Abstract
Introduction
Globally, the prevalence of overweight and obesity is increasing, predisposing females to health hazards including compromised reproductive capacity. Our objective was to investigate the effect of ad libitum, isocalorically and hypocalorically restricted high-fat diet (HFD) feeding on reproductive function in diet-induced obese female rats.
Material and methods
Twenty female albino Sprague Dawley rats were used; 5 rats were kept on a standard pellet animal diet to serve as a control group (A) and 15 rats were fed a HFD for 9 weeks to induce obesity. The HFD fed animals were equally divided into three groups: an ad libitum HFD group (B), an isocalorically restricted HFD group (C), and a hypocalorically restricted HFD group (D). Estrous cyclicity, hormonal levels, ovarian histopathology and caspase-3 immunoreactivity were evaluated.
Results
The HFD-fed rats in groups B, C and D had significant irregularity in estrous cyclicity Vs group A (p = 0.001, 0.003 and 0.034 respectively). Groups C and D had significant reduction in serum progesterone level (p = 0.006 and 0.018 Vs A). Isocaloric restriction of HFD feeding significantly increased serum LH. Groups B and C had a significant increase in caspase-3 expression in the ovary (p < 0.001).
Conclusions
Ad libitum HFD interfered with the normal estrous cycle and enhanced apoptosis of luteal cells in obese female rats. The HFD restriction interfered with the normal estrous cycle and caused functional insufficiency of the corpus luteum in obese female rats. These results suggest that HFD feeding determinately affects female reproductive function independently of caloric intake.
Keywords: obesity, calorie restriction, female reproduction, fat
Introduction
The prevalence of overweight and obesity is increasing globally, predisposing females to health hazards including compromised reproductive capacity. Formerly considered a problem of developed countries, this epidemic is now dramatically on the rise in low- and middle-income countries [1]. Anovulation, irregular menses, subfertility, miscarriage, and adverse pregnancy outcomes, with lasting effects for children, are reproductive sequelae of obesity among women of reproductive age. On the other hand, not all obese women experience poor reproductive health. Because of this, it is important to recognize factors other than obesity that affect reproductive function in obese women. Nutrition is one of them [2].
A handful of studies have previously reported altered reproductive functions in diet-induced obese animal models [3], and several studies have addressed the effects of dietary restriction using standard rodent diets on reproductive function of female rats [4–6]. Currently, dietary restriction using a high-fat diet (HFD) is more relevant to dietary status in most world countries [7].
Leptin is an adipocyte hormone that functions as an afferent signal in a negative feedback loop regulating body weight. Since its discovery in 1995, several studies have suggested a pivotal role of leptin in reproductive function [8–10].
Programmed cell death or apoptosis is attributed to the exhaustion of the oocyte reserve through germ cell death and follicular atresia, so apoptosis is an essential component of normal reproductive function and it has been proposed to be the major mechanism that determines female reproductive life span [11].
The corpus luteum is primarily responsible for the synthesis and release of progesterone, a hormone essential for the establishment and maintenance of pregnancy in mammals. In the absence of pregnancy, the corpus luteum will cease to synthesize progesterone, and the bulk of the luteal tissue will decrease in mass. This process is termed luteolysis [12]. The luteolytic process is typically subdivided, whereby the decline in progesterone is described as functional luteolysis and the structural involution is described as structural luteolysis [13]. Luteolysis, at least in part, is mediated by apoptosis in essentially all mammalian species. It has become evident that apoptosis is triggered by the activation of cysteine aspartate-specific proteases (caspases). A cascade of proteolysis beginning with the activation of caspase-8 leads to activation of caspase-3, which is the principal downstream effector enzyme of cell death, and activation of caspase-3 is considered by many as a final executioner of the apoptotic cell death program [12, 14].
To our knowledge, no studies have been carried out to investigate the effect of HFD feeding independently from caloric intake on reproductive function in obese female rats. So our objective was to investigate the effect of ad libitum, isocalorically restricted and hypocalorically restricted HFD feeding on reproductive function in diet-induced obese female rats.
Material and methods
This study was performed at the Physiology and Clinical Pathology departments, Faculty of Medicine, Suez Canal University, Ismailia, Egypt. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Suez Canal University. All efforts were made to minimize animal suffering and to reduce the number of animals used. The “Principles of laboratory animal care” were followed, as well as specific national laws where applicable.
