Abstract
Objective
Short endoscopic secretin tests for exocrine pancreatic function are not properly evaluated in cystic fibrosis (CF).
Methods
Patients with CF and healthy controls (HCs) underwent endoscopic collection of duodenal juice between 30 and 45 minutes after secretin stimulation. Duodenal juice was analyzed for HCO3− concentration and pancreatic enzyme activities. Stool was analyzed for fecal elastase.
Results
Thirty-one patients with CF and 25 HCs were tested. Patients were classified as exocrine pancreatic sufficient (n = 13) or insufficient (n = 18). Both bicarbonate concentrations and enzyme activities in duodenal juice differentiated patients with CFI from patients with CFS and HC (P < 0.001). The population displays strong correlation between severe CF genotype in both alleles and pancreatic insufficient phenotype (P < 0.001).
Conclusions
Pancreatic exocrine insufficient CF patients could be differentiated from exocrine sufficient patients and HCs using short endoscopic secretin test.
Key Words: pancreas, cystic fibrosis, exocrine pancreatic function, secretin, endoscopy
Cystic fibrosis (CF) is the most common, fatal autosomal recessive disease among white populations, with a frequency of 1 to 4-5000 live births.1 After the discovery of the CF transmembrane regulator (CFTR) gene in 1989 and the linking of the disease to changes in the CFTR protein,2–4 the CF genotype has been mapped in details. The CF Mutation Database lists more than 1900 different mutations in the CFTR gene.5 The CFTR protein is a complex chloride channel and regulatory protein found in all exocrine tissues. Various CFTR defects cause disturbed transport of chloride, sodium, bicarbonate, and water leading to thick, viscous secretions in affected organs. The phenotypic expression of disease varies widely as a function of the specific mutations present.6
Pancreatic function testing has played an important role in the discovery of the mechanism of CF pancreatic pathophysiology.6–8 Population studies have indicated that 72% to 88% of patients with CF develop exocrine pancreatic insufficiency.9,10 Most patients with CF develop insufficiency prenatally or during first year of infancy.11 Recent studies indicate lower prevalence of pancreatic insufficiency in CF populations indicating a higher frequency of milder mutations,9 but still, 87% receives pancreas enzyme therapy.12 A small proportion of patients with exocrine pancreatic sufficient CF develop insufficiency later in life,13 leading to a need for regular follow-up of exocrine pancreatic function in patients with pancreatic sufficient CF.
Fecal elastase (FE) is the most widespread tool for screening for exocrine function in patients with CF.13,14 This test is noninvasive, cheap, and validated for screening.14 The CF foundation has published guidelines for pancreatic enzyme substitution based on FE levels.15 There are, however, some problems using FE. Firstly, FE has low sensitivity and specificity in detecting mild to moderate pancreatic failure compared with direct testing.16 Secondly, FE, like all other indirect tests, is unable to assess acinar reserve capacity or detect ductal dysfunction.17 The test is also affected by the liquid content in the stool, giving raise to false positives in patients with watery diarrhea. The pitfalls of FE diagnostics may reduce the value of such screening in pancreatic sufficient adults. Some still advocate the use of 3-day fecal fat as the only option to follow the development toward pancreatic exocrine insufficiency in patients with CF.18
The “criterion standard” direct pancreatic function tests have good diagnostic accuracy17 but are cumbersome, time consuming, and technically difficult. These tests have not reached widespread use and are not suitable as screening tools. New short direct endoscopic tests have been evaluated in other pancreatic diseases.19–21 Short endoscopic tests have been criticized for measuring peak concentrations and not outputs of bicarbonate and digestive enzymes. Hence, the validity of such tests in evaluating CF pancreatic disease, where hyperconcentration is a part of the mechanism, has been questioned.17 We aimed to evaluate the diagnostic accuracy of our timed short endoscopic secretin test (EST) using FE as a standard for exocrine failure in a population of patients with CF with a high prevalence of pancreatic sufficiency and healthy controls (HCs).
MATERIAL AND METHODS
Subjects
During a 2-year period (December 2010–May 2014), consecutive patients with CF older than 15 years attending a regular follow-up in the CF clinic at Haukeland University Hospital, Bergen, Norway, were offered a detailed evaluation of exocrine pancreatic function. Patients with lung-transplanted CF and patients with CF considered for lung transplantation were not included. Forty-one patients agreed to be included for prospective data collection. Nine patients did not perform EST. Cystic fibrosis diagnosis was evaluated according to the diagnostic criteria for CF defined in the CF foundation consensus report,22 discovering doubts about the correct CF diagnosis in 3 patients who either had sweat tests in the area between 40 and 60 mmol/L or lacked exact information of childhood sweat tests. One patient with intermediate range sweat test (46 mmol/L) and weak CF phenotypic characteristics was excluded. The other 2 patients had typical CF phenotype and were included. We thus included 40 patients for overview of the relation between genotype and exocrine status and 31 patients with CF for evaluation of EST.
