes (Subba Rao 2007). Therefore, DNA damage and unrepaired DNA lesions have been recognized as the leading component of many neurological disorders (Suberbielle et al. 2013). Currently, the search for biomarkers for DNA damage in psychiatric diseases is gaining increased attention and several appeared evidences suggest the contribution of this pathogenic mechanism to psychiatric diseases. However, to date very few reviews focus on DNA damage and repair in major psychiatric disorders. In this review we hypothesize that oxidative DNA damage plays an important role in the pathophysiology of psychiatric disorders and then address these issues in MDD, bipolar disorder and schizophrenia that represent the common and major psychiatric diseases.
2. DNA damage and cellular balance between oxidative stress and antioxidant processes
DNA damage, mainly in the form of oxidative DNA damage and cytogenetic damage, means any modification of DNA leading to changes of its coding properties and functions. This DNA lesion can be formed through intracellular and extracellular processes after exposure to various exogenous and endogenous insults. The exogenous insults include environmental DNA-damaging agents such as ionizing radiation, ultraviolent light, drugs and other chemicals (Ward 1988, McMillan et al. 2008, O'Neill & Wardman 2009). The endogenous sources of damage can be replication stress and oxidative stress which result in free radicals from oxygen metabolism as by-products such as reactive oxygen species (ROS), including hydroxyl (OH−), superoxide (O2−) and hydrogen peroxide (H2O2) (Cooke et al. 2003, Valko et al. 2007); reactive nitrogen species (RNS), including nitric oxide (NO) and nitrogen dioxide; and superoxide radicals from inflammatory responses (Kryston et al. 2011). While these free radicals can attack all cellular vital components like DNA, proteins and lipids, the nuclear and mitochondrial DNA damage has caught much attention, as it can block genome replication and transcription, further leads to mutations or genome aberration that threaten cell or organism viability (Uttara et al. 2009). The DNA lesions can occur in several types such as small base chemical alteration, helix distorting lesions, single-strand break and double-strand break (Rao 1993, Katyal & McKinnon 2008), all of which can interfere normal transcription or replication and incur a challenge for repair processes (Hoeijmakers 2001). DNA damage triggers a number of cellular responses including activation of cell cycle checkpoints which delay cell cycle progression in order to facilitate DNA repair or eliminate damaged cells through apoptosis (Zhou & Elledge 2000, Rouse & Jackson 2002). Coincidently, DNA damage also activates signaling pathways that serve to halt the cell cycle, when DNA repair occurs. Therefore, DNA damage is always synchronously followed by DNA repair, which involves the detection, repair and restoration of the damaged DNA (Hoeijmakers 2001). There are at least four active DNA repair pathways in nervous system, each specific to particular kind of DNA damage. For example, base excision repair is specific to correction of DNA base modifications created by reactive oxygen species. Nucleotide excision repair removes DNA lesions which distort its helical structure and creates cross links. Mismatch repair is essential for post-replication repair of misincorporated bases. Moreover, pathways like single-strand break repair and double-strand break repair are instrumental in repairing DNA-strand breaks. Specifically, there could be two options for a cell to repair double-strand breaks: nonhomologous end joining (NHEJ) or homologous recombination (HR) repair (Dexheimer 2013, Madabhushi et al. 2014).
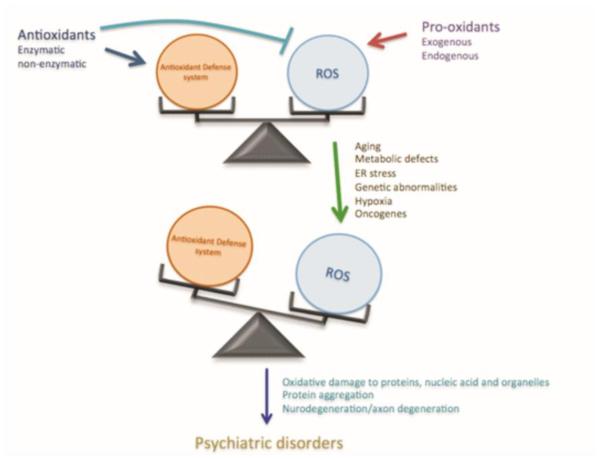
Normally there is a balance between oxidative and reductive processes and their regulation contributes to the mechanisms in neuronal development and vesicular transport (Wilson & Gonzalez-Billault 2015). The reductive processes include several antioxidant enzymes such as catalase, superoxide dismutase (SOD), glutathione peroxidase and glutathione reductase (Krohne-Ehrich et al. 1977, McCord & Fridovich 1988, Chelikani et al. 2004). This built-in clearance system provides protection against ROS and other free radicals. Both exogenous (natural/synthetic) and endogenous antioxidants are responsible for inhibiting formation of ROS, binding metal ions needed for ROS production, and removing ROS and their precursors (Gilgun-Sherki et al. 2001). However, in case of metabolic stress the intracellular environment may face accumulation of these free radicals after the clearance pathways get exhausted. As such, imbalanced redox processes and oxidative stress will damage cellular vital components including DNA, and lead to the development and pathogenesis of several biological disorders (Fig. 1). The neuron loss in neural disorders can be the result of a complex interplay among oxidative injury, excitatory stimulation, dysfunction of key proteins and genetic factors. Similarly important, a perturbation of the redox homeostasis including a reduction of cellular antioxidant defenses and DNA repair capabilities also contribute to the development of these disorders. Therefore, DNA damage and DNA repair defects can lead to a variety of consequences, such as impaired DNA replication and transcription processes (Saxowsky & Doetsch 2006, Katyal & McKinnon 2008), which certainly contributes to aging and neurodegenerative diseases (Katyal & McKinnon 2008, Martin 2008, Coppede & Migliore 2015). Likewise, giving oxidative stress has been implicated as a potentially pathophysiological factor in a range of psychiatric disorders (Ng et al. 2008, Pandya et al. 2013), the coexistence of DNA damage and impaired DNA repair have been identified in these diseases, though still an emerging research area.
Fig.1. Role of imbalanced ROS production in psychiatric disorders.
In normal states there is a balance between ROS production and antioxidant systems inside cells. Different factor can alter this ROS homeostasis, which has damaging effect on intracellular contents that leads to development of different psychiatric conditions.
It has been reported that there are higher levels of oxidative products and lower levels of antioxidants in the brain and peripheral tissues of the patients suffering from psychiatric diseases (Smaga et al. 2015). There are two common biomarkers of oxidative damage: 8-oxo-7, 8-dihydro-2’-dihydroguanosine (8-oxodG, also called 8-OHdG) and 8-oxo-7,8-dihydroguanosine (8-oxoGuo). 8-oxodG is the end product of the hydroxylation of guanine, and represents major oxidative DNA damage products (Hu et al. 2010). Guanine base is known to be the most susceptible to oxidative damage (Spassky & Angelov 1997, Radak & Boldogh 2010). Specifically, ROS such as H2O2, hydroxyl radicals, singlet oxygen, and one-electron oxidants react with guanine residues in cellular DNA, leading to the formation of 8-oxodG (Kohen & Nyska 2002, Cadet et al. 2008). 8-oxoGuo is a ribonucleoside analogue of 8-oxodG and considered as a marker of RNA oxidation (Henriksen et al. 2009). These biomarkers have been commonly used to estimate the DNA damage in humans after exposure to cancer-causing agents, such as tobacco smoke, asbestos fibers, heavy metals, and polycyclic aromatic hydrocarbons. However, in recent years, 8-oxodG and 8-oxoGuo levels in the urine, blood and brain tissues have been widely used as not only an endogenous oxidative nucleotide damage biomarker (Evans et al. 2004, Nishioka & Arnold 2004, Hu et al. 2010, Sertan Copoglu et al. 2015), but also as a risk factor for many diseases (Wei et al. 2009).
3. DNA damage in MDD
MDD is one of the most prevalent and costly psychiatric disorder and has been estimated to be the second cause of disability worldwide (Malberg & Blendy 2005), with 10-30% of women and 7-15% of men likely to suffer from general depression in their life-time (Malberg & Blendy 2005). MDD is characterized by lowered mood, an inability to experience pleasure (anhedonia), combined by cognitive and vegetative symptoms such as changes in sleep, appetite, sexual and gastrointestinal motility (Belmaker & Agam 2008). Due to a higher suicidal rate than non-depressed subjects, MDD is associated with severe morbidity and an increased mortality (Cuijpers et al. 2014). As mentioned above, there have been several hypotheses for the pathophysiology of MDD. However, the exact etiology and mechanisms underlying MDD have not been determined. Nevertheless, a growing body of studies demonstrated that oxidative damage in the brain of the patients suffering from major mental illnesses is one of the major pathological process (Glaser et al. 1985, Flint et al. 2005). As such, in recent years, while general depression as a symptom may be caused by different alterations in the brains such as deficiency of neurotransmitters, inflammation, or glucocorticoid receptors, etc., an increased severity of major depression has been hypothesized to be associated with an increase of oxidative DNA and RNA damage, which is attenuated by an effective antidepressant treatment (Jorgensen et al. 2013b).