Animals and nutritional management
Twenty female albino Sprague Dawley rats (7–9 weeks of age, weighing 137–176 g at the beginning of the experiment) purchased from the center for experimental animals, Faculty of Veterinarian Medicine, Zagazig University, were used in the study. All rats were left to acclimatize for one week prior to the experiment and were housed in plastic cages maintained at controlled room temperature (22–24°C), 60% humidity with 12-hour day/night cycle with free access to a standard pellet animal diet (purchased from El Gomhorya company, Ismailia, Egypt) containing 67% carbohydrates, 10% fat, and 23% protein as the energy sources (overall calories: 3.6 kcal/g) and tap water. After weight matching, 5 rats were kept on a standard pellet animal diet to serve as a control group (group A) and 15 rats were fed a HFD consisting of 88% of standard pellet animal diet, 10% lard and 2% cholesterol for 9 weeks to induce obesity. The HFD was composed of the following energy sources: 52% carbohydrates, 30% fat, and 18% protein (overall calories: 4.8 kcal/g). The amount of calories in each gram was calculated in the Nutrition and Food Science Department, Faculty of Education, Suez Canal University. The major composition of the diets used in this study is presented in Table I [15, 16]. Next the HFD fed animals were equally divided into three weight-matched groups: the ad libitum HFD group (group B), which was freely maintained on the same composition of HFD for another 4 weeks; the isocaloric restricted HFD group (group C), which was fed 75% of their average food intake before restriction (overall calories: 3.6 kcal/g) for another 4 weeks; and the hypocaloric restricted HFD group (group D), which was fed 50% of their average food intake before restriction (overall calories: 2.4 kcal/g) for another 4 weeks. Food intake was measured per cage to avoid the stress of individual housing [16]. Food intake was measured daily for 1 week before the start of food restriction, and the average amount was calculated [17].
Table I.
Major components of experimental diets (g/100 g diet)
| Component | Standard pellet animal diet | High-fat diet |
|---|---|---|
| Carbohydrates | 60 | 52.8 |
| Cholesterol | 0 | 2 |
| Protein | 20 | 17.6 |
| Fats | 4 | 13.5 |
| Fatty acid composition | 16.4% Saturated fatty acids 54.3% Polyunsaturated fatty acids 29.3% Monounsaturated fatty acids |
32.2% Saturated fatty acids 22.4% Polyunsaturated fatty acids 43.8% Monounsaturated fatty acids 1.6% Other fatty acids |
Methods
All animals were weekly weighed from the first week of study onwards. Estrous cyclicity was daily examined (5 days per week) for all animals from the start of HFD restriction until the end of the study. Estrous cyclicity monitoring was done by vaginal smear between 9 and 11 a.m. The vaginal smears were examined by standard light microscope to observe proportions of cells to determine the phase of rat estrous cycle. The rat estrous cycle takes 4–5 days and consists of four phases: diestrus, proestrus, estrus and metestrus. The diestrus phase is characterized by predominance of leucocytes, the proestrus phase is characterized by appearance of predominant nucleated epithelial cells, the estrus phase is characterized by appearance of clustered large cornified epithelial cells, and the metestrus phase is characterized by appearance of equal proportions of these cells [18, 19]. Irregular estrous cycles were characterized by keeping in the same phase during 4–5 days or when the alternation among the phases did not follow the sequence of proestrus, estrus, metestrus and diestrus [18].
At the end of the study the following procedures were done for all animals:
Measuring the body weight and length. Body mass index (BMI) was calculated according to the formula: BMI = body weight (g)/length2 (cm2). The Lee index was calculated according to the formula: Lee index = cubic root of body weight in grams divided by length in centimeters [20]. Corrected ovarian weight was calculated as % of ovarian weight/body weight [19].
After 5 h of food withdrawal [21], blood samples were taken from the retro-bulbar venous plexus from anesthetized rats to measure the concentrations of serum glucose, cholesterol, triglycerides, free fatty acids, follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, progesterone and leptin.
Assay of fasting serum concentrations of glucose, cholesterol, triglycerides and free fatty acids: glucose, cholesterol and triglyceride measurements were performed with commercial kits on the Cobas c311 analyzer (Roche Diagnostics, Germany). The free fatty acid assay was performed with BioVision's Free Fatty Acid Quantification Colorimetric Kit.
Assay of FSH, LH, estradiol, progesterone and leptin: the serum was obtained by centrifugation (2400 rpm, for 20 min at 4°C) and the concentrations of estradiol, LH, progesterone and FSH were determined by (Vidas, BioMerieux). Assays of serum leptin concentrations were performed using ELISA kits (R&D Systems) according to the manufacturer's manual. The sensitivity of the assay is 22 pg/ml with an interassay coefficient of variation ∼5%.
Anesthetized rats were sacrificed and dissected for collection of both ovaries, which were weighed then processed for subsequent histopathological studies.
- Histopathological studies:
- Hematoxylin and eosin study: ovaries were stored in 10% neutral buffered formalin, embedded in paraffin blocks, and sectioned (5 µm). Ovarian sections were stained with hematoxylin and eosin. Histological analysis of the ovarian sections was done by a professional pathologist. The follicles and corpora lutea were counted. Graafian follicles were characterized by the presence of a confluent antral space filled with fluid with a diameter greater than 350 µm [3].
