Abstract
Objective
Chronic obstructive pulmonary disease (COPD) is a common co-morbidity in HIV, with prevalence and severity of disease incompletely explained by risk factors such as smoking and age. Unique HIV-associated factors, including microbial translocation, monocyte activation, and endothelial dysfunction, have been described in other co-morbidities, but have not been investigated in relation to pulmonary abnormalities in HIV. This study assessed the relationship of these pathologic processes to pulmonary function in HIV-infected and uninfected individuals and determined if relationships were unique to HIV.
Design
Longitudinal observational study
Methods
274 participants completed pulmonary function testing. Markers of inflammation (IL-6, IL-8, TNF-α), microbial translocation (lipopolysaccharide, sCD14), monocyte activation (sCD163, sCD14, and IL-2 receptor), and endothelial dysfunction (endothelin-1) were measured at baseline. Cross-sectional and longitudinal analyses were performed, adjusting for pertinent covariates.
Results
In HIV-infected individuals, higher IL-6 and endothelin-1 associated with worse FEV1 percent-predicted, and higher sCD163 associated with worse FEV1/FVC. IL-6, TNF-α, lipopolysaccharide, sCD163, IL-2 receptor, and endothelin-1 associated with diffusing impairment. sCD163 and endothelin-1 interacted with HIV status in relationship to pulmonary function. In HIV-infected individuals only, baseline endothelin-1 was associated with lower FEV1, and sCD163 and endothelin-1 were associated with lower diffusing capacity during follow-up.
Conclusions
Circulating markers of HIV-associated humoral abnormalities are associated with airflow obstruction and diffusing impairment, and baseline measures of monocyte activation and endothelial dysfunction associate with lower pulmonary function over time in HIV-infected persons. These findings suggest mechanisms of the disproportionate burden of COPD in HIV-infected persons.
Keywords: HIV, Pulmonary Disease, Chronic Obstructive, Pulmonary Diffusing Capacity, Macrophage activation, Endothelin-1
Introduction
Abnormal pulmonary function is well-documented in persons with chronic human immunodeficiency virus (HIV), and specific pulmonary diseases such as chronic obstructive pulmonary disease (COPD), asthma, and pulmonary vascular disease are also disproportionately frequent[1–15]. HIV appears to be an independent risk factor for these diseases, as these associations have been demonstrated despite consideration of known risk factors for chronic pulmonary disease such as smoking, prior pneumonia history, or injection drug use[2–4, 7, 14]. The biologic mechanisms that underlie pulmonary function abnormalities in this vulnerable population remain incompletely described.
Features unique to chronic HIV infection are likely to contribute to the unexplained differences in pulmonary disease incidence, prevalence, and progression between HIV-infected persons and the general population. One potential contributor is enhanced inflammation, generally in the context of persistent immune activation[16–18]. We have recently demonstrated cross-sectional relationships between peripheral T-lymphocyte activation and senescence and classic inflammatory markers interleukin (IL)-6 and C-reactive protein with pulmonary function testing (PFT) abnormalities in HIV[19], but longitudinal analyses are lacking. Additionally, these markers explain only a small percentage of the variance in pulmonary function among HIV-infected persons. Related and alternative processes characterizing chronic HIV that would plausibly associate with COPD include monocyte activation, microbial translocation, and endothelial dysfunction. These conditions have been related to other HIV-associated non-AIDS comorbidities, but have not been explored in HIV-associated pulmonary disease.
Abnormally elevated measures of monocyte activation, which persist despite anti-retroviral therapy (ART), have been linked to HIV progression, mortality, and HIV-associated cardiovascular and neurocognitive conditions[20–29]. Given the centricity of the alveolar macrophage to pulmonary immunity, monocyte activation may be expected to relate to HIV-associated pulmonary disease. The potential causes of immune activation (T-lymphocyte or monocyte) in HIV are myriad, but one potential specific driver is microbial translocation (MT) of bacteria across the gut epithelial barrier, with bacterial cell wall elements serving as immunogenic triggers[30–33]. Finally, endothelial damage and dysfunction are consequences of HIV infection that have been associated with cardiovascular and other HIV-associated comorbidities[34–40]. As translational and epidemiologic data suggest a link between endothelial damage and apoptosis with COPD in the general population[41–46], endothelial factors may be expected to play a role in PFT abnormalities in persons with HIV.
We investigated these three constructs (microbial translocation, macrophage activation, and endothelial dysfunction) in conjunction with classic inflammatory markers to describe their relationships to one another and to determine their potential role in HIV-related pulmonary disease. We measured markers of Th1 inflammation (IL-6, IL-8, tumor necrosis factor alpha [TNF-α]), microbial translocation (sCD14, lipopolysaccharide [LPS]), monocyte activation (sCD163, sCD14, and IL-2 soluble receptor alpha [IL-2sRa]), and endothelial dysfunction (endothelin [ET]-1) in a prospective cohort of HIV-infected and HIV-uninfected men. Using cross-sectional and longitudinal analyses, we determined which biomarkers independently associated with PFTs in HIV-infected participants, to what degree biologic markers from related pathways correlate in this population, and whether specific associations were unique to HIV-infected versus HIV-uninfected persons.
Methods
Participants
Participants included 293 men in the Lung HIV Study enrolled from 2009–2011 at 2 participating sites[47]. Recruitment of Lung HIV Study participants occurred from the Pittsburgh and Los Angeles sites of the Multicenter AIDS Cohort Study (MACS)[48], which enrolls and prospectively follows men with or at risk for HIV. Results of baseline PFTs from a subset of these men have previously been reported[4]. All participants provided informed consent, and study protocols were approved by the Institutional Review Boards of participating sites (University of Pittsburgh; University of California, Los Angeles).
Participants were seen at a baseline visit and at 18 and 36 months. Clinical characteristics including age, HIV status, race, body mass index (BMI), cigarette smoking status, cumulative pack-years of cigarettes smoked (pack-years), history of injection drug use, use of ART, and history of Pneumocystis or bacterial pneumonia were recorded at each study visit. The most proximal CD4+ T-cell counts and HIV viral loads were included from the 12 months preceding PFTs.
Pulmonary function testing
Spirometry was performed before and after bronchodilator administration, and diffusing capacity for carbon monoxide (DLCO) was measured following American Thoracic Society (ATS)/European Respiratory Society (ERS) standards[49, 50]. Spirometry reference values were determined from the third National Health and Nutrition Examination Survey equations[51], and reference values for DLCO used Neas et al. equations[52] that were adjusted for hemoglobin and carboxyhemoglobin[50]. Bronchodilator response was defined as improvement in either FEV1 or FVC of at least 200 mL and 12% between administration of bronchodilator[53]. DLCO was not adjusted for alveolar volume. Trained research technicians performed PFTs, and site pulmonologists confirmed quality compatible with ATS guidelines. If PFTs did not meet ATS guidelines, they were reviewed by site pulmonologists who determined if they were acceptable for inclusion in the study.