Twenty-six HCs without gastrointestinal disease were also examined with EST. One HC was unable to fulfill the EST protocol and was excluded. Twenty-five HCs were included. The inclusion flow chart is illustrated in Figure 1. There was an age difference between the HC and CF groups performing EST as displayed in Table 1. The protocol was approved by the local ethics committee (approval number: REK 2010/2857-7) and the study was performed in accordance with the Helsinki Declaration. All subjects signed an informed consent. The protocol adheres to the STARD (STAndards for the Reporting of Diagnostic accuracy studies) statement.23
FIGURE 1.
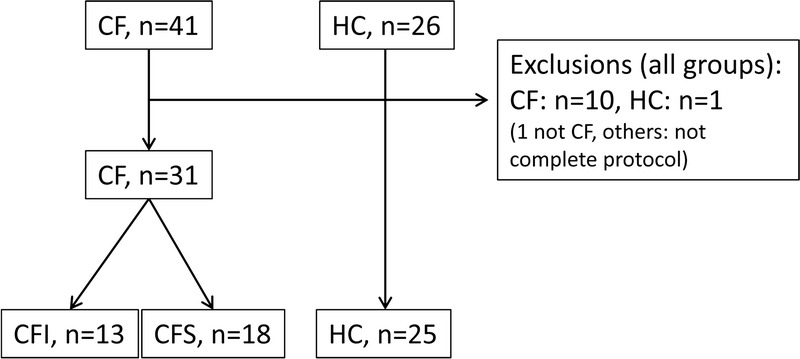
Inclusion flow chart.
TABLE 1.
Results
Methods
Before the examination medication, smoking habits, alcohol consumption, and body mass index were documented in all subjects. A review of the electronic patient journal was performed, and information on CFTR mutation and sweat-test values (Na+ and Cl−) were extracted from patient journals as documentation for the CF diagnosis. The genetic testing was performed over time, mostly by CF v3 Genotyping kit screening for 33 known CFTR mutations and collected retrospectively. Additional testing was performed for the CFTR mutations 4005+2T>C and R117H. Patients with unconfirmed mutation on screening performed whole gene sequencing for known CFTR mutations.
Fecal Elastase
Fecal elastase 1 (FE-1) was measured by a commercial monoclonal analysis kit (ScheBo Biotech, Giessen, Germany). We classified patients as exocrine pancreatic sufficient or insufficient by FE-1 concentration. Patients with FE less than 100 μg/g were considered pancreas insufficient.15
Short Endoscopic Secretin Test
The details of duodenal aspiration, duodenal juice handling, and analysis are described elsewhere19,20 but will be briefly described later.
The test procedure is illustrated in Figure 2. All procedures were performed by 4 experienced operators. Procedures were performed blinded to knowledge of pancreatic function status. Blinding to diagnosis and patient appearance was not possible. The patients received topical pharyngeal lidocaine (XYLOCAIN; AstraZeneca AB, Sweden) and conscious sedation with intravenous midazolam (MIDAZOLAM; Actavis Group HF, Island) 2 to 5 mg before the procedure. The study participants fasted for 8 hours before Secretin (Secrelux Sanochemia Diagnostics, Neuss, Germany; 1 CU/kg, maximum dose 70 CU) was administered intravenously for 1 minute. Twenty-five minutes after secretin administration, we performed gastroscopy and carefully emptied the stomach and duodenum of fluid. Thirty minutes after secretin injection, the tip of the endoscope was placed distal to the papilla of Vateri and duodenal juice was aspirated through the working channel of the endoscope in three 5-minute sequences between 30 and 45 minutes after secretin stimulation. The samples were immediately stored on ice.
FIGURE 2.
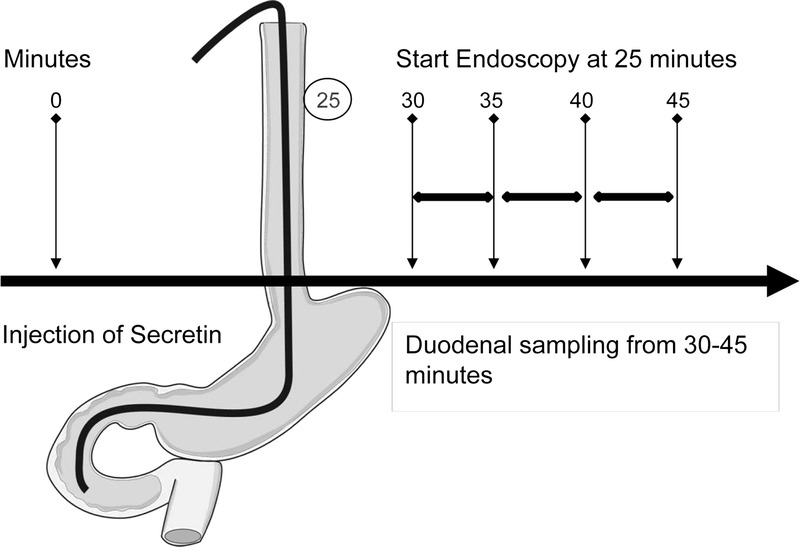
The figure displays the examination procedure for EST.