- Caspase-3 immunohistochemistry: the detection of caspase-3 protein expression in the ovarian sections relied on immunohistochemistry, which was based on a streptavidin biotin peroxidase method (Biogenex, San Ramon, CA, USA). Ovarian paraffin sections and polyclonal antibody to caspase-3 (Santa Cruz, USA, at dilution 1 : 150) were incubated for 1 h at room temperature. Diaminobenzidine (Sigma Fast 3,3’-diaminobenzidine tablets, D-4293; Sigma, St. Louis, MO, USA) was used as the chromogen. Cytoplasmic staining for caspase-3 was considered positive. The integral optical density (IOD) of immunohistochemical intensity was then calculated using Image-Pro plus 6.0 software. Each value represents IOD counted at a high-power view (400×) by a microscope. The mean value represents the average number derived from the 5 high-power fields of each case [22].
Statistical analysis
All the data were expressed as mean ± standard error of mean (SEM) and analyzed using the program Statistical Package for the Social Sciences (SPSS) (IBM SPSS Statistics, Version 20). All the comparisons among groups were carried out using one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test to test the significance difference among group means. Data were considered statistically significant at p ≤ 0.05.
Results
Characteristics of the animals
Ad libitum HFD feeding for 13 weeks significantly increased the body weight and BMI in group B vs. groups A, C and D and significantly increased the Lee index in group B vs. groups A and D. Isocalorically restricted HFD for 4 weeks significantly reduced the ovarian weight in group C vs. groups A and B and significantly reduced the corrected ovarian weight in group C vs. group A. Hypocalorically restricted HFD for 4 weeks significantly reduced the body and ovarian weights in group D vs. group A (Table II).
Table II.
Comparison of the mean body weight, BMI, Lee index, ovarian weight and corrected ovarian weight in the study groups
| Group | Body weight [g] | BMI [g/cm2] | Lee index [g/cm] | Ovarian weight [mg] | Corrected ovarian weight |
|---|---|---|---|---|---|
| A | 166 ±4.7 | 0.47 ±0.01 | 0.29 ±0.003 | 74.1 ±6.4 | 0.045 ±0.004 |
| B | 198.6 ±8.7a | 0.61 ±0.02a | 0.32 ±0.006c | 69 ±8.8 | 0.034 ±0.003 |
| C | 159 ±3.6 | 0.5 ±0.01 | 0.3 ±0.002 | 39.2 ±4.65d | 0.026 ±0.002e |
| D | 134.8 ±3b | 0.45 ±0.03 | 0.29 ±0.008 | 46.4 ±4.5b | 0.036 ±0.002 |
Mean ± SEM.
Significant difference between group B vs. groups A, C and D.
Significant difference between group D vs. group A.
Significant difference between group B vs. groups A and D.
Significant difference between group C vs. groups A and B.
Significant difference between group C vs. group
Serum concentrations of glucose, cholesterol, triglycerides and free fatty acids
Ad libitum HFD for 13 weeks caused no significant changes in the fasting serum levels of glucose, triglycerides, cholesterol or free fatty acids. Isocalorically restricted HFD for 4 weeks significantly increased the fasting serum cholesterol level in group C vs. groups A and B. Hypocaloric restricted HFD for 4 weeks caused no significant changes in fasting serum levels of glucose, triglycerides, cholesterol or free fatty acids (Table III).
Table III.
Comparison of mean fasting levels of serum glucose, triglycerides, cholesterol and free fatty acids in the study groups
| Group | Serum glucose [mg/dl] | Serum triglycerides [mg/dl] | Serum cholesterol [mg/dl] | Serum free fatty acids [mmol/l] |
|---|---|---|---|---|
| A | 64.2 ±6.4 | 68.8 ±9.5 | 91 ±10.8 | 0.76 ±0.13 |
| B | 57 ±14.4 | 56 ±1.8 | 86.4 ±8.8 | 1 ±0.3 |
| C | 88.4 ±8.2 | 85.8 ±17.3 | 163.4 ±24a | 0.88 ±0.21 |
| D | 52.2 ±1.7 | 63 ±4.3 | 117.4 ±7.4 | 1.1 ±0.24 |
Mean ± SEM.
Significant difference between group C vs. groups A and B.