Biomarker measurements
Protein levels of biomarkers of IL-8, sCD14, sCD163, IL-2sRα, ET-1 were measured in serum via ELISA (R&D, Minneapolis, MN), and IL-6 and TNF-α were measured via Luminex (Luminex corporation, Austin, TX). Serum aliquots obtained at baseline visits were stored at −80°C and were assayed after a single thaw. Technical replicates were performed in triplicate, and replicates with coefficients of variation greater than 20% were rejected and the assay repeated. LPS was assayed via Limulus Amebocyte Lysate assay (Lonza; Walkersville, MD).
Statistical analysis
Variables were transformed as necessary using natural logarithm or square root to approximate normality. Baseline clinical, demographic and laboratory markers as well as PFT measures were compared between HIV-infected and HIV-uninfected groups using Student’s t-test or Chi-square test. Pearson correlation coefficients between different biomarkers were calculated in all participants and separately by HIV status. Associations between serum biomarkers and PFT measures were calculated in HIV-infected and HIV-uninfected subgroups using standardized beta in linear regression analysis (for continuous measures) or odds ratio in logistic regression (for binomial variables). We then introduced an interaction term between each serum marker and HIV status in the model to test the difference of these associations between HIV-infected and HIV-uninfected individuals.
In each model, clinical covariates entered the model if bivariate analysis demonstrated p-value of <0.2, and were maintained in the model if p-values remained at <0.05 after adjustment. Given the known strong association between smoking exposure and pulmonary function, pack-years smoked were forced into the models regardless of significance of association.
Longitudinal analyses assessing the association of baseline biomarkers with pulmonary function decline over time (18–36 months of follow-up) were performed for the HIV-infected subgroup using mixed-effect models (with random intercept for each participant). Participants were included in the analysis if they returned for one or both longitudinal visits. Time-biomarker interaction terms were tested in each mixed-effects model to measure the effect of biomarker on change of PFT during the time of follow up. Statistical analyses were performed using Stata version 14.0 (StataCorp; College Station, TX).
Results
Characteristics of the cohort
274 of 293 men performed acceptable PFTs. 124 (45.3%) were HIV-infected, and the majority of those infected were prescribed ART (87%) with clinically undetectable HIV viral load (81%)(Table 1). The median age of the cohort was 54 and was similar between HIV-infected and HIV-uninfected individuals. More than half of the cohort had history of smoking, with higher cumulative pack-years smoked among those with HIV infection. HIV-infected participants also had lower BMI, greater frequency of ever using injection drugs, and greater frequency of Pneumocystis pneumonia history.
Table 1.
Baseline characteristics of study participants
| Overall Cohort (n = 274) |
HIV-infected (n = 124) |
HIV-uninfected (n = 150) |
p-value* | |
|---|---|---|---|---|
| Age (years), median (IQR) | 54 (48–61) | 53 (48–59) | 55 (49–62) | 0.1 |
| Race/ethnicity | 0.7 | |||
| - Caucasian, n (%) | 213 (78%) | 98 (79%) | 115 (77%) | |
| - Black, n (%) | 47 (17%) | 22 (18%) | 25 (17%) | |
| - Hispanic, n (%) | 8 (3%) | 2 (2%) | 6 (4%) | |
| - Other, n (%) | 6 (2%) | 2 (2%) | 4 (3%) | |
| Body mass index, median (IQR) | 26.1 (23.7–29.7) | 25.0 (23.1–27.9) | 26.7 (24.1–30.6) | 0.001 |
| Smoking history (pack years), median (IQR) | 1.9 (0–22.1) | 5.7 (0–30.8) | 0 (0–16.5) | 0.01 |
| Smoking history, categorized | ||||
| - Ever smoker, n (%) | 148 (54%) | 74 (57%) | 74 (49%) | 0.1 |
| Injection drug use | ||||
| - Last 6 months, n (%) | 1 (3%) | 1 (6%) | 0 | 0.9 |
| - Ever, n (%) | 28 (10%) | 18 (15%) | 10 (7%) | 0.03 |
| Prior pneumonia history | ||||
| - Bacterial, n (%) | 22 (8%) | 14 (11%) | 8 (5%) | 0.1 |
| - Pneumocystis, n (%) | 4 (1%) | 4 (3%) | 0 | 0.04 |
| Using ART, n (%) | n/a | 108 (87%) | n/a | n/a |
| HIV viral load, median (IQR) | na | 49 (49–49) | n/a | n/a |
| Viral load undetectable, n (%) | n/a | 97 (81%) | n/a | n/a |
| CD4 count, median (IQR) | n/a | 560 (431–804) | n/a | n/a |
| CD4 count < 200 cells/µl , n (%) | n/a | 2 (2%) | n/a | n/a |
| FEV1%predicted, pre-BD, median (IQR) | 98 (87–110) | 98 (86–109) | 99 (89–110) | 0.3 |
| FEV1%predicted, post-BD, median (IQR) | 103 (91–112) | 101 (90–111) | 104 (92–113) | 0.3 |
| FEV1/FVC ratio, pre-BD, median (IQR) | 77 (71–80) | 77 (70–80) | 77 (72–80) | 0.9 |
| FEV1/FVC ratio, post-BD, median (IQR) | 79 (74–84) | 80 (74–84) | 79 (74–83) | 0.4 |
| FEV1/FVC ratio < 70% | ||||
| - Pre-BD n (%) | 62 (23%) | 33 (27%) | 29 (19%) | 0.1 |
| - Post-BD, n (%) | 41 (15%) | 19 (15%) | 22 (15%) | 0.9 |
| Positive bronchodilator response**, n (%) | 22 (8%) | 13 (11%) | 9 (6%) | 0.2 |
| DLCO %predicted, median (IQR) | 82 (71–92) | 77 (68–92) | 84 (73–92) | 0.03 |
| DLCO < 80 %predicted, n (%) | 123 (45%) | 69 (56%) | 54 (36%) | 0.001 |
ART: antiretroviral therapy, BD: bronchodilator, DLCO: diffusing capacity for carbon monoxide, FEV1: forced expiratory volume in one second, FVC: forced vital capacity, IQR: interquartile range
From comparison of HIV- and HIV+ group
“Bronchodilator response” defined as change in FEV1 or FVC by 12% and 200 mL followed administration of 400 mcg albuterol, as per ATS/ERS criteria
Pulmonary function testing
Cross-sectional PFT analyses including men from this cohort have previously been reported[4], and data are summarized here. In general, spirometry was normal, with median FEV1 percent-predicted values of 98% and 103% before and after the administration of bronchodilator. FEV1/FVC ratios were similarly normal, on average, but 15% of the cohort demonstrated an abnormal post-bronchodilator FEV1/FVC ratio (less than 0.7), consistent with COPD. A substantially higher number of participants (123 [45%]) had diffusing impairment (DLCO < 80% predicted), and HIV-infected persons had higher odds of diffusing impairment and significantly lower diffusing capacity on average (Table 1).
Biomarker Data
Data were available for the majority of participants, with some data incomplete due to absence of available serum or plasma samples (Supplemental Table 1). In unadjusted comparisons, biomarkers sCD163, sCD14, and IL-2sRα were significantly higher among participants with HIV (Supplemental Table 1a). One outlying influential point for IL-6 (> 500 pg/mL, in an HIV-uninfected participant) was excluded from the PFT analyses, with the rationale that it was highly irregular in the cohort. Comparisons of biomarkers based on ART status in HIV-infected persons found that ET-1 was significantly lower among persons not receiving ART, but otherwise there were no differences in biomarkers (Supplemental Table 1b). The sample size (16 persons not receiving ART) was not adequate to draw conclusions from these data.