There is an extensive body of evidence to support the involvement of DNA damage in MDD. First, the patients of MDD exhibit an increased level of biological marker of DNA damage. For example, higher concentrations of serum 8-oxodG were observed in patients with clinical depression (Forlenza & Miller 2006) and in depressive patients with gastric adenocarcinoma (Wei et al. 2009), compared to their non-depressive controls. There is a positive association between depressive symptoms and 8-oxodG levels in peripheral leukocytes in a non-clinical population (Irie et al. 2003), as well as in clinically depressed individual (Irie et al. 2005). Furthermore, association between increased levels of urinary 8-oxodG and major depression was also detected in patients with myalgic encephalomyelitis or chronic fatigue syndrome (Maes et al. 2009a). The meta-analysis carried out recently confirmed that 8-oxodG and F2-isoprostanes as the oxidative stress markers were increased in patients with MDD and bipolar disorder, indicating that depression is associated with increased oxidative damage to DNA and lipids (Palta et al. 2014, Black et al. 2015). However, one study did not find any significant association between urinary 8-oxodG and depressive symptoms in a large, non-clinical population of Japanese office workers (Yi et al. 2012). Another cross sectional study found a slight correlation between depressive symptoms and oxidative stress in college students (Matsushita et al. 2010). Second, the peripheral blood leukocytes showed significantly lower copy number of mitochondrial DNA (mtDNA) and higher oxidative damage due to higher oxidative stress in MDD patients (Chang et al. 2015). Similarly, another study reported higher number of single and double stranded breaks in DNA along with oxidative damage with reduced DNA repair activity in patients with depressive disorder (Czarny et al. 2015b). The same group further genotyped single nucleotide polymorphisms (SNPs) of three genes encoding glycosylases, which are essential enzymes for adequate operation of base excision repair (BER). They found a stronger association of certain SNPs with early-onset depression than with late-onset depression supporting the hypothesis that altered DNA repair may be involved in pathogenesis of depression (Czarny et al. 2015a). Other studies also showed an increased level of telomere shortening (Simon et al. 2006) and an increased frequency of DNA damage (Andreazza et al. 2007). Moreover, depression can alter the ability of a cell to repair damaged DNA (Czarny et al. 2015b, Forsberg et al. 2015), including a reduced plasma antioxidants, antioxidant enzyme function and total antioxidant capacity (Maes et al. 2011). There is a significant decreased activity of antioxidants such as whole-blood reduced glutathione, and decreased levels of antioxidant enzymes such as glutathione peroxidase, erythrocyte SOD and serum heme oxygenase 1 in depressed patients (Rybka et al. 2013). Besides DNA damage, depressed individuals also exhibit increased peripheral markers of oxidative damage to lipid and proteins, (Maes et al. 2011), as indicated by increased levels of malondialdehyde, a byproduct of polyunsaturated fatty acid peroxidation and arachidonic acid (Khanzode et al. 2003, Ozcan et al. 2004, Galecki et al. 2009a, Galecki et al. 2009b, Rybka et al. 2013), and F2 isoprostanes, an oxidized derivative of arachidonic acid (Yager et al. 2010, Chung et al. 2013). It is noteworthy that there is a clear clinical difference between MDD and general depression. Long history of depressive episodes and their severity are the most important differential features in MDD. This may be the reason why alterations listed above for the involvement of DNA damage are mostly from the studies on MDD, rather than general depression. This is in line with the concept of allostasis and allostatic load (McEwen 1998), as neuronal damage observed in MDD is the result of the maladaptation to oxidative stress and dysfunction of DNA repair mechanisms after prolonged and severe depression episodes.
DNA damage observed in MDD may mainly be caused by oxidative stress, which has been hypothesized to constitute a pathogenic mechanism in depression (Ng et al. 2008), and supported by many evidences, including increased peripheral markers of oxidative damage to lipids, proteins and DNA, as well as a reduced plasma antioxidants level, activity and total antioxidant capacity (Maes et al. 2011). In a human study on gene expression profiles in cells from autopsies of the frontal cortex, MDD was associated with molecular signs of inflammation, apoptosis and oxidative stress (Shelton et al. 2011). Also, there are reports related to increased plasma levels of peroxides and xanthine oxidase (Herken et al. 2007, Maes et al. 2010) and increased xanthine oxidase activity in post-mortem brain tissue of patients with recurrent depression (Michel et al. 2010). Furthermore, oxidative stress impacts telomere stability and functions to damage telomeric DNA, rendering the cell susceptible to apoptosis (Opresko et al. 2005, Zhang et al. 2007). Moreover, oxidative stress stimulates and amplifies inflammatory responses (Krishnadas & Cavanagh 2012). Increased exposure and potency of oxidative stress to neural cells leads to stress-induced neural dysfunction and neuronal cell death (Jesberger & Richardson 1991). In patients with MDD, this is especially true to neurons in the hippocampus (Sapolsky 2000), as there is a decrease in blood flow and glucose uptake by the hippocampus (Kalia 2005). This is supported by the marked decrease in hippocampal volume caused by substantial loss of cells via neuronal cell death (Sapolsky 2000). Much of this volume loss and cell death can be directly attributed to oxidative stress-induced apoptosis of the hippocampal neurons (Bremner et al. 2000).
Stress is often a predisposing factor in the development of MDD (Sapolsky 1996). Prolonged elevations of glucocorticoids are often observed in patients with MDD, which may also contribute to an increased oxidative damage found in MDD (Joergensen et al. 2011). Many in vivo and in vitro studies support this notion. For example, stress-induced higher glucocorticoids negatively interfere mitochondrial function (Madrigal et al. 2001, Du et al. 2009), increase DNA strand breaks, decrease total repair capacity and influence a number of genes related to DNA damage signaling pathways (Flint et al. 2007). Both acute and chronic stress significantly elevates 8-oxodG levels and DNA strand breaks in the frontal cortex, amygdala and hippocampus (Adachi et al. 1993, Liu et al. 1996, Consiglio et al. 2010). Human studies also showed that even a non-pathological stress like heavy workload and fatigue or psychological stress positively correlated with 8-oxodG levels in the peripheral blood leukocytes among the female workers (Irie et al. 2001, Irie et al. 2002, Inoue et al. 2009).
Under severe stress, the body begins to produce ROS that go unchecked due to unbalanced antioxidant levels seen in MDD. Buildup of ROS has deleterious consequences involving hemolysis, producing excessive amounts of heme iron, which acts as a pro-oxidant (Friedman & Caflisch 2010). The heme iron can react with the hydroxyl radical produced in the Fenton Reaction and causes both nuclear and mtDNA damage (via single or double stranded breaks) (Lloyd et al. 1997). A separate study that measured antioxidant enzymes and non-enzymatic molecules in addition to total antioxidant potential and plasma H2O2, reported a correlation between amount of oxidative stress and the severity of depression, determined by the total number of depressive episodes. In the plasma, these authors measured increased H2O2, which is lipid soluble and can diffuse out of the erythrocytes into the plasma. They also measured a decrease in antioxidative status, including a significant decrease detected in plasma uric acid levels (Yanik, Erel, & Kati, 2004), which is the most prevalent antioxidant molecules without enzymatic properties.
4. DNA damage in bipolar disorder
Bipolar disorder is the second most studied psychiatric disorder after schizophrenia and characterized by recurrent episodes of mania or hypomania along with general depression. Depression appears to be more common and longer lasting in bipolar disorder patients cultivating suicidal tendencies in affectees (Anderson et al. 2012). As a highly disabling psychiatric disorder, bipolar disorder has been considered as one of the leading causes of disability and is associated with increased morbidity and mortality (Kupfer 2005). Pathologically, this disorder has been associated with greater cognitive impairment, elevated levels of inflammatory markers, brain atrophy and poorer response to treatment (Robinson & Ferrier 2006, Berk 2009). However, the molecular mechanisms underlying pathogenesis of bipolar disorder remain unclear.
Similar to MDD, bipolar disorder has recently been linked to an increased DNA damage, possibly due to increased oxidative stress (Andreazza et al. 2007). For example, a meta-analysis revealed the presence of oxidative damage in bipolar disorder, showing higher levels of lipid peroxidation and DNA/RNA damage, as well as increased levels of NO in bipolar disorder patients (Ozcan et al. 2004, Brown et al. 2014). Biochemical measurements using peripheral samples showed that serum 8-oxodG levels (Soeiro-de-Souza et al. 2013), and urinary 8-oxodG and 8-oxoGuo levels (Munkholm et al. 2015) were higher in bipolar disorder patients compared to healthy controls and positively correlated with the number of manic episodes. The increased oxidatively generated nucleoside damage was present through all affective phases of the illness (Munkholm et al. 2015). Similarly, post-mortem studies demonstrated a higher DNA fragmentation in the anterior cingulate cortex of bipolar disorder patients (Buttner et al. 2007) and increased levels of single-strand and double-strand breaks in selective brain areas like the frontal cortex, hypothalamus, thalamus, cerebellum, pons and medulla in bipolar disorder patients, but not in the hippocampus (Mustak et al. 2010). However, another study revealed that damage in the hippocampus occurs predominantly in the cytoplasm of cells and thus affects RNA more than DNA (Che et al. 2010). The increase in DNA damage may lead to increased cell necrosis and subsequent inflammation of nearby tissues. In addition, patients suffering from bipolar disorder exhibited altered antioxidant systems (Ozcan et al. 2004), and a significantly decreased levels of poly ADP-ribose polymerase, a DNA repair enzyme, as well as SOD and catalase in the post-mortem brain hippocampus (Benes et al. 2006). Consistent to the above findings, neuronal loss has been reported in the medial prefrontal cortex, a key system in the regulation of emotional, behavioral, endocrine and innate immunological responses to stress (Guidotti et al. 2000, Savitz et al. 2014), the paraventricular nucleus and supraoptic nucleus of patients suffering from bipolar disorder (Manaye et al. 2005). The psychosocial stress, which results due to dysfunctional hypothalamic-pituitary-adrenal (HPA) axis, releasing high levels of glucocorticoids, also has role in bipolar disorder (Leverich et al. 2002, Brown et al. 2005, Johnson 2005, Bender & Alloy 2011). These higher levels of glucocorticoids negatively affect DNA repair machinery and antioxidant enzyme efficiency, which could be attributed to increased DNA damage in this disorder and moreover causes neuronal loss in the hippocampus, cingulate gyrus, striatum, amygdala and subcortical regions in the brain of bipolar patients (Cecil et al. 2002, Bertolino et al. 2003, Berretta et al. 2007, Reynolds & Reynolds 2011).
In a case report of monozygotic twins with bipolar disorder diagnosed via the DSM-IV, the authors examined the role of oxidative damage (Frey et al. 2007) and measured serum levels of thiobarbituric acid reactive substances, H2O2, as well as enzymatic activity levels of SOD and catalase. They found increased levels of SOD but lower levels of catalase along with increase in plasma levels of H2O2. Interestingly, they found that following a two-week treatment with lithium, one of the patients with bipolar disorder had levels of SOD similar to the control. However, her twin counterpart who did not undergo treatment had remained high levels of H2O2 and SOD. Furthermore, the treated patient’s levels of catalase remained low and her DNA damage levels remained high (Frey et al. 2007). This suggests that the damage to DNA in patients with bipolar disorder is a trait that is continuous and unchanging regardless of treatment and is not a state that can be altered with antioxidants. A similar study measured the methylation patterns of monozygonic twins discordant for bipolar disorder and found differences in four of the 10 explored regions (Kuratomi et al. 2008), showing a possible pathophysiological role of altered DNA methylation in Bipolar disorder.