Estrous cyclicity results
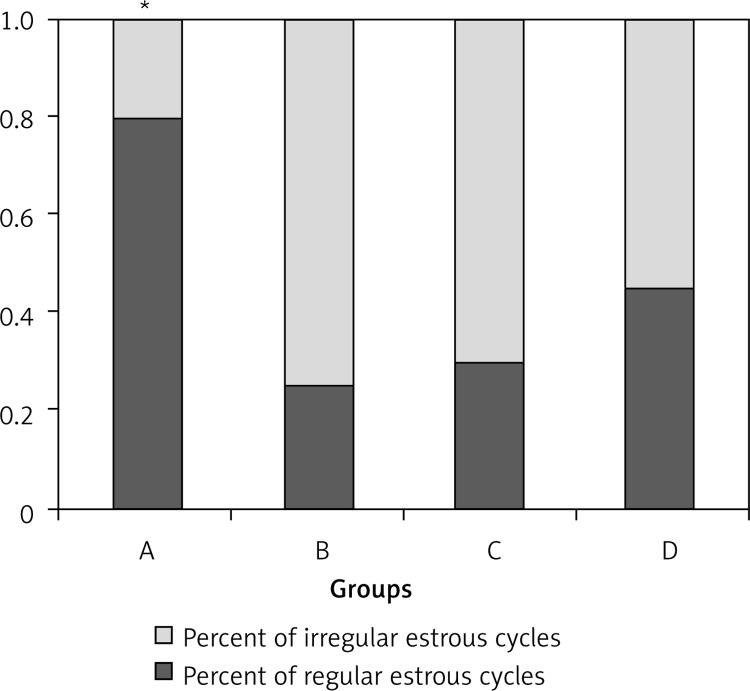
In group A, 80% of female rats had regular estrous cycles with mean duration of 4–5 days and with the sequence of proestrus, estrus, metestrus and diestrus. The HFD feeding independently of caloric intake in groups B, C and D significantly reduced this percentage. Most of the irregular cycles were characterized by prolonged diestrus. During the last week before the sacrifice of rats, 20% of rats in groups C and D and 0% of rats in group B had regular cycles. Most of the irregular cycles were characterized by persistent diestrus (Figure 1).
Figure 1.
Estrous cyclicity in the study groups
*Significant difference between group A vs. groups B, C and D.
Serum FSH, LH, estradiol, progesterone and leptin results
Ad libitum HFD for 13 weeks caused no significant changes in serum levels of FSH, LH, estradiol, or progesterone in group B. Isocalorically restricted HFD for 4 weeks significantly increased the serum LH level in group C vs. groups A and B, and significantly reduced the serum progesterone level in group C vs. groups A and B. Hypocalorically restricted HFD for 4 weeks significantly reduced the serum progesterone level in group D vs. group A. There was no significant change in serum leptin among the study groups (Table IV).
Table IV.
Comparison of mean levels of serum FSH, LH, estradiol, progesterone and leptin in the study groups
| Group | Serum FSH [mIU/ml] | Serum LH [mIU/ml] | Serum estradiol [pg/ml] | Serum progesterone [pg/ml] | Serum leptin [ng/ml] |
|---|---|---|---|---|---|
| A | 3.4 ±0.28 | 2.3 ±0.16 | 10.1 ±0.25 | 4.6 ±0.5 | 4.3 ±1.2 |
| B | 3.3 ±0.25 | 2.6 ±0.21 | 12.1 ±1.37 | 3.9 ±0.46 | 7.6 ±1.7 |
| C | 8.7 ±1.8 | 6.5 ±1.7a | 13.3 ±1.8 | 1.8 ±0.6a | 3.4 ±0.7 |
| D | 8.2 ±2.4 | 2.9 ±0.27 | 10.2 ±0.8 | 2.2 ±0.33b | 4.9 ±1.1 |
Mean ± SEM.
Significant difference between group C vs. groups A and B.
Significant difference between group D vs. group A.
Histopathological results
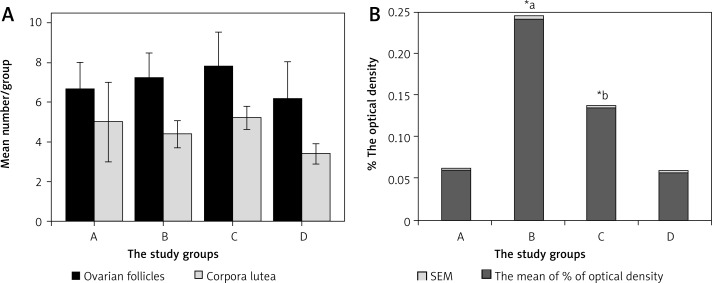
There was no significant difference between the study groups in the count of ovarian follicles or corpora lutea (Figure 2 A). The ovaries of rats in group B showed a significant increase in immunoreactivity for caspase-3 versus groups A, C and D. The ovaries of rats in group C showed a significant increase in immunoreactivity for caspase-3 versus groups A and D (Figures 2 B, Figure 3).
Figure 2.