Duodenal Juice Handling
Each sample was split into 3 aliquots. One aliquot was either stored on ice for bicarbonate analysis less than 3 hours after collection or snap frozen on liquid nitrogen and stored at less than 80°C until the day of analysis. The other 2 aliquots were snap frozen more than 5 minutes after collection and stored on liquid nitrogen until the day of analysis. Before snap freezing, a protease inhibitor was added to one of these aliquots (Complete; Roche Diagnostics, Mannheim, Germany; 0.2 mL solution of 1 tablet per 1.5 mL water added per milliliter duodenal juice).
Duodenal Juice Analysis
Bicarbonate concentration (mEq/L) in duodenal juice was analyzed by the back titration method24 in each of the 3 samples. Enzyme activity analyses (U/mL) were performed in 96-well micro plate assays, using a Tecan Infinite M200 Pro microplate reader with iControl Tecan software (Tecan Group Ltd., Mannedorf, Switzerland). We used aliquots treated with protease inhibitors for activity analyses of pancreas lipase and amylase. Chymotrypsin and elastase activities were measured in the untreated duodenal juice. Samples were diluted as appropriate to fit into the measuring range for each assay and analyzed in triplicate. We used kinetic, fluorescent assays to determine the activities of elastase, chymotrypsin, and pancreas lipase. The increase in relative fluorescence units was measured every 60 seconds after contact between samples and substrate. We determined activity by relating the slope of increase in relative fluorescence unit in the samples to the slopes of enzyme standards. We used a colorimetric endpoint assay to determine amylase activity. The highest values of bicarbonate and enzymes were considered to be the peak level and used as an estimate of exocrine pancreatic function. Patient with dry tap or low volumes unfit to analyze bicarbonate by back titration (n = 3) had bicarbonate value defined as zero. The most widespread cutoff for duodenal bicarbonate concentration in other pancreatic disease is 80 meq/L.25,26 Others have suggested a cutoff value of 60 mEq/L as a lower limit conservative cutoff.21 We chose 80 mEq/L in our calculations. Validated cutoff levels for pancreatic insufficiency based on duodenal concentration/activities in patients with CF are not established.
Statistical Analysis
All statistics where calculated in SigmaPlot 11, (Copyright 2011 Systat Software Inc., San Jose, Calif). Normal distribution of the samples was tested by Kolmogorov-Smirnov test. The results are expressed as median values with range. Simple comparisons between groups were made by student t tests or Mann-Whitney U test when samples were not normally distributed. Receiver operator curves (ROC curves) were drawn and accuracy data were calculated. Variance is expressed through 95% confidence intervals. Five percent level of statistical significance was used.
Outliers were not excluded. Patients with missing data in single analyses were not excluded. Missing data were not analyzed. Subjects with missing index analysis (not performed EST) were excluded from the final diagnostic evaluation.
RESULTS
Fecal Elastase
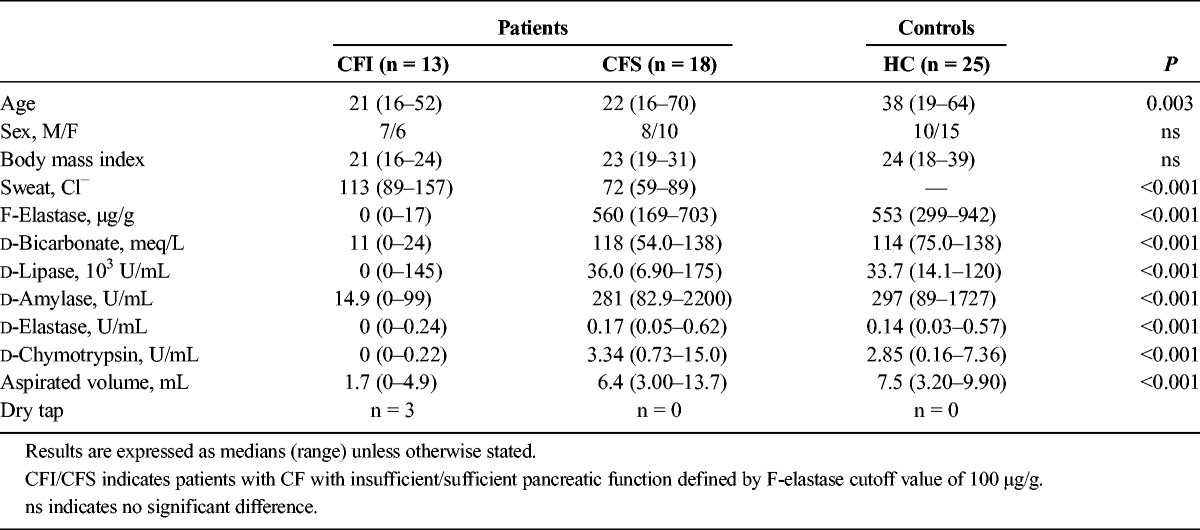
When sorted by results of FE, the patients undergoing EST were grouped as follows: CF pancreatic insufficient (CFI, n = 13) and CF pancreatic sufficient (CFS, n = 18). Demographic data and FE results are displayed in Table 1.