Correlations between biomarkers
In the overall cohort, moderate associations were found between IL-6 and TNF-α (r = 0.67, p <0.0001) and between sCD163 and IL-2sRα (r = 0.39, p<0.0001); significant but weaker associations between other markers are shown in Supplemental Table 2a. Among HIV-infected individuals, associations were more robust, with a strong association between IL-6 and TNF-α (r = 0.87, p <0.0001), moderate associations between IL-8 and sCD14 (r = 0.32, p <0.001), IL-8 and sCD163 (r = 0.30, p <0.001), TNF-α and LPS (r = 0.31, p <0.001), and sCD163 and IL-2sRα (r = 0.38, p <0.0001). Weaker associations reaching significance are shown in Supplemental Table 2b. LPS and sCD14, both included as potential markers of microbial translocation, did not correlate, nor were there strong correlations among all of the Th1 biomarkers or between the monocyte activation markers sCD163 and sCD14.
Biomarker associations with pulmonary function
Spirometry, cross-sectional analyses
Results of analyses for pre-bronchodilator and post-bronchodilator spirometry were not meaningfully different; therefore, we report only post-bronchodilator analyses. Pack-years smoked retained significance in nearly all multivariable models. In only one instance did an additional variable enter a model: age retained significance in the model examining sCD163 and FEV1/FVC ratio in HIV-infected persons, but did not significantly change the association.
In the HIV-infected group, several significant associations were found. Higher IL-6 was associated with lower post-bronchodilator FEV1 percent-predicted (beta = −0.24, p = 0.01). Higher sCD163 was associated with lower post-bronchodilator FEV1/FVC ratios (beta = −0.23, p = 0.01) and greater odds of bronchodilator response (OR 4.9, p = 0.04). Higher ET-1 was also associated with lower post-bronchodilator FEV1 percent-predicted values (beta = −0.36, p < 0.001), greater odds of post-bronchodilator airflow obstruction (FEV1/FVC < 0.7; OR 4.8, p = 0.04), and greater odds of bronchodilator response (OR 7.6, p = 0.03). Among HIV-uninfected participants, higher IL-6 (beta = −0.20, p = 0.01) and IL-2sRα (beta = −0.20, p = 0.02) were associated with lower post-bronchodilator FEV1 percent-predicted (Table 2).
Table 2.
Adjusted associations between circulating biomarkers and pulmonary function testing in HIV-infected and uninfected participants
| IL-6 | HIV-infected | HIV-uninfected | p-int HIV | ||
|---|---|---|---|---|---|
| beta | p-value | beta | p-value | ||
| FEV1%predicted, post-BD | −0.24 | 0.01 | −0.20 | 0.01 | 0.6 |
| FEV1/FVC ratio, post-BD | −0.13 | 0.2 | 0.02 | 0.8 | 0.3 |
| DLCO %predicted | −0.35 | <0.001 | −0.37 | <0.001 | 0.7 |
| OR | p-value | OR | p-value | ||
| FEV1/FVC < 70%, post-BD | 1.04 | 0.09 | 1.0 | 0.9 | 0.2 |
| Positive BD response | 1.06 | 0.051 | 1.0 | 0.9 | 0.1 |
| IL-8 | |||||
| beta | p-value | beta | p-value | ||
| FEV1%predicted, post-BD | −0.17 | 0.06 | 0.05 | 0.6 | 0.08 |
| FEV1/FVC ratio, post-BD | −0.16 | 0.08 | 0.04 | 0.7 | 0.1 |
| DLCO %predicted | −0.12 | 0.2 | 0.0 | n/a | 0.3 |
| OR | p-value | OR | p-value | ||
| FEV1/FVC < 70%, post-BD | 2.4 | 0.1 | 1.0 | 0.9 | 0.3 |
| Positive BD response | 1.8 | 0.4 | 1.3 | 0.8 | 0.8 |
| TNF-α | |||||
| beta | p-value | beta | p-value | ||
| FEV1%predicted, post-BD | −0.18 | 0.07 | −0.05 | 0.5 | 0.3 |
| FEV1/FVC ratio, post-BD | −0.04 | 0.7 | 0.10 | 0.2 | 0.3 |
| DLCO %predicted | −0.38 | <0.001 | −0.32 | <0.001 | 0.6 |
| OR | p-value | OR | p-value | ||
| FEV1/FVC < 70%, post-BD | 1.01 | 0.2 | 1.0 | 0.9 | 0.3 |
| Positive BD response | 1.02 | 0.03 | 1.0 | 0.6 | 0.06 |
| LPS | |||||
| beta | p-value | beta | p-value | ||
| FEV1%predicted, post-BD | 0.07 | 0.5 | −0.06 | 0.5 | 0.5 |
| FEV1/FVC ratio, post-BD | 0.07 | 0.5 | −0.01 | 0.9 | 0.7 |
| DLCO %predicted | −0.19 | 0.03 | −0.24 | 0.004 | 0.9 |
| OR | p-value | OR | p-value | ||
| FEV1/FVC < 70%, post-BD | 0.7 | 0.4 | 1.0 | 0.9 | 0.7 |
| Positive BD response | 1.2 | 0.7 | 1.0 | 0.9 | 0.8 |
| sCD14 | |||||
| beta | p-value | beta | p-value | ||
| FEV1%predicted, post-BD | −0.08 | 0.5 | −0.11 | 0.2 | 0.7 |
| FEV1/FVC ratio, post-BD | −0.12 | 0.2 | 0.04 | 0.6 | 0.2 |
| DLCO %predicted | −0.06 | 0.5 | −0.05 | 0.5 | 0.9 |
| OR | p-value | OR | p-value | ||
| FEV1/FVC < 70%, post-BD | 2.4 | 0.4 | 0.5 | 0.5 | 0.2 |
| Positive BD response | 0.2 | 0.09 | 0.6 | 0.7 | 0.5 |
| sCD163 | |||||
| beta | p-value | beta | p-value | ||
| FEV1%predicted, post-BD | −0.13 | 0.2 | −0.12 | 0.2 | 0.8 |
| FEV1/FVC ratio, post-BD | −0.23 | 0.01 | −0.07 | 0.4 | 0.2 |
| DLCO %predicted | −0.18 | 0.04 | −0.15 | 0.07 | 0.6 |
| OR | p-value | OR | p-value | ||
| FEV1/FVC < 70%, post-BD | 3.8 | 0.06 | 1.2 | 0.7 | 0.2 |
| Positive BD response | 4.9 | 0.04 | 0.6 | 0.5 | 0.05 |
| IL-2sRα | |||||
| beta | p-value | beta | p-value | ||
| FEV1%predicted, post-BD | −0.12 | 0.2 | −0.20 | 0.02 | 0.4 |
| FEV1/FVC ratio, post-BD | −0.04 | 0.6 | −0.07 | 0.4 | 0.8 |
| DLCO %predicted | −0.25 | 0.005 | −0.24 | 0.004 | 0.9 |
| OR | p-value | OR | p-value | ||
| FEV1/FVC < 70%, post-BD | 1.4 | 0.6 | 1.6 | 0.5 | 0.9 |
| Positive BD response | 1.4 | 0.6 | 1.4 | 0.7 | 0.9 |
| ET-1 | |||||
| beta | p-value | beta | p-value | ||
| FEV1%predicted, post-BD | −0.36 | <0.001 | −0.04 | 0.6 | 0.003 |
| FEV1/FVC ratio, post-BD | −0.14 | 0.1 | −0.11 | 0.2 | 0.8 |
| DLCO %predicted | −0.32 | <0.001 | −0.07 | 0.4 | 0.02 |
| OR | p-value | OR | p-value | ||
| FEV1/FVC < 70%, post-BD | 4.8 | 0.04 | 1.9 | 0.3 | 0.3 |
| Positive BD response | 7.6 | 0.03 | 4.9 | 0.053 | 0.8 |
BD: bronchodilator, DLCO: diffusing capacity for carbon monoxide, ET-1: endothelin-1, FEV1: forced expiratory volume in one second, FVC: forced vital capacity, IL: interleukin, IL-2sRα: IL-2 soluble receptor alpha, LPS: lipopolysaccharide, TNF-α: tumor necrosis factor alpha
p-int HIV: p-value for interaction of biomarker and HIV status with pulmonary function outcome
Diffusing Capacity, cross-sectional analyses
Among HIV-infected participants, several variables were found to associate independently with lower DLCO percent-predicted values, including IL-6 (beta = −0.35, p < 0.001), TNF-α (beta = −0.38, p < 0.001), LPS (beta = −0.19, p = 0.03), sCD163 (beta = −0.18, p = 0.04), IL-2sRα (beta = −0.25, p = 0.005), and ET-1 (beta = −0.32, p <0.001). In the HIV-uninfected group, IL-6, TNF-α, LPS, and IL-2sRα demonstrated similar associations with DLCO percent-predicted (Table 2).
