Abstract
Pathogens inflict a wide variety of disease manifestations on their hosts, yet the impacts of disease on the behaviour of infected hosts are rarely studied empirically and are seldom accounted for in mathematical models of transmission dynamics. We explored the potential impacts of one of the most common disease manifestations, fever, on a key determinant of pathogen transmission, host mobility, in residents of the Amazonian city of Iquitos, Peru. We did so by comparing two groups of febrile individuals (dengue-positive and dengue-negative) with an afebrile control group. A retrospective, semi-structured interview allowed us to quantify multiple aspects of mobility during the two-week period preceding each interview. We fitted nested models of each aspect of mobility to data from interviews and compared models using likelihood ratio tests to determine whether there were statistically distinguishable differences in mobility attributable to fever or its aetiology. Compared with afebrile individuals, febrile study participants spent more time at home, visited fewer locations, and, in some cases, visited locations closer to home and spent less time at certain types of locations. These multifaceted impacts are consistent with the possibility that disease-mediated changes in host mobility generate dynamic and complex changes in host contact network structure.
Keywords: activity space, contact, dengue, infection, movement, network
1. Background
It is well known that pathogens are capable of effecting a wide range of disease manifestations in their hosts. Disease manifestations can significantly alter behaviour in human and non-human animal hosts [1], leading to important consequences for the ecology of both host [2] and pathogen [3] populations. They can also mediate behavioural changes in susceptible, uninfected hosts seeking to avoid infection and illness [4]. Such behavioural responses by susceptible hosts have been recently shown to exert important dynamic feedbacks on the course of epidemics [4–6].
One effect of many disease manifestations on host behaviour is modification of host mobility; e.g. changes in home range, distance and speed of movement, or locations visited. Although pathogens such as Toxoplasma gondii have been shown to greatly increase host mobility in rodents [7], it is likely that disease manifestations more typically lead to an overall reduction in mobility—anyone who has ever stayed at home from work or school owing to illness is a case in point. Such effects have potentially broad significance throughout epidemiology, ecology and evolution. Hypothesized consequences of disease-mediated reductions in mobility range from shifts in the seasonality of avian influenza outbreaks in waterfowl [8] to the evolution of virulence attenuation in directly transmitted pathogens when compared with vector-borne pathogens, such as Plasmodium spp. [9]. More generally, how disease impacts host mobility and contact has been proposed as a key determinant of how within-host infection dynamics affect population-level transmission dynamics [10].
Despite the expected ubiquity of disease impacts on mobility and their documentation in animal systems [11], they have seldom been measured empirically in humans. One of the few studies of this phenomenon in humans was based on self-completed written questionnaires administered by mail to 179 Britons diagnosed with swine flu [12]. That study, which focused on the measurement of contacts suitable for influenza transmission, showed that illness reduced contacts by approximately 50%. A follow-up study [13] estimated that reductions in contact owing to the impacts of disease on behaviour caused a 71% reduction in the pathogen's basic reproductive number, R0, compared with what it would have been had sick people engaged in as many contacts as healthy people. Thus, it is clear that the pervasive effects of disease manifestations on host behaviour could have important epidemiological consequences, yet these effects are almost completely absent from transmission models of infectious diseases of humans (although see [14]).
Quantifying the impacts of illness on direct contacts [12,13] was a significant advance for understanding the epidemiology of influenza and other directly transmitted pathogens. Nonetheless, there remain a number of gaps in knowledge about the impacts of illness on contact that merit additional study. First, for indirectly transmitted pathogens and for exposure to environmentally mediated agents of disease, a highly relevant concept is that of the activity space [15,16], which is a description of a person's time allocation across the set of all locations that she or he frequents [17], similar to the concept of a utilization distribution in the animal movement literature [18]. Establishing that illness reduces direct contacts does not address how illness might affect time allocation at key locations for exposure to environmental contaminants or biting by insect vectors. Second, a gap that could not possibly be filled by the aforementioned study [12] alone is that it pertained to a single geographical and cultural context, leaving open the possibility of different behavioural consequences of illness under different circumstances. People in resource-poor, tropical environments, for example, are known to have comparatively complex patterns of intra-urban mobility, which has important consequences for transmission dynamics [19]. Third, the nature and severity of disease manifestations vary considerably across different diseases and could, therefore, impact behaviour, mobility and contact in different ways.
To begin to address these gaps in the understanding of disease impacts on mobility, we used retrospective, semi-structured interviews of 926 participants in a longitudinal study of dengue epidemiology in the Amazonian city of Iquitos, Peru. Because these data came from a longitudinal study of dengue, a disease caused by viruses transmitted by day-biting mosquitoes, interviews were designed to characterize individuals' activity spaces rather than their contacts with other people [20,21]. Symptoms associated with dengue are classically described as acute fever, headache, musculoskeletal pain and rash [22,23]. For analytical tractability, however, we restricted our analysis to a binary classification of study participants as either febrile or afebrile based on a threshold body temperature of 38°C. Nonetheless, there is extensive variation in the severity of fever and other symptoms of dengue, which allowed disease to impact multiple features of the measured activity spaces of our study participants. Additionally, while some febrile participants had laboratory confirmed dengue virus infections (DENV+), others did not (DENV−) and were instead probably infected with some other pathogen, such as influenza or a bacterial agent. This allowed us to explore the possibility that impacts of fever on mobility depend on the fever's aetiology.