Duodenal Bicarbonate and Enzymes
The values of duodenal bicarbonate concentration and lipase, amylase, elastase, and chymotrypsin activities are presented in Table 1 and Figure 3.
FIGURE 3.
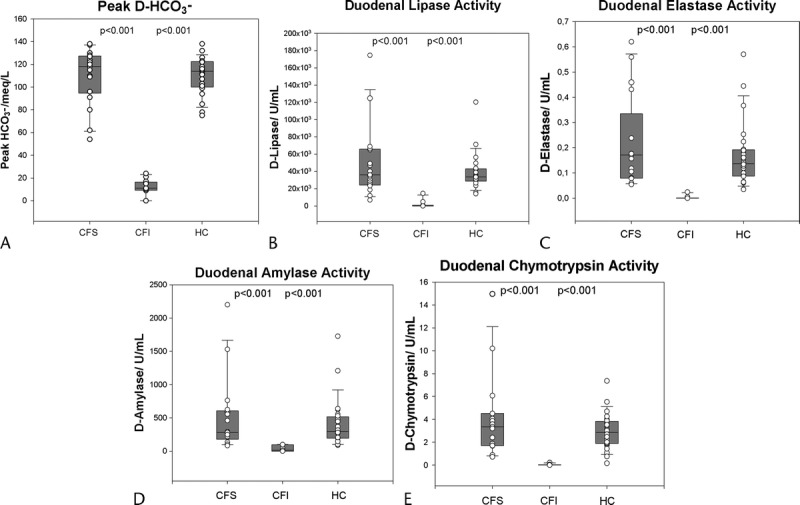
Box and scatter plots for peak bicarbonate concentrations and peak duodenal pancreatic enzyme activities divided by groups.
Using peak bicarbonate concentrations and peak duodenal digestive enzyme activity levels, we were able to differentiate the CFI group from both HCs and CFS patients (P < 0.001). The differences between CFS group and HC group were nonsignificant.
Duodenal bicarbonate correlates with volume aspirated in EST. Duodenal bicarbonate concentration is the most direct CFTR function measure. If we define an intermediate range of CFTR function between 60 and 90 mEq/L, we identify 2 patients with CF in this group. Both these subjects are classified as pancreas sufficient by the FE cutoff value of 100 μg/g. The plot of bicarbonate against lipase activity demonstrates hyperconcentration of the duodenal enzymes in the 2 patients with CF in the intermediate bicarbonate level (Fig. 4).
FIGURE 4.
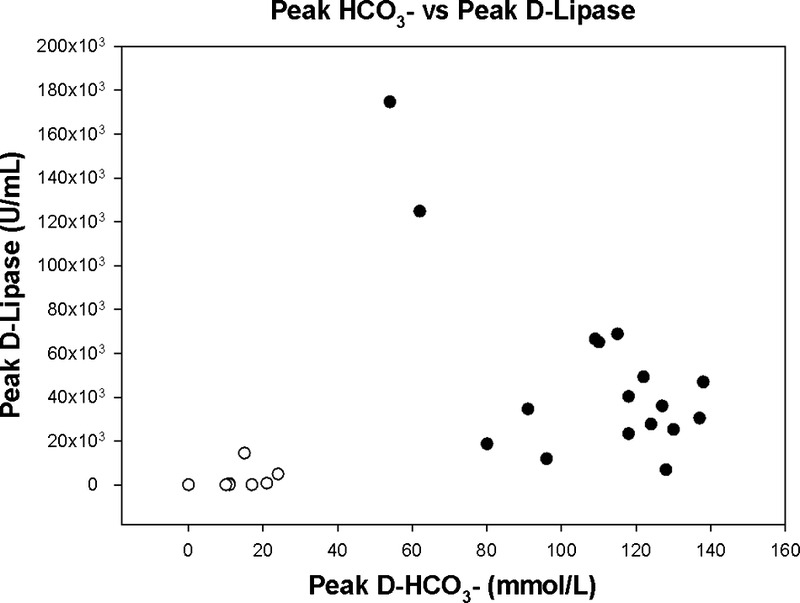
The figure illustrates the correlation between peak duodenal bicarbonate concentration and duodenal lipase activity in the patients with CF. Mark especially the 2 patients with intermediate bicarbonate concentrations, aspirated volumes in the lower normal range and hyperconcentrated duodenal enzymes. White circles indicates CFI; black circles, CFS.