A – The mean of number of ovarian follicles and corpora lutea in the study groups; B – the percent of optical density of immunoreactive cells
*aSignificant increase in the percent of optical density of ovarian caspase-3 immunoreactivity in group B vs. groups A, C and D
*bSignificant increase in the percent of optical density of ovarian caspase-3 immunoreactivity in group C vs. groups A and D
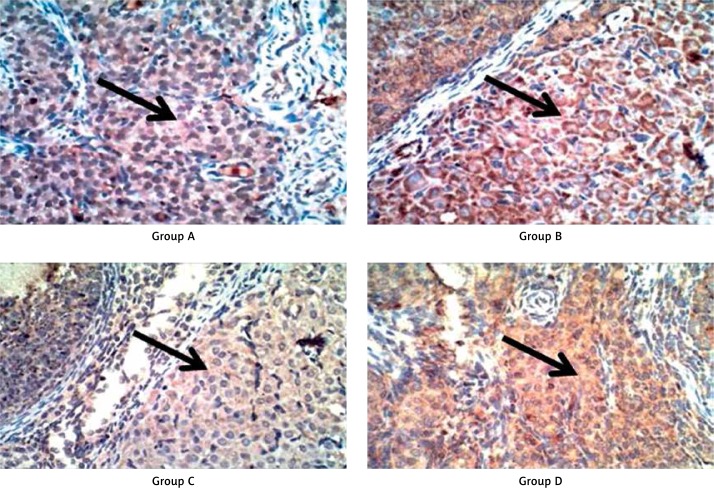
Figure 3.
Caspase-3 immunostaining of luteal cells, showing strong cytoplasmic staining for caspase-3 in groups B and C and weak cytoplasmic staining in groups A and D. Photographed at magnification, 400×
Discussion
“The reproductive function in women is impaired by a negative and positive nutritional status” [6]. In the current study, ad libitum HFD for 13 weeks significantly increased the body weight, BMI and Lee index. Isocaloric HFD restriction caused the body weight of obese rats to decrease to a mean value that did not significantly differ from that of the control group. Hypocaloric HFD restriction significantly reduced the body weight of obese rats. These results are in agreement with the results of Park et al.; they found that rats gradually developed obesity when placed on a HFD, and when rats were subjected to 40% restricted HFD treatment, they had a significant reduction in body weights [23].
In the current study, ovarian weight of restricted HFD fed rats was significantly decreased. A similar result was obtained by other studies that subjected rats to different protocols of dietary restriction using standard rodents’ diets [5, 9, 19]. Concerning corrected ovarian weight, we found a significant decrease in corrected ovarian weight of the rats that were subjected to isocaloric HFD restriction only. This finding may be due to the fact that the body weights of these rats were not significantly different from control rats, and at the same time they had a significant decrease in ovarian weight. Hypocaloric HFD restriction caused a proportional significant decrease in both rats’ body weights and ovarian weights, and this resulted in no significant change in corrected ovarian weight in rats treated with hypocaloric HFD restriction. This result is in agreement with Dos Santos et al., as they found that ovarian weight when corrected to the body weight did not differ in dietary restricted rats [19].
In the current study we found that ad libitum HFD caused no significant change in fasting concentrations of blood glucose, cholesterol, triglycerides, or free fatty acids when compared to standard diet fed rats. This result was also found in lean mice under a HFD [24]. Restricted HFD had no effect on blood glucose triglycerides and free fatty acids. Similar results were obtained by Meidenbauer et al., as they found that mice, when treated with a lard-based HFD in restricted amounts, exhibited decreased glucose levels and a normolipidemic profile [25]. On the other hand, we found that serum cholesterol level was significantly increased with isocaloric HFD restriction. This effect may be explained by the fact that we used lard- based diets which are rich in saturated fatty acids and poor in polyunsaturated fatty acids [26]. Saturated fatty acids have a strong cholesterol-raising effect when isocalorically replacing carbohydrates in the diet [27].
In the current study, there was no significant difference in the ovarian follicles count among the studied groups. A similar result was obtained by Akamine et al., who found that HFD for 120 days did not promote a significant change in ovarian morphology [21]. In the present study, ad libitum HFD feeding interfered with the normal estrous cycle, significantly increased luteal cell apoptosis, and caused no significant effect on the serum levels of FSH, LH, estradiol or progesterone. Akamine et al. found that HFD extended the estrous cycle in rats, caused no significant effect on serum FSH or estradiol, significantly changed serum progesterone after HFD for 120 and 180 days, and changed serum LH after HFD for 120 days only. The difference between our results and those results may be due to the difference in duration of HFD treatment: 90 days in the present study versus 120 and 180 days in the Akamine et al. study [21].