Interaction with HIV infection
Given the observed differences in relationships of several biomarker levels and PFTs between HIV-infected and uninfected participants, interaction testing was performed. ET-1 demonstrated interaction with HIV in relationship to FEV1 percent-predicted and DLCO percent-predicted values, and sCD163 demonstrated interaction with HIV related to bronchodilator response (Table 2, Figure 1).
Figure 1.
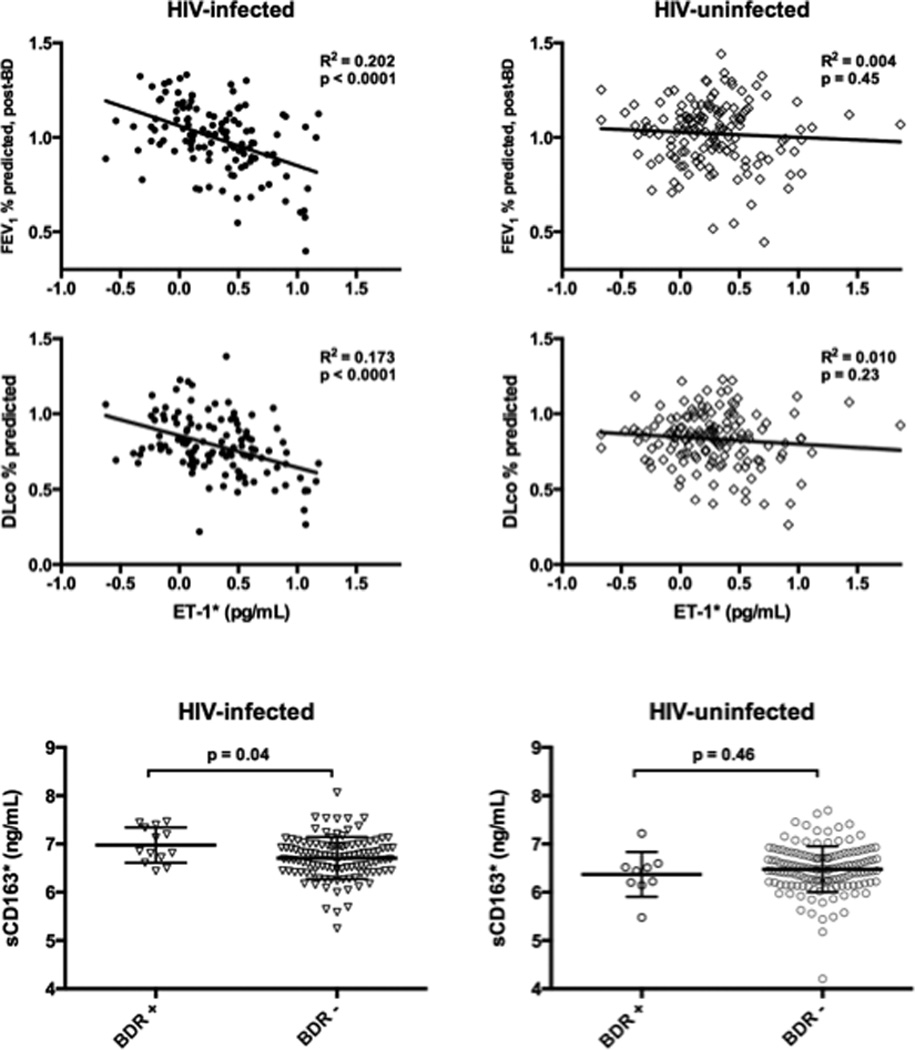
Unadjusted relationships between biomarkers and pulmonary function in instances where HIV status modifies the association.
BDR: bronchodilator response, DLco: diffusing capacity for carbon monoxide, FEV1: forced expiratory volume in one second
ET-1: endothelin-1 * log transformation
Line and whiskers represent mean and standard deviation.
Longitudinal Analyses
Seventy of 124 HIV-infected participants and 72 of 150 HIV-uninfected persons with biomarker data returned for at least one planned follow-up visit and were included in the longitudinal analyses. Compared to those lost to follow up, those who returned for scheduled visits were younger, more frequently of black race, had worse baseline pulmonary function on all measures, and had higher levels of several serum inflammatory markers. There were no differences in follow-up by HIV status, and no difference in HIV-related variables between those who did and did not return (Supplemental Table 3).
In those with longitudinal data, post-bronchodilator FEV1 percent-predicted values decreased over time, and trends for decrease were similar among HIV-infected and HIV-uninfected individuals (Figure 2). Percent-predicted diffusing capacity appeared to increase over time (similarly in both groups, Figure 2), though this was likely due to surveillance bias or regression to the mean, as including all participants at each time point (i.e. not excluding participants’ values from the baseline visit if they had no subsequent visit) revealed that DLCO may in fact remain stable, as persons who did not follow up tended to have better DLCO percent-predicted at baseline than those who did not (Supplemental Figure 1).
Figure 2.