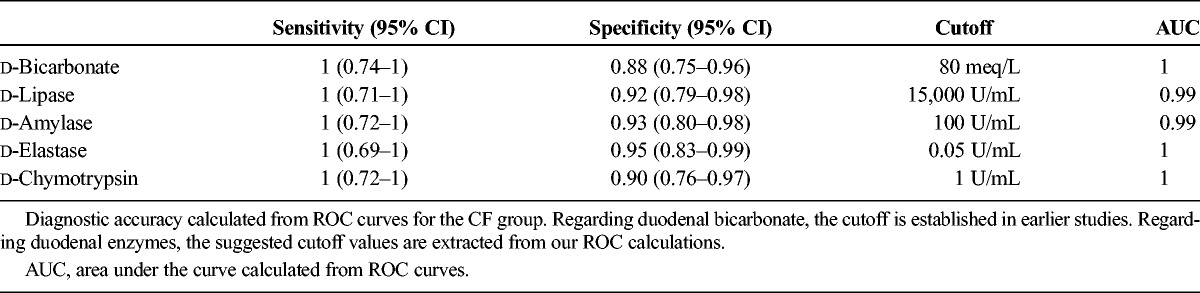
Diagnostic Accuracy
Receiver operator curves were calculated for bicarbonate concentrations and enzyme activities. Sensitivity and specificity corresponded to suggested cutoff values are displayed in Table 2. Compared with FE as standard for exocrine function, d-bicarbonate has a sensitivity of 100% and a specificity of 88% with the usual cutoff value set to 80 meq/L (P < 0.001). The positive predictive value of the test was 91% and the negative predictive value was 100% compared with the same standard. d enzymes have slightly lower sensitivities and specificities compared with the FE standard, but the differences in ROC areas between enzymes and bicarbonate are nonsignificant (Table 2).
TABLE 2.
Test Accuracy
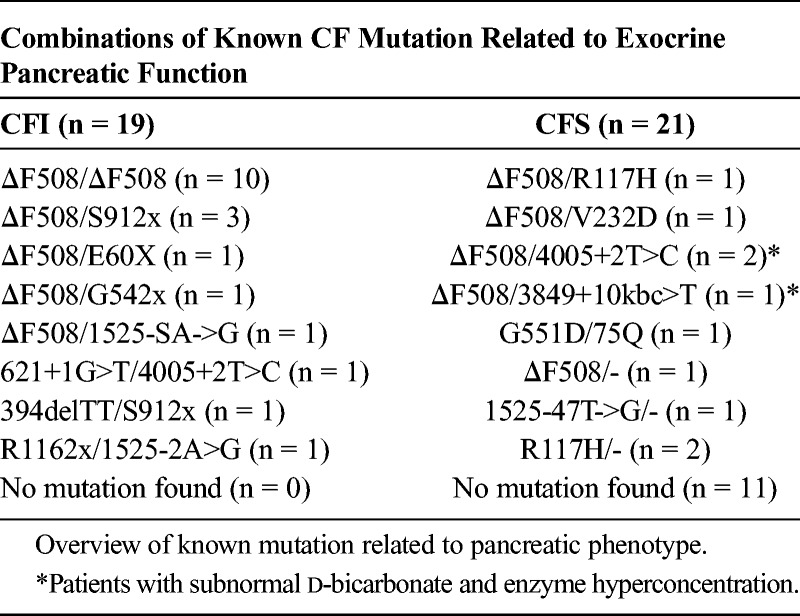
Genotype/Phenotype Analysis
A list of known mutations organized according to pancreatic phenotype for the whole group (n = 40) is displayed in Table 3. There is a strong tendency toward pancreatic insufficient phenotype in the group with known, severe CF mutations on both alleles (P < 0.001). The group with pancreatic sufficient phenotype has the highest number of patients without a known CF mutation. As noted earlier, the quality review of the CF diagnosis is according to diagnostic criteria,22 discovering doubts about the correct CF diagnosis in 3 patients (one excluded). Neither of these patient had pancreatic insufficient phenotype. One patient had a pathogenic mutation in one of the CFTR alleles; the other two had no known CFTR mutation.
TABLE 3.
CFTR Mutations
Adverse Events
The subjects reported mild abdominal discomfort and sensation of hunger connected to the secretin infusion. Most subjects also reported mild to moderate discomfort during the endoscopy. Heart rhythm, pulse, and oxygen saturation were observed in all subjects during the procedure. No complications or severe adverse events were reported.
DISCUSSION
In the present study, we demonstrate that a new, short EST is well tolerated and has a good diagnostic accuracy in assessing exocrine pancreatic function in patients with CF. We demonstrated a good correspondence between duodenal bicarbonate after secretin stimulation and the FE standards in the pancreas insufficient patients. Furthermore, duodenal enzyme concentrations also had fair correspondence with FE in both the pancreas sufficient groups and the patients with severe insufficiency. Among patients with CF with intermediate bicarbonate levels, we discovered 2 patients with CF with marked elevated pancreatic enzyme activity levels in all enzyme groups compared with other patients and controls. This phenomenon might prove to be a marker to identify patients with pancreatic sufficient CF at risk of or in the process of developing pancreatic insufficiency. Exocrine pancreatic test results were well correlated to the severity of CF genotype in our population.