The seeming discrepancy between enhanced apoptosis in luteal cells which signifies ageing of the corpus luteum in female rats [11] and absence of any significant effect on progesterone concentrations in ad libitum HFD treatment may be explained by the fact that steroidogenic cells of the corpus luteum which produce progesterone lose their capacity via mechanisms independent of those responsible for executing apoptosis and that structural and functional luteolysis might be separately regulated [12, 28].
In the current study, we observed that isocaloric HFD restriction partially but significantly ameliorated the enhanced ovarian apoptosis, and hypocaloric HFD restriction completely ameliorated this effect. On the other hand, isocaloric HFD restriction interfered with the normal estrous cycle and caused a significant decrease in serum progesterone and a significant increase in serum LH. The significant increase in LH concentrations in isocaloric HFD restriction may be explained by withdrawal of progesterone's negative feedback effect on LH secretion [29]. Also hypocaloric HFD restriction interfered with the normal estrous cycle and caused a significant decrease in serum progesterone only. This finding may be attributed to the presence of two antagonizing factors that controlled LH secretion in hypocalorically restricted HFD fed rats. The first one is withdrawal of progesterone's negative feedback effect, and this factor tends to increase LH secretion. The second factor is energy restriction, which tends to reduce LH secretion [30–32], so our hypothesis is that both effects blunted each other and resulted in no significant change in LH concentrations in hypocalorically restricted HFD fed rats.
In the present study we found a tendency of serum leptin to increase with ad libitum HFD, but this increase failed to reach a significant level. The results of leptin concentration changes with a HFD are conflicting. Some studies have found that leptin concentrations significantly increased with a HFD due to increased adiposity [3, 23]. In contrast, Ainslie et al. found that leptin level significantly decreased after a HFD protocol for 4 weeks, and they found no change in leptin concentrations after a HFD protocol for 14 weeks. The authors suggested that the decrease in leptin concentrations is the cause of weight gain occurring with the HFD [33]. The duration of the HFD protocol may be responsible for this different result, as Soulis et al. found that with longer HFD protocols in female rats, leptin levels were decreased [34]. In the current study we found no significant effect on leptin levels in HFD restriction. Shi et al. also found the same result in female mice with moderate food restriction [35]. On the other hand, some studies have found a significant decrease in serum leptin concentrations [7, 36]. In the present study, animal feeding and sampling were done in the morning. Schlitt and Schulz suggested that detection of the effects of feeding restriction on leptin concentrations is strongly dependent on the timing of both feeding and sampling and their interaction and that the circadian rhythms of leptin release result in a decline in leptin concentrations in the morning, leading to an inability to detect differences between the ad libitum and restricted groups during the morning [36]. According to the present results concerning leptin concentrations, it is difficult to relate serum leptin concentrations to reproductive effects of ad libitum and restricted HFD, and more work is needed to elucidate this relation.
In the current study, the HFD contained an increased ratio of saturated fatty acids and a decreased ratio of polyunsaturated fatty acids. This change in the composition of the dietary fats may be responsible for the observed reproductive effects, as many previous studies have reported [37–39].
Obesity has a constantly growing prevalence worldwide, and it represents one of the most important health risks of our time [40–42], so it is recommended that studies that deal with obesity effects on different aspects of health must be continued.
Our results suggest that HFD feeding determinately affects female rats’ reproductive function independently of caloric intake. Further studies are required to address the role of leptin, increased saturated fatty acid and decreased polyunsaturated fatty acid concentrations to clarify the mechanism of the caloric-independent effect of HFD on reproductive function.
In conclusion, the ad libitum HFD interfered with the normal estrous cycle and caused enhanced apoptosis of luteal cells in obese female rats. The HFD restriction interfered with the normal estrous cycle and caused functional insufficiency of the corpus luteum in obese female rats. These results suggest that HFD feeding determinately affects female reproductive function independently of caloric intake.
Acknowledgments
We thank Dr. Rasha Farghaly (School of Medicine, Suez Canal University, Egypt) for helping in statistical analyses. This work did not receive funds from any organizations.
Conflict of interest
The authors declare no conflict of interests.
