Summary
As part of the innate host response neutrophils release neutrophil extracellular traps (NETs), protein:DNA complexes that contain a number of antimicrobial peptides (AMPs), such as cathelicidin. Human cathelicidin in its active form, LL37, has potent antimicrobial activity against bacteria. However, whether LL37 derived from NETs contributes to antimicrobial activity against intracellular pathogens remains unclear. Here, we report that NETs induced by mycobacteria contain cathelicidin. Human macrophages internalized NET‐bound cathelicidin, which is transported to lysosomal compartments. Furthermore, using a model of in vitro‐generated LL37:DNA complexes we found that LL37 derived from such complexes attacks mycobacteria in macrophage phagolysosomes resulting in antimicrobial activity. Taken together, our results suggest a mechanism by which LL37 in complex with DNA contributes to host defence against intracellular bacteria in human macrophages.
Keywords: cathelicidin, LL37, macrophage, mycobacteria, neutrophil extracellular trap
Abbreviations
- AMP
antimicrobial peptide
- BBG
brilliant blue G
- BCG
Mycobacterium bovis bacillus Calmette–Guérin Pasteur
- BCG‐GFP
Mycobacterium bovis bacillus Calmette–Guérin Pasteur expressing GFP
- CaI
calcium ionophore A23187
- CFU
colony‐forming unit
- CLP
chlorpromazine hydrochloride
- DPI
diphenyleneiodonium
- hCAP18
human cationic antimicrobial protein
- MDM
monocyte‐derived macrophages
- MOI
multiplicity of infection
- NET
neutrophil extracellular trap
- PFA
paraformaldehyde
- P2X7R
P2X7 receptor
- ROS
reactive oxygen species
Introduction
Neutrophils eject antimicrobial peptide (AMP):DNA complexes as part of neutrophil extracellular traps (NETs), agglomerates of proteins and DNA, in response to different pathogens.1, 2, 3, 4, 5, 6 Because NETs were shown to trap bacteria in vitro and contain DNA and many proteins with antimicrobial properties, e.g. neutrophil elastase and cathelicidin,1, 2, 7 antimicrobial activity of NETs has been intensively studied. Several studies report that bacteria are killed in NETs; however, others questioned these findings.1, 8, 9, 10, 11, 12 Moreover, several pathogens have developed strategies to escape from NETs, such as secretion of DNases,10, 13, 14, 15 or inhibition of cathelicidin activity.16, 17, 18 Notably, antimicrobial activity of NETs against intracellular mycobacteria has only been tested in extracellular conditions, revealing no microbial killing.9
Cathelicidins are broadly active against extracellular and intracellular pathogens.19, 20, 21, 22 The only human cathelicidin is produced as an inactive precursor (hCAP18) and processed to the active LL37 via cleavage by extracellular proteases.23, 24 LL37 kills bacteria by binding of its amphipathic helix to membranes.25, 26, 27 The ability of human macrophages to up‐regulate a vitamin‐D‐dependent host defence pathway resulting in the intracellular induction of cathelicidin is crucial for antimicrobial activity against mycobacteria.21, 28, 29, 30, 31 The mouse homologue of cathelicidin, CRAMP, was also shown to play an important role against intracellular infection with salmonella,32 yet in mice CRAMP is not induced by vitamin D.33
Neutrophils express large amounts of hCAP18 in secondary granules,34 which are released and activated via exocytosis or in complex with DNA as part of NETs.3, 7, 24, 35 Consistently, NETs contain both hCAP18 and LL37.3, 35 Exogenous free LL37 is internalized by human macrophages, which is facilitated by the P2X7 receptor and clathrin‐mediated endocytosis pathways, and provides antimicrobial activity against intracellular pathogens, such as mycobacteria and Staphylococcus aureus.22, 36 Nevertheless, the role of LL37:DNA complexes in human host defence is unclear, because LL37 loses its antimicrobial activity when bound to DNA.37, 38 Given the pivotal role of human cathelicidin/LL37 in the macrophage host response against intracellular bacteria,19, 20, 21, 22, 28 we investigated whether extracellular NETs and LL37:DNA complexes are internalized by macrophages, transported to and processed in bacterial phagolysosomes, and whether LL37 derived from such complexes provides direct antimicrobial activity against intracellular microbes inside infected macrophages.
Materials and methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki and approved by the local Ethic Committee (Ethikkommission) of the University of Cologne, Germany. All donors provided written informed consent.
Isolation of primary human cells
Peripheral blood mononuclear cells were isolated from blood or ‘buffy coats’ of healthy donors by Ficoll‐Paque separation (GE Healthcare, Chalfont St Giles, UK). Monocytes were isolated via CD14+ MACS cell separation (Miltenyi Biotec, Bergisch Gladbach, Germany) and differentiated into monocyte‐derived macrophages (MDM) for 4–7 days in the presence of 50 ng/ml macrophage colony‐stimulating factor (Miltenyi Biotec). Neutrophils were isolated with the MACSxpress Neutrophil Isolation Kit (Miltenyi Biotec). In some experiments granulocytes were isolated from the Ficoll‐Paque bottom phase by dextran separation followed by incubation with ACK lysis buffer.
Staining of NETs
Neutrophils were grown on poly‐l‐lysine‐coated cover slides in RPMI supplemented with 10% human AB serum and stimulated with 5 μm calcium ionophore A23187 (CaI) or left untreated for 1·5 hr. When NETosis was induced with bacteria, cells were infected with Mycobacterium bovis bacillus Calmette–Guérin Pasteur (BCG) for 4 hr at a multiplicity of infection (MOI) of 10. Then, cells were washed twice and fixed with methanol at −20° or with 4% paraformaldehyde (PFA) at room temperature. PFA‐fixed cells were permeabilized with 0·1% Triton X‐100 for 2 min. Blocking was performed for 30 min in PBS containing 2% BSA and all antibodies were diluted in PBS with 0·1% BSA. Monoclonal antibodies against cathelicidin (clone 3D11; Hycult Biotech Inc., Plymouth Meeting, PA), myeloperoxidase (clone SP72; Abcam, Cambridge, UK), neutrophil elastase (clone EPR7479; Abcam), as well as secondary antibodies coupled to Alexa Fluor 488 (Life Technologies, Carlsbad, CA) were used. Nuclei were visualized with 300 nm DAPI (Invitrogen, Carlsbad, CA). Slides were mounted with Immu‐Mount (Thermo Fisher Scientific, Waltham, MA) and analysed with a BZ‐9000 (Keyence, Osaka, Japan), an Eclipse E800 (Nikon, Tokyo, Japan), or a Fluoview FV 1000 (Olympus, Tokyo, Japan) microscope.
Isolation of NETs
Neutrophils were activated with 5 μm CaI or left untreated in RPMI supplemented with 10% human AB serum. After 1·5 hr, cells were washed and fresh medium containing 5 U/ml of the restriction enzyme AluI (NEB, Ipswich, MA) was added for 20 min at 37°.39 Supernatants were harvested by centrifugation at 300 g for 5 min at 4° and then immediately used for macrophage stimulation or stored at −20° for analysis.
SDS–PAGE and Western blot analysis
NET supernatant was precipitated with TCA and SDS–PAGE was applied. Samples were run in denaturing and reducing conditions on an 18% acrylamide gel (Thermo Fisher Scientific). Then, proteins were transferred to a 0·2 μm nitrocellulose membrane (Life Sciences) for 40 min applying semi‐dry blotting. To study protein expression, membranes were incubated with 0·1% Ponceau S solution (Sigma, St Louis, MO). For Western blot analysis, blocking was performed with 5% milk powder in PBS‐T for 1 hr. Then, anti‐cathelicidin antibody recognizing both hCAP18 and LL37 (clone OSX 12; Abcam) was diluted 1 : 1000 in PBS‐T containing 5% milk powder and membranes were incubated overnight at 4°. Detection was performed with anti‐mouse IgG‐coupled to horseradish peroxidase (Cell Signaling, Danvers, MA) diluted 1 : 2000 PBS‐T containing 5% milk powder and visualized by enhaniced chemiluminescence (Thermo Fisher Scientific).
Quantification of neutrophil DNA release by spectrofluorometry
Neutrophils were stimulated with 25 nm PMA, 5 μm CaI, BCG (at an MOI of 10) or left untreated in RPMI supplemented with 10% human AB serum. Ten minutes before the stimuli, 10 μm diphenyleneiodonium (DPI, Sigma) was added, or not. After 4 hr, 5 μm SYTOX Green (Life Technologies) was added and fluorescence intensity was measured at 485/535 nm (excitation/emission).
Macrophage internalization of NETs
Neutrophils were activated with 5 μm CaI or left untreated in macrophage serum‐free medium (SFM, Life Technologies). Anti‐cathelicidin antibody (clone 3D11; Hycult) or IgG1 (clone 11711; R&D Systems, Minneapolis, MN) was added immediately after CaI‐stimulation of neutrophils (NETα‐cath and NETisotype, respectively) or to untreated neutrophils (Ctrα‐cath and Ctrisotype). After 1·5 hr, cells were washed and NETs were isolated as described above. Then, MDMs were stimulated with 100 μl antibody‐stained NET and control supernatant in macrophage SFM with or without addition of 100 nm LysoTracker Red DND‐99 (Thermo Fisher). After 1 hr, 5 μg/ml DNase I was added for 10 min. MDMs were washed twice and fixed with 4% PFA at room temperature. Cells were permeabilized with 0·1% Triton X‐100 for 2 min. Next, blocking was performed for 30 min in PBS with 2% BSA followed by incubation with secondary antibodies coupled to Alexa Fluor 488 (Life Technologies) diluted in PBS with 0·1% BSA. Nuclei were visualized with 300 nm DAPI (Invitrogen) and the cytoskeleton was visualized with rabbit anti‐β‐actin coupled to Alexa Fluor 594 (Life Technologies). Slides were mounted with Immu‐Mount (Thermo Scientific) and analysed with an Eclipse E800 (Nikon) microscope. Quantification was performed using automated counting with imageJ applying an individual threshold for each experiment. A minimum of 100 macrophage cells was counted per condition and analysed for the number of individually detectable FITC‐positive dots (ΔFITC‐positive dots/100 cells). Uptake of NETs was quantified as follows:
(FITC‐positive dots in NETα‐cath.) – (FITC‐positive dots in NETistoype) or (FITC‐positive dots in Ctrα‐cath.) –(FITC‐positive dots in Ctristoype).
DNA analysis
Human genomic DNA or isopropanol precipitated NET DNA was run on a 0·8% agarose gel supplemented with SYBR Safe DNA Gel Stain (Invitrogen).
DNA labelling
Human genomic DNA was labelled with Alexa Fluor 594 (DNA594) or 647 (DNA647) dyes using the Ulysis Alexa Fluor Nucleic Acid Labeling Kit (Thermo Fisher).
Generation of LL37:DNA complexes
LL37 (500 μg/ml; Invitrogen) and 100 ng/μl fractionated human genomic DNA (Bioline, Luckenwalde, Germany) were incubated for 30 min at room temperature and diluted 1 : 10 (50 μg/ml LL37 and 10 ng/μl DNA),40 1 : 50 (10 μg/ml LL37 and 2 ng/μl DNA), 1 : 100 (5 μg/ml LL37 and 1 ng/μl DNA) or 1 : 1000 (0·5 μg/ml LL37 and 0·1 ng/μl DNA) in RPMI supplemented with 10% human AB serum or in SFM.
Analysis of LL37:DNA internalization into macrophages by flow cytometry
MDMs were incubated with medium alone, 10 μg/ml LL37:FAM or LL37:FAM:DNA complexes (10 μg/ml LL37 and 2 ng/μl DNA; equates 1 : 50 dilution, see above) for 15 min to 1 hr at 4° or 37°. In some experiments 10 μm brilliant blue G (BBG, Sigma) or 50 μm chlorpromazine hydrochloride (CLP, Sigma) were added 1 hr in advance. Cells were washed three times with ice cold PBS, scraped and transferred to FACS tubes for immediate analysis with a FACSCalibur (BD).
Analysis of LL37:DNA complexes in BCG‐infected macrophages by immunofluorescence microscopy
MDMs were infected with single‐cell suspensions of log‐phase BCG or BCG‐GFP (kindly provided by Georg Plum, University of Cologne, Cologne, Germany) at a MOI of 10. In some conditions, 10 μm chloroquine (InvivoGen, San Diego, CA) or 50 nm bafilomycin A1 (Sigma) were added 30 min before stimulation with complexes. Cells were washed twice and stimulated with complexes of LL37‐biotin (Innovagen, Lund, Sweden) and DNA594 (LL37‐biotin:DNA594) or LL37‐TAMRA (Innovagen) and DNA647 (LL37‐TAMRA:DNA647) (complexes were diluted 1 : 10 in RPMI supplemented with 10% human AB serum) with or without re‐addition of chloroquine or bafilomycin A1 for 3–4 hr. MDMs were washed twice and fixed with methanol at −20°. Next, blocking was performed for 30 min in PBS with 2% BSA and all antibodies were diluted in PBS with 0·1% BSA. Monoclonal antibodies against LAMP‐1 (Cell Signaling, clone D2D11), as well as secondary antibodies coupled to Alexa Fluor 350 (Life Technologies) were used. Slides were mounted with Immu‐Mount (Thermo Scientific) and analysed with a Fluoview FV 1000 (Olympus) microscope.
CFU assay
MDMs were infected with single‐cell suspensions of log‐phase BCG (MOI 2·5). After 3 hr, MDMs were washed twice with PBS. Then, infected cells were stimulated with DNA (0·1 ng/μl), LL37 (0·5 μg/ml), LL37:DNA complexes (0·5 μg/ml LL37 and 0·1 ng/μl DNA; equates to 1 : 1000 dilution, see above) or medium alone for 1 hr in macrophage SFM. Cells were washed twice and incubated in fresh medium under optimized serum conditions in the presence of 10 μg/ml gentamicin (Sigma). After 3 days, cells were lysed with 0·05% SDS (Serva Electrophoresis, Heidelberg, Germany). The lysates were resuspended vigorously and diluted in 0·05% Tyloxapol (Sigma Aldrich). Three to five dilutions of each sample were plated on 7H10 agar plates (Becton Dickinson, Franklin Lakes, NJ and Merck Millipore, Billerica, MA) and incubated for 21 days at 37°.
Electron microscopy
MDMs were grown on ACLAR films and infected with BCG (MOI 2·5) for 3 hr, then washed twice. LL37‐biotin:DNA complexes (1 : 100 diluted in SFM) were washed twice with ddH2O and used for macrophage stimulation. After 1 hr, fresh RPMI supplemented with 10% human AB serum was added and cells were incubated for additional 17 hr. Then, streptavidin‐collodial gold conjugate (Sigma, 10 nm) was added to the cells for one additional hour. Cells were washed twice and fixed with 2% PFA and 2% glutardialdehyde (Merck, Darmstadt, Germany) in 0·1 m cacodylate buffer (Sigma) for 15 min. Then, cells were washed twice followed by 2% osmium tetroxide in Sörensen buffer for 1 hr, and treated with 1% uranyl acetate in 70% ethanol for 30 min. The specimens were subsequently dehydrated in a graded series of ethanol and embedded in Epon (Electron Microscopy Sciences, Hatfield, PA). Ultrathin sections (90 nm) were cut with a diamond knife on an ultramicrotome and placed on copper grids. Transmission electron microscopy was performed using a Zeiss 109 electron microscope (Carl Zeiss, Oberkochen, Germany).
Statistical analysis
P‐values were calculated by two‐tailed Student's t‐tests. In some cases measured values were log transformed to align data points to normal distribution.
Results
Cathelicidin expression in human NETs
Because of the importance of cathelicidin in human host defence,19, 20, 21, 22, 28 we studied its expression in neutrophil‐ejected protein:DNA complexes. Therefore, we induced release of protein:DNA complexes by neutrophils with CaI. Neutrophil‐ejected DNA webs contained cathelicidin consistent with previous reports,3, 7, 35, 41, 42 as well as neutrophil elastase and myeloperoxidase, thereby formally identifying the ejected DNA webs as NETs1, 2, 43 (Fig. 1a). The cathelicidin antibody used recognizes a sequence in LL37, hence, stains cleaved LL37, as well as uncleaved hCAP18. To further characterize cathelicidin expression in neutrophil‐ejected protein:DNA complexes, we separated CaI‐induced NETs from neutrophil cell bodies in vitro.39 Isolated NETs contained large DNA fragments with DNA sizes up to ~10 kbp (Fig. 1b). Ponceau staining showed a spectrum of proteins in isolated NETs (Fig. 1c) and Western blot analysis using an antibody that also recognizes both hCAP18 and LL37 indicated that both the cathelicidin precursor hCAP18 and the active AMP LL37 are complexed to the ejected DNA (Fig. 1d).3, 35 Taken together, our data confirm the presence of both hCAP18 and the active LL37 in NETs.
Figure 1.

Neutrophil extracellular traps (NETs) contain cathelicidin in vitro and in vivo. (a) Neutrophils stimulated with CaI or left untreated for 1·5 hr were stained with DAPI and antibodies against cathelicidin (Cath.), myeloperoxidase (MPO) or neutrophil elastase (NE) and analysed by fluorescent microscopy. (b–d) Isolated NETs were analysed for (b) DNA expression on an agarose gel, (c) protein expression in a nitrocellulose membrane and (d) expression of cathelicidin by Western blot. LL37: recombinant LL37; Lysate: neutrophil cell lysate. (b/w): black and white.
Cathelicidin expression in mycobacteria‐induced NETs
To investigate whether NETs induced by intracellular pathogens5 express cathelicidin, we infected neutrophils with BCG. After 4 hr, cells were fixed, stained with a monoclonal cathelicidin antibody and DAPI. Immunofluorescence analysis showed DNA fibres coated with cathelicidin (Fig. 2a), demonstrating NET induction by infection with BCG. Given that NET formation upon stimulation with many extra‐ and intracellular bacteria is reactive oxygen species (ROS)‐dependent,8, 12, 44 we tested whether neutrophil DNA release by BCG is dependent on ROS. We stimulated neutrophils in the presence or absence of DPI, an NADPH oxidase inhibitor.45 The release of extracellular DNA was quantified using SYTOX Green, a non‐membrane permeable fluorescent dye that binds to extracellular DNA.46 Stimulation with CaI, PMA12, 35, 39 and BCG resulted in a significant increase of fluorescence compared with unstimulated cells (Fig. 2b). DPI blocked PMA‐induced8, 12, 47 and BCG‐induced DNA release, yet, consistent with previous studies, DPI had no effect on CaI‐induced DNA release, which is ROS independent.12 Taken together, our data indicate that BCG induces ejection of cathelicidin:DNA complexes by neutrophils in a ROS‐dependent manner.
Figure 2.
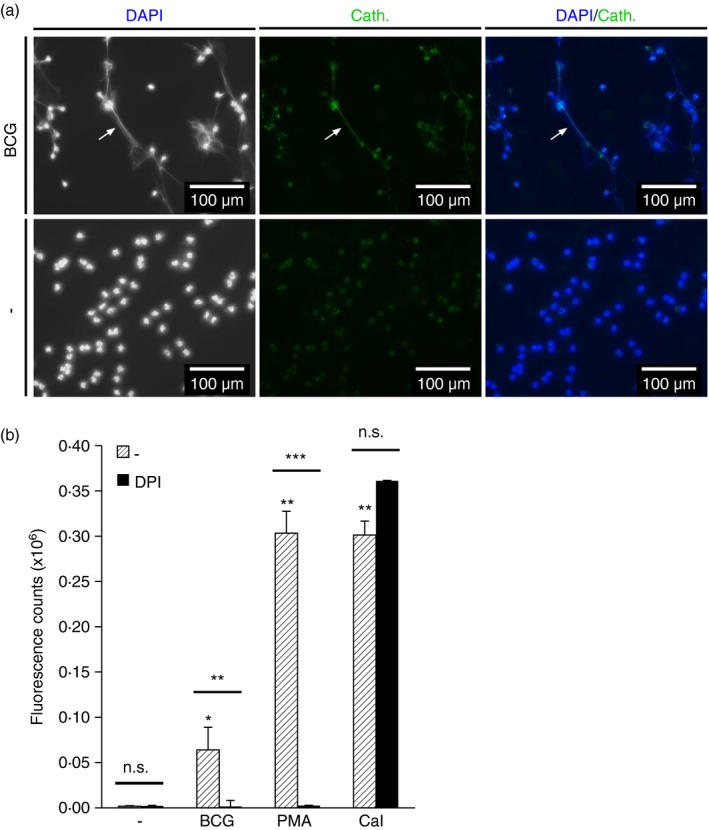
Mycobacterium bovis bacillus Calmette–Guérin Pasteur (BCG) infection triggers NETosis. (a) Neutrophils were infected with BCG for 4 hr. Cathelicidin (Cath.) was detected by a monoclonal antibody and DNA was stained with DAPI. Cells were analysed by fluorescent microscopy. (b) Neutrophils were stimulated with BCG, PMA or CaI with or without addition of the NADPH‐oxidase inhibitor DPI 10 min before stimulation. SYTOX Green was added after 4 hr and fluorescence was measured by spectrofluorometry. Fluorescence intensity of wells treated the same way, but lacking neutrophils were subtracted from the values to compensate for release of bacterial DNA. Bars represent mean Δfluorescent counts ± SEM (n = 3; *P < 0·05, **P < 0·01, ***P < 0·001).
NET cathelicidin/LL37:DNA complexes are internalized and processed in macrophage phagolysosomes
To study whether NET cathelicidin is internalized by macrophages we added isolated NETs to primary human macrophages and monitored internalization of cathelicidin and DNA. To detect cathelicidin from NETs and avoid staining of macrophage‐derived cathelicidin we used antibody pre‐stained NETs (NETα‐cath.). Furthermore, we added DNase I to the cultures to degrade extracellular DNA.48 Macrophage cytoskeletons were stained with an anti‐actin antibody. Immunofluorescence analysis revealed cathelicidin that was associated with macrophages and local actin accumulations were observed in cells that internalized NET cathelicidin (Fig. 3a), indicating active endocytosis.48 Moreover, we detected cathelicidin, which was associated with extranuclear DNA, indicating NET uptake48 (Fig. 3a). To exclude uptake of NETs mediated by antibodies, we added isolated NETs not stained with antibodies (NET(−ab)) to macrophages and still observed extranuclear DNA (see Supplementary material, Fig. S1). We quantified cathelicidin internalization into macrophages (cathelicidin‐positive dots per 100 cells ± SEM: 6·2 ± 1·0, P < 0·001) (Fig. 3b). Furthermore, application of NETsα‐cath on lysotracker‐stained macrophages showed accumulation of NET cathelicidin in lysosomes (Fig. 3c). Taken together, our results show that NETs are internalized by macrophages and cathelicidin derived from NETs is transported into macrophage lysosomes.
Figure 3.
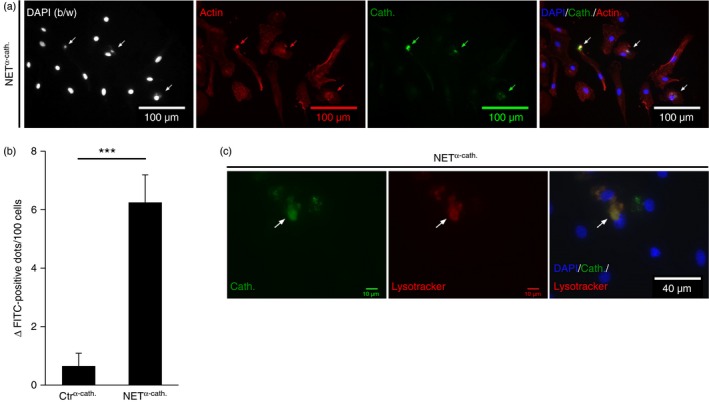
Neutrophil extracellular trap (NET) cathelicidin is internalized into human macrophages. Human macrophages were cultured with isolated NETs or supernatants from unstimulated neutrophils (Ctr) stained with antibodies against cathelicidin (NET α‐cath. or Ctr α‐cath.) for 1 hr. (a) NET α‐cath. was stained with a respective secondary antibody. Cells were co‐stained with DAPI, as well as anti‐actin antibody and were analysed by fluorescence microscopy. Representative images are shown. (b/w): black and white. Arrows point towards sites of NET α‐cath internalization. (b) Shows quantification of internalized NET cathelicidin. Bars represent mean ΔFITC‐positive dots/100 cells ± SEM (n = 9; ***P < 0·001). (c) Macrophages were incubated with NET α‐cath. supernatants for 1 hr and stained with lysotracker and DAPI. NET α‐cath. was stained with a respective secondary antibody and cells were analysed by fluorescence microscopy. Representative images are shown.
NETs contain a number of proteins, peptides and DNA with antimicrobial properties. Efficient knock down of cathelicidin in primary neutrophils is hardly feasible. Moreover, in HL‐60, a neutrophil‐like cell line, we did not detect cathelicidin in ejected DNA complexes (data not shown). Hence, specific effects of human NET cathelicidin are difficult to measure. We and others show that NETs express both hCAP18 and LL37, thus NETs are a source of LL37:DNA complexes.3, 35 We decided to use a model of in vitro‐generated complexes of human LL37 and human genomic DNA to study in detail the uptake and intracellular fate of LL37 complexed with DNA in macrophages. To visualize LL37, we used LL37‐TAMRA or LL37‐FAM, DNA was labelled with Alexa Fluor 594 or 647. Complexes were generated by incubating LL37 and DNA with different fragment sizes reflecting DNA purified from NETs, resulting in large accumulations of LL37 and DNA forming complexes40 (see Supplementary material, Fig. S2a,b). We incubated macrophages with medium alone, LL37‐FAM or complexes of LL37‐FAM and DNA and studied binding at 4° and internalization at 37° by flow cytometry. Incubation with LL37‐FAM or LL37‐FAM:DNA resulted in increased fluorescence compared with medium control, which was higher at 37° as compared with 4°, indicating both binding and uptake of LL37‐FAM or LL37‐FAM:DNA by macrophages (Fig. 4a). Previously, it was shown that LL37 internalization into macrophages is dependent on the P2X7 receptor (P2X7R) and clathrin‐mediated endocytosis.36 Hence, we investigated whether internalization of LL37:DNA complexes into human macrophages is dependent on the P2X7R or clathrin‐mediated endocytosis. Therefore, we treated macrophages with the P2X7R inhibitor BBG and applied LL37‐FAM, complexes of LL37‐FAM and DNA, or medium alone.49, 50 As expected, BBG inhibited internalization of LL37‐FAM (Fig. 4b, left graph). BBG also inhibited internalization of LL37‐FAM:DNA complexes into human macrophages (Fig. 4b, right graph, Fig. 4c). To investigate whether clathrin‐mediated endocytosis mediates internalization of LL37‐FAM:DNA complexes, we inhibited clathrin‐mediated endocytosis with CLP.36, 51, 52, 53 CLP decreased internalization of LL37‐FAM:DNA complexes into human macrophages (Fig. 4d). In summary, our data indicate that internalization of LL37:DNA complexes into human macrophages is at least in part mediated by P2X7R and suggest contribution of clathrin‐dependent endocytosis.
Figure 4.
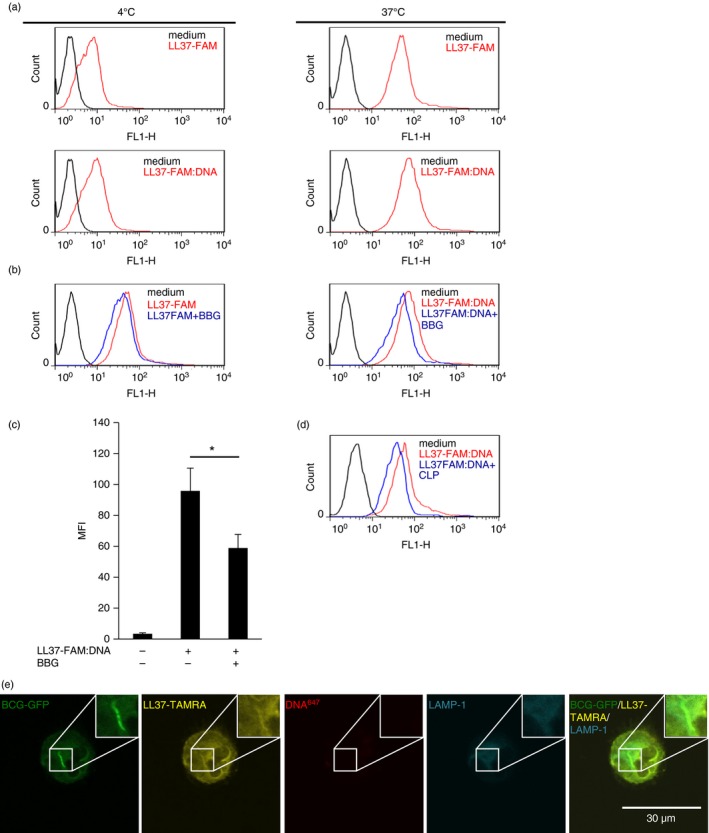
Uptake of LL37:DNA complexes into human macrophages. (a) Monocyte‐derived macrophages (MDMs) were incubated with medium alone, LL37‐FAM or LL37‐FAM:DNA complexes for 1 hr at 4° or 37°. Fluorescence intensity was analysed by flow cytometry. (b, c) MDMs were incubated with medium alone, LL37‐FAM or LL37‐FAM:DNA complexes for 1 hr with or without addition of brilliant blue G (BBG) and analysed as described in (a). In (c) bars show mean fluorescence intensity (MFI) ± SEM (n = 3; *P < 0·05). (d) MDMs were incubated with medium alone, LL37‐FAM or LL37‐FAM:DNA complexes for 15 min with or without addition of chlorpromazine hydrochloride (CLP) and analysed as described in (a). (e) Human macrophages were infected with bacillus Calmette–Guérin Pasteur expressing GFP (BCG‐GFP) and incubated with LL37‐TAMRA:DNA 647 for 4 hr. Lysosomal compartments were stained with a monoclonal antibody against LAMP‐1. Cells were analysed by confocal microscopy. One representative cell is shown. (Pictures shown in Figure 4(e) and 5(a) were taken from the same experiment).
Next, we studied the subcellular localization of LL37:DNA complexes in BCG‐infected macrophages after uptake. Therefore, we infected human macrophages with BCG conjugated to green fluorescent protein (BCG‐GFP) and subsequently added LL37‐TAMRA:DNA647 complexes. Confocal microscopy revealed that LL37‐TAMRA accumulated in close proximity to bacteria after uptake by macrophages (Fig. 4e; Fig. 5a,b, upper rows). Staining of LAMP‐1 showed that a large portion of LL37‐TAMRA together with the bacteria was associated with lysosomal compartments (Fig. 4e; Fig. 5a,b, upper rows). DNA647 was distributed primarily in the cell periphery and was not associated with lysosomes (Fig. 5a,b, upper rows). However, when we blocked lysosome biology with chloroquine or bafilomycin A1,54, 55 large amounts of DNA647 were observed in macrophage lysosomes (Fig. 5a,b, lower rows). Moreover, LL37‐TAMRA that localized with DNA in the chloroquine‐treated or bafilomycin A1‐treated samples was less closely associated with bacteria and distributed more randomly in the cytosol as LL37‐TAMRA in the samples, in which lysosome function was not blocked (Fig. 5a,b). Consistently, we observed an increase of DNA594‐positive macrophages after incubation with LL37:DNA594 complexes when cells were treated with chloroquine compared with non‐chloroquine‐treated cells, indicating degradation of complex‐derived DNA in lysosomal compartments (see Supplementary material, Fig. S3). We also observed an increase of DNA594‐positive cells when DNA was complexed to LL37 compared with the application of DNA594 alone (see Supplementary material, Fig. S3), indicating that complex generation results in enhanced DNA uptake, an effect that was observed by others.40, 56 Taken together, our data demonstrate that NET cathelicidin/LL37:DNA complexes are internalized by human macrophages, subsequently DNA is degraded in lysosomes, LL37 is released and co‐localizes with intracellular mycobacteria.
Figure 5.
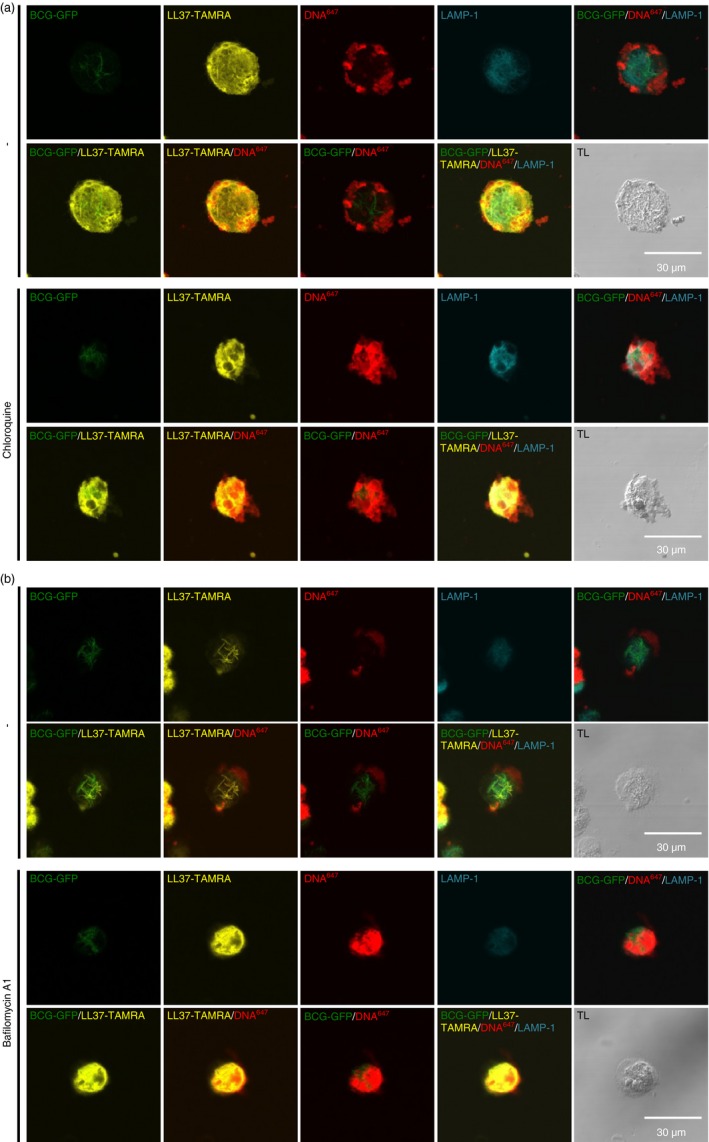
LL37:DNA complexes are processed in human macrophages. Human macrophages were infected with bacillus Calmette–Guérin Pasteur expressing GFP (BCG‐GFP) and incubated with LL37‐TAMRA:DNA 647 for 4 hr. Cells were cultured with or without (a) chloroquine or (b) bafilomycin A1. Lysosomal compartments were stained with a monoclonal antibody against LAMP‐1. Cells were analysed by confocal microscopy. One representative cell of each condition is shown. Transmission light (TL) images show position of cells on the respective image. In (b), complexes were washed before application on macrophages.
LL37 derived from LL37:DNA complexes attacks intracellular bacteria inside macrophages
Antimicrobial activity of NETs and AMP:DNA complexes had not been tested against intracellular pathogens intracellularly. To investigate whether complexes composed of LL37 and DNA contribute to antimicrobial activity against intracellular pathogens in macrophages we infected macrophages with BCG, stimulated the infected cells with DNA, LL37 or LL37:DNA complexes for 1 hr and measured bacterial growth after 3 days by colony forming unit assay (CFU). We observed a trend towards bacterial growth inhibition when LL37 was applied alone and a significant growth inhibition when LL37 was complexed to DNA compared with cells stimulated with medium alone (growth inhibition ± SEM: 24·2 ± 3·0%, P < 0·05) (Fig. 6a). The results indicate that application of LL37:DNA complexes to human macrophages results in growth inhibition of internalized BCG. Next, we tested whether LL37 directly targets BCG in macrophages. We incubated BCG‐infected macrophages with complexes of biotinylated LL37 and DNA (LL37‐biotin:DNA) or not, then added gold‐labelled streptavidin. Electron microscopy analysis showed gold particles associated with bacterial membranes after application of complexes (Fig. 6b). Together, our data indicate that LL37 from LL37:DNA complexes targets mycobacteria inside human macrophages and inhibits growth of intracellular mycobacteria via integration into bacterial membranes.
Figure 6.
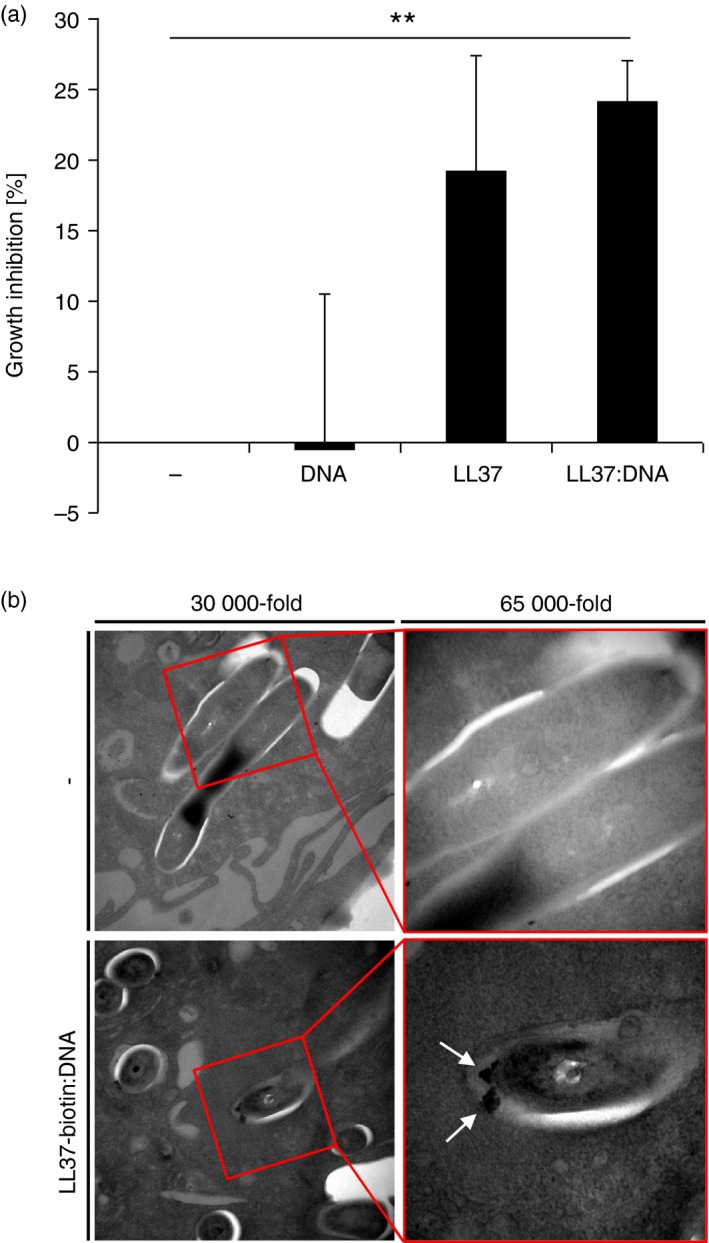
LL37 derived from LL37:DNA complexes attacks mycobacteria in human macrophages. (a) Mycobacterium bovis Bacillus Calmette–Guérin Pasteur (BCG) ‐infected human macrophages were incubated with DNA, LL37, LL37:DNA complexes or medium alone. After 3 days, cells were lysed and viable bacteria were quantified by colony‐forming units. Bars represent means of growth inhibition in % ± SEM (n = 3; **P < 0·01). (b) Human macrophages were infected with BCG and incubated with LL37‐biotin:DNA complexes. Streptavidin colloidal gold conjugate was added to the living cells, which were then fixed and prepared for electron microscopy. Representative electron microscopy images are shown.
Discussion
In this study we demonstrate that NET cathelicidin, as well as LL37:DNA complexes, are internalized by human macrophages. Furthermore, we provide evidence that LL37:DNA complexes are processed in macrophages enabling LL37 dissociation from DNA and integration of LL37 into bacterial membranes, resulting in growth inhibition of bacteria intracellularly. Hence, our study suggests a novel mechanism by which AMP:DNA complexes, and potentially full NETs, mediate antimicrobial activity against intracellular pathogens.
Our data indicate that the underlying mechanism of LL37 release from DNA before binding to bacteria is mediated via DNA‐degradation in lysosomes, because inhibition of lysosome biology with chloroquine or bafilomycin A1 resulted in persistence of DNA and hence co‐localization of DNA and LL37 in lysosomes. In non‐chloroquine/bafilomycin A1‐treated cells, DNA was almost absent in lysosomes and LL37 was associated with and concentrated in close proximity to BCG in lysosomes, suggesting binding of LL37 to the bacteria in lysosomes. Furthermore, integration of complex‐derived LL37 into mycobacterial membranes was demonstrated by electron microscopy. Of relevance, binding of LL37 to DNA inhibits its extracellular bactericidal activity in vitro, which seems to be reversible by DNase‐mediated degradation of DNA.38 Furthermore, in mouse neutrophils, CRAMP mediated intracellular activity against bacteria in phagolysosomes. However, when CRAMP was associated with NETs, its activity against extracellular bacteria was reduced.57
Lysosomes are well known to degrade both LL37 and DNA.36, 58, 59, 60, 61, 62 The entry of cathelicidin:DNA complexes into the endolysosomal pathway was described by a recent publication showing uptake of DNA in complex with chicken cathelicidin or human LL37. This study showed that cathelicidin was degraded in endosomes, subsequently DNA was liberated, after which Toll‐like receptor 2/1 was activated.51 Moreover, Tang et al.36 observed degradation of LL37 in macrophages infected with S. aureus. This study shows direct co‐localization of internalized LL37 and bacteria in macrophage lysosomes resulting in enhanced antimicrobial activity. The authors concluded that LL37 exerts its antibacterial activity after internalization and is then degraded to prevent damaging effects of the peptide on the host cells. In our study we detected the TAMRA‐tag at the N‐terminus of the LL37 peptide in LL37‐TAMRA:DNA647 complexes and observed co‐localization with bacteria in lysosomes. This is consistent with the previous study by Tang et al.,36 in which co‐localization of LL37‐TAMRA and FITC‐labelled bacteria was detected. We cannot completely rule out that the peptide was cleaved off the tag before detection and unspecifically binds bacteria. Nevertheless, the electron microscopy images, in which LL37‐biotin detected with gold beads was used, provide further evidence that LL37 targets infected mycobacteria. With respect to DNA, lysosomal degradation, e.g. by DNase II in macrophages, is well characterized.59, 60, 61, 62 We observed that LL37‐TAMRA dissociated from DNA labelled with an Alexa Fluor 647 reagent that forms a stable adduct with the N7 position of guanine residues. In our experiments the Alexa Fluor 647 signal was absent in lysosomes in macrophages not treated with chloroquine or bafilomycin A1 indicating that DNA gets depredated. In summary, it is tempting to speculate that endolysosomal processing of LL37:DNA complexes promotes macrophage host defence by two means: first, released LL37 could directly target bacteria as suggested by our study, meanwhile innate immune receptor‐mediated activation by DNA could lead to induction of macrophage autonomous host defence pathways, and maybe both mechanisms cooperate to fight intracellular infection.
Our results show that internalization of LL37:DNA complexes into human macrophages was dependent on the P2X7R and clathrin‐mediated endocytosis. This is consistent with studies showing that internalization of LL37 into human macrophages is dependent on the P2X7R and clathrin‐mediated endocytosis.36 Of note, involvement of P2X7R in NET‐mediated activation of caspase‐1 and subsequent secretion of interleukin‐1β and interleukin‐18 by macrophages has been suggested previously.35 Additionally, in chicken macrophages, internalization of complexes composed of chicken cathelicidin and DNA was found to be clathrin‐dependent,51 consistent with our results in human macrophages.
We found that cathelicidin is expressed in mycobacteria‐induced NETs, as has been reported for chemically induced and spontaneously released NETs.35, 41, 42 Moreover, consistent with previous studies investigating NET release by extracellular and intracellular bacteria, we demonstrate that ejection of neutrophil DNA triggered by BCG is ROS‐dependent.8, 9, 12, 44 Of note, it was shown that Mycobacterium tuberculosis induced NETosis via secretion of early secreted antigenic target‐663 and that M. tuberculosis‐induced NETosis was dependent on ROS production and phagocytosis, but not dependent on the bacterial 19 kDa lipoprotein.44
The observation that macrophages internalized NETs is supported by previous findings.64 One study suggested NET phagocytosis by macrophages as a mechanism that removes NETs from the extracellular space, thereby preventing NET‐induced inflammation in autoimmunity.48 Uptake of NETs was demonstrated by increase of neutrophil elastase and extranuclear DNA in macrophages after incubation with purified NETs or NETting neutrophils.44, 48 Besides, interaction of NETs with macrophages was detected by enhanced secretion of cytokines upon NET stimulation.35, 44, 65 Moreover, in monocytes/macrophages, LL37:DNA complexes induced DNA‐mediated secretion of interferons and cytokines.51, 56 Of relevance, in a recent study, labelled neutrophils were activated to undergo NETosis and the fluorescent label was detected in macrophages after co‐culture with the pre‐stained neutrophils, indicating internalization of neutrophil components into macrophages.66
There is a growing body of evidence for the concept that the ability of macrophages to kill intracellular bacteria is supported by uptake of neutrophil‐derived exogenous proteins. Neutrophil granule proteins were shown to enhance mycobacterial killing in macrophages.67, 68, 69, 70, 71 Moreover, exogenous free LL37, that is not complexed with DNA, was shown to reach lysosomal compartments and trigger macrophage activation72 or enhanced antimicrobial activity against mycobacteria.22 Our study, by showing that LL37:DNA complexes are internalized by human macrophages, after which LL37 dissociates from DNA attacking intracellular mycobacteria and promoting antimicrobial activity, suggests a novel link between antimicrobial functions of NETs and uptake of neutrophil components by macrophages. Given that NETs also contain full hCAP18, future studies are required to examine the role of hCAP18 in complex with DNA in assisting macrophage antimicrobial activity. When taken together, our and the previous studies22, 72 suggest an important role of cathelicidin in two distinct neutrophil defence programmes, degranulation and NETosis, that assist macrophages in antimicrobial host defence. It remains to be investigated whether other NET‐associated proteins contribute to intracellular antimicrobial activity. To note, neutrophil elastase and myeloperoxidase, both expressed in NETs, enhanced killing of intracellular bacteria in macrophages.69, 70, 71
Disclosures
The authors state no conflict of interest.
Supporting information
Figure S1. Neutrophil extracellular traps (NETs) are internalized into human macrophages. Human macrophages were cultured with isolated NETs and stained with DAPI. Cells were analysed by fluorescence microscopy. White arrow points to NET DNA. (b/w): black and white.
Figure S2. Cell‐free LL37:DNA complexes. (a) Human genomic, labelled DNA (DNA594) was visualized on an agarose gel (left). DNA was complexed with LL37 or not and analysed by confocal microscopy (right). (b) Confocal microscopy analyses of LL37‐TAMRA, DNA647, as well as complexes of LL37 and DNA647 and complexes of LL37‐TAMRA and DNA647.
Figure S3. Human macrophages internalize LL37:DNA complexes. Human macrophages were incubated with DNA594 or LL37:DNA594 complexes with or without the addition of chloroquine. A minimum of 79 cells were counted and analysed for the number of DNA594 positive cells. Bars represent mean of one experiment analysed by four individual investigators. Error bars show mean of DNA594 positive cells in % ± SEM.
Acknowledgements
We thank Janina Quennet, Ben Twardoz and Mojgan Ghilav for excellent technical assistance, Marina Alber for assistance with mycobacterial experiments, Birgit Gathof for providing buffy coats, and all blood donors who participated in this study. This work was funded by the Ministry of Innovation, Science, Research and Technology of the German State of North Rhine‐Westphalia, the Centre for Molecular Medicine (B4), and the Deutsche Forschungsgemeinschaft (SFB829 and SFB670).
References
- 1. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–5. [DOI] [PubMed] [Google Scholar]
- 2. Urban CF, Ermert D, Schmid M, Abu‐Abed U, Goosmann C, Nacken W et al Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans . PLoS Pathog 2009; 5:e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J et al Neutrophils activate plasmacytoid dendritic cells by releasing self‐DNA‐peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011; 3:73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol 2012; 198:773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD et al Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 2014; 15:1017–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White P, Cooper P, Milward M, Chapple I. Differential activation of neutrophil extracellular traps by specific periodontal bacteria. Free Radic Biol Med 2014; 75(Suppl 1):S53. [DOI] [PubMed] [Google Scholar]
- 7. Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z et al Netting neutrophils in autoimmune small‐vessel vasculitis. Nat Med 2009; 15:623–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V et al Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ramos‐Kichik V, Mondragon‐Flores R, Mondragon‐Castelan M, Gonzalez‐Pozos S, Muniz‐Hernandez S, Rojas‐Espinosa O et al Neutrophil extracellular traps are induced by Mycobacterium tuberculosis . Tuberculosis 2009; 89:29–37. [DOI] [PubMed] [Google Scholar]
- 10. Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques‐Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 2006; 16:401–7. [DOI] [PubMed] [Google Scholar]
- 11. Menegazzi R, Decleva E, Dri P. Killing by neutrophil extracellular traps: fact or folklore? Blood 2012; 119:1214–6. [DOI] [PubMed] [Google Scholar]
- 12. Parker H, Albrett AM, Kettle AJ, Winterbourn CC. Myeloperoxidase associated with neutrophil extracellular traps is active and mediates bacterial killing in the presence of hydrogen peroxide. J Leukoc Biol 2012; 91:369–76. [DOI] [PubMed] [Google Scholar]
- 13. Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M et al DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol 2006; 16:396–400. [DOI] [PubMed] [Google Scholar]
- 14. Seper A, Hosseinzadeh A, Gorkiewicz G, Lichtenegger S, Roier S, Leitner DR et al Vibrio cholerae evades neutrophil extracellular traps by the activity of two extracellular nucleases. PLoS Pathog 2013; 9:e1003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mollerherm H, Neumann A, Schilcher K, Blodkamp S, Zeitouni NE, Dersch P et al Yersinia enterocolitica‐mediated degradation of neutrophil extracellular traps (NETs). FEMS Microbiol Lett 2015; 362: fnv192. doi: 10.1093/femsle/fnv192 [DOI] [PubMed] [Google Scholar]
- 16. Lauth X, von Kockritz‐Blickwede M, McNamara CW, Myskowski S, Zinkernagel AS, Beall B et al M1 protein allows Group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J Innate Immun 2009; 1:202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cole JN, Pence MA, vonKockritz‐Blickwede M , Hollands A, Gallo RL, Walker MJ et al M protein and hyaluronic acid capsule are essential for in vivo selection of covRS mutations characteristic of invasive serotype M1T1 group A streptococcus. MBio 2010; 1(4). pii: e00191–10. doi: 10.1128/mBio.00191‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LaRock CN, Dohrmann S, Todd J, Corriden R, Olson J, Johannssen T et al Group A streptococcal M1 protein sequesters cathelicidin to Evade innate immune killing. Cell Host Microbe 2015; 18:471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL‐37, a cathelin‐associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother 1998; 42:2206–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun 2005; 73:6771–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D‐mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol 2007; 179:2060–3. [DOI] [PubMed] [Google Scholar]
- 22. Sonawane A, Santos JC, Mishra BB, Jena P, Progida C, Sorensen OE et al Cathelicidin is involved in the intracellular killing of mycobacteria in macrophages. Cell Microbiol 2011; 13:1601–17. [DOI] [PubMed] [Google Scholar]
- 23. Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL‐37 in granulocytes. Eur J Biochem 1996; 238:325–32. [DOI] [PubMed] [Google Scholar]
- 24. Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS et al Human cathelicidin, hCAP‐18, is processed to the antimicrobial peptide LL‐37 by extracellular cleavage with proteinase 3. Blood 2001; 97:3951–9. [DOI] [PubMed] [Google Scholar]
- 25. Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL‐37 in phospholipid membranes: relevance to the molecular basis for its non‐cell‐selective activity. Biochem J 1999; 341(Pt 3):501–13. [PMC free article] [PubMed] [Google Scholar]
- 26. Thennarasu S, Tan A, Penumatchu R, Shelburne CE, Heyl DL, Ramamoorthy A. Antimicrobial and membrane disrupting activities of a peptide derived from the human cathelicidin antimicrobial peptide LL37. Biophys J 2010; 98:248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vandamme D, Landuyt B, Luyten W, Schoofs L. A comprehensive summary of LL‐37, the factotum human cathelicidin peptide. Cell Immunol 2012; 280:22–35. [DOI] [PubMed] [Google Scholar]
- 28. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR et al Toll‐like receptor triggering of a vitamin D‐mediated human antimicrobial response. Science 2006; 311:1770–3. [DOI] [PubMed] [Google Scholar]
- 29. Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M et al T‐cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci USA 2010; 107:22593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S et al Vitamin D is required for IFN‐γ‐mediated antimicrobial activity of human macrophages. Sci Transl Med 2011; 3:104ra2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klug‐Micu GM, Stenger S, Sommer A, Liu PT, Krutzik SR, Modlin RL et al CD40 ligand and interferon‐γ induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology 2013; 139:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci USA 2004; 101:2422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up‐regulated in myeloid cells by 1,25‐dihydroxyvitamin D3. FASEB J 2005; 19:1067–77. [DOI] [PubMed] [Google Scholar]
- 34. Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP‐18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 1997; 90:2796–803. [PubMed] [Google Scholar]
- 35. Kahlenberg JM, Carmona‐Rivera C, Smith CK, Kaplan MJ. Neutrophil extracellular trap‐associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J Immunol 2013; 190:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang X, Basavarajappa D, Haeggstrom JZ, Wan M. P2X7 receptor regulates internalization of antimicrobial peptide LL‐37 by human macrophages that promotes intracellular pathogen clearance. J Immunol 2015; 195:1191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neumann A, Vollger L, Berends ET, Molhoek EM, Stapels DA, Midon M et al Novel role of the antimicrobial peptide LL‐37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J Innate Immun 2014; 6:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weiner DJ, Bucki R, Janmey PA. The antimicrobial activity of the cathelicidin LL37 is inhibited by F‐actin bundles and restored by gelsolin. Am J Respir Cell Mol Biol 2003; 28:738–45. [DOI] [PubMed] [Google Scholar]
- 39. Barrientos L, Marin‐Esteban V, de Chaisemartin L, Le‐Moal VL, Sandre C, Bianchini E et al An improved strategy to recover large fragments of functional human neutrophil extracellular traps. Front Immunol 2013; 4:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B et al Plasmacytoid dendritic cells sense self‐DNA coupled with antimicrobial peptide. Nature 2007; 449:564–9. [DOI] [PubMed] [Google Scholar]
- 41. Garcia‐Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z et al Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011; 3:73ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM et al Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 2011; 187:538–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 2010; 191:677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Braian C, Hogea V, Stendahl O. Mycobacterium tuberculosis‐ induced neutrophil extracellular traps activate human macrophages. J Innate Immun 2013; 5:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. O'Donnell BV, Tew DG, Jones OT, England PJ. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 1993; 290(Pt 1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp 2010; Feb 24;(36). pii: 1724. doi: 10.3791/1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lim MB, Kuiper JW, Katchky A, Goldberg H, Glogauer M. Rac2 is required for the formation of neutrophil extracellular traps. J Leukoc Biol 2011; 90:771–6. [DOI] [PubMed] [Google Scholar]
- 48. Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol 2013; 191:2647–56. [DOI] [PubMed] [Google Scholar]
- 49. Tomasinsig L, Pizzirani C, Skerlavaj B, Pellegatti P, Gulinelli S, Tossi A et al The human cathelicidin LL‐37 modulates the activities of the P2X7 receptor in a structure‐dependent manner. J Biol Chem 2008; 283:30471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant blue G selectively blocks ATP‐gated rat P2X(7) receptors. Mol Pharmacol 2000; 58:82–8. [PubMed] [Google Scholar]
- 51. Coorens M, van Dijk A, Bikker F, Veldhuizen EJ, Haagsman HP. importance of endosomal cathelicidin degradation to enhance DNA‐induced chicken macrophage activation. J Immunol 2015; 195:3970–7. [DOI] [PubMed] [Google Scholar]
- 52. Rejman J, Bragonzi A, Conese M. Role of clathrin‐ and caveolae‐mediated endocytosis in gene transfer mediated by lipo‐ and polyplexes. Mol Ther 2005; 12:468–74. [DOI] [PubMed] [Google Scholar]
- 53. Ghigo E, Kartenbeck J, Lien P, Pelkmans L, Capo C, Mege JL et al Ameobal pathogen mimivirus infects macrophages through phagocytosis. PLoS Pathog 2008; 4:e1000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Umata T, Moriyama Y, Futai M, Mekada E. The cytotoxic action of diphtheria toxin and its degradation in intact Vero cells are inhibited by bafilomycin A1, a specific inhibitor of vacuolar‐type H+‐ATPase. J Biol Chem 1990; 265:21940–5. [PubMed] [Google Scholar]
- 55. Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y. Bafilomycin A1, a specific inhibitor of vacuolar‐type H+‐ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 1991; 266:17707–12. [PubMed] [Google Scholar]
- 56. Chamilos G, Gregorio J, Meller S, Lande R, Kontoyiannis DP, Modlin RL et al Cytosolic sensing of extracellular self‐DNA transported into monocytes by the antimicrobial peptide LL37. Blood 2012; 120:3699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jann NJ, Schmaler M, Kristian SA, Radek KA, Gallo RL, Nizet V et al Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap‐associated cathelicidin. J Leukoc Biol 2009; 86:1159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Singh D, Vaughan R, Kao CC. LL‐37 peptide enhancement of signal transduction by Toll‐like receptor 3 is regulated by pH: identification of a peptide antagonist of LL‐37. J Biol Chem 2014; 289:27614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fujiwara Y, Kikuchi H, Aizawa S, Furuta A, Hatanaka Y, Konya C et al Direct uptake and degradation of DNA by lysosomes. Autophagy 2013; 9:1167–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y et al Requirement of DNase II for definitive erythropoiesis in the mouse fetal liver. Science 2001; 292:1546–9. [DOI] [PubMed] [Google Scholar]
- 61. Kawane K, Fukuyama H, Yoshida H, Nagase H, Ohsawa Y, Uchiyama Y et al Impaired thymic development in mouse embryos deficient in apoptotic DNA degradation. Nat Immunol 2003; 4:138–44. [DOI] [PubMed] [Google Scholar]
- 62. Evans CJ, Aguilera RJ. DNase II: genes, enzymes and function. Gene 2003; 322:1–15. [DOI] [PubMed] [Google Scholar]
- 63. Francis RJ, Butler RE, Stewart GR. Mycobacterium tuberculosis ESAT‐6 is a leukocidin causing Ca2+ influx, necrosis and neutrophil extracellular trap formation. Cell Death Dis 2014; 5:e1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boe DM, Curtis BJ, Chen MM, Ippolito JA, Kovacs EJ. Extracellular traps and macrophages: new roles for the versatile phagocyte. J Leukoc Biol 2015; 97:1023–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015; 349:316–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nakazawa D, Shida H, Kusunoki Y, Miyoshi A, Nishio S, Tomaru U et al The responses of macrophages in interaction with neutrophils that undergo NETosis. J Autoimmun 2016; 67:19–28. [DOI] [PubMed] [Google Scholar]
- 67. Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT et al Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol 2006; 177:1864–71. [DOI] [PubMed] [Google Scholar]
- 68. Jena P, Mohanty S, Mohanty T, Kallert S, Morgelin M, Lindstrom T et al Azurophil granule proteins constitute the major mycobactericidal proteins in human neutrophils and enhance the killing of mycobacteria in macrophages. PLoS One 2012; 7:e50345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ribeiro‐Gomes FL, Moniz‐de‐Souza MC, Alexandre‐Moreira MS, Dias WB, Lopes MF, Nunes MP et al Neutrophils activate macrophages for intracellular killing of Leishmania major through recruitment of TLR4 by neutrophil elastase. J Immunol 2007; 179:3988–94. [DOI] [PubMed] [Google Scholar]
- 70. Mathy‐Hartert M, Deby‐Dupont G, Melin P, Lamy M, Deby C. Bactericidal activity against Pseudomonas aeruginosa is acquired by cultured human monocyte‐derived macrophages after uptake of myeloperoxidase. Experientia 1996; 52:167–74. [DOI] [PubMed] [Google Scholar]
- 71. Marodi L, Tournay C, Kaposzta R, Johnston RB Jr, Moguilevsky N. Augmentation of human macrophage candidacidal capacity by recombinant human myeloperoxidase and granulocyte–macrophage colony‐stimulating factor. Infect Immun 1998; 66:2750–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nakagawa Y, Gallo RL. Endogenous intracellular cathelicidin enhances TLR9 activation in dendritic cells and macrophages. J Immunol 2015; 194:1274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Neutrophil extracellular traps (NETs) are internalized into human macrophages. Human macrophages were cultured with isolated NETs and stained with DAPI. Cells were analysed by fluorescence microscopy. White arrow points to NET DNA. (b/w): black and white.
Figure S2. Cell‐free LL37:DNA complexes. (a) Human genomic, labelled DNA (DNA594) was visualized on an agarose gel (left). DNA was complexed with LL37 or not and analysed by confocal microscopy (right). (b) Confocal microscopy analyses of LL37‐TAMRA, DNA647, as well as complexes of LL37 and DNA647 and complexes of LL37‐TAMRA and DNA647.
Figure S3. Human macrophages internalize LL37:DNA complexes. Human macrophages were incubated with DNA594 or LL37:DNA594 complexes with or without the addition of chloroquine. A minimum of 79 cells were counted and analysed for the number of DNA594 positive cells. Bars represent mean of one experiment analysed by four individual investigators. Error bars show mean of DNA594 positive cells in % ± SEM.