Fecal elastase has previously demonstrated to be an excellent tool in repeated evaluation of exocrine pancreatic function in patients with CF and has more or less prevented the need for more complex, direct pancreatic function tests.11,14,27 However, FE has demonstrated weaknesses compared with the secretin-cholecystokinin test when aiming to follow progress in pancreatic failure or to detect early failure.16 Fecal elastase is not a tool to directly assess pancreatic ductal function.17 Development of the new, expensive CFTR modulators28 will increase the need of a clinical test to evaluate pancreatic CFTR function. Evaluation of the new, timed ESTs19–21,29 has to our knowledge previously not been performed in patients with CF. Schibli et al17 stated that tests with short sampling periods, concentration-based end points, and lacking evaluation of intestinal losses could create considerable errors when estimating pancreatic function in patients with CF, especially in the intermediate range. However, the short period analyzed in their study was confined to samples collected during the first 20 minutes after secretin stimulation, which later has been shown to be before the peak bicarbonate level in the duodenum in other studies including chronic pancreatitis patients.25,29 We claim that bicarbonate-concentration measure in duodenal juice is a physiological correct way to assess failure in CFTR function, because ductal secretion of water and bicarbonate is dependent of CFTR.8 Challenges in timing of duodenal collection and pollution from gastric ventricular fluid with low pH have been addressed in earlier works validating ESTs.21,30 The complex tube-based tests probably have better accuracy than shorter, concentration-based tests and should still be considered as criterion standard, but the fact that these tests have existed for decades without achieving widespread use justifies the search for simplified methods. We believe that our timed protocol can achieve acceptable results comparable with the more cumbersome and complex direct tests and that EST can prove to be a valuable direct CFTR-function test. To make a final conclusion on this issue, an evaluation of the FE and EST against the old quantitative direct tests must be conducted.
We chose FE as a standard for exocrine pancreatic function in our protocol because this is the simplest and most widespread test; thus, the protocol was not designed to compare the diagnostic accuracies of EST and FE. The conservative FE cutoff value of 100 μg/g gives a firm definition for the pancreas insufficient group. The influence of the possible low sensitivity in the FE test of early exocrine failure cannot be decided and specificity levels achieved for EST are influenced from reduced sensitivity of FE. Another accepted criterion standard for exocrine function in CF is the 3-day fecal-fat sample. Because of the lack of sensitivity of this test for mild to moderate failure, this standard will suffer from the same pitfalls as FE.
Analyses of pancreatic enzymes infer numerous practical pitfalls and require conscious handling of the duodenal samples.20 These analyses demonstrate lower diagnostic accuracy compared with bicarbonate. Still, we propose that pancreatic enzyme activity assessment gives valuable, additional information. Firstly, our procedure for analyzing duodenal enzymes requires only small amounts of fluid; thus, we are able perform analyses in patients with CF with severe volume output failure. Secondly, the phenomenon of enzyme hyperconcentration illustrates an etiological factor postulated to pre-exist the pancreatic destruction in CF. This may prove to be a sign of early volume output failure.
The correlation between CF genotype and pancreatic insufficient phenotype is well established in several studies.31–33 In the present study, the information on genotype was collected retrospectively from a patient registry, and the tests were performed for a period. The population has a number of unidentified CF mutations in the CFS group. Patients with CF in western Norway are earlier described to have a low prevalence of pancreatic insufficiency, possibly due to regional variations in genotype with a higher prevalence of milder CF mutations.34 We did not include patients with lung transplants or severe lung disease. Thus, the population presented is not a representative cohort of the whole CF population in our region. It is still interesting to note that also in our population, patients with CF with severe CF genotype have a high rate of exocrine pancreatic insufficiency and those milder mutations or unknown mutation status is a predictor pancreatic sufficiency; thus, the EST results fit the expectations when correlated to genotype.
We find all of the patients with CF-related disorder or doubtful CF diagnosis in the CFS group. We excluded one but chose to include the 2 others because of the presence of significant clinical signs of CF phenotype represented by classical organ manifestations and colonization from CF-associated pathogens strongly suggestive of CF. As noted by Ooi et al,35 the diagnostic guidelines provide guidance and promote rigorous evaluation for the diagnosis of CF but neither guideline should be regarded as dogma. The genetic diversity combined with a remaining uncertainty of the diagnosis in the CFS group may explain why we are not able to identify reduced bicarbonate levels in this group compared with HCs. Whether the presence of milder or heterozygote CFTR mutation can give partly reduced CFTR function and reduced duodenal bicarbonate compared with subjects with normal CFTR status remains unanswered.