Human mtDNA is prone to oxidative injury because mitochondria generate reactive oxygen species during the respiratory chain reactions (Yamada et al. 2006). Oxidative damage induces both point mutations and large deletions in mtDNA. The relative amount of mtRNA is reported to decrease with age in bipolar disorder (Kakiuchi et al. 2005, Vawter et al. 2006), though others found no differences compared to controls (Sabunciyan et al. 2007). Leukocytes have been shown to be a good cell model for studies of mitochondrial function (Liu et al. 2003, Leuner et al. 2012). The leukocyte mtDNA copy number of the bipolar disorder group was significantly lower than that of the control subjects, except for one report that no change was observed in this marker (de Sousa et al. 2014), indicating that more studies are warranted to evaluate mtDNA content. However, another study on bipolar patients showed a significantly higher mitochondrial oxidative damage than compared groups (Chang et al. 2014). Bipolar disorder has been associated with decreased mitochondrial electron transport chain activity and increased oxidative stress. Also, mtDNA encodes mitochondrial electron transport chain proteins and has been associated with altered oxidative stress.
Through comet assay, a sensitive method to assess DNA damage in single cells (Tice et al. 2000), it was shown that higher DNA damage appeared to be associated with the severity of manic or depressive episodes, suggesting that high oxidative stress found in the bipolar disorder may be responsible for elevated DNA damage (Andreazza et al. 2007). Supported evidence for the involvement of oxidative stress in DNA damage, found in bipolar disorder, came from an increased telomere shortening among patients with bipolar disorder (Simon et al. 2006). Consistent with these observations, the mood stabilizer lithium that is typically effective in bipolar disorder patients significantly decreased the SOD/catalase ratio similar to healthy subjects (Khairova et al. 2012). Preclinical studies showed that lithium treatment increased mtDNA content (Vawter et al. 2006). In addition, those patients who had been treated with lithium and valproate showed lower levels of oxidation related DNA damage, suggesting a role of oxidative stress induced DNA damage in bipolar disorder (Shao et al. 2005, Andreazza et al. 2007). In an animal model of mania, lithium and valproate were able to prevent and/or reverse DNA damage and antioxidant enzyme changes caused by amphetamine treatment (Andreazza et al. 2008).
5. DNA damage in schizophrenia
Schizophrenia is a chronic and severe mental illness that affects about 1% of world population (Schultz et al. 2007). It is characterized by positive symptoms (disturbances of thought, hallucinations and delusions), negative symptoms (anhedonia, social withdrawal, and apathy), and cognitive dysfunctions (Ettinger et al. 2014, Rosell et al. 2014). Etiologically these symptoms can be related to the neurodevelopmental, neurodegenerative, and structural abnormalities, which may originate from the interactions between malfunctioning genetic (variation of genes) and non-genetic (environmental stimuli) factors (Weinberger 1987, Lewis & Lieberman 2000, Lewis & Levitt 2002, Sawa & Snyder 2002, Harrison & Weinberger 2005). In addition, an integration of treatment studies, postmortem and animal investigations reveal that schizophrenia is also related to dysregulation of dopaminergic and glutamatergic neurotransmission, as well as increased pro-inflammatory status of the brain (Stevens 1982, Howes et al. 2009, Anticevic et al. 2012, Schwartz et al. 2012). This may be the reason why the majority of antipsychotic interventions target on dopaminergic and monoaminergic receptor systems (Taly 2013). Nevertheless, the clear mechanisms underlying the pathogenesis of schizophrenia remain to be elucidated.
Schizophrenia exhibits peripheral and cerebral mitochondrial dysfunctions (Prabakaran et al. 2004, Regenold et al. 2009, Rosenfeld et al. 2011), decreases in antioxidant defenses (Do et al. 2000, Reddy et al. 2003, Gysin et al. 2007) and increases in a range of oxidative stress markers (Yao et al. 2004, Zhang et al. 2006, Gama et al. 2008). As such, oxidative DNA damage has been proposed as culprits in the most recent hypotheses concerning the pathophysiology of schizophrenia and its associated symptoms (Zhang et al. 2010). This alteration has been recognized in treated, untreated and early-stage schizophrenic patients (Boskovic et al. 2011). For example, a higher level of 8-oxodG in the blood has been reported in non-remission schizophrenia patients (Sertan Copoglu et al. 2015). Also, both urinary 8-oxodG and 8-oxoGuo levels were significantly increased in the schizophrenia patients (Jorgensen et al. 2013a). The higher rate of these two DNA/RNA damage markers persists in these patients despite some controlling manipulations such as smoking and alcohol intake were applied. Postmortem studies reveal a higher level of 8-oxodG in the midbrain, caudate putamen and hippocampus in schizophrenia patients compared to the control, even up to10-folds higher (Cardozo-Pelaez et al. 2000, Nishioka & Arnold 2004, Che et al. 2010). In addition, using the comet assay technique, a significantly greater DNA damage level has been demonstrated in the blood samples from drug naïve schizophrenia patients, compared to matched controls (Muraleedharan et al. 2015). Paralleling to an increased DNA damage, schizophrenia patients also exhibited the decrease in antioxidant defenses such a lower level of glutathione peroxidase (Altuntas et al. 2000, Ranjekar et al. 2003), which has been recognized as a contributing factor for brain structural abnormalities found in schizophrenia (Buckman et al. 1987). All these studies suggested that DNA damage has been a common pathogenic process that contributes to declining course and poor outcome in schizophrenia. To support this notion, postmortem analysis shows that there is a significant neuronal loss in the prefrontal cortex and mammillary bodies of schizophrenia patients (Guidotti et al. 2000, Bernstein et al. 2007b, Bernstein et al. 2007a, Cabungcal et al. 2013). This neuronal loss may also be contributed by psychosocial stress, which has been recognized to trigger the onset and initial recurrence of schizophrenia (Nuechterlein et al. 1992, Norman & Malla 1994, Benes 1997, Walker & Diforio 1997, Gispen-de Wied 2000). Stress-induced hypercortisolemia may be one possible cause for the damage in the brain of schizophrenia patients.
Most likely, these nucleic lesions can result from oxidative stress, a common pathogenic process occurring in schizophrenia (Mahadik & Mukherjee 1996, Michel et al. 2004). An elevated level of oxidative stress markers such as ROS has been found in the samples of peripheral blood and cerebrospinal fluid of schizophrenia patients (Do et al. 2000, Sirota et al. 2003, Dietrich-Muszalska & Olas 2009, Korotkova et al. 2011). Post-mortem study demonstrated that the levels of SOD, nitric oxide, Cu, Zn- and MnSOD in the frontal cortex, caudate, and substantia innominate from schizophrenia patients were significantly increased (Michel et al. 2004, Yao et al. 2004). It was reported that the oxidative stress in the brain not only leads to DNA damage, but downstream can further cause hypoactive NMDA receptors, mitochondrial dysfunction and impaired inflammatory responses (Bitanihirwe & Woo 2011). The cognitive deficits accompanying schizophrenia are found to be caused by an increase in ROS and could be subject to modification via medications that lower oxidative stress, such as antidepressants, or those that directly reduce DNA damage, like mood stabilizers (Massaad & Klann 2011). In addition to inflammatory similarities to the other psychiatric disorders, patients with schizophrenia also presented with increased levels of malondialdehyde, a lipid peroxidation product (Loidl-Stahlhofen et al. 1994). Unlike the others, however, schizophrenia is associated with a decrease in SOD in neuroleptic-free patients, while catalase and glutathione peroxidase remain unaffected (Zhang et al. 2010). Nevertheless, chronic schizophrenic patients taking long-term neuroleptics showed increased levels of SOD (Raffa et al. 2009), indicating these drugs could have effects to normalize levels via endogenous antioxidative mechanisms (Padurariu et al. 2010).
In addition to the general oxidative markers like SOD, nitric oxide, catalase and malondialdehyde, patients with schizophrenia also show an imbalance in neurotransmitter levels, including dopamine and glutamate. Particularly important is the auto-oxidation of dopamine, which leads to production of hydrogen peroxide, quinones and superoxide, resulting in an increase of oxidant levels and stress (Fleckenstein et al. 2007). These auto-oxidation induced products cause further deterioration of antioxidant systems trying to combat high oxidant levels. Dopamine alone can reduce levels of reduced glutathione by 40% (Grima et al. 2003). Dopamine auto-oxidation with low levels of antioxidants can lead to retrograde degeneration of the mediodorsal nucleus of the thalamus (Popken et al. 2000). As neurons from the mediodorsal nucleus project to the dorsolateral prefrontal cortex, a substantial neuronal loss in this nucleus contributes to the cognitive impairment found in schizophrenia (Weinberger et al. 1986, Maher et al. 1995). Since glutamate is the major excitatory neurotransmitter of the brain, it must be balanced by its counterpart, γ-amino-butyric acid (GABA). Any interference to normal GABA secretion, or over-excitation of glutamate will turn to glutamate excitotoxicity, which is heavily associated with schizophrenia (Olney et al. 1999). Excess glutamate leads to calcium influx and release from internal sources, which in turn, can lead to generation of ROS (Hirose & Chan 1993) and subsequently oxidative stress and DNA damage in these brain regions (Lidow 2003).
6. RNA damage and mental illnesses
RNA is a single-stranded nucleic acid and is mostly located in cytoplasm in the proximity of the mitochondria. Thereby cytoplasmic RNA is especially vulnerable to the hydroxylation and may be at higher risk of being oxidatively modified than DNA (Nunomura A et al., 2009). The reason is possibly that the nitrogen bases in single-strand RNA are not protected by hydrogen bonding as in double-stranded DNA (Bregeon & Sarasin 2005, Li et al. 2006, Nunomura et al. 2009). As such, the oxidation of mRNA may cause improper translation and protein aggregation (Shan et al. 2007, Tanaka et al. 2007). Similarly, the oxidation of non-coding RNA species could affect the regulation of protein translation, gene expression, and neural synaptic plasticity (Satterlee et al. 2007). There was equal amount of damaged RNA along with damaged DNA in psychiatric diseases (Munkholm et al. 2015), indicating that the oxidative stress can interfere with the stability of all nucleic acids and that oxidatively generated nucleoside damage plays an important role in the development of these diseases. The consequences can be severe, which range from altering the gene expression to death of neurons. It can even have diagnostic implications as in some studies oxidative RNA damage increases at the early stages of neurodegenerative disease progression (Bradley-Whitman & Lovell 2013).