References
- 1.Nteeba J, Ross JW, Perfield JW, 2nd, Keating AF. High fat diet induced obesity alters ovarian phosphatidylinositol-3 kinase signaling gene expression. Reprod Toxicol. 2013;42:68–77. doi: 10.1016/j.reprotox.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jungheim E, Travieso J, Hopeman M. Weighing the impact of obesity on female reproductive function and fertility. Nutr Rev. 2013;71(Suppl. 1):S3–8. doi: 10.1111/nure.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balasubramanian P, Jagannathan L, Subramanian M, Gilbreath E, MohanKumar P, MohanKumar S. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J Neuroendocrinol. 2012;24:748–55. doi: 10.1111/j.1365-2826.2011.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McShane TM, Wise PM. Life-long moderate caloric restriction prolongs reproductive life span in rats without interrupting estrous cyclicity: effects on the gonadotropin-releasing hormone/luteinizing hormone axis. Biology of Reproduction. 1996;54:70–5. doi: 10.1095/biolreprod54.1.70. [DOI] [PubMed] [Google Scholar]
- 5.DiMarco N, Dart L, Sanborn C. Modified activity-stress paradigm in an animal model of the female athlete triad. J Appl Physiol. 2007;103:1469–78. doi: 10.1152/japplphysiol.01137.2005. [DOI] [PubMed] [Google Scholar]
- 6.Lie ME, Overgaard A, Mikkelsen JD. Effect of a postnatal high-fat diet exposure on puberty onset, estrous cycle regularity, and kisspeptin expression in female rats. Reprod Biol. 2013;13:298–308. doi: 10.1016/j.repbio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Duivenvoorde L, van Schothorst E, Bunschoten A, Keijer J. Dietary restriction of mice on a high-fat diet induces substrate efficiency and improves metabolic health. JME. 2011;47:81–97. doi: 10.1530/JME-11-0001. [DOI] [PubMed] [Google Scholar]
- 8.Friedman J. The function of leptin in nutrition, weight, and physiology. Nutr Rev. 2002;60:S1–14. doi: 10.1301/002966402320634878. [DOI] [PubMed] [Google Scholar]
- 9.Roman EA, Ricci AG, Faletti AG. Leptin enhances ovulation and attenuates the effects produced by food restriction. Mol Cell Endocrinol. 2005;242:33–41. doi: 10.1016/j.mce.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Norambuena MC, Silva M, Urra F, et al. Effects of nutritional restriction on metabolic, endocrine and ovarian function in llamas (Lama glama) Anim Reprod Sci. 2013;138:252–60. doi: 10.1016/j.anireprosci.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 11.Slot K, Voorendt M, de Boer-Brouwer M, van Vugt H, Teerds K. Estrous cycle dependent changes in expression and distribution of Fas, Fas ligand, Bcl-2, Bax, and pro- and active caspase-3 in the rat ovary. J Endocrinol. 2006;188:179–92. doi: 10.1677/joe.1.06165. [DOI] [PubMed] [Google Scholar]
- 12.Carambula S, Matikainen T, Lynch M, et al. Caspase-3 is a pivotal mediator of apoptosis during regression of the ovarian corpus luteum. Endocrinology. 2002;1:1495–501. doi: 10.1210/endo.143.4.8726. [DOI] [PubMed] [Google Scholar]
- 13.Davis J, Rueda B. The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Frontiers in Bioscience. 2002;7:1949–78. doi: 10.2741/davis1. [DOI] [PubMed] [Google Scholar]
- 14.Boone D, Tsang B. Caspase-3 in the rat ovary: localization and possible role in follicular atresia and luteal regression. Biology of Reproduction. 1998;58:1533–9. doi: 10.1095/biolreprod58.6.1533. [DOI] [PubMed] [Google Scholar]
- 15.De Meijer V, Le H, Meisel J, et al. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism. 2010;59:1092–105. doi: 10.1016/j.metabol.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Z, Fan J, Ding X, Qiao L, Wang G. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig Dis Sci. 2010;55:931–40. doi: 10.1007/s10620-009-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang P, Li S, Shao M, et al. Calorie restriction and endurance exercise share potent anti-inflammatory function in adipose tissues in ameliorating diet-induced obesity and insulin resistance in mice. Nutr Metab (Lond) 2010;16:7–59. doi: 10.1186/1743-7075-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcodens FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62(4A):609–14. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 19.Dos Santos ZA, Da Silva RJ, Bacurau RF, Tirapegui J, Ribeiro SM. Effect of food restriction and intense physical training on estrous cyclicity and plasma leptin concentrations in rats. J Nutr Sci Vitaminol (Tokyo) 2011;57:1–8. doi: 10.3177/jnsv.57.1. [DOI] [PubMed] [Google Scholar]
- 20.Novelli E, Diniz Y, Galhardi C, et al. Anthropometrical parameters and markers of obesity in rats. Laboratory Animals. 2007;41:111–9. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- 21.Akamine EH, Marc AC, Camporez JP, et al. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol. 2010;206:65–74. doi: 10.1677/JOE-09-0461. [DOI] [PubMed] [Google Scholar]