In the present study, we aimed to evaluate our short EST in a group of patients with CF with a well-established clinical diagnosis. Despite the fact that EST is an invasive test, the current version using less than 20-minute sampling time is feasible and can be performed in patients with CF with a low risk of patient hazard. Our study is the first evaluation of a timed short EST in patients with CF compared with HCs. Because of costs, test simplicity, and lack of patient hazard, FE will remain the first choice for assessing exocrine pancreatic function in patients with CF. We believe that the EST can be a valuable clinical supplement where the FE result leaves doubt whether there is pancreatic insufficiency or not. Particularly, the test might prove useful in selected patients with CF at risk if a combination of bicarbonate and enzyme values can give an indication on early or forthcoming exocrine pancreatic insufficiency. Whether the test can identify early or the risk of future pancreatic insufficiency remains unanswered. The effect of enzyme hyperconcentration in the phase of early pancreatic failure needs further confirmation in a protocol with a higher number of patients in this phase of disease progression. An important near-future perspective can be to identify subjects to be targeted for therapeutic interventions with new CFTR modulators to preserve pancreatic function.
ACKNOWLEDGMENTS
The authors give their special acknowledgement to the supporting laboratory technicians Liv Aasmul and Aud Sissel Hjartholm for preserving and running analyses on duodenal juice and to Line Lærum for always keeping the spirit up in the attending patients with CF.
Footnotes
Deceased 2014.
The study was supported by MedViz (http://medviz.uib.no/), an interdisciplinary research cluster from Haukeland University Hospital, University of Bergen, and Christian Michelsen Research AS. The authors have received independent travel grants and scholarships from Abbott, the Norwegian Gastroenterology association, and the Norwegian CF foundation.
The authors are receiving salaries from the affiliated institutions. The research group has received funding from the Norwegian Cystic Fibrosis foundation. In addition, authors T.E., F.E., and G.D. have received limited research grants from Abbott Norway and the Norwegian Gastroenterology Association.
REFERENCES
- 1. O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet. 2009; 373: 1891– 1904. [DOI] [PubMed] [Google Scholar]
- 2. Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989; 245: 1073– 1080. [DOI] [PubMed] [Google Scholar]
- 3. Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989; 245: 1066– 1073. [DOI] [PubMed] [Google Scholar]
- 4. Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989; 245: 1059– 1065. [DOI] [PubMed] [Google Scholar]
- 5.Cystic fibrosis genetic analysis consortioum. CF Mutation Database. [Database Online]. Available at: http://www.genet.sickkids.on.ca/app. Accessed May 9, 2014.
- 6. Wilschanski M, Durie PR. Patterns of GI disease in adulthood associated with mutations in the CFTR gene. Gut. 2007; 56: 1153– 1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kopelman H, Durie P, Gaskin K, et al. Pancreatic fluid secretion and protein hyperconcentration in cystic fibrosis. N Engl J Med. 1985; 312: 329– 334. [DOI] [PubMed] [Google Scholar]
- 8. Kopelman H, Corey M, Gaskin K, et al. Impaired chloride secretion, as well as bicarbonate secretion, underlies the fluid secretory defect in the cystic fibrosis pancreas. Gastroenterology. 1988; 95: 349– 355. [DOI] [PubMed] [Google Scholar]
- 9. Augarten A, Ben Tov A, Madgar I, et al. The changing face of the exocrine pancreas in cystic fibrosis: the correlation between pancreatic status, pancreatitis and cystic fibrosis genotype. Eur J Gastroenterol Hepatol. 2008; 20: 164– 168. [DOI] [PubMed] [Google Scholar]
- 10. Borowitz D, Baker SS, Duffy L, et al. Use of fecal elastase-1 to classify pancreatic status in patients with cystic fibrosis. J Pediatr. 2004; 145: 322– 326. [DOI] [PubMed] [Google Scholar]
- 11. O'Sullivan BP, Baker D, Leung KG, et al. Evolution of pancreatic function during the first year in infants with cystic fibrosis. J Pediatr. 2013; 162: 808– 812. [DOI] [PubMed] [Google Scholar]
- 12.Cystic Fibrosis Foundation Patient Registry. 2012 Annual data report. Available at: http://www.cff.org/UploadedFiles/aboutCFFoundation/AnnualReport/2012-Annual-Report.pdf. Accessed May 9, 2014.