2. Material and methods
(a). Overview
Our primary goal was to identify aspects of human mobility for which there are clear and unambiguous effects of fever. To do so, we used interview data collected from residents of Iquitos, Peru (salient characteristics of which are described in the electronic supplementary material, Methods). Interview data were stratified by demographic status, fever status and fever aetiology to perform separate comparisons among these strata for each aspect of mobility that we considered. We performed comparisons under a hypothesis-testing framework, e.g. is a given aspect of mobility perceptibly different between febrile and afebrile people? We prioritized the identification of effects over detailed quantification of effect sizes or probabilistic statements about what level of detail best explains the data. Although effect sizes or probabilistic statements could be insightful, we restricted our analysis to a series of hypothesis tests because of strengths and limitations of our interview data and because of the need to establish basic knowledge about the impacts of fever on human mobility.
(b). Mobility data collection and processing
We collected data on intra-urban mobility by characterizing study participants' activity spaces with retrospective, semi-structured movement interviews (SSIs). The SSI was designed in accordance with focus group discussions with local residents [20], validated against comparable data collected using global positioning system data-loggers [21] and used to parametrize models of intra-urban human mobility [24]. A detailed description of the SSI is available in the electronic supplementary material, Methods.
At its conclusion, each interview yielded a table describing the activity space of a study participant in the two-week period prior to the interview. Each row of this table corresponded to a location visited by the study participant, and respective columns of this table contained a unique identifier for each location, its latitude, its longitude, its designation as belonging to one of the eight aforementioned land-use types, the frequency per day of visits to the location by the study participant and the average duration of each visit to the location by the study participant. These tables were then used to derive tables that aggregated specific features of the activity spaces of all study participants, including: (i) a table containing the total number of locations visited by each study participant; (ii) eight tables containing the number of locations of each type visited by each study participant; (iii) eight tables containing the geographical coordinates of each location of a given type that was visited by a given study participant, as well as, the geographical coordinates of that study participant's home; (iv) nine tables containing the frequency per day of visits to each study participant's home and to locations of each type visited by each study participant; and (v) nine tables containing the average duration of visits to each study participant's home and to locations of each type visited by each study participant.
(c). Study participant enrollment and classification
Participants in our study were enrolled as part of a longitudinal, cluster-based investigation of dengue epidemiology. In total, that study involved 2444 participants aged 5 years or older living in two intensively studied neighbourhoods, Maynas and Tupac Amaru (electronic supplementary material, figure S1). This longitudinal cohort study has been described in full detail by Stoddard et al. [25]. Salient details of that study include that febrile participants were identified by members of the field team who actively monitored for fever (defined as body temperature ≥38°C) in each house, which was visited at least three times per week during the study period. Blood was drawn upon detection of fever (acute sample) and approximately 15 days later (convalescent sample), and, for participants who were willing, an SSI was performed within 24 h of detection of fever in 92% of cases. In addition, residents of other houses visited by febrile participants were recruited into the study and asked to participate in an SSI upon initiation, rather than requiring detection of fever. We designated study participants who were interviewed without a detectable fever as ‘afebrile’ and those who participated in an SSI at the time at which fever was detected as ‘febrile’.
In addition to being febrile or afebrile, we further classified study participants according to two dimensions (table 1). First, febrile participants were classified as either having an active dengue infection at the time of the SSI (DENV+), or having a fever for presumably some other reason (DENV−). This determination was made by testing acute and convalescent blood samples for dengue-specific IgM antibodies by antibody-capture ELISA and by testing acute samples for dengue viruses by real-time polymerase chain reaction [26]. Second, we categorized study participants according to four demographic classifications that we presume account for a large portion of inter-individual variation in mobility. Classifications included: school-age children aged 5–17 (whose mobility is probably dominated by school, home and neighbours' houses), college students aged more than or equal to 18 (whose mobility is probably dominated by attending college but may have more variability than children), homemakers and unemployed adults (who presumably spend more time at home than other adults) and adults aged more than or equal to 18 who work outside the home (who probably spend a great deal of time outside the home at diverse locations).
Table 1.
Numbers of study participants who provided information about time spent at home or time spent at locations other than home, stratified by fever status and demographic category.
| school-age children | college student | homemaker/unemployed adult | working adult | |
|---|---|---|---|---|
| time at home | ||||
| afebrile | 34 | 14 | 36 | 44 |
| febrile, DENV+ | 91 | 15 | 24 | 41 |
| febrile, DENV– | 214 | 53 | 94 | 116 |
| time elsewhere | ||||
| afebrile | 43 | 25 | 52 | 53 |
| febrile, DENV+ | 143 | 25 | 32 | 59 |
| febrile, DENV– | 241 | 66 | 121 | 128 |
The high degree of informality in the Iquitos labour market [19] precluded further refinement of the working adult population, because a large proportion of the population has more than one job, leading to over 150 self-reported occupations among our study participants. Although impacts of fever on mobility were our primary interest, accounting for other sources of variation in mobility was important, because it reduced the potential for confounding, e.g. there were relatively more school-age children among febrile participants than there were among afebrile participants (table 1). Had we not disaggregated study participants according to these demographic categories, differences in movement attributable solely to demographic differences could have been mistakenly attributed to differences associated with fever or its aetiology.