- 22.Salakou S, Kardamakis D, Tsamandas AC, et al. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In Vivo. 2007;21:123–32. [PubMed] [Google Scholar]
- 23.Park S, Park NY, Valacchi G, Lim Y. Calorie restriction with a high-fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediators Inflamm. 2012;2012:984643. doi: 10.1155/2012/984643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asakawa A, Inui A, Kaga T, et al. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947–52. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meidenbauer JJ, Ta N, Seyfried TN. Influence of a ketogenic diet, fish-oil, and calorie restriction on plasma metabolites and lipids in C57BL/6J mice. Nutrition Metabolism. 2014;11:23. doi: 10.1186/1743-7075-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizunoya W, Iwamoto Y, Shirouchi B, et al. Dietary fat influences the expression of contractile and metabolic genes in rat skeletal muscle. PLOS ONE. 2013;8:e80152. doi: 10.1371/journal.pone.0080152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mensink RP, Katan MP. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb Vasc Biol. 1992;12:911–9. doi: 10.1161/01.atv.12.8.911. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira-Dias G, Mateus L, Costa AS, et al. Progesterone and caspase-3 activation in equine cyclic corpora lutea. Reprod Dom Anim. 2007;42:380–6. doi: 10.1111/j.1439-0531.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 29.Messinis IE. Ovarian feedback, mechanism of action and possible clinical implications. Human Reproduction Update. 2006;12:557–71. doi: 10.1093/humupd/dml020. [DOI] [PubMed] [Google Scholar]
- 30.Howland BE. Effect of restricted feed intake on LH levels in female rats. J Anim Sci. 1972;34:445–7. doi: 10.2527/jas1972.343445x. [DOI] [PubMed] [Google Scholar]
- 31.Recabarren SE, Lobos A, Torres V, Oyarzo R, Sir-Petermann T. Secretory patterns of leptin and luteinizing hormone in food-restricted young female sheep. Biol Res. 2004;37:371–84. doi: 10.4067/s0716-97602004000300003. [DOI] [PubMed] [Google Scholar]
- 32.Omayma KF. Effect of long term food-restriction (low-caloric diet) on pars distalis of the anterior pituitary gland of adult male albino rats: a biochemical and histological study. Egypt J Histol. 2010;33:236–44. [Google Scholar]
- 33.Ainslie DA, Proietto J, Fam BC, Thorburn AW. Short-term, high-fat diets lower circulating leptin concentrations in rats. Am J Clin Nutr. 2000;71:438–42. doi: 10.1093/ajcn/71.2.438. [DOI] [PubMed] [Google Scholar]
- 34.Soulis G, Kitraki E, Gerozissis K. Early neuroendocrine alterations in female rats following a diet moderately enriched in fat. Cell Mol Neurobiol. 2005;25:869–80. doi: 10.1007/s10571-005-4943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H, Strader AD, Woods SC, Seeley RJ. Sexually dimorphic responses to fat loss after caloric restriction or surgical lipectomy. Am J Physiol Endocrinol Metab. 2007;293:E316–26. doi: 10.1152/ajpendo.00710.2006. [DOI] [PubMed] [Google Scholar]
- 36.Schlitt JM, Schulz LC. The source of leptin, but not leptin depletion in response to food restriction, changes during early pregnancy in mice. Endocrine. 2012;41:227–35. doi: 10.1007/s12020-011-9548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams GL, Stanko RL. Dietary fats as reproductive nutraceuticals in beef cattle. J Anim Sci. 2000;77:1–12. [Google Scholar]
- 38.Zeron Y, Ocheretny A, Kedar O, Borochov A, Sklan D, Arav A. Seasonal changes in bovine fertility: relation to developmental competence of oocytes, membrane properties and fatty acid composition of follicles. Reproduction. 2001;121:447–54. [PubMed] [Google Scholar]
- 39.Herrera-Camacho J, Soberano-Martinez A, Duran KEO, Aguilar-Perez C, Ku-Vera JC. Milad Manafi., editor. Effect of fatty acids on reproductive performance of ruminants. Artificial insemination in farm animals. 2011. ISBN: 978-953-307-312-5, InTech, Available from: http://www.intechopen.com/books/artificial-insemination-in-farm-animals/effect-of-fatty-acids-on-reproductive-performance-of-ruminants.
- 40.Nikolic D, Cvjeticanin S, Petronic I, et al. Population genetic analyses of susceptibility to increased body weight. Arch Med Sci. 2012;8:998–1002. doi: 10.5114/aoms.2012.32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Januszek-Trzciąkowska A, Małecka-Tendera E, Klimek K, Matusik P. Obesity risk factors in a representative group of Polish prepubertal children. Arch Med Sci. 2014;10:880–5. doi: 10.5114/aoms.2013.33328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kocełak P, Żak-Gołąb A, Rzemieniuk A, et al. The influence of oral water load on energy expenditure and sympatho-vagal balance in obese and normal weight women. Arch Med Sci. 2012;8:1003–8. doi: 10.5114/aoms.2012.32406. [DOI] [PMC free article] [PubMed] [Google Scholar]