- 13. Couper RT, Corey M, Moore DJ, et al. Decline of exocrine pancreatic function in cystic fibrosis patients with pancreatic sufficiency. Pediatr Res. 1992; 32: 179– 182. [DOI] [PubMed] [Google Scholar]
- 14. Walkowiak J, Nousia-Arvanitakis S, Agguridaki C, et al. Longitudinal follow-up of exocrine pancreatic function in pancreatic sufficient cystic fibrosis patients using the fecal elastase-1 test. J Pediatr Gastroenterol Nutr. 2003; 36: 474– 478. [DOI] [PubMed] [Google Scholar]
- 15. Borowitz D, Robinson KA, Rosenfeld M, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009; 155: S73– S93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Walkowiak J, Cichy WK, Herzig KH. Comparison of fecal elastase-1 determination with the secretin-cholecystokinin test in patients with cystic fibrosis. Scand J Gastroenterol. 1999; 34: 202– 207. [DOI] [PubMed] [Google Scholar]
- 17. Schibli S, Corey M, Gaskin KJ, et al. Towards the ideal quantitative pancreatic function test: analysis of test variables that influence validity. Clin Gastroenterol Hepatol. 2006; 4: 90– 97. [DOI] [PubMed] [Google Scholar]
- 18. Weintraub A, Blau H, Mussaffi H, et al. Exocrine pancreatic function testing in patients with cystic fibrosis and pancreatic sufficiency: a correlation study. J Pediatr Gastroenterol Nutr. 2009; 48: 306– 310. [DOI] [PubMed] [Google Scholar]
- 19. Erchinger F, Engjom T, Tjora E, et al. Quantification of pancreatic function using a clinically feasible short endoscopic secretin test. Pancreas. 2013; 42: 1101– 1106. [DOI] [PubMed] [Google Scholar]
- 20. Tjora E, Wathle G, Engjom T, et al. Severe pancreatic dysfunction but compensated nutritional status in monogenic pancreatic disease caused by carboxyl-ester lipase mutations. Pancreas. 2013; 42: 1078– 1084. [DOI] [PubMed] [Google Scholar]
- 21. Jensen NM, Larsen S. A rapid, endoscopic exocrine pancreatic function test and the Lundh test: a comparative study. Pancreatology. 2008; 8: 617– 624. [DOI] [PubMed] [Google Scholar]
- 22. Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008; 153: S4– S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. STARD. Statement Standards for the Reporting of Diagnostic accuracy studies. Available at: http://www.stard-statement.org/. Accessed May 9, 2014.
- 24. Segel IH. Acid-Base chemistry. In: Segel IH, ed. Biochemical Calculations. 2nd ed New York: Wiley; 1976: 1– 91. [Google Scholar]
- 25. Conwell DL, Zuccaro G, Jr, Vargo JJ, et al. An endoscopic pancreatic function test with synthetic porcine secretin for the evaluation of chronic abdominal pain and suspected chronic pancreatitis. Gastrointest Endosc. 2003; 57: 37– 40. [DOI] [PubMed] [Google Scholar]
- 26. Stevens T, Conwell DL, Zuccaro G, Jr, et al. A prospective crossover study comparing secretin-stimulated endoscopic and Dreiling tube pancreatic function testing in patients evaluated for chronic pancreatitis. Gastrointest Endosc. 2008; 67: 458– 466. [DOI] [PubMed] [Google Scholar]
- 27. Walkowiak J, Sands D, Nowakowska A, et al. Early decline of pancreatic function in cystic fibrosis patients with class 1 or 2 CFTR mutations. J Pediatr Gastroenterol Nutr. 2005; 40: 199– 201. [DOI] [PubMed] [Google Scholar]
- 28. Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011; 365: 1663– 1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stevens T, Conwell DL, Zuccaro G, Jr, et al. The efficiency of endoscopic pancreatic function testing is optimized using duodenal aspirates at 30 and 45 minutes after intravenous secretin. Am J Gastroenterol. 2007; 102: 297– 301. [DOI] [PubMed] [Google Scholar]
- 30. Stevens T, Conwell DL, Zuccaro G, Jr, et al. A randomized crossover study of secretin-stimulated endoscopic and dreiling tube pancreatic function test methods in healthy subjects. Am J Gastroenterol. 2006; 101: 351– 355. [DOI] [PubMed] [Google Scholar]
- 31. Ahmed N, Corey M, Forstner G, et al. Molecular consequences of cystic fibrosis transmembrane regulator (CFTR) gene mutations in the exocrine pancreas. Gut. 2003; 52: 1159– 1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Durno C, Corey M, Zielenski J, et al. Genotype and phenotype correlations in patients with cystic fibrosis and pancreatitis. Gastroenterology. 2002; 123: 1857– 1864. [DOI] [PubMed] [Google Scholar]
- 33. Ooi CY, Dorfman R, Cipolli M, et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology. 2011; 140: 153– 161. [DOI] [PubMed] [Google Scholar]
- 34. Dorlöchter L, Aksnes L, Fluge G. Faecal elastase-1 and fat-soluble vitamin profiles in patients with cystic fibrosis in Western Norway. Eur J Nutr. 2002; 41: 148– 152. [DOI] [PubMed] [Google Scholar]
- 35. Ooi CY, Dupuis A, Ellis L, et al. Comparing the American and European diagnostic guidelines for cystic fibrosis: same disease, different language? Thorax. 2012; 67: 618– 624. [DOI] [PubMed] [Google Scholar]