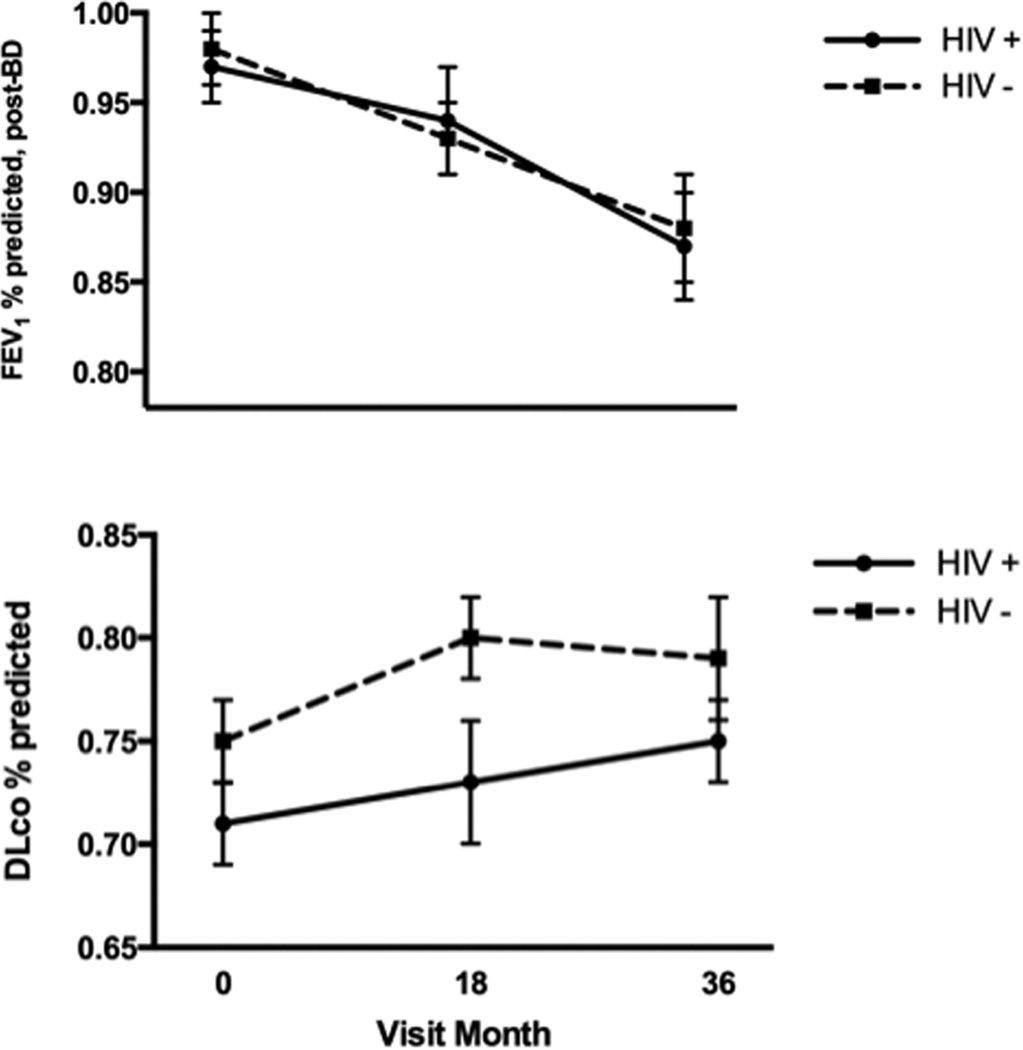
Unadjusted trends of pulmonary function over longitudinal follow-up in HIV-infected and uninfected persons
Data are included only for persons who returned for at least one follow-up visit.
HIV-positive, n = 70; HIV-negative, n = 98
Errors bars represent standard error of the mean.
In HIV-infected participants with repeated measures of PFTs, in models adjusting for time constants and pack-years smoked, two circulating biomarkers contributed significantly to lower PFT. Baseline levels of ET-1 significantly independently associated with lower post-bronchodilator FEV1 percent-predicted over time, and baseline levels of sCD163 and ET-1 independently associated with lower diffusing capacity percent-predicted over time (Table 3). Time-biomarker interaction terms were all non-significant (ET-1 with FEV1 percent-predicted: p=0.16; ET-1 with diffusing capacity percent-predicted: p=0.9: sCD163 with diffusing capacity percent-predicted: p=0.3), suggesting that while higher baseline biomarker levels are associated with lower pulmonary function over time, they do not independently predict a more rapid rate of decline of FEV1 or DLCO percent-predicted. No associations were found between baseline biomarkers and PFT changes in HIV-uninfected persons.
Table 3.
Multivariable models for baseline biomarkers significantly associated with lower pulmonary function in HIV-infected participants during follow-up
| Post-bronchodilator FEV1 percent-predicted | beta (95% CI) | p-value | |
|---|---|---|---|
| ET-1* | −0.20 (−0.30 – −0.11) | <0.001 | |
| Pack-years smoked** | −0.01 (−0.03 – −0.002) | 0.02 | |
| Time (per year) | −0.03 (−0.04 – −0.02) | <0.001 | |
| Interaction term between ET-1 and time | ---- | 0.16 | |
| DLCO percent-predicted | beta (95% CI) | p-value | |
| sCD163* | −0.08 (−0.16 – −0.001) | 0.047 | |
| Pack-years smoked** | −0.02 (−0.03 – −0.005) | 0.005 | |
| Time (per year) | 0.02 (0.006 – 0.04) | 0.005 | |
| Interaction term between sCD163 and time | ---- | 0.90 | |
| DLCO percent-predicted | beta (95% CI) | p-value | |
| ET-1* | −0.10 (−0.20 – −0.006) | 0.04 | |
| Pack-years smoked** | −0.01 (−0.03 – −0.001) | 0.04 | |
| Time (per year) | 0.02 (0.007 – 0.04) | 0.005 | |
| Interaction term between ET-1 and time | ---- | 0.30 | |
CI: confidence interval, DLCO: diffusing capacity for carbon monoxide, ET-1: endothelin-1, FEV1: forced expiratory volume in one second
Log transformation
Square-root transformation
To further evaluate the impact of smoking status on the relationship between biomarkers and pulmonary function in HIV-infected participants, we compared the longitudinal relationships between biomarkers and PFTs between never-smoker and smokers (current or former). While the relationships between ET-1 and FEV1 percent-predicted did not vary by smoking status, the relationships between both ET-1 and sCD163 with DLCO percent-predicted appeared to be limited to smokers; though inferences should be made with caution given the small number (n = 28) of HIV-infected non-smokers with longitudinal follow-up (Supplemental Table 4).
Exploratory multivariable regression path analyses were performed to assess whether the effects of either sCD163 or ET-1 on pulmonary function in HIV-infected participants were mediated by the other. In these models, we assumed that each biomarker had two distinct effects on pulmonary function, one direct and one indirect. Models were adjusted for pack-years smoked. We also performed cross-sectional cyclic path analyses to explore the converse hypothesis that impaired pulmonary function may be driving the biomarker abnormalities – these models had fewer significant associations for direct and indirect effects and were not as well-fitted as the models describing a path proceeding from biomarker to pulmonary function. The best-fitted models suggested that part of the effects of sCD163 on diffusing capacity and post-bronchodilator FEV1 percent-predicted may be mediated through ET-1 (Supplemental Figure 2).