(d). Models and statistical analyses
For each aspect of mobility encoded in the aforementioned tables, we used models with the same level of detail and parametric forms used in the analysis by Perkins et al. [24], with some minor modifications. For the number of locations visited, we assumed a negative binomial distribution for the total number of locations of all types, but a Poisson distribution for the number of locations of each type. We modelled the probability of choosing a location of a given distance from home d as the product of the number of locations at distance d weighted by an exponential function exp(−μ d) with a unique parameter μ for each location type. For the frequency and duration of periods of time at home and at locations of each type, we assumed separate bivariate normal distributions on a log-log scale with non-zero correlations, and we assumed that distance from home had no effect on the frequency or duration of visits.
To test hypotheses about differences in mobility attributable to fever, its aetiology, and demographic category, we fitted models for each aspect of mobility with study participants agglomerated in different ways. For example, in the simplest model, a given aspect of mobility for all study participants was assumed to be described by a single set of parameters. By contrast, in the most complex model, a given aspect of mobility was described by distinct parameter sets for each of 12 groups: four demographic categories by three fever statuses (i.e. febrile and DENV+, febrile and DENV−, afebrile). With respect to fever status, we considered models denoted F3 (mobility of each group explained by distinct parameters), F2 (mobility of individuals with no fever distinct from those with any fever) and F1 (mobility indistinguishable with respect to fever status). With respect to demographic category, we considered models denoted C4 (mobility of individuals in each group explained by distinct parameters) and C1 (mobility of individuals indistinguishable with respect to demographic category). The letter in each of these codes denotes the factor (i.e. F = febrile status, C = demographic category), whereas the numeral denotes the number of distinct subgroups considered in the corresponding model, e.g. 2 = each individual fell into one of two possible categories. Combining models of fever status and demographic category yielded the following set of models describing both: F3,C4; F2,C4; F1,C4; F3,C1; and F2,C1.
To compare these models, and thereby to test hypotheses about the influence of fever status and demographic category on each aspect of mobility, we performed a series of pairwise likelihood ratio tests for each aspect of mobility. Likelihood ratio tests are applicable to nested models and quantify the probability that the observed deviance between two models is due to chance [27]. For example, F1,C4 is nested within F2,C4, because the former is a special case of the latter, but with some of the parameters constrained to be equal. For each aspect of mobility, we fitted all candidate models by likelihood maximization and performed a likelihood ratio test for each pair of nested models in the R language [28]. Given 12 pairs of nested models for each aspect of mobility, we addressed each hypothesis by considering the results of those statistical tests on the whole rather than by parsing each statistical test as corresponding to a meaningful and distinct hypothesis. For example, if a difference in mobility attributable to fever was detected when all participants were pooled, but not when they were disaggregated by demographic category (e.g. F2,C1 > F1,C1 but F2,C4 = F1,C4), then our overall conclusion was that there was no difference in mobility attributable to fever because the difference that was detected could be attributable solely to demographic differences. Furthermore, because we performed 12 separate tests for each aspect of mobility, we regarded support for significance between two models as strongest if the p-value of a test was less than α = 0.05/12 = 4.17 × 10−3, which was obtained by applying a Bonferroni correction [29]. Although our analysis could have been performed under alternative frameworks, such as information theoretic or Bayesian approaches, our choice of a series of nested likelihood ratio tests was appropriate given our hypothesis testing goals and the nested relationships among our models [30].
3. Results
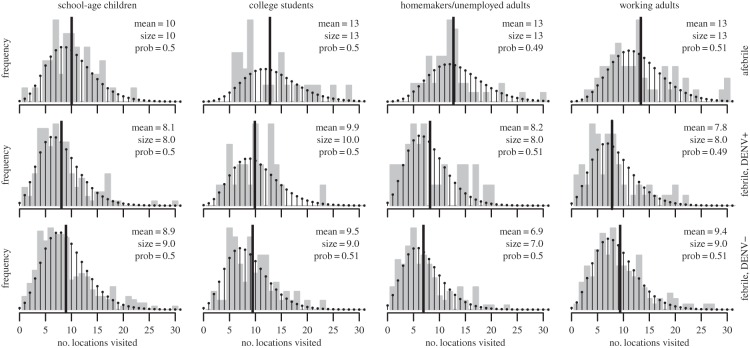
Fever had consistent and discernable effects on all aspects of mobility that we examined (summarized in table 2). For the total number of locations that people visited, febrile study participants visited 30% fewer locations on average than afebrile participants (figure 1), with all pairwise tests of models with F3 or F2 against models with F1 having p < 10−13 (electronic supplementary material, table S1). This difference could be owing to febrile participants visiting fewer locations of many types, but given the available data and the statistical approach that we used, commercial locations were the only location type for which there were consistent reductions across all demographic categories (electronic supplementary material, figure S5). All pairwise tests of models with F3 or F2 against F1 had p < 10−10 (electronic supplementary material, table S3). This effect was weaker for school-age children, but for other demographic categories, febrile participants visited at least one fewer commercial location on average (electronic supplementary material, figure S5).
Table 2.