There are studies on RNA damage occurring in MDD, bipolar disorder and schizophrenia, respectively. It was reported that systemic RNA damage, as measured by urinary 8-oxoGuo excretion, was higher with increasing severity of depression, which was even further increased after therapies like electroconvulsive therapy (Jorgensen et al. 2013c, Jorgensen et al. 2013b). Similarly, urinary 8-oxoGuo excretion was increased in patients suffered from schizophrenia (Jorgensen et al. 2013a) or bipolar disorder (Munkholm et al. 2015), compared to healthy controls. However, more comprehensive studies reveal that RNA damage appears in all three mental illnesses mentioned above. Post-mortem studies demonstrated that hippocampal levels of intracellular DNA and RNA oxidation levels were increased in all schizophrenia, bipolar disorder and MDD patients (Che et al. 2010). Brain autopsies of dead patients suffering from MDD, bipolar disorder and schizophrenia have shown RNA oxidative damage (Joergensen et al. 2011, Jorgensen et al. 2013a). The magnitude of RNA oxidative damage was highest in patients with schizophrenia, whereas it was more modestly increased in patients with bipolar disorder and less so in patients with major depression. These findings suggest that RNA oxidative damage may be associated with the severity of these mental illnesses. The pattern of predominant oxidative damage to cytoplasmic RNA rather than nuclear DNA may be reversible after pharmacological intervention.
7. Effects of antidepressants against oxidative DNA damage
Antidepressants are mainly used for the treatment of MDD and for depressive symptoms appearing in many other psychiatric and degenerative disorders (Briley & Moret 1993, Martin 2008). They can be used alone or in combination with other medications. The most important classes of antidepressants are the selective serotonin reuptake inhibitors (Geddes & Cipriani 2004), serotonin and norepinephrine reuptake inhibitors and tricyclic antidepressants. Serotonin, dopamine and norepinephrine are the most important neurotransmitters primarily involved in regulation of mood and emotions (Butler & Meegan 2008). Selective serotonin reuptake inhibitors, norepinephrine reuptake inhibitors and most tricyclic antidepressants are believed to increase the extracellular level of serotonin and norepinephrine by inhibiting their reuptake into the presynaptic neurons. As a consequence, treatments with most of these antidepressants are believed to lead to an elevation in intra-synaptic concentration of these neurotransmitters and improve neurotransmission (Tatsumi et al. 1997, Gillman 2007), which also is the base for the hypothesis of the “monoamine theory” for etiology of major depression (Schildkraut 1965).
As mentioned above, a number of proposed mechanisms for major depression are brought into sight. However, none of them has been widely accepted. Recent years’ studies showed that general depression is accompanied by mitochondrial dysfunctions. DNA damage caused from ROS, RNS, and oxidative and nitrosative stress (O&NS), as well as lowered levels of antioxidants and antioxidant enzymes are involved in the pathophysiology of major depression (Maes et al. 2009b, Maes et al. 2011). Therefore, some studies have revealed the new targets for antidepressants. For example, antidepressants may protect neurons against neurotoxicity caused by several toxic compounds. Fluoxetine suppresses kainic acid-induced neuronal loss in the rat hippocampus, and the neuroprotective effect might be associated with its anti-inflammatory effects. It was reported that both R and S isomers of fluoxetine attenuated chronic neurodegeneration induced by a commonly used inflammogen lipopolysaccharide (LPS) (Zhang et al. 2012).
Furthermore, antidepressants may exert their therapeutic effects through targeting on oxidative and nitrosative mechanisms (Lee et al. 2013). Some studies suggest that conventional antidepressants and mood stabilizers may act in part through antioxidant mechanisms (Berk et al. 2011, Maes et al. 2011), and conversely that antioxidants have antidepressant properties (Berk et al. 2008, Scapagnini et al. 2012). The increasing evidence showed antioxidant effects of antidepressants and the relevance of intracellular oxidative pathways in the pathophysiology of major depression (Michel et al. 2007, Maes et al. 2009b, Maes et al. 2011, Behr et al. 2012, Michel et al. 2012). A few antidepressants have come up as potential treatment options to reduce oxidative damage in major depression. For example, beneficial effects of fluoxetine in reducing oxidative stress in brains (Omar M.E. Abdel-Salam 2011), shielding effects of desipramine against ischemia/reperfusion-induced oxidative stress in mice brain(Gaur & Kumar 2010), the protective role of venlafaxine against stress-induced oxidative neuronal DNA damage (Abdel-Wahab & Salama 2011), and the effect of deprenyl in protecting substantia nigra neurons from oxidative stress (Wu et al. 1993) have been reported. Coenzyme Q10 is a fat-soluble vitamin-like substance and possesses antidepressant activity. It was reported that coenzyme Q10 can protect chronic restraint stress-induced hippocampal DNA damage which is possible through in part by maintaining mitochondrial function and its well documented antioxidant properties (Aboul-Fotouh 2013). Similar observations also showed that treatment with antidepressants can normalize stress-induced increased levels of malondialdehyde and protein carbonyl accumulations in the brain, suggesting that these drugs attenuate lipid peroxidation (Bilici et al. 2001, Khanzode et al. 2003, Galecki et al. 2009a, Zafir et al. 2009).
Our previous studies showed that almost all tested antidepressants with different action mechanisms can prevent or reverse DSP4/CPT-induced DNA damage response and cell cycle arrest, indicating that the effects of antidepressants against DNA damage may be one of the common mechanisms of action for their clinical use to relieve depressive symptoms that appear in psychiatric diseases (Wang et al. 2015). These findings may explain why antidepressants with different mechanisms can generally relieve symptoms of depression. These effects may be ascribed to the abilities of some antidepressants in scavenging hydroxyl radicals or the up-regulation of the expression of antioxidant defense enzymes and may add a new feature to the neuroprotective potency of these antidepressants. Further exploration of these underlying mechanisms may shed light on therapeutic strategies for treatment of these diseases.
8. Conclusion
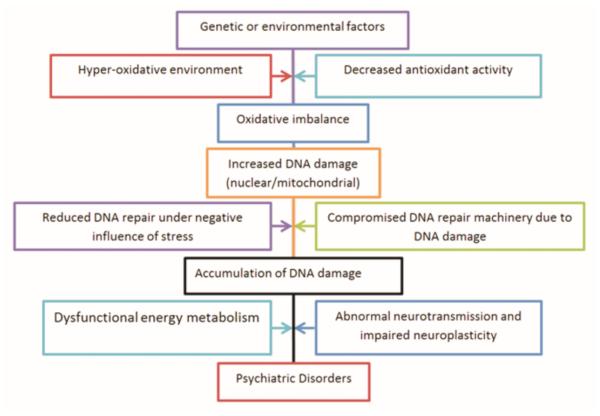
Previously, articles have described the importance of oxidative stress in psychiatric disorders and highlighted its implications in therapeutics (Ng et al. 2008, Smaga et al. 2015), but its important association as a symptomatic factor in almost all psychiatric conditions has not been discussed in detail. In this review we have described that DNA damage is involved in MDD, bipolar disorder and schizophrenia (Table 1). Oxidative damage has been strongly associated with the development of psychiatric symptoms. It produces an unfavorable intracellular environment to work properly for DNA repair enzymes and can even halt the process. The accumulation of mutations load in nuclear genome can not only be a driving force for neurodegeneration but also initiate tumorigenesis. So DNA damage acts like a double edged sword, making conditions worse for cells. It is noteworthy that psychiatric disorders are complex multifactorial illness comprising changes in neural structures and functions. Genetic factors are important in the etiology of most psychiatric disorders too, which is also related to DNA damage. For example, an impaired capacity to synthesize glutathione, an important antioxidant component, due to genetic polymorphism is a vulnerability factors for schizophrenia (Gysin et al. 2007). Also, study in schizophrenia patients showed a positive association between schizophrenia and a functional polymorphism in the gene for manganese SOD (Boskovic et al. 2011). On the other hand, environmental factors such as psychosocial and oxidative stress play an important role in the onset and progression of these diseases. Striking evidence has revealed that as the consequence of exposure to these environmental insults, maladaptation-induced abnormalities including changes in gene expression are a source of epigenetic modifications in specific brain regions. Therefore, abnormal transcriptional and epigenetic regulation may be a unifying theme in these psychiatric disorders, which may share common molecular mediators encoded by a subset of interrelated genes, controlling neurotransmission and survival signaling (Fig. 2).
Table 1.
Studies reporting DNA damage in major psychiatric disorders.
| Psychiatric disorder | Biomarkers | Source | References |
|---|---|---|---|
|
Major Depressive
Disorder |
8-oxodG increase | Serum | Forlenza & Miller 2006, Maes et al. 2009 |
| Leukocytes | Irie et al. 2003 | ||
| mtDNA damage | Leukocytes | Chang et al. 2015 | |
| ss & dsDNA breaks | Leukocytes | Czarny et al. 2015 | |
| Telomere shortening | Leukocytes | Simon et al. 2006 | |
| Bipolar Disorder | 8-oxodG increases | Serum | Soeiro-de-Souza et al. 2013 |
| 8-oxodG and 8-oxoGuo increases |
Urine | Munkholm et al. 2015 | |
| More DNA fragmentation | Brain tissue | Buttner et al. 2007 | |
| ss & dsDNA breaks | Brain tissue | Mustak et al. 2010 | |
| RNA damage | Brain tissue | Che et al. 2010 | |
| mtDNA copy number decrease and damage |
Leukocytes | Chang et al. 2014 | |
| Telomere shortening | Simon et al. 2006 | ||
| Schizophrenia | 8-oxodG increase | Serum | Sertan Co-poglu et al. 2015 |
| 8-oxodG and 8-oxoGuo increase |
Urine | Jorgensen et al. 2013 | |
| 8-oxodG increase | Brain tissue |
Cardozo-Pelaez et al. 2000. Nishioka & Arnold 2004, Che et al. 2010 |
|
| ss & dsDNA breaks | Leukocytes | Muraleedharan et al. 2015 |
ss & dsDNA: single strand &double strand DNA; mtDNA: mitochondrial DNA; 8-oxodG: 8-oxo-7, 8-dihydro-2-dihydroguanonine; 8-oxi-Guo: 8-oxo-7,8-dihydroguanonine.
Fig. 2.
Schematic diagram showing proposed etiological mechanism for psychiatric symptoms in major psychiatric disorders.
Furthermore, mitochondrial dysfunction is at the base of development and progression of MDD, bipolar disorder and schizophrenia. While mtDNA damage, overproduction of free radicals, abnormal mitochondrial dynamics and other factors can be the cause of mitochondrial malfunction. The overall consequence of this may result in the impairment of mitochondrial electron transport chain and cellular energy crises in the central nervous system in these psychiatric diseases. For example, there is a significant decrease of mitochondrial ATP production rates and mitochondrial enzyme ratios in MDD patients (Gardner et al. 2003). As such, regional abnormalities in the frontal lobe have been found in the subjects with MDD (Lee et al. 2008). The postmortem brain of schizophrenia showed reduced activities and expression of mitochondrial respiratory chain components (Maurer et al. 2001), reduced ATP levels (Rezin et al. 2009) reduced expression of genes involved in the regulation of arnithine and polyamine metabolism (Middleton et al. 2002) and complex I abnormalities (Rosenfeld et al. 2011). Similarly, in the brain of subjects with bipolar disorder, there are disrupted intracellular calcium homeostasis, and decreased mitochondrial respiration complex I in the prefrontal cortex (Andreazza et al. 2010, Uemura et al. 2011). Energy metabolism impairment would lead cells to apoptosis and neuronal loss occurs.