Discussion
In a well-characterized cohort of both HIV-infected and HIV-uninfected men, we found cross-sectional associations of several inflammatory biomarkers (IL-6, TNF-α, LPS, sCD163, and IL-2sRa) and the endothelial mediator ET-1 with worse pulmonary function in the cohort. Additionally, we identified interactions between HIV seropositivity and two mediators (sCD163 and ET-1) with measures of obstruction or diffusing impairment. Finally, we demonstrated that among HIV-infected participants with serial pulmonary function testing, baseline sCD163 and ET-1 continued to be associated with lower pulmonary function in longitudinal analysis.
Prior studies have demonstrated associations between chronic HIV infection and pulmonary dysfunction; with reports of more frequent respiratory symptoms[14], worse diffusing capacity[4, 7], and more frequent COPD diagnoses and radiographic emphysema[1–3, 14, 54] among persons with HIV. These observations are in part related to known exposures, most notably high frequency and intensity of cigarette smoking[55, 56], but analyses in well-conducted studies have established that a gap persists between HIV-infected and uninfected groups independent of age, smoking, and prior pulmonary infection[1–3, 14, 54]. We have previously shown an association between systemic inflammation and T-lymphocyte activation with HIV-associated COPD[19], but data are lacking regarding integration with specific pathways. Features relatively unique to persons with HIV that may contribute to pulmonary pathophysiology include persistent immune activation, microbial translocation, and endothelial dysfunction.
A major finding of this study is the novel discovery that endothelin-1 is independently associated with worse airflow obstruction and diffusing capacity impairment in persons with HIV, and that it is associated with lower FEV1 percent-predicted and lower DLCO percent-predicted over time. Endothelial dysfunction is a complication of acute and chronic HIV[35, 36], and markers of endothelial activation (including cellular adhesion molecules, methylarginines, fibrinogen, and D-dimer) have been associated with cardiovascular disease in HIV[34, 37, 39, 40]. Endothelial damage markers have also been associated with COPD in the general population[42–46]. Interestingly, parallel associations between ET-1 and PFTs were not observed in the HIV-uninfected participants, despite similar median levels of ET-1 in HIV-infected and uninfected persons. The lack of association in the HIV-uninfected subset is unlikely to be explained by lower statistical power due to sample size or precision relative to HIV-infected, given that the HIV-uninfected group was larger, and the statistical variances of affected PFT values were similar between the two groups. Interaction testing confirmed significant interactions between ET-1 levels and HIV serostatus with post-bronchodilator FEV1 percent-predicted and DLCO percent-predicted in this cohort, supporting a larger effect size of ET-1 relative to PFT outcomes in HIV-infected persons.
The finding of circulating ET-1 in association with PFT abnormalities adds to the existing literature describing a relationship between endothelial dysfunction and pulmonary disease, though in our cohort the association was only seen in HIV-infected persons. In a small study of individuals with COPD, plasma ET-1 negatively correlated with FEV1, and ET-1 was increased in sputum and plasma during exacerbations, compared to controls during clinical stability[45]. Additionally, ET-1 is increased in exhaled breath condensate in exercise-induced bronchospasm and asthma[57], and has been related to the development of bronchiolitis obliterans syndrome in pulmonary allografts[58]. Recently, a very large population study (not selecting for persons with respiratory disease) found ET-1 levels to negatively correlate with FEV1[44]. Other investigations have related pulmonary outcomes to vascular measures of endothelial function; one such study demonstrated cross-sectional associations between brachial artery flow-mediated dilation, FEV1, and quantitative CT emphysema scores[41]. Another recent investigation found a relationship between quantitative CT measure of lung total tissue volume and DLCO, further supporting that the pulmonary microvasculature plays a significant role in the gas exchange abnormalities of COPD[59].
The drivers of ET-1 expression are not completely elucidated, but include reactive oxygen species, inflammatory mediators, and hypoxia-related factors[60]. In addition to its most clearly-defined function as a potent vasoregulator, ET-1 is a bronchoreactive mediator [61, 62] and an inflammatory regulator, having been found in vivo to affect regional expression of IL-6[63, 64], monocyte chemoattractant protein-1[63], CX3CL1[65], and other inflammatory cytokines[66]. Animal and in vivo studies suggest potential mechanisms of endothelial-pulmonary associations, including emphysema secondary to endothelial apoptosis and endothelial inflammation, regional inflammation and chemotaxis, and matrix metalloproteinase production[67–70] [43, 46].
The greater relationship between ET-1 and PFTs in HIV-infected persons, despite similar circulating ET-1 levels to HIV-uninfected persons in the cohort, is not explained. It is possible that persons with HIV demonstrate more local variation in ET-1 regulation or receptor expression – for instance, one study has found that in response to vasoreactive challenge, local ET-1 remained elevated in persons with hypertension, but not in healthy volunteers, suggesting regional dysregulation with lack of adaptation[71]. Whether a similar local adaptation failure may be present in HIV is unknown. Additionally, the source of elevated circulating ET-1 in our participants is not known. Although pulmonary vascular endothelium is the most common location of ET-1 synthesis, ET-1 can be produced by many cell types, notably epithelium, smooth muscle, and immune cells, including alveolar macrophages[72, 73]. ET-1 expression from alveolar macrophages has been shown to be increased in congestive heart failure patients, although the enhanced elaboration from macrophages did not correlate with serum levels[73]. Future studies measuring ET-1 levels from the pulmonary compartment or assessing regional ET-B receptor expression may help explain the mechanism of its differential effect in HIV-infected versus uninfected persons.
The second novel finding of this study, equally important, is the association between markers of monocyte activation with pulmonary function in persons with HIV. Monocyte activation is a feature of both acute and chronic HIV infection[20] and is an important correlate of HIV-associated morbidity in the ART era[21]. Commonly measured markers of monocyte activation include sCD163, a circulating molecule which is enzymatically cleaved from monocytes upon activation[74], and sCD14. Both markers are elevated in HIV infection, including among persons with viral control[24, 25, 27, 28]. Additionally, both are increased in inflammatory HIV-associated non-AIDS conditions such as cardiovascular disease, atherosclerosis,[20, 29, 75–78] and HIV-associated neurocognitive disorders[22, 23, 26, 79]. Soluble IL-2 receptor alpha (IL-2sRα, or sCD25), the circulating cleavage product of the IL-2 receptor (CD25), is a marker of disease activity in chronic inflammatory diseases[80–82]. CD25 is present on many leukocytes, including activated T-lymphocytes, and IL-2sRα has thus been used as a circulating surrogate of T-lymphocyte activation[83, 84]; as such, it has been found to associate with cardiovascular risk among women with HIV[84]. Of interest, however, monocytes also elaborate CD25, and IL-2sRα levels have been associated with percentage of non-classical monocytes while failing to associate with T-lymphocyte subsets[81]. In this cohort, regardless of HIV serostatus, IL-2sRα significantly correlated with sCD163 and sCD14, suggesting that it may be linked to monocyte activation. Given the importance of macrophages to pulmonary immunity and their relationship to COPD[85], monocyte contributions to pulmonary dysfunction may also be significant among persons with HIV, but these markers had not previously been investigated with reference to PFTs.