Summary of differences between febrile and afebrile study participants with respect to different aspects of mobility.
| aspect of mobility | impact of fever | tables and figures |
|---|---|---|
| total number of locations visited | febrile study participants from all demographic categories consistently visited fewer locations | electronic supplementary material, table S1; figure 1 |
| types of locations visited | febrile study participants from all demographic categories consistently visited fewer commercial locations. Consistent differences between the numbers of locations of all other types that febrile and afebrile participants visited were not as perceptible | electronic supplementary material, tables S2–S9 and figures S4–S11 |
| distance from home of locations visited | on the whole, there were significant differences between the distances from home to locations visited by febrile and afebrile study participants, particularly when accounting for demographic category. Notable differences included that homemakers with dengue fever visited residential and commercial locations closer to home, and febrile children and college students visited recreational locations closer to home | electronic supplementary material, table S10 and figures S12 and S13 |
| time spent at home | febrile study participants from all demographic categories consistently spent more time at home overall, which resulted from coming and going from home less frequently and from staying there longer each time they were at home | electronic supplementary material, table S12; figure 2 |
| time spent per location at locations other than home | the most perceptible difference in time spent per location at locations other than home was that febrile school-aged children spent less time at educational locations. This difference appeared to be driven more by a lower frequency of visits than by a shorter duration | electronic supplementary material, tables S12–S19 and figures S14–S21 |
Figure 1.
Number of locations visited by study participants, stratified by participant fever status (rows) and demographic category (columns). Grey bars show the empirical distribution, the black line shows the empirical mean (numerical value printed in each panel), and the balls and stems show the fitted negative binomial distribution with maximum-likelihood estimates of parameter values (size and prob as in dnbinom function in R, values indicated in each panel).
There were also differences in the tendencies for febrile and afebrile participants to visit locations of varying distances from home. After removing the effect of the distribution of locations within a given distance of a person's home, parameters describing the strength of one's preference for visiting locations closer to home differed significantly between febrile and afebrile participants and between those who were DENV+ and DENV−, with all pairwise tests of models with F3 against F2 or F1 having p < 10−4 (electronic supplementary material, table S10). We could not identify systematic differences (e.g. we could not conclude that febrile participants consistently exhibited a stronger preference for visiting locations closer to home), but instead detected wholesale differences between multiple sets of eight location-type-specific parameters (electronic supplementary material, figure S12). Although not tested statistically on an individual basis, effects that appeared particularly notable and that seemed logical included that febrile, DENV+ adults who do not work outside the home tended to restrict their movements to residential and commercial locations closer to home than their afebrile counterparts did, and school-age children and college students visited recreational locations closer to home when febrile (electronic supplementary material, figures S12 and S13).
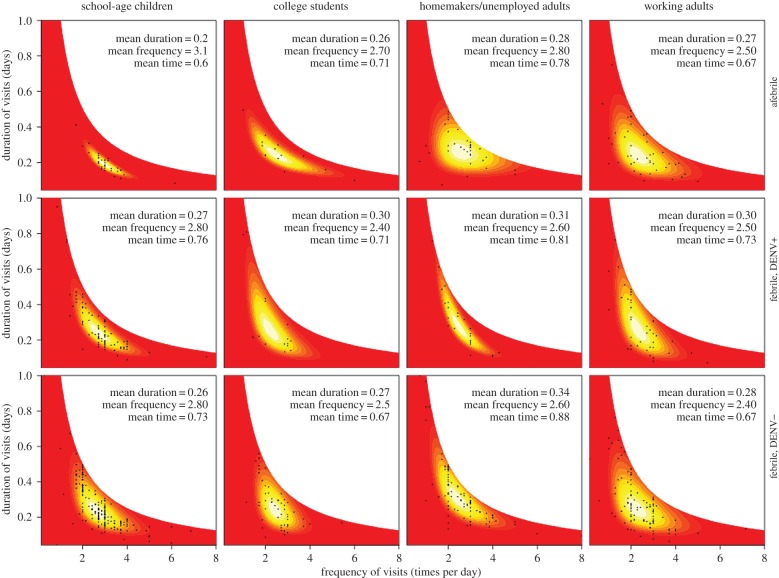
Febrile study participants generally spent more time at home than afebrile participants, although the extent of this effect varied across demographic categories (figure 2). Febrile school-age children spent 26.6% more time at home than their afebrile counterparts when DENV+ and 21.6% more when DENV−. Among adults, those who do not work outside the home exhibited the greatest increase in time spent at home due to fever (DENV+: 3.8% more than afebrile, DENV−: 12.8% more than afebrile), whereas differences in time spent at home between febrile and afebrile college students and adults working outside the home were less consistent (figure 2). A pairwise test of F2,C1 and F1,C1 models was statistically significant (p = 0.003), as were tests of F2,C4 against F1,C4 (p < 10−12) and tests comparing models with F3 against those with F2 (p < 10−3) (electronic supplementary material, table S11). The only location type besides home for which there was a significant difference in time spent per location was the educational location type. Febrile school-age children spent less time at each educational location they visited than afebrile children did, primarily due to a difference in the frequency of visits rather than the duration of visits (electronic supplementary material, figure S17). Pairwise tests of models with differences in both fever status and demographic category were statistically significant with p < 0.01 (electronic supplementary material, table S15).
Figure 2.
Joint probability distributions of the frequency and mean duration of periods of time spent at home. Colours represent continuous probabilities that range low (red), medium (orange), and high (bright yellow and white). Parameter values of each distribution were fitted by likelihood maximization to data from individuals from each of four groups (children, students, homemakers, others) using interviews conducted when those individuals either had no fever, dengue fever, or fever due to some other cause. Sample means of duration, frequency and proportion of total time spent at home for each group are noted in each panel.