Moreover, any biomarkers of oxidative damage mentioned in this manuscript are from peripheral samples, possibly due to the fact that no information is available about the endogenous antioxidant status of brain in patients suffering from psychiatric disorder. Also, the expression of oxidative stress-response gene was not activated in the prefrontal cortex of patients with MDD (Teyssier et al. 2011), which lead to the notion that peripheral markers may not necessary correlate with change in the central nervous system. However, there is evidence that the antioxidant transcriptional response of the cortical cells is impaired due to a deficit in neurotrophic factors such as brain-derived neurotrophic factor, which plays a major role in the redox control of neuronal cells by up-regulating the expression of antioxidant enzymes (Gardiner et al. 2009), but is down-regulated in the hippocampal formation and prefrontal cortex of depressed patients (Hu & Russek 2008). Furthermore, while the assessment of peripheral analytes is comprised to increase the confidence in the target (Carboni 2013), peripheral biomarkers detected during acute mood episodes could in fact constitute markers of disease activity (Kapczinski et al. 2010, Kapczinski et al. 2011).
As we have mentioned earlier, many psychiatric disorders have recurrent episodes of depression and anxiety along with other chronic symptoms like cognitive decline. The disease progression accompanies with increased severity of these symptoms. As most antipsychotic drugs tend to relieve the symptoms by altering the neuron signaling, there is a need for inclusion of multimodal therapies including drugs that boost up the DNA repair process and relieve the psychiatric symptoms. A more thorough approach will help in unraveling disease pathogenesis, facilitating prediction and prevention, as well as a better treatment of psychiatric disorders.
Acknowledgements
This work is supported by NIH grant MH080323.
Abbreviations
- H2O2
hydrogen peroxide
- 8-oxodG
8-oxo-7,8-dihydro-2’-dihydroguanosine
- 8-oxoGuo
8-oxo-7,8-dihydroguanosine
- MDD
major depression disorder
- mtDNA
mitochondrial DNA
- NO
nitric oxide
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
The authors declare no conflict of interest regarding the work reported here.
REFERENCES
- Abdel-Wahab BA, Salama RH. Venlafaxine protects against stress-induced oxidative DNA damage in hippocampus during antidepressant testing in mice. Pharmacol Biochem Behav. 2011;100:59–65. doi: 10.1016/j.pbb.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Aboul-Fotouh S. Coenzyme Q10 displays antidepressant-like activity with reduction of hippocampal oxidative/nitrosative DNA damage in chronically stressed rats. Pharmacol Biochem Behav. 2013;104:105–112. doi: 10.1016/j.pbb.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Adachi S, Kawamura K, Takemoto K. Oxidative damage of nuclear DNA in liver of rats exposed to psychological stress. Cancer Res. 1993;53:4153–4155. [PubMed] [Google Scholar]
- Altuntas I, Aksoy H, Coskun I, Caykoylu A, Akcay F. Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin Chem Lab Med. 2000;38:1277–1281. doi: 10.1515/CCLM.2000.201. [DOI] [PubMed] [Google Scholar]
- Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ. 2012;345:e8508. doi: 10.1136/bmj.e8508. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, Goncalves CA, Kapczinski F. DNA damage in bipolar disorder. Psychiatry Res. 2007;153:27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Andreazza AC, Kauer-Sant'Anna M, Frey BN, et al. Effects of mood stabilizers on DNA damage in an animal model of mania. J Psychiatry Neurosci. 2008;33:516–524. [PMC free article] [PubMed] [Google Scholar]
- Andreazza AC, Shao L, Wang JF, Young LT. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch Gen Psychiatry. 2010;67:360–368. doi: 10.1001/archgenpsychiatry.2010.22. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012;109:16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr GA, Moreira JC, Frey BN. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxidative medicine and cellular longevity. 2012;2012:609421. doi: 10.1155/2012/609421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Bender RE, Alloy LB. Life stress and kindling in bipolar disorder: review of the evidence and integration with emerging biopsychosocial theories. Clinical psychology review. 2011;31:383–398. doi: 10.1016/j.cpr.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. The role of stress and dopamine-GABA interactions in the vulnerability for schizophrenia. J Psychiatr Res. 1997;31:257–275. doi: 10.1016/s0022-3956(96)00044-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Matzilevich D, Burke RE, Walsh J. The expression of proapoptosis genes is increased in bipolar disorder, but not in schizophrenia. Mol Psychiatry. 2006;11:241–251. doi: 10.1038/sj.mp.4001758. [DOI] [PubMed] [Google Scholar]
- Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. Int J Neuropsychopharmacol. 2009;12:441–445. doi: 10.1017/S1461145708009498. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI. N-acetyl cysteine for depressive symptoms in bipolar disorder--a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–475. doi: 10.1016/j.biopsych.2008.04.022. [DOI] [PubMed] [Google Scholar]
- Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Krause S, Krell D, et al. Strongly reduced number of parvalbumin-immunoreactive projection neurons in the mammillary bodies in schizophrenia: further evidence for limbic neuropathology. Ann N Y Acad Sci. 2007a;1096:120–127. doi: 10.1196/annals.1397.077. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Sahin J, Smalla KH, Gundelfinger ED, Bogerts B, Kreutz MR. A reduced number of cortical neurons show increased Caldendrin protein levels in chronic schizophrenia. Schizophrenia research. 2007b;96:246–256. doi: 10.1016/j.schres.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Lange N. Neuron numbers and volume of the amygdala in subjects diagnosed with bipolar disorder or schizophrenia. Biol Psychiatry. 2007;62:884–893. doi: 10.1016/j.biopsych.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Frye M, Callicott JH, Mattay VS, Rakow R, Shelton-Repella J, Post R, Weinberger DR. Neuronal pathology in the hippocampal area of patients with bipolar disorder: a study with proton magnetic resonance spectroscopic imaging. Biol Psychiatry. 2003;53:906–913. doi: 10.1016/s0006-3223(02)01911-x. [DOI] [PubMed] [Google Scholar]
- Bilici M, Efe H, Koroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Woo TU. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- Boskovic M, Vovk T, Kores Plesnicar B, Grabnar I. Oxidative stress in schizophrenia. Current neuropharmacology. 2011;9:301–312. doi: 10.2174/157015911795596595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley-Whitman M, Lovell M. Increased oxidative dramage in RNA in alzheimer's disease progression. J. Anal. Bioanal. Tech. 2013;S2(004):1–9. [Google Scholar]
- Bregeon D, Sarasin A. Hypothetical role of RNA damage avoidance in preventing human disease. Mutat Res. 2005;577:293–302. doi: 10.1016/j.mrfmmm.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Briley M, Moret C. Neurobiological mechanisms involved in antidepressant therapies. Clin Neuropharmacol. 1993;16:387–400. doi: 10.1097/00002826-199310000-00002. [DOI] [PubMed] [Google Scholar]
- Brown GR, McBride L, Bauer MS, Williford WO. Impact of childhood abuse on the course of bipolar disorder: a replication study in U. J Affect Disord. 2005;89:57–67. doi: 10.1016/j.jad.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218:61–68. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Buckman TD, Kling AS, Eiduson S, Sutphin MS, Steinberg A. Glutathione peroxidase and CT scan abnormalities in schizophrenia. Biol Psychiatry. 1987;22:1349–1356. doi: 10.1016/0006-3223(87)90069-2. [DOI] [PubMed] [Google Scholar]
- Butler SG, Meegan MJ. Recent developments in the design of anti-depressive therapies: targeting the serotonin transporter. Current medicinal chemistry. 2008;15:1737–1761. doi: 10.2174/092986708784872357. [DOI] [PubMed] [Google Scholar]
- Buttner N, Bhattacharyya S, Walsh J, Benes FM. DNA fragmentation is increased in non-GABAergic neurons in bipolar disorder but not in schizophrenia. Schizophrenia research. 2007;93:33–41. doi: 10.1016/j.schres.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal JH, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-life insults impair parvalbumin interneurons via oxidative stress: reversal by N-acetylcysteine. Biol Psychiatry. 2013;73:574–582. doi: 10.1016/j.biopsych.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Cadet J, Douki T, Ravanat JL. Oxidatively generated damage to the guanine moiety of DNA: mechanistic aspects and formation in cells. Accounts of chemical research. 2008;41:1075–1083. doi: 10.1021/ar700245e. [DOI] [PubMed] [Google Scholar]
- Carboni L. Peripheral biomarkers in animal models of major depressive disorder. Disease markers. 2013;35:33–41. doi: 10.1155/2013/284543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo-Pelaez F, Brooks PJ, Stedeford T, Song S, Sanchez-Ramos J. DNA damage, repair, and antioxidant systems in brain regions: a correlative study. Free Radic Biol Med. 2000;28:779–785. doi: 10.1016/s0891-5849(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Cecil KM, DelBello MP, Morey R, Strakowski SM. Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disord. 2002;4:357–365. doi: 10.1034/j.1399-5618.2002.02235.x. [DOI] [PubMed] [Google Scholar]
- Chang CC, Jou SH, Lin TT, Lai TJ, Liu CS. Mitochondria DNA change and oxidative damage in clinically stable patients with major depressive disorder. PloS one. 2015;10:e0125855. doi: 10.1371/journal.pone.0125855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Jou SH, Lin TT, Liu CS. Mitochondrial DNA variation and increased oxidative damage in euthymic patients with bipolar disorder. Psychiatry and clinical neurosciences. 2014;68:551–557. doi: 10.1111/pcn.12163. [DOI] [PubMed] [Google Scholar]
- Che Y, Wang JF, Shao L, Young T. Oxidative damage to RNA but not DNA in the hippocampus of patients with major mental illness. J Psychiatry Neurosci. 2010;35:296–302. doi: 10.1503/jpn.090083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelikani P, Fita I, Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CP, Schmidt D, Stein CM, Morrow JD, Salomon RM. Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatry Res. 2013;206:213–216. doi: 10.1016/j.psychres.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consiglio AR, Ramos AL, Henriques JA, Picada JN. DNA brain damage after stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:652–656. doi: 10.1016/j.pnpbp.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- Coppede F, Migliore L. DNA damage in neurodegenerative diseases. Mutat Res. 2015;776:84–97. doi: 10.1016/j.mrfmmm.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171:453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- Czarny P, Kwiatkowski D, Galecki P, et al. Association between single nucleotide polymorphisms of MUTYH, hOGG1 and NEIL1 genes, and depression. J Affect Disord. 2015a;184:90–96. doi: 10.1016/j.jad.2015.05.044. [DOI] [PubMed] [Google Scholar]
- Czarny P, Kwiatkowski D, Kacperska D, et al. Elevated level of DNA damage and impaired repair of oxidative DNA damage in patients with recurrent depressive disorder. Medical science monitor : international medical journal of experimental and clinical research. 2015b;21:412–418. doi: 10.12659/MSM.892317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa RT, Uno M, Zanetti MV, Shinjo SM, Busatto GF, Gattaz WF, Marie SK, Machado-Vieira R. Leukocyte mitochondrial DNA copy number in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:32–35. doi: 10.1016/j.pnpbp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Dexheimer TS. DNA repai pathways and mechanisms. In: Mathews LA, Cabarcas s.M., Hurt EM, editors. DNA Repair of Cancer Stem Cells. Springer Netherlands; 2013. pp. 19–32. [Google Scholar]
- Dietrich-Muszalska A, Olas B. Modifications of blood platelet proteins of patients with schizophrenia. Platelets. 2009;20:90–96. doi: 10.1080/09537100802641499. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuenod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Du J, Wang Y, Hunter R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci U S A. 2009;106:3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N. A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2012;367:2475–2484. doi: 10.1098/rstb.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Meyhofer I, Steffens M, Wagner M, Koutsouleris N. Genetics, cognition, and neurobiology of schizotypal personality: a review of the overlap with schizophrenia. Frontiers in psychiatry. 2014;5:18. doi: 10.3389/fpsyt.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MD, Dizdaroglu M, Cooke MS. Oxidative DNA damage and disease: induction, repair and significance. Mutat Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Flint MS, Baum A, Chambers WH, Jenkins FJ. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology. 2007;32:470–479. doi: 10.1016/j.psyneuen.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Flint MS, Carroll JE, Jenkins FJ, Chambers WH, Han ML, Baum A. Genomic profiling of restraint stress-induced alterations in mouse T lymphocytes. J Neuroimmunol. 2005;167:34–44. doi: 10.1016/j.jneuroim.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2'-deoxyguanosine in clinical depression. Psychosomatic medicine. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- Forsberg K, Aalling N, Wortwein G, Loft S, Moller P, Hau J, Hageman I, Jorgensen MB, Jorgensen A. Dynamic regulation of cerebral DNA repair genes by psychological stress. Mutation research. Genetic toxicology and environmental mutagenesis. 2015;778:37–43. doi: 10.1016/j.mrgentox.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Kunz M, Gomes FA, Quevedo J, Salvador M, Goncalves CA, Kapczinski F. Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:283–285. doi: 10.1016/j.pnpbp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Friedman R, Caflisch A. On the orientation of the catalytic dyad in aspartic proteases. Proteins. 2010;78:1575–1582. doi: 10.1002/prot.22674. [DOI] [PubMed] [Google Scholar]
- Galecki P, Szemraj J, Bienkiewicz M, Florkowski A, Galecka E. Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacological reports : PR. 2009a;61:436–447. doi: 10.1016/s1734-1140(09)70084-2. [DOI] [PubMed] [Google Scholar]
- Galecki P, Szemraj J, Bienkiewicz M, Zboralski K, Galecka E. Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients. Hum Psychopharmacol. 2009b;24:277–286. doi: 10.1002/hup.1014. [DOI] [PubMed] [Google Scholar]
- Gama CS, Salvador M, Andreazza AC, Lobato MI, Berk M, Kapczinski F, Belmonte-de-Abreu PS. Elevated serum thiobarbituric acid reactive substances in clinically symptomatic schizophrenic males. Neurosci Lett. 2008;433:270–273. doi: 10.1016/j.neulet.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Gardiner J, Barton D, Overall R, Marc J. Neurotrophic support and oxidative stress: converging effects in the normal and diseased nervous system. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2009;15:47–61. doi: 10.1177/1073858408325269. [DOI] [PubMed] [Google Scholar]
- Gardner A, Johansson A, Wibom R, Nennesmo I, von Dobeln U, Hagenfeldt L, Hallstrom T. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord. 2003;76:55–68. doi: 10.1016/s0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- Gaur V, Kumar A. Protective effect of desipramine, venlafaxine and trazodone against experimental animal model of transient global ischemia: possible involvement of NO-cGMP pathway. Brain Res. 2010;1353:204–212. doi: 10.1016/j.brainres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Cipriani A. Selective serotonin reuptake inhibitors. BMJ. 2004;329:809–810. doi: 10.1136/bmj.329.7470.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–975. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007;151:737–748. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen-de Wied CC. Stress in schizophrenia: an integrative view. Eur J Pharmacol. 2000;405:375–384. doi: 10.1016/s0014-2999(00)00567-7. [DOI] [PubMed] [Google Scholar]
- Glaser R, Thorn BE, Tarr KL, Kiecolt-Glaser JK, D'Ambrosio SM. Effects of stress on methyltransferase synthesis: an important DNA repair enzyme. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 1985;4:403–412. doi: 10.1037//0278-6133.4.5.403. [DOI] [PubMed] [Google Scholar]
- Grima G, Benz B, Parpura V, Cuenod M, Do KQ. Dopamine-induced oxidative stress in neurons with glutathione deficit: implication for schizophrenia. Schizophrenia research. 2003;62:213–224. doi: 10.1016/s0920-9964(02)00405-x. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, et al. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Henriksen T, Hillestrom PR, Poulsen HE, Weimann A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2'-deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radic Biol Med. 2009;47:629–635. doi: 10.1016/j.freeradbiomed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Herken H, Gurel A, Selek S, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Archives of medical research. 2007;38:247–252. doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hirose K, Chan PH. Blockade of glutamate excitotoxicity and its clinical applications. Neurochem Res. 1993;18:479–483. doi: 10.1007/BF00967252. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- Hu CW, Chao MR, Sie CH. Urinary analysis of 8-oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanosine by isotope-dilution LC-MS/MS with automated solid-phase extraction: Study of 8-oxo-7,8-dihydroguanine stability. Free Radic Biol Med. 2010;48:89–97. doi: 10.1016/j.freeradbiomed.2009.10.029. [DOI] [PubMed] [Google Scholar]
- Hu Y, Russek SJ. BDNF and the diseased nervous system: a delicate balance between adaptive and pathological processes of gene regulation. J Neurochem. 2008;105:1–17. doi: 10.1111/j.1471-4159.2008.05237.x. [DOI] [PubMed] [Google Scholar]
- Inoue A, Kawakami N, Ishizaki M, Tabata M, Tsuchiya M, Akiyama M, Kitazume A, Kuroda M, Shimazu A. Three job stress models/concepts and oxidative DNA damage in a sample of workers in Japan. Journal of psychosomatic research. 2009;66:329–334. doi: 10.1016/j.jpsychores.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Irie M, Asami S, Ikeda M, Kasai H. Depressive state relates to female oxidative DNA damage via neutrophil activation. Biochem Biophys Res Commun. 2003;311:1014–1018. doi: 10.1016/j.bbrc.2003.10.105. [DOI] [PubMed] [Google Scholar]
- Irie M, Asami S, Nagata S, Miyata M, Kasai H. Relationships between perceived workload, stress and oxidative DNA damage. International archives of occupational and environmental health. 2001;74:153–157. doi: 10.1007/s004200000209. [DOI] [PubMed] [Google Scholar]
- Irie M, Asami S, Nagata S, Miyata M, Kasai H. Psychological mediation of a type of oxidative DNA damage, 8-hydroxydeoxyguanosine, in peripheral blood leukocytes of non-smoking and non-drinking workers. Psychotherapy and psychosomatics. 2002;71:90–96. doi: 10.1159/000049351. [DOI] [PubMed] [Google Scholar]
- Irie M, Miyata M, Kasai H. Depression and possible cancer risk due to oxidative DNA damage. J Psychiatr Res. 2005;39:553–560. doi: 10.1016/j.jpsychires.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Jesberger JA, Richardson JS. Oxygen free radicals and brain dysfunction. The International journal of neuroscience. 1991;57:1–17. doi: 10.3109/00207459109150342. [DOI] [PubMed] [Google Scholar]
- Joergensen A, Broedbaek K, Weimann A, Semba RD, Ferrucci L, Joergensen MB, Poulsen HE. Association between urinary excretion of cortisol and markers of oxidatively damaged DNA and RNA in humans. PloS one. 2011;6:e20795. doi: 10.1371/journal.pone.0020795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL. Life events in bipolar disorder: towards more specific models. Clinical psychology review. 2005;25:1008–1027. doi: 10.1016/j.cpr.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen A, Broedbaek K, Fink-Jensen A, et al. Increased systemic oxidatively generated DNA and RNA damage in schizophrenia. Psychiatry Res. 2013a;209:417–423. doi: 10.1016/j.psychres.2013.01.033. [DOI] [PubMed] [Google Scholar]
- Jorgensen A, Krogh J, Miskowiak K, et al. Systemic oxidatively generated DNA/RNA damage in clinical depression: associations to symptom severity and response to electroconvulsive therapy. J Affect Disord. 2013b;149:355–362. doi: 10.1016/j.jad.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Jorgensen A, Krogh J, Miskowiak K, et al. Systemic oxidatively generated DNA/RNA damage in clinical depression: Associations to symptom severity and response to electroconvulsive therapy. J Affect Disord. 2013c doi: 10.1016/j.jad.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Kakiuchi C, Ishiwata M, Kametani M, Nelson C, Iwamoto K, Kato T. Quantitative analysis of mitochondrial DNA deletions in the brains of patients with bipolar disorder and schizophrenia. Int J Neuropsychopharmacol. 2005;8:515–522. doi: 10.1017/S1461145705005213. [DOI] [PubMed] [Google Scholar]
- Kalia M. Neurobiological basis of depression: an update. Metabolism. 2005;54:24–27. doi: 10.1016/j.metabol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Dal-Pizzol F, Teixeira AL, et al. Peripheral biomarkers and illness activity in bipolar disorder. J Psychiatr Res. 2011;45:156–161. doi: 10.1016/j.jpsychires.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Dal-Pizzol F, Teixeira AL, et al. A systemic toxicity index developed to assess peripheral changes in mood episodes. Mol Psychiatry. 2010;15:784–786. doi: 10.1038/mp.2009.112. [DOI] [PubMed] [Google Scholar]
- Katyal S, McKinnon PJ. DNA strand breaks, neurodegeneration and aging in the brain. Mech Ageing Dev. 2008;129:483–491. doi: 10.1016/j.mad.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Khairova R, Pawar R, Salvadore G, et al. Effects of lithium on oxidative stress parameters in healthy subjects. Molecular medicine reports. 2012;5:680–682. doi: 10.3892/mmr.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanzode SD, Dakhale GN, Khanzode SS, Saoji A, Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox report : communications in free radical research. 2003;8:365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- Kohen R, Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Korotkova EI, Misini B, Dorozhko EV, Bukkel MV, Plotnikov EV, Linert W. Study of OH radicals in human serum blood of healthy individuals and those with pathological schizophrenia. International journal of molecular sciences. 2011;12:401–410. doi: 10.3390/ijms12010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, Cavanagh J. Depression: an inflammatory illness? Journal of neurology, neurosurgery, and psychiatry. 2012;83:495–502. doi: 10.1136/jnnp-2011-301779. [DOI] [PubMed] [Google Scholar]
- Krohne-Ehrich G, Schirmer RH, Untucht-Grau R. Glutathione reductase from human erythrocytes. Isolation of the enzyme and sequence analysis of the redox-active peptide. Eur J Biochem. 1977;80:65–71. doi: 10.1111/j.1432-1033.1977.tb11856.x. [DOI] [PubMed] [Google Scholar]
- Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- Kuratomi G, Iwamoto K, Bundo M, Kusumi I, Kato N, Iwata N, Ozaki N, Kato T. Aberrant DNA methylation associated with bipolar disorder identified from discordant monozygotic twins. Mol Psychiatry. 2008;13:429–441. doi: 10.1038/sj.mp.4002001. [DOI] [PubMed] [Google Scholar]
- Lee BT, Seok JH, Lee BC, Cho SW, Yoon BJ, Lee KU, Chae JH, Choi IG, Ham BJ. Neural correlates of affective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:778–785. doi: 10.1016/j.pnpbp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee SJ, Han C, Patkar AA, Masand PS, Pae CU. Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:224–235. doi: 10.1016/j.pnpbp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Leuner K, Schulz K, Schutt T, et al. Peripheral mitochondrial dysfunction in Alzheimer's disease: focus on lymphocytes. Mol Neurobiol. 2012;46:194–204. doi: 10.1007/s12035-012-8300-y. [DOI] [PubMed] [Google Scholar]
- Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biol Psychiatry. 2002;51:288–297. doi: 10.1016/s0006-3223(01)01239-2. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Li Z, Wu J, Deleo CJ. RNA damage and surveillance under oxidative stress. IUBMB Life. 2006;58:581–588. doi: 10.1080/15216540600946456. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Brain Res Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- Liu CS, Tsai CS, Kuo CL, Chen HW, Lii CK, Ma YS, Wei YH. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic Res. 2003;37:1307–1317. doi: 10.1080/10715760310001621342. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang X, Shigenaga MK, Yeo HC, Mori A, Ames BN. Immobilization stress causes oxidative damage to lipid, protein, and DNA in the brain of rats. FASEB J. 1996;10:1532–1538. [PubMed] [Google Scholar]
- Lloyd RV, Hanna PM, Mason RP. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic Biol Med. 1997;22:885–888. doi: 10.1016/s0891-5849(96)00432-7. [DOI] [PubMed] [Google Scholar]
- Loidl-Stahlhofen A, Hannemann K, Spiteller G. Generation of alpha-hydroxyaldehydic compounds in the course of lipid peroxidation. Biochim Biophys Acta. 1994;1213:140–148. doi: 10.1016/0005-2760(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Madabhushi R, Pan L, Tsai LH. DNA damage and its links to neurodegeneration. Neuron. 2014;83:266–282. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology. 2001;24:420–429. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis / chronic fatigue syndrome. Neuro endocrinology letters. 2009a;30:715–722. [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: markers that further explain the higher incidence of neurodegeneration and coronary artery disease. J Affect Disord. 2010;125:287–294. doi: 10.1016/j.jad.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metabolic brain disease. 2009b;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Mukherjee S. Free radical pathology and antioxidant defense in schizophrenia: a review. Schizophrenia research. 1996;19:1–17. doi: 10.1016/0920-9964(95)00049-6. [DOI] [PubMed] [Google Scholar]
- Maher BA, Manschreck TC, Woods BT, Yurgelun-Todd DA, Tsuang MT. Frontal brain volume and context effects in short-term recall in schizophrenia. Biol Psychiatry. 1995;37:144–150. doi: 10.1016/0006-3223(94)00203-F. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Blendy JA. Antidepressant action: to the nucleus and beyond. Trends Pharmacol Sci. 2005;26:631–638. doi: 10.1016/j.tips.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Manaye KF, Lei DL, Tizabi Y, Davila-Garcia MI, Mouton PR, Kelly PH. Selective neuron loss in the paraventricular nucleus of hypothalamus in patients suffering from major depression and bipolar disorder. J Neuropathol Exp Neurol. 2005;64:224–229. doi: 10.1093/jnen/64.3.224. [DOI] [PubMed] [Google Scholar]
- Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, Chen G. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- Martin LJ. DNA damage and repair: relevance to mechanisms of neurodegeneration. J Neuropathol Exp Neurol. 2008;67:377–387. doi: 10.1097/NEN.0b013e31816ff780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaad CA, Klann E. Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxidants & redox signaling. 2011;14:2013–2054. doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Kumano-Go T, Suganuma N, et al. Anxiety, neuroticism and oxidative stress: cross-sectional study in non-smoking college students. Psychiatry and clinical neurosciences. 2010;64:435–441. doi: 10.1111/j.1440-1819.2010.02109.x. [DOI] [PubMed] [Google Scholar]
- Maurer I, Zierz S, Moller H. Evidence for a mitochondrial oxidative phosphorylation defect in brains from patients with schizophrenia. Schizophrenia research. 2001;48:125–136. doi: 10.1016/s0920-9964(00)00075-x. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968-1988) Free Radic Biol Med. 1988;5:363–369. doi: 10.1016/0891-5849(88)90109-8. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McMillan TJ, Leatherman E, Ridley A, Shorrocks J, Tobi SE, Whiteside JR. Cellular effects of long wavelength UV light (UVA) in mammalian cells. J Pharm Pharmacol. 2008;60:969–976. doi: 10.1211/jpp.60.8.0004. [DOI] [PubMed] [Google Scholar]
- Michel TM, Camara S, Tatschner T, Frangou S, Sheldrick AJ, Riederer P, Grunblatt E. Increased xanthine oxidase in the thalamus and putamen in depression. World J Biol Psychiatry. 2010;11:314–320. doi: 10.3109/15622970802123695. [DOI] [PubMed] [Google Scholar]
- Michel TM, Frangou S, Thiemeyer D, Camara S, Jecel J, Nara K, Brunklaus A, Zoechling R, Riederer P. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder--a postmortem study. Psychiatry Res. 2007;151:145–150. doi: 10.1016/j.psychres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Michel TM, Pulschen D, Thome J. The role of oxidative stress in depressive disorders. Curr Pharm Des. 2012;18:5890–5899. doi: 10.2174/138161212803523554. [DOI] [PubMed] [Google Scholar]
- Michel TM, Thome J, Martin D, Nara K, Zwerina S, Tatschner T, Weijers HG, Koutsilieri E. Cu, Zn- and Mn-superoxide dismutase levels in brains of patients with schizophrenic psychosis. J Neural Transm (Vienna) 2004;111:1191–1201. doi: 10.1007/s00702-004-0160-9. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Mirnics K, Pierri JN, Lewis DA, Levitt P. Gene expression profiling reveals alterations of specific metabolic pathways in schizophrenia. J Neurosci. 2002;22:2718–2729. doi: 10.1523/JNEUROSCI.22-07-02718.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Poulsen HE, Kessing LV, Vinberg M. Elevated levels of urinary markers of oxidatively generated DNA and RNA damage in bipolar disorder. Bipolar Disord. 2015;17:257–268. doi: 10.1111/bdi.12245. [DOI] [PubMed] [Google Scholar]
- Muraleedharan A, Menon V, Rajkumar RP, Chand P. Assessment of DNA damage and repair efficiency in drug naive schizophrenia using comet assay. J Psychiatr Res. 2015;68:47–53. doi: 10.1016/j.jpsychires.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Mustak MS, Hegde ML, Dinesh A, Britton GB, Berrocal R, Subba Rao K, Shamasundar NM, Rao KS, Sathyanarayana Rao TS. Evidence of altered DNA integrity in the brain regions of suicidal victims of Bipolar Depression. Indian journal of psychiatry. 2010;52:220–228. doi: 10.4103/0019-5545.70974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Arnold SE. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2004;12:167–175. [PubMed] [Google Scholar]
- Norman RM, Malla AK. A prospective study of daily stressors and symptomatology in schizophrenic patients. Soc Psychiatry Psychiatr Epidemiol. 1994;29:244–249. doi: 10.1007/BF00802047. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, Yee CM, Mintz J. Developmental Processes in Schizophrenic Disorders: longitudinal studies of vulnerability and stress. Schizophrenia bulletin. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta neuropathologica. 2009;118:151–166. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- O'Neill P, Wardman P. Radiation chemistry comes before radiation biology. International journal of radiation biology. 2009;85:9–25. doi: 10.1080/09553000802640401. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Omar ME, Abdel-Salam SMYM, Sleem Amany A. The effect of different antidepressant drugs on oxidative stress after lipopolysaccharide administration in mice. EXCLI Journal. 2011:290–302. [PMC free article] [PubMed] [Google Scholar]
- Opresko PL, Fan J, Danzy S, Wilson DM, 3rd, Bohr VA. Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 2005;33:1230–1239. doi: 10.1093/nar/gki273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. International clinical psychopharmacology. 2004;19:89–95. doi: 10.1097/00004850-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Padurariu M, Ciobica A, Dobrin I, Stefanescu C. Evaluation of antioxidant enzymes activities and lipid peroxidation in schizophrenic patients treated with typical and atypical antipsychotics. Neurosci Lett. 2010;479:317–320. doi: 10.1016/j.neulet.2010.05.088. [DOI] [PubMed] [Google Scholar]
- Palta P, Samuel LJ, Miller ER, 3rd, Szanton SL. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosomatic medicine. 2014;76:12–19. doi: 10.1097/PSY.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya CD, Howell KR, Pillai A. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:214–223. doi: 10.1016/j.pnpbp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popken GJ, Bunney WE, Jr., Potkin SG, Jones EG. Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci U S A. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697. doi: 10.1038/sj.mp.4001511. 643. [DOI] [PubMed] [Google Scholar]
- Radak Z, Boldogh I. 8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress. Free Radic Biol Med. 2010;49:587–596. doi: 10.1016/j.freeradbiomed.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A. Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1178–1183. doi: 10.1016/j.pnpbp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- Rao KS. Genomic damage and its repair in young and aging brain. Mol Neurobiol. 1993;7:23–48. doi: 10.1007/BF02780607. [DOI] [PubMed] [Google Scholar]
- Reddy R, Keshavan M, Yao JK. Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophrenia research. 2003;62:205–212. doi: 10.1016/s0920-9964(02)00407-3. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Phatak P, Marano CM, Sassan A, Conley RR, Kling MA. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol Psychiatry. 2009;65:489–494. doi: 10.1016/j.biopsych.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LM, Reynolds GP. Differential regional N-acetylaspartate deficits in postmortem brain in schizophrenia, bipolar disorder and major depressive disorder. J Psychiatr Res. 2011;45:54–59. doi: 10.1016/j.jpsychires.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res. 2009;34:1021–1029. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Ferrier IN. Evolution of cognitive impairment in bipolar disorder: a systematic review of cross-sectional evidence. Bipolar Disord. 2006;8:103–116. doi: 10.1111/j.1399-5618.2006.00277.x. [DOI] [PubMed] [Google Scholar]
- Rosell DR, Futterman SE, McMaster A, Siever LJ. Schizotypal personality disorder: a current review. Curr Psychiatry Rep. 2014;16:452. doi: 10.1007/s11920-014-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M, Brenner-Lavie H, Ari SG, Kavushansky A, Ben-Shachar D. Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry. 2011;69:980–988. doi: 10.1016/j.biopsych.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Rouse J, Jackson SP. Interfaces between the detection, signaling, and repair of DNA damage. Science. 2002;297:547–551. doi: 10.1126/science.1074740. [DOI] [PubMed] [Google Scholar]
- Rybka J, Kedziora-Kornatowska K, Banas-Lezanska P, Majsterek I, Carvalho LA, Cattaneo A, Anacker C, Kedziora J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med. 2013;63C:187–194. doi: 10.1016/j.freeradbiomed.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Sabunciyan S, Kirches E, Krause G, Bogerts B, Mawrin C, Llenos IC, Weis S. Quantification of total mitochondrial DNA and mitochondrial common deletion in the frontal cortex of patients with schizophrenia and bipolar disorder. J Neural Transm (Vienna) 2007;114:665–674. doi: 10.1007/s00702-006-0581-8. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Satterlee JS, Barbee S, Jin P, Krichevsky A, Salama S, Schratt G, Wu DY. Noncoding RNAs in the brain. J Neurosci. 2007;27:11856–11859. doi: 10.1523/JNEUROSCI.3624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Price JL, Drevets WC. Neuropathological and neuromorphometric abnormalities in bipolar disorder: view from the medial prefrontal cortical network. Neurosci Biobehav Rev. 2014;42:132–147. doi: 10.1016/j.neubiorev.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- Saxowsky TT, Doetsch PW. RNA polymerase encounters with DNA damage: transcription-coupled repair or transcriptional mutagenesis? Chemical reviews. 2006;106:474–488. doi: 10.1021/cr040466q. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Davinelli S, Drago F, De Lorenzo A, Oriani G. Antioxidants as antidepressants: fact or fiction? CNS Drugs. 2012;26:477–490. doi: 10.2165/11633190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Schultz SH, North SW, Shields CG. Schizophrenia: a review. American family physician. 2007;75:1821–1829. [PubMed] [Google Scholar]
- Schwartz TL, Sachdeva S, Stahl SM. Genetic data supporting the NMDA glutamate receptor hypothesis for schizophrenia. Curr Pharm Des. 2012;18:1580–1592. doi: 10.2174/138161212799958594. [DOI] [PubMed] [Google Scholar]
- Sertan Copoglu U, Virit O, Hanifi Kokacya M, et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res. 2015;229:200–205. doi: 10.1016/j.psychres.2015.07.036. [DOI] [PubMed] [Google Scholar]
- Shan X, Chang Y, Lin CL. Messenger RNA oxidation is an early event preceding cell death and causes reduced protein expression. FASEB J. 2007;21:2753–2764. doi: 10.1096/fj.07-8200com. [DOI] [PubMed] [Google Scholar]
- Shao L, Young LT, Wang JF. Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry. 2005;58:879–884. doi: 10.1016/j.biopsych.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry. 2011;16:751–762. doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiwaku H, Okazawa H. Impaired DNA damage repair as a common feature of neurodegenerative diseases and psychiatric disorders. Current molecular medicine. 2015;15:119–128. doi: 10.2174/1566524015666150303002556. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60:432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Sirota P, Gavrieli R, Wolach B. Overproduction of neutrophil radical oxygen species correlates with negative symptoms in schizophrenic patients: parallel studies on neutrophil chemotaxis, superoxide production and bactericidal activity. Psychiatry Res. 2003;121:123–132. doi: 10.1016/s0165-1781(03)00222-1. [DOI] [PubMed] [Google Scholar]
- Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek J, Przegalinski E, Pera J, Filip M. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacological reports : PR. 2015;67:569–580. doi: 10.1016/j.pharep.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Soeiro-de-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA. Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. Int J Neuropsychopharmacol. 2013;16:1505–1512. doi: 10.1017/S1461145713000047. [DOI] [PubMed] [Google Scholar]
- Spassky A, Angelov D. Influence of the local helical conformation on the guanine modifications generated from one-electron DNA oxidation. Biochemistry. 1997;36:6571–6576. doi: 10.1021/bi962761d. [DOI] [PubMed] [Google Scholar]
- Stevens JR. Neuropathology of schizophrenia. Arch Gen Psychiatry. 1982;39:1131–1139. doi: 10.1001/archpsyc.1982.04290100011003. [DOI] [PubMed] [Google Scholar]
- Subba Rao K. Mechanisms of disease: DNA repair defects and neurological disease. Nat Clin Pract Neurol. 2007;3:162–172. doi: 10.1038/ncpneuro0448. [DOI] [PubMed] [Google Scholar]
- Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, Devidze N, Kreitzer AC, Mucke L. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-beta. Nat Neurosci. 2013;16:613–621. doi: 10.1038/nn.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A. Novel approaches to drug design for the treatment of schizophrenia. Expert opinion on drug discovery. 2013;8:1285–1296. doi: 10.1517/17460441.2013.821108. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Chock PB, Stadtman ER. Oxidized messenger RNA induces translation errors. Proc Natl Acad Sci U S A. 2007;104:66–71. doi: 10.1073/pnas.0609737104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Teyssier JR, Ragot S, Chauvet-Gelinier JC, Trojak B, Bonin B. Expression of oxidative stress-response genes is not activated in the prefrontal cortex of patients with depressive disorder. Psychiatry Res. 2011;186:244–247. doi: 10.1016/j.psychres.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environmental and molecular mutagenesis. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Uemura T, Green M, Corson TW, Perova T, Li PP, Warsh JJ. Bcl-2 SNP rs956572 associates with disrupted intracellular calcium homeostasis in bipolar I disorder. Bipolar Disord. 2011;13:41–51. doi: 10.1111/j.1399-5618.2011.00897.x. [DOI] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current neuropharmacology. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Tomita H, Meng F, et al. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: implications for brain disorders. Mol Psychiatry. 2006;615:663–679. doi: 10.1038/sj.mp.4001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychological review. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hilton BA, Cui K, Zhu MY. Effects of Antidepressants on DSP4/CPT-Induced DNA Damage Response in Neuroblastoma SH-SY5Y Cells. Neurotoxicity research. 2015;28:154–170. doi: 10.1007/s12640-015-9534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Wei YC, Zhou FL, He DL, Bai JR, Ding H, Wang XY, Nan KJ. Oxidative stress in depressive patients with gastric adenocarcinoma. Int J Neuropsychopharmacol. 2009;12:1089–1096. doi: 10.1017/S1461145709000091. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Wilson C, Gonzalez-Billault C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Frontiers in cellular neuroscience. 2015;9:381. doi: 10.3389/fncel.2015.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RM, Chiueh CC, Pert A, Murphy DL. Apparent antioxidant effect of l-deprenyl on hydroxyl radical formation and nigral injury elicited by MPP+ in vivo. Eur J Pharmacol. 1993;243:241–247. doi: 10.1016/0014-2999(93)90181-g. [DOI] [PubMed] [Google Scholar]
- Yager S, Forlenza MJ, Miller GE. Depression and oxidative damage to lipids. Psychoneuroendocrinology. 2010;35:1356–1362. doi: 10.1016/j.psyneuen.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Yamada S, Nomoto S, Fujii T, Kaneko T, Takeda S, Inoue S, Kanazumi N, Nakao A. Correlation between copy number of mitochondrial DNA and clinico-pathologic parameters of hepatocellular carcinoma. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2006;32:303–307. doi: 10.1016/j.ejso.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy RD. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophrenia bulletin. 2004;30:923–934. doi: 10.1093/oxfordjournals.schbul.a007142. [DOI] [PubMed] [Google Scholar]
- Zafir A, Ara A, Banu N. Invivo antioxidant status: a putative target of antidepressant action. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:220–228. doi: 10.1016/j.pnpbp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zhang F, Zhou H, Wilson BC, Shi JS, Hong JS, Gao HM. Fluoxetine protects neurons against microglial activation-mediated neurotoxicity. Parkinsonism & related disorders. 2012;18(Suppl 1):S213–217. doi: 10.1016/S1353-8020(11)70066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhao Z, He L, Wan C. A meta-analysis of oxidative stress markers in schizophrenia. Science China. Life sciences. 2010;53:112–124. doi: 10.1007/s11427-010-0013-8. [DOI] [PubMed] [Google Scholar]
- Zhang P, Dilley C, Mattson MP. DNA damage responses in neural cells: Focus on the telomere. Neuroscience. 2007;145:1439–1448. doi: 10.1016/j.neuroscience.2006.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Cao LY, Wu GY, Xu Q, Shen Y, Zhou DF. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophrenia research. 2006;81:291–300. doi: 10.1016/j.schres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]