Abstract
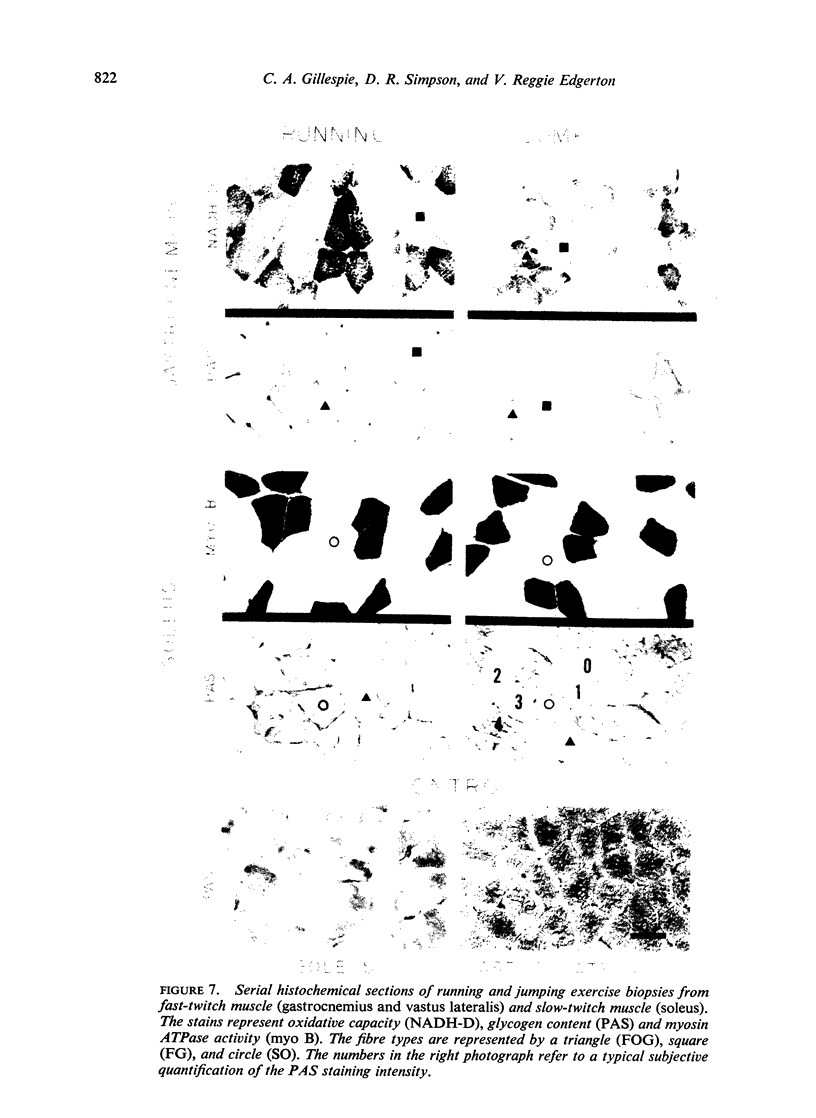
A qualitative histochemical assessment of glycogen loss in biopsies was made in bush-babies after running and jumping. Glycogen loss was related to the specific type of exercise. After running, glycogen loss was greatest in the slow-twitch oxidative fibre and depletion in the fast-twitch oxidative glycolytic fibres was similarly greater than in the fast-twitch glycolytic fibres. After jumping, the opposite pattern of glycogen utilization occurred (FG>FOG>SO).
Full text
PDF
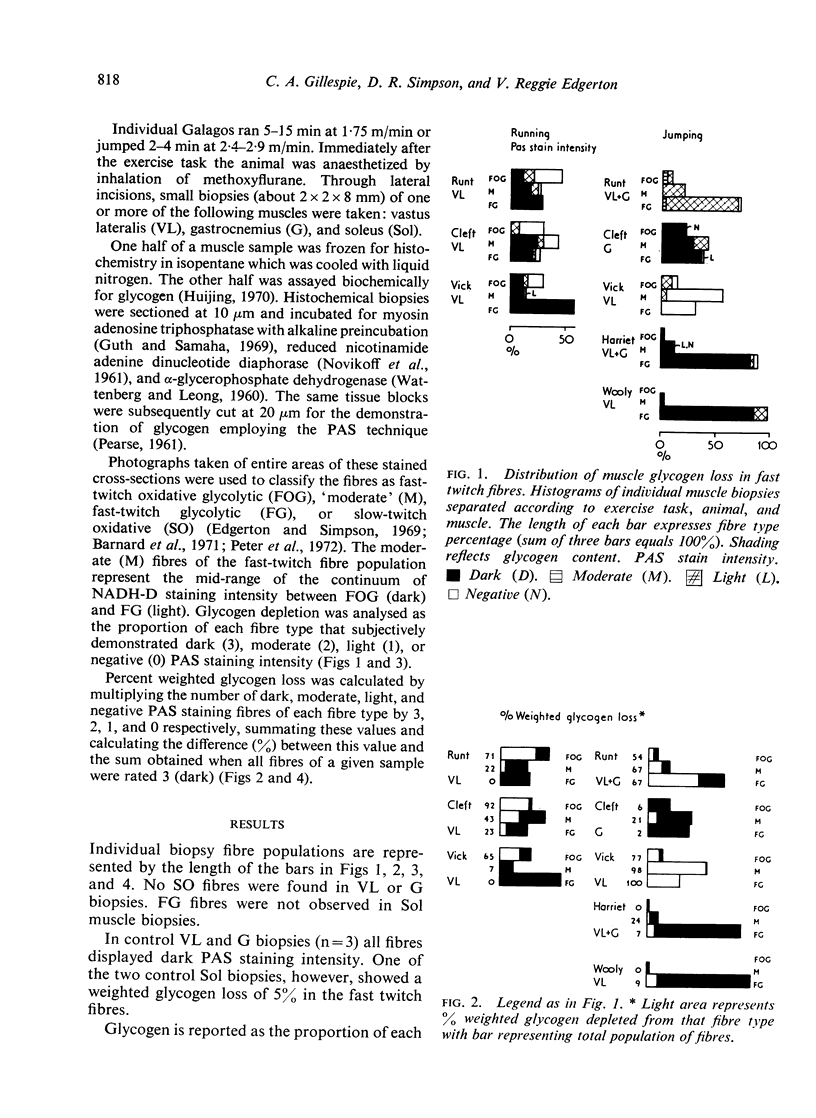
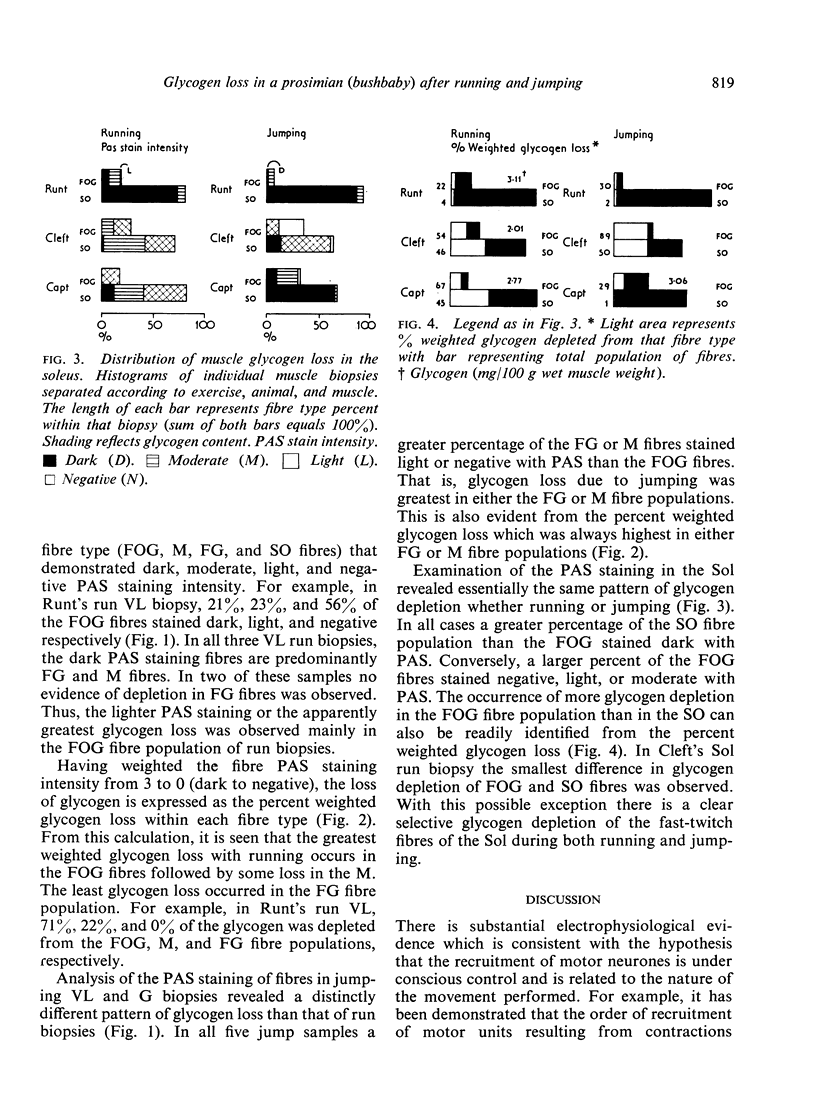
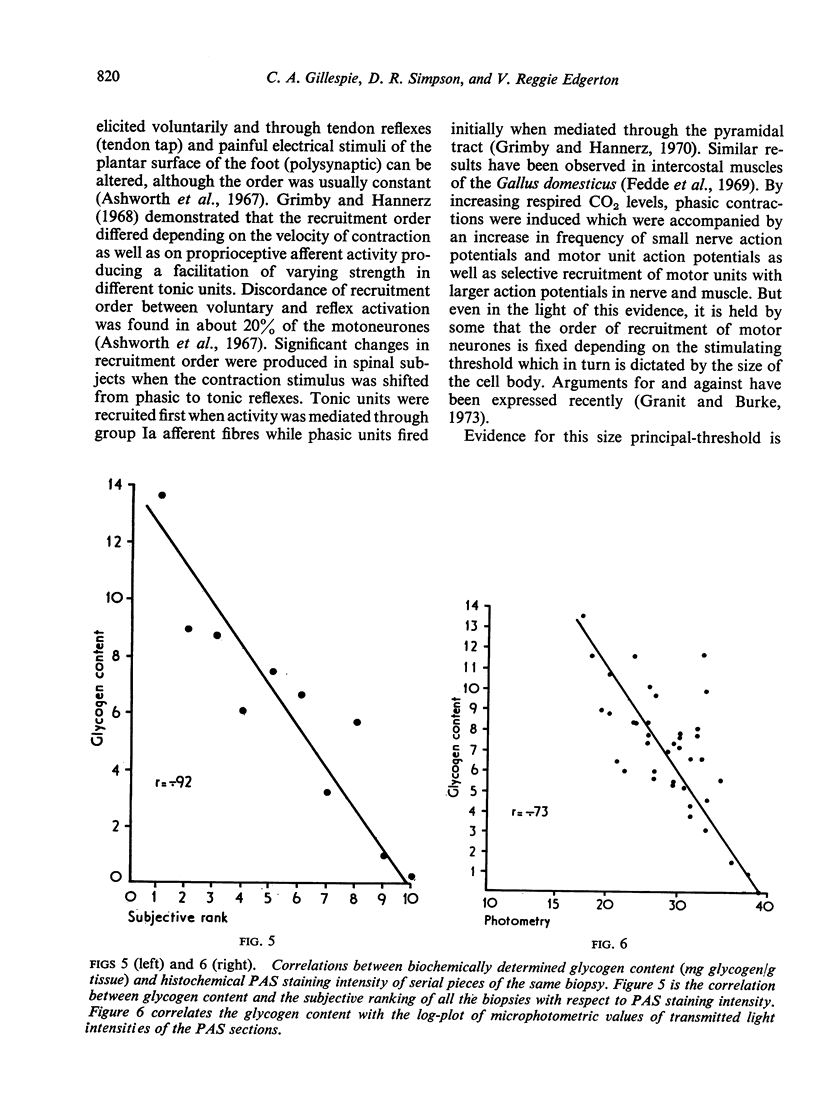



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth B., Grimby L., Kugelberg E. Comparison of voluntary and reflex activation of motor units. Functional organization of motor neurones. J Neurol Neurosurg Psychiatry. 1967 Apr;30(2):91–98. doi: 10.1136/jnnp.30.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R. J., Edgerton V. R., Furukawa T., Peter J. B. Histochemical, biochemical, and contractile properties of red, white, and intermediate fibers. Am J Physiol. 1971 Feb;220(2):410–414. doi: 10.1152/ajplegacy.1971.220.2.410. [DOI] [PubMed] [Google Scholar]
- Bergström J., Hultman E. A study of the glycogen metabolism during exercise in man. Scand J Clin Lab Invest. 1967;19(3):218–228. doi: 10.3109/00365516709090629. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Zajac F. E., 3rd Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971 Nov 12;174(4010):709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- DANFORTH W. H., HELMREICH E., CORICF The effect of contraction and of epinephrine on the phosphorylase activity of frog sartorius muscle. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1191–1199. doi: 10.1073/pnas.48.7.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANFORTH W. H., HELMREICH E. REGULATION OF GLYCOLYSIS IN MUSCLE. I. THE CONVERSION OF PHOSPHORYLASE BETA TO PHOSPHORYLASE ALPHA IN FROG SARTORIUS MUSCLE. J Biol Chem. 1964 Oct;239:3133–3138. [PubMed] [Google Scholar]
- Edgerton V. R., Barnard R. J., Peter J. B., Gillespie C. A., Simpson D. R. Overloaded skeletal muscles of a nonhuman primate (Galago senegalensis). Exp Neurol. 1972 Nov;37(2):322–339. doi: 10.1016/0014-4886(72)90077-5. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Barnard R. J., Peter J. B., Simpson D. R., Gillespie C. A. Response of muscle glycogen and phosphorylase to electrical stimulation in trained and nontrained guinea pigs. Exp Neurol. 1970 Apr;27(1):46–56. doi: 10.1016/0014-4886(70)90200-1. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Simpson D. R. The intermediate muscle fiber of rats and guinea pigs. J Histochem Cytochem. 1969 Dec;17(12):828–838. doi: 10.1177/17.12.828. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Simpson D., Barnard R. J., Peter J. B. Phosphorylase activity in acutely exercised muscle. Nature. 1970 Feb 28;225(5235):866–867. doi: 10.1038/225866a0. [DOI] [PubMed] [Google Scholar]
- Fedde M. R., DeWet P. D., Kitchell R. L. Motor unit recruitment pattern and tonic activity in respiratory muscles of Gallus domesticus. J Neurophysiol. 1969 Nov;32(6):995–1004. doi: 10.1152/jn.1969.32.6.995. [DOI] [PubMed] [Google Scholar]
- Granit R., Burke R. E. The control of movement and posture. Brain Res. 1973 Apr 13;53(1):1–28. doi: 10.1016/0006-8993(73)90763-4. [DOI] [PubMed] [Google Scholar]
- Grimby L., Hannerz J. Differences in recruitment order of motor units in phasic and tonic flexion reflex in "spinal man". J Neurol Neurosurg Psychiatry. 1970 Oct;33(5):562–570. doi: 10.1136/jnnp.33.5.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimby L., Hannerz J. Recruitment order of motor units on voluntary contraction: changes induced by proprioceptive afferent activity. J Neurol Neurosurg Psychiatry. 1968 Dec;31(6):565–573. doi: 10.1136/jnnp.31.6.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- Hartley L. H., Mason J. W., Hogan R. P., Jones L. G., Kotchen T. A., Mougey E. H., Wherry F. E., Pennington L. L., Ricketts P. T. Multiple hormonal responses to prolonged exercise in relation to physical training. J Appl Physiol. 1972 Nov;33(5):607–610. doi: 10.1152/jappl.1972.33.5.607. [DOI] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965 May;28(3):599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Huijing F. A rapid enzymic method for glycogen estimation in very small tissue samples. Clin Chim Acta. 1970 Dec;30(3):567–572. doi: 10.1016/0009-8981(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Keul J., Doll E., Keppler D. The substrate supply of the human skeletal muscle at rest, during and after work. Experientia. 1967 Nov 15;23(11):974–979. doi: 10.1007/BF02136259. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):415–423. doi: 10.1136/jnnp.31.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B., SHIN W. Y., DRUCKER J. Mitochondrial localization of oxidative enzymes: staining results with two tetrazolium salts. J Biophys Biochem Cytol. 1961 Jan;9:47–61. doi: 10.1083/jcb.9.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- WATTENBERG L. W., LEONG J. L. Effects of coenzyme Q10 and menadione on succinic dehydrogenase activity as measured by tetrazolium salt reduction. J Histochem Cytochem. 1960 Jul;8:296–303. doi: 10.1177/8.4.296. [DOI] [PubMed] [Google Scholar]