Beyond differences attributable to fever on the whole, there were no clear differences in any aspect of mobility between febrile study participants with differing aetiologies. The majority of pairwise comparisons of models with F3 against those with F2 were not significant (electronic supplementary material, tables S1–S19), and those that were significant tended to support differences that were relatively weak or equivocal compared with differences attributable to fever on the whole (figures 1 and 2, electronic supplementary material, S12). For example, although there were statistically significant differences in the tendency to visit locations closer to home among afebrile, DENV+ febrile, and DENV− febrile study participants (p < 10−3; electronic supplementary material, table S10), there were no clear, systematic differences across all location types (electronic supplementary material, figures S12 and S13).
Irrespective of fever status, there were substantial differences in mobility among participants from different demographic categories. School-age children tended to visit fewer unique locations overall (figure 1), spend more time at home (figure 2), visit locations closer to home (electronic supplementary material, figures S12 and S13), visit more residential locations (electronic supplementary material, figure S4) and spend more time at each residential (electronic supplementary material, figure S14) and educational location that they visited (electronic supplementary material, figure S17) than participants from the other demographic categories. College students also spent more time at educational locations that they visited (electronic supplementary material, figure S17), but were otherwise similar to other adults. Adults who did not work outside the home were distinguished by spending more time at home than participants from other categories (figure 2).
There also appeared to be a select few interactions between the effects of fever and demographic category on mobility. School-age children tended to visit approximately the same number of commercial locations (2.5–2.7) regardless of their febrile status, whereas febrile participants from other demographic categories consistently visited on average one fewer commercial location when febrile (electronic supplementary material, figure S5 and table S3). Differences between participants' overall preferences for visiting locations closer to home also depended on demographic category, as stated earlier (electronic supplementary material, figure S12 and table S10). Time spent at home by college students did not appear to depend on fever status, whereas febrile participants from other demographic categories spent significantly more time at home than their afebrile counterparts (figure 2).
4. Discussion
We used retrospective, semi-structured interviews to assess a very basic, but surprisingly neglected, question about host–pathogen interaction in humans: what is the impact of fever on an infected person's mobility? Our results revealed differences in all aspects of mobility between study participants who were febrile during the time they were interviewed and those who were afebrile. Reductions in the number of locations visited and time spent outside the home were among the most unambiguous differences between febrile and afebrile participants, regardless of their demographic category. These and other effects, such as visiting locations closer to home and changes in the types of locations visited, are logically consistent with one another and with previous reports from urban settings in developed countries [12,13]. These results are also consistent with the concept of ‘sickness behaviours’ in the animal behaviour literature, where impaired mobility and other effects are better studied and are thought to have important adaptive consequences [11,31]. Together, our results suggest that fever significantly impairs human mobility and, by extension, could modulate the potential for infectious people with clinically apparent infections to engage in contacts and contribute to onward transmission.
The effects that we observed pertained to a two-week period prior to presentation of fever, which exceeds the duration of fever for people infected with dengue, influenza and many other pathogens [32,33]. Thus, it is most likely that participants in our study experienced fever for only a few days prior to their interviews. If so, it would imply that impacts of fever on mobility during the time when study participants were actually febrile may have been stronger than the effects that we were able to measure. For example, rather than spending 26.6% more time at home (figure 2), this would suggest that DENV+ febrile children may have spent nearly all of their time at home on days when they experienced fever. Similarly, some effects could have gone undetected altogether. For example, it is noteworthy that we detected no increase in visits to healthcare locations among febrile individuals. This is not altogether surprising, however, given the common occurrence of relatively mild disease associated with dengue infection [34]. This result could also be an artefact of our study design, as some study participants may have waited for a visit from a project physician rather than seeking healthcare at a local clinic or hospital. For these and similar reasons, our quantitative results should be interpreted as conservative underestimates of the impacts of fever on mobility.
Although our results are of limited value quantitatively, they provide an important starting point for determining the extent to which the impacts of disease manifestations on mobility are perceptible with interviews, one of several common instruments for measuring detailed, individual-level mobility [35,36]. One limitation of the retrospective SSI that we used is that it relied on study participant recall, which is subject to recall bias. Even so, in a previous study in Iquitos [21], we compared data obtained from the SSI with comparable data obtained from wearable GPS data-loggers and found that, overall, the SSI was a superior means of measuring activity spaces due to behavioural limitations associated with GPS methods; e.g. forgetting to wear the GPS data-logger. With regard to this study, it is possible that recall bias may have impaired our ability to detect the full extent of the true effect of fever on mobility, in which case our estimates of the impact of fever on mobility are conservative. This could be so if individuals who have visited more locations (i.e. afebrile individuals) are less likely to recall all the locations they have visited compared with those who have visited fewer locations; i.e. febrile individuals. Addressing this and other open questions—such as the impact of other disease manifestations on mobility or how suppression of fever with antipyretics affects mobility during key times in the course of a person's infectiousness—was beyond the scope of our study, but constitutes an important direction for future studies designed with similar objectives in mind.
Future studies should examine behavioural modification owing to fever across the period of time that a person is infectious. For dengue, infectiousness often lasts longer than fever. In experimental feeding studies, infection of mosquitoes was documented up to 2 days before [37] and 2 days after [38] the period of fever, which typically lasts between 2 and 7 days [32]. In a more recent study [39], at a given viremia level, dengue virus infected people with no detectable symptoms or whose symptoms had not yet begun were more infectious to mosquitoes than people who were bitten when they were symptomatic. Fully addressing how fever and other disease symptoms affect an individual's contribution to transmission will require combining data on human mobility with data on infectiousness at multiple time points over the course of infection [10,40].
Pathogen natural history, particularly mode of transmission, will also affect how impacts of fever on mobility influence impacts fever on contact and opportunities for pathogen transmission. For dengue and other vector-borne diseases, the impact of spending more time at home and visiting fewer locations on vector–human contact will depend on the densities of competent vectors at those locations [15]. If there are more vectors at a person's home than at the locations where they spend less time due to fever, then they could effectively be engaging in more mosquito contacts than if fever had not reduced their mobility. Conversely, if there are fewer vectors at home than elsewhere in a person's activity space, the converse would be true. Densities of the DENV mosquito vector Aedes aegypti in Iquitos are sufficiently heterogeneous in time and space that both of these possibilities probably happen [38]. For directly transmitted pathogens, spending more time at home and visiting fewer locations should result in fewer opportunities for transmission, on average [13]. Other effects of fever could counteract this tendency, including increased visitation by members of a sick person's family or social network [6]. Furthermore, because the impacts of fever on an infected person's mobility are inherently dynamic and, as we have shown, multifaceted, they are likely to generate dynamic and complex changes in contact network structure over time. For both directly and indirectly transmitted pathogens, mathematical modelling will play an essential role in future studies seeking to elucidate how disease-mediated changes in the mobility of infected hosts affect the dynamic nature of contact networks and pathogen transmission.
Accurately quantifying a realistic balance between the various effects of fever on contact and the relationship between fever and infectiousness is important for applications of population-level models to forecast future dynamics, infer information about key parameters from historical dynamics, and assess intervention impacts [5,41]. In addition to disease-mediated changes in contact rates within a population, it is also likely that fever and other disease manifestations could modify mobility and contact between humans and mosquitoes. Spatial models used for forecasting the spread of pandemics [42] and for estimating sources and sinks of transmission [43] routinely use movement data from flight records, call data records and other sources, or a continuum of models derived from physical principles [44], such as gravity [45] and radiation [46] models. These approaches rely on data or assumptions about healthy individuals and do not consider how disease manifestations might impact mobility and, consequently, spatial transmission dynamics. Based on effects that we detected—such as a tendency for febrile people to visit fewer locations and to spend more time at home—spatial models ignoring these effects could overestimate the potential for a disease to become pandemic or overemphasize the importance of mobility in identifying sources and sinks of transmission. Better understanding the impacts of disease manifestations on mobility during times when hosts are infectious merits increased attention in infectious disease epidemiology.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the residents of Iquitos for their support and participation in this study. We thank our Peruvian field team (Helvio Astete Vega, Wilder Carrasco Huaman, Ester Jennifer Rios Lopez, Shirley Maribel Guedez Gonzales, Wendy Lorena Quiroz Flores) and data management personnel (Jimmy Espinosa Benavides, John Ramirez, Angelo Mitidieri, Rommel Vasquez Alves, Diana Bazan Ferrando, Gabriela Vasquez La Torre, Alan Lozano). We also thank Elizabeth Archie, Shweta Bansal, and Bobby Reiner for thoughtful discussions about this work.
Ethics
The procedures for enrollment of participants, dengue diagnosis, semi-structured interviews and participant follow-up were approved by the Institutional Review Boards (IRB) of the University of California at Davis (2007.15244), Emory University (IRB9162) and Tulane University through an inter-institutional IRB agreement with the United States Naval Medical Research Center Unit No. 6 (NAMRU-6). The study protocol (NMRCD 2007.0007) was reviewed and approved by the NAMRU-6 IRB, which includes Peruvian (host country) IRB representation, and the Loreto Regional Health Department, which oversees health research in Iquitos. Study participants provided written consent.
Data accessibility
All data used in this study are available on Dryad (http://dx.doi.org/10.5061/dryad.rd66f) with the exception of study participant age, sex, interview date and spatial coordinates of home locations and locations visited, owing to the personal nature of that information. Data, as well as code for fully reproducing the analyses, are also available on GitHub (https://github.com/TAlexPerkins/fever_mobility).
Authors' contributions
T.A.P., V.A.P.-S., S.T.S. and G.M.V.-P. conceived of the study; V.A.P.-S., S.T.S., A.C.M., B.M.F., K.C.L., E.S.H., T.J.K., J.P.E., U.K., T.W.S., and G.M.V.-P. designed field studies and carried out data collection; T.A.P. performed the statistical analyses and made the tables and figures; T.A.P., V.A.P.-S. and G.M.V.-P. wrote the manuscript. All authors provided substantial feedback on revisions of the manuscript and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by grants from the US National Institutes of Health-National Institute of Allergy and Infectious Diseases (NIH/NIAID) awards R01 AI069341-01 and 1P01AI098670-01A1 (to T.W.S.) and by the Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directory, Department of Homeland Security, and Fogarty International Center, National Institutes of Health. Authors E.S.H. and T.J.K. were US Military service members. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a US Government work as a work prepared by military service members or employees of the US Government as part of those persons' official duties.
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense nor the US Government.
References
- 1.Adamo SA. 2012. The strings of the puppet master: how parasites change host behaviour. In Host manipulation by parasites (eds Hughes DP, Brodeur J, Thomas F), pp. 36–51. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Sargent LW, Baldridge AK, Vega-Ross M, Towle KM, Lodge DM. 2014. A trematode parasite alters growth, feeding behavior, and demographic success of invasive rusty crayfish (Orconectes rusticus). Oecologia 175, 947–958. ( 10.1007/s00442-014-2939-1) [DOI] [PubMed] [Google Scholar]
- 3.Pfennig KS. 2000. Evolution of pathogen virulence: the role of variation in host phenotype. Proc. R. Soc. Lond. B 268, 755–760. ( 10.1098/rspb.2000.1582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funk S, Salathe M, Jansen VAA. 2010. Modelling the influence of human behavior on the spread of infectious diseases: a review. J. R. Soc. Interface 7, 1247–1256. ( 10.1098/rsif.2010.0142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fenichel EP, et al. 2011. Adaptive human behavior in epidemiological models. Proc. Natl Acad. Sci. USA 108, 6306–6311. ( 10.1073/pnas.1011250108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manfredi P, D'Onofrio A. 2013. Modeling the interplay between human behavior and the spread of infectious diseases. Berlin, Germany: Springer. [Google Scholar]
- 7.Worth AR, Andrew Thompson RC, Lymbery AJ. 2014. Reevaluating the evidence for Toxoplasma gondii-induced behavioral changes in rodents. Adv. Parasitol. 85, 109–142. ( 10.1016/B978-0-12-800182-0.00003-9) [DOI] [PubMed] [Google Scholar]
- 8.Galsworthy SJ, ten Bosch QA, Hoye BJ, Heesterbeek JAP, Klaassen M, Klinkenberg D. 2011. Effects of infection-induced migration delays on the epidemiology of avian influenza in wild mallard populations. PLoS ONE 6, e26118 ( 10.1371/journal.pone.0026118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ewald PW. 1994. Evolution of infectious disease. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Handel A, Rohani P. 2015. Crossing the scale from within-host infection dynamics to between-host transmission fitness: a discussion of current assumptions and knowledge. Phil. Trans. R. Soc. B 370, 20140302 ( 10.1098/rstb.2014.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghai RR, Fugere V, Chapman CA, Goldberg TL, Davies TJ. 2015. Sickness behaviour associated with non-lethal infections in wild primates. Proc. R. Soc. B 282, 20151436 ( 10.1098/rspb.2015.1436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eames KT, Tilston NL, White PJ, Adams E, Edmunds WJ. 2010. The impact of illness and the impact of school closure on social contact patterns. Health Technol. Assess. 14, 267–312. ( 10.3310/hta14340-04) [DOI] [PubMed] [Google Scholar]
- 13.Van Kerckhove K, Hens N, Edmunds WJ, Eames KT. 2013. The impact of illness on social networks: implications for transmission and control of influenza. Am. J. Epidemiol. 178, 1655–1662. ( 10.1093/aje/kwt196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balcan D, Goncalves B, Hu H, Ramasco JJ, Colizza V, Vespignani A. 2010. Modeling the spatial spread of infectious diseases: the global epidemic and mobility computational model. J. Comput. Sci. 1, 132–145. ( 10.1016/j.jocs.2010.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoddard ST, Morrison AC, Vazquez-Prokopec GM, Paz-Soldan VA, Kochel TJ, Kitron U, Elder JP, Scott TW. 2009. The role of human movement in the transmission of vector-borne pathogens. PLoS Neglected Trop. Dis. 3, e481 ( 10.1371/journal.pntd.0000481) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perchoux C, Chaix B, Cummins S, Kestens Y. 2013. Conceptualization and measurement of environmental exposure in epidemiology: accounting for activity space related to daily mobility. Health and Place 21, 86–93. ( 10.1016/j.healthplace.2013.01.005) [DOI] [PubMed] [Google Scholar]
- 17.Horton FE, Reynolds DR. 1971. Effects of urban spatial structure on individual behavior. Econ. Geogr. 47, 36–48. ( 10.2307/143224) [DOI] [Google Scholar]
- 18.Worton BJ. 1989. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70:164–168. ( 10.2307/1938423) [DOI] [Google Scholar]
- 19.Vazquez-Prokopec G, et al. 2013. Using GPS technology to quantify human mobility, dynamic contacts and infectious disease dynamics in a resource-poor urban environment. PLoS ONE 8, e58802 ( 10.1371/journal.pone.0058802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paz-Soldan VA, Stoddard ST, Vazquez-Prokopec GM, Morrison AC, Elder JP, Kitron U, Kochel TJ, Scott TW. 2010. Assessing and maximizing the acceptability of global positioning system device use for studying the role of human movement in dengue virus transmission in Iquitos, Peru. Am. J. Trop. Med. Hyg. 82, 723–730. ( 10.4269/ajtmh.2010.09-0496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paz-Soldan VA, et al. 2014. Strengths and weaknesses of global positioning system (GPS) data-loggers and semi-structured interviews for capturing fine-scale human mobility: findings from Iquitos, Peru. PLoS Neglected Trop. Dis. 8, e2888 ( 10.1371/journal.pntd.0002888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalayanarooj SD, et al. 1997. Early clinical and laboratory indicators of acute dengue illness. J. Infect. Dis. 176, 313–321. ( 10.1086/514047) [DOI] [PubMed] [Google Scholar]
- 23.Libraty DH, et al. 2002. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 185, 1213–1221. ( 10.1086/340365) [DOI] [PubMed] [Google Scholar]
- 24.Perkins TA, et al. 2014. Theory and data for simulating fine-scale human movement in an urban environment. J. R. Soc. Interface 11, 20140642 ( 10.1098/rsif.2014.0642) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoddard ST, et al. 2013. House-to-house human movement drives dengue virus transmission. Proc. Natl Acad. Sci. USA 110, 994–999. ( 10.1073/pnas.1213349110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forshey BM, et al. 2010. Arboviral etiologies of acute febrile illnesses in western South America, 2000–2007. PLoS Neglected Trop. Dis. 4, e787 ( 10.1371/journal.pntd.0000787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolker BM. 2008. Ecological models and data in R. Princeton, NJ: Princeton University Press. [Google Scholar]
- 28.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 29.Sokal RR, Rohlf FJ. 1995. Biometry: the principles and practices of statistics in biological research, 3rd edn. New York, NY: WH Freeman. [Google Scholar]
- 30.Stephens PA, Buskirk SW, Hayward GD, Martinez del Rio C.. 2005. Information theory and hypothesis testing: a call for pluralism. J. Appl. Ecol. 42, 4–12. ( 10.1111/j.1365-2664.2005.01002.x) [DOI] [Google Scholar]
- 31.Adelman JS, Martin LB. 2009. Vertebrate sickness behaviors: adaptive and integrative neuroendocrine immune responses. Integr. Comp. Biol. 49, 202–214. ( 10.1093/icb/icp028) [DOI] [PubMed] [Google Scholar]
- 32.Nishiura H, Halstead SB. 2007. Natural history of dengue virus (DENV)-1 and DENV-4 infections: reanalysis of classic studies. J. Infect. Dis. 195, 1007–1013. ( 10.1086/511825) [DOI] [PubMed] [Google Scholar]
- 33.Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, Valleron AJ. 2008. Time lines of infection and disease in human influenza: a review of volunteer challenge studies. Am. J. Epidemiol. 167, 775–785. ( 10.1093/aje/kwm375) [DOI] [PubMed] [Google Scholar]
- 34.Grange L, Simon-Loriere E, Sakuntabhai A, Gresh L, Paul R, Harris E. 2014. Epidemiological risk factors associated with high global frequency of inapparent dengue virus infections. Front. Immunol. 5, 280 ( 10.3389/fimmu.2014.00280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pindolia DK, Garcia AJ, Wesolowski A, Smith DL, Buckee CO, Noor AM, Snow RW, Tatem AJ. 2012. Human movement data for malaria control and elimination strategic planning. Malar. J. 11, 205 ( 10.1186/1475-2875-11-205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Read JM, Edmunds WJ, Riley S, Lessler J, Cummings DAT. 2012. Close encounters of the infectious kind: methods to measure social mixing behaviour. Epidemiol. Infect. 140, 2117–2130. ( 10.1017/S0950268812000842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen NM, et al. 2013. Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc. Natl Acad. Sci. USA 110, 9072–9077. ( 10.1073/pnas.1303395110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Con G, et al. 2014. Shifting patterns of Aedes aegypti fine scale spatial clustering in Iquitos, Peru. PLoS Neglected Trop. Dis. 8, e3038 ( 10.1371/journal.pntd.0003038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duoung V, et al. 2015. Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl Acad. Sci. USA 112, 14 688–14 693. ( 10.1073/pnas.1508114112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vazquez-Prokopec GM, Perkins TA, Waller LA, Lloyd AL, Reiner RC Jr, Scott TW, Kitron U. 2016. Coupled heterogeneities and their impact on parasite transmission and control. Trends Parasitol. 32, 356–367. ( 10.1016/j.pt.2016.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkins TA, Scott TW, Le Menach A, Smith DL. 2013. Heterogeneity, mixing, and the spatial scales of mosquito-borne pathogen transmission. PLoS Comput. Biol. 9, e1003327 ( 10.1371/journal.pcbi.1003327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brockmann D, Hebling D. 2013. The hidden geometry of complex, network-driven contagion phenomena. Science 342, 1337–1342. ( 10.1126/science.1245200) [DOI] [PubMed] [Google Scholar]
- 43.Wesolowski A, Eagle N, Tatem AJ, Smith DL, Noor AM, Snow RW, Buckee CO. 2012. Quantifying the impact of human mobility on malaria. Science 338, 267–270. ( 10.1126/science.1223467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simini F, Maritan A, Neda Z. 2013. Human mobility in a continuum approach. PLoS ONE 8, e60069 ( 10.1371/journal.pone.0060069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zipf GK. 1946. The p1p2/d hypothesis: on the intercity movement of persons. Am. Sociol. Rev. 11, 677–686. ( 10.2307/2087063) [DOI] [Google Scholar]
- 46.Simini F, Gonzalez MC, Maritan A, Barabasi AL. 2012. A universal model for mobility and migration patterns. Nature 484, 96 ( 10.1038/nature10856) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are available on Dryad (http://dx.doi.org/10.5061/dryad.rd66f) with the exception of study participant age, sex, interview date and spatial coordinates of home locations and locations visited, owing to the personal nature of that information. Data, as well as code for fully reproducing the analyses, are also available on GitHub (https://github.com/TAlexPerkins/fever_mobility).