In the current study, median sCD163, sCD14, and IL-2sRα values were significantly higher in the HIV-infected than uninfected group, in keeping with prior data[24, 28, 29, 84]. sCD163 independently associated with worse pulmonary dysfunction, including lower FEV1/FVC post-bronchodilator ratio, greater odds of bronchodilator responsiveness, and lower DLCO percent-predicted in HIV-infected participants. Similar associations were not demonstrated in the HIV-uninfected group, though there were fewer persons with a bronchodilator response (9/150 [6%] versus 13/124 [11%]), which reduced power to detect an association. Nonetheless, a statistical interaction was detected between HIV seropositivity and positive bronchodilator response. IL-2sRα was also associated with DLCO percent-predicted among HIV-infected persons in this cohort, but unlike sCD163, relationships were nearly identical in the HIV-uninfected group. Importantly, sCD163 baseline levels also were associated with lower DLCO over the period of follow-up, though no significant longitudinal association was found with FEV1. Exploratory path analyses supported that the effect of sCD163 on FEV1 and DLCO percent-predicted are at least partially mediated by ET-1, suggesting that monocyte activation and endothelial dysfunction may be linked with regard to pulmonary disease in HIV-infected persons. These findings require further exploration in mechanistic studies.
Of the humoral inflammatory markers investigated, IL-6 and TNF-α were higher in HIV-infected individuals with worse pulmonary function. Higher plasma IL-6 levels were associated with both lower post-bronchodilator FEV1 percent-predicted and diffusing impairment and, and TNF-α was associated with bronchodilator response and lower DLCO percent-predicted. Relationships were similar between HIV-infected and uninfected participants. IL-6 and TNF-α levels were closely correlated in this cohort, suggesting they represent the same Th1 inflammatory pathway. Consistent with findings in some general population COPD analyses[86, 87], these markers associated with spirometry in the HIV-uninfected group. We have found similar associations with IL-6 and with C-reactive protein (not measured in this study) with PFTs in a different HIV-infected cohort[19], and these inflammatory markers have been associated with other HIV-associated chronic diseases, including cardiovascular disease[17, 88], and with COPD outcomes in case of IL-6[87, 89]. One prior HIV study comparing IL-6 to radiographic emphysema found no relationship between the two, though that study did not include PFTs to allow for direct comparison to our data[54]. Notably, despite previous findings of IL-8 associating with elevated pulmonary artery systolic pressure and dyspnea and HIV-infected persons[12], IL-8 did not significantly associate with PFTs in this cohort. This finding is in keeping with our previous data in a different HIV-infected cohort[19] and with available COPD literature[86].
Microbial translocation is a feature of HIV infection[33] and has been linked to chronic immune activation despite ART[30–32]; due to this association with systemic inflammation, potential for contribution to pulmonary disease, particularly COPD, is compelling. A previous investigation of potential microbial translocation markers in persons with HIV found an independent association between sCD14 and risk of radiographic emphysema, though this study did not assess spirometry[54]. In our analysis, we measured circulating levels of microbial translocation markers LPS and the soluble form of its ligand (sCD14). LPS was associated with lower DLCO in both HIV-infected and uninfected groups, but there was no association with obstruction. There were no associations between sCD14 levels and any pulmonary function outcome in either HIV-infected or HIV-uninfected participants. In the current study, with mostly well-controlled HIV (81% of HIV-infected participants virally-suppressed, 2% with CD4+ T-cell counts <200 cells/uL), LPS and sCD14 did not correlate. This lack of association suggests that in this cohort, sCD14 does not specifically represent the construct of “microbial translocation,” but may represent non-specific monocyte activation, as discussed above. Although prior microbial translocation data have demonstrated that high levels of sCD14 in the plasma reflect LPS exposure[30], the two markers have not been reliably correlated in persons without significant immune suppression[90]; furthermore, in vivo work has shown IL-6 or IL-1β stimulation can induce sCD14 production comparable to that induced by LPS[91], suggesting inflammatory signaling independent of microbial translocation. Microbial translocation cannot be discounted as a factor in HIV-associated pulmonary disease based on this relatively small sample, but the contributions compared to other pathways appear to be limited.
We did not find differences in PFT decline (FEV1 or DLCO percent-predicted) between HIV-infected and –uninfected participants in this cohort, possibly due to the relatively limited number of participants who returned for serial testing. Our results were similar to those from another longitudinal cohort, where no differences were found in longitudinal spirometry between HIV-infected and –uninfected groups unless participants were analyzed in strata of poor viral control or CD4+ cell deficiency[92]. We did not have an adequate number of poorly controlled participants to independently assess this factor in this study. In addition, because the MACS was started in the 1980s, the cohort may suffer from survivor bias, and current individuals may have better pulmonary function than those who have died.
This study has several limitations. First, only circulating biomarkers were assessed. Evaluation of biomarker and receptor concentrations from respiratory samples or human bronchial epithelial cells with similar correlations to circulating markers and to pulmonary function will be revealing regarding systemic and local relationships of these molecules. Additionally, complementary vascular mediators (such as endothelial growth factors, cellular adhesion molecules, and methylarginines) and other markers of endothelial apoptosis, such as endothelial microparticles, were not examined in this cohort. Further studies of the associations between levels of ET-1 and other vascular mediators and pulmonary outcomes in HIV-infected populations are warranted. A final important limitation of this study is that the findings are limited to men. COPD phenotypes and pathogenesis demonstrate sex variation[93, 94]; additionally, biomarker levels and activity may vary between males and females. ET-1 demonstrates sex differences in the cardiovascular system[95] and kidney[96] and sCD163 has been found to be higher in women[97, 98], with possible modulation by sex hormones[99]. These associations require confirmation in a cohort including female participants.
A major strength of our current study is the longitudinal follow-up, which is more strongly suggestive of a relationship between immune cell activation and endothelial dysfunction with reduction in pulmonary function than are prior cross-sectional analyses. Additionally, including both HIV-infected and –uninfected participants allowed direct comparison between groups and identification of pathophysiology that may be particularly enhanced in persons with HIV. Finally, assessing multiple biomarkers in one cohort permitted comparison of their relationships, allowing hypothesis-generating inferences for possible interactions between biologic pathways. In conclusion, this study discovered two new potential arenas contributing to the disproportionate burden of pulmonary dysfunction among persons with HIV. Further exploration of monocyte activation and endothelial dysfunction in the role of HIV-associated lung disease and symptoms may lead to new implications for treatment in this population.
Supplementary Material
Acknowledgments
Sources of Support: F32 HL114426 (MF); K24 HL123342, P01 HL103455, R01 HL090339 (AM)
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
Authors’ Contributions to the Study:
MEF: acquisition and interpretation of data for the work, drafting and revision of the work for intellectual content
MN: analysis of data for the work, drafting and revision of the work for intellectual content
MRG: acquisition of data for the work, revision of the work for intellectual content
DC: acquisition of data for the work, revision of the work
CK: acquisition of data for the work, revision of the work
JBS: acquisition of data for the work, revision of the work
AC: acquisition of data for the work, revision of the work
JWR: acquisition of data for the work, revision of the work
ECK: conception of the work, revision of the work for intellectual content
LK: conception of the work, revision of the work for intellectual content
AM: conception and design of the work, critical review of analysis and interpretation of data for the work, revision of the work for intellectual content
REFERENCES
- 1.Diaz PT, King MA, Pacht ER, Wewers MD, Gadek JE, Nagaraja HN, et al. Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med. 2000;132:369–372. doi: 10.7326/0003-4819-132-5-200003070-00006. [DOI] [PubMed] [Google Scholar]
- 2.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 3.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crothers K, McGinnis K, Kleerup E, Wongtrakool C, Hoo GS, Kim J, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr. 2013;64:271–278. doi: 10.1097/QAI.0b013e3182a9215a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui Q, Carruthers S, McIvor A, Smaill F, Thabane L, Smieja M. Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Res Ther. 2010;7:6–16. doi: 10.1186/1742-6405-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drummond MB, Kirk GD, Astemborski J, Marshall MM, Mehta SH, McDyer JF, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012;67:309–314. doi: 10.1136/thoraxjnl-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzpatrick ME, Gingo MR, Kessinger C, Lucht L, Kleerup E, Greenblatt RM, et al. HIV infection is associated with diffusing capacity impairment in women. J Acquir Immune Defic Syndr. 2013;64:284–288. doi: 10.1097/QAI.0b013e3182a9213a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George MP, Kannass M, Huang L, Sciurba FC, Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4:e6328. doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, Weinman R, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirani A, Cavallazzi R, Vasu T, Pachinburavan M, Kraft WK, Leiby B, et al. Prevalence of obstructive lung disease in HIV population: a cross sectional study. Respir Med. 2011;105:1655–1661. doi: 10.1016/j.rmed.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, et al. High Prevalence of Echocardiographic Abnormalities among HIV-infected Persons in the Era of Highly Active Antiretroviral Therapy. Clin Infect Dis. 2011;52:378–386. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 12.Morris A, Gingo MR, George MP, Lucht L, Kessinger C, Singh V, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2012;26:731–740. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, De Zuttere D, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 14.Gingo MR, Balasubramani GK, Rice TB, Kingsley L, Kleerup EC, Detels R, et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm Med. 2014;14:75. doi: 10.1186/1471-2466-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gingo MR, Wenzel SE, Steele C, Kessinger CJ, Lucht L, Lawther T, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy Clin Immunol. 2012;129:708–714. doi: 10.1016/j.jaci.2011.11.015. e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 17.Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55:126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fitzpatrick ME, Singh V, Bertolet M, Lucht L, Kessinger C, Michel J, et al. Relationships of pulmonary function, inflammation, and T-cell activation and senescence in an HIV-infected cohort. AIDS. 2014;28:2505–2515. doi: 10.1097/QAD.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Justice AC, Freiberg MS, Tracy R, Kuller L, Tate JP, Goetz MB, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. doi: 10.1093/cid/cir989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson AM, Harezlak J, Bharti A, Mi D, Taylor MJ, Daar ES, et al. Plasma and Cerebrospinal Fluid Biomarkers Predict Cerebral Injury in HIV-Infected Individuals on Stable Combination Antiretroviral Therapy. J Acquir Immune Defic Syndr. 2015;69:29–35. doi: 10.1097/QAI.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KC. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS. 2013;27:1387–1395. doi: 10.1097/QAD.0b013e32836010bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castley A, Berry C, French M, Fernandez S, Krueger R, Nolan D. Elevated plasma soluble CD14 and skewed CD16+ monocyte distribution persist despite normalisation of soluble CD163 and CXCL10 by effective HIV therapy: a changing paradigm for routine HIV laboratory monitoring? PLoS One. 2014;9:e115226. doi: 10.1371/journal.pone.0115226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26:843–853. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 26.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, et al. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, et al. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One. 2013;8:e55279. doi: 10.1371/journal.pone.0055279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendez-Lagares G, Romero-Sanchez MC, Ruiz-Mateos E, Genebat M, Ferrando-Martinez S, Munoz-Fernandez MA, et al. Long-term suppressive combined antiretroviral treatment does not normalize the serum level of soluble CD14. J Infect Dis. 2013;207:1221–1225. doi: 10.1093/infdis/jit025. [DOI] [PubMed] [Google Scholar]
- 29.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 31.Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–733. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 32.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 2013;26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14:385–390. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arildsen H, Sorensen KE, Ingerslev JM, Ostergaard LJ, Laursen AL. Endothelial dysfunction, increased inflammation, and activated coagulation in HIV-infected patients improve after initiation of highly active antiretroviral therapy. HIV Med. 2013;14:1–9. doi: 10.1111/j.1468-1293.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 36.Graham SM, Rajwans N, Jaoko W, Estambale BB, McClelland RS, Overbaugh J, et al. Endothelial activation biomarkers increase after HIV-1 acquisition: plasma vascular cell adhesion molecule-1 predicts disease progression. AIDS. 2013;27:1803–1813. doi: 10.1097/QAD.0b013e328360e9fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jang JJ, Berkheimer SB, Merchant M, Krishnaswami A. Asymmetric dimethylarginine and coronary artery calcium scores are increased in patients infected with human immunodeficiency virus. Atherosclerosis. 2011;217:514–517. doi: 10.1016/j.atherosclerosis.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Parikh RV, Scherzer R, Grunfeld C, Nitta EM, Leone A, Martin JN, et al. Elevated levels of asymmetric dimethylarginine are associated with lower CD4+ count and higher viral load in HIV-infected individuals. Atherosclerosis. 2013;229:246–252. doi: 10.1016/j.atherosclerosis.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh RV, Scherzer R, Nitta EM, Leone A, Hur S, Mistry V, et al. Increased levels of asymmetric dimethylarginine are associated with pulmonary arterial hypertension in HIV infection. AIDS. 2014;28:511–519. doi: 10.1097/QAD.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross AC, Rizk N, O’Riordan MA, Dogra V, El-Bejjani D, Storer N, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barr RG, Mesia-Vela S, Austin JH, Basner RC, Keller BM, Reeves AP, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176:1200–1207. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carratu P, Scoditti C, Maniscalco M, Seccia TM, Di Gioia G, Gadaleta F, et al. Exhaled and arterial levels of endothelin-1 are increased and correlate with pulmonary systolic pressure in COPD with pulmonary hypertension. BMC Pulm Med. 2008;8:20. doi: 10.1186/1471-2466-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon C, Gudi K, Krause A, Sackrowitz R, Harvey BG, Strulovici-Barel Y, et al. Circulating endothelial microparticles as a measure of early lung destruction in cigarette smokers. Am J Respir Crit Care Med. 2011;184:224–232. doi: 10.1164/rccm.201012-2061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oelsner EC, Pottinger TD, Burkart KM, Allison M, Buxbaum SG, Hansel NN, et al. Adhesion molecules, endothelin-1 and lung function in seven population-based cohorts. Biomarkers. 2013;18:196–203. doi: 10.3109/1354750X.2012.762805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roland M, Bhowmik A, Sapsford RJ, Seemungal TA, Jeffries DJ, Warner TD, et al. Sputum and plasma endothelin-1 levels in exacerbations of chronic obstructive pulmonary disease. Thorax. 2001;56:30–35. doi: 10.1136/thorax.56.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomashow MA, Shimbo D, Parikh MA, Hoffman EA, Vogel-Claussen J, Hueper K, et al. Endothelial microparticles in mild chronic obstructive pulmonary disease and emphysema. The Multi-Ethnic Study of Atherosclerosis Chronic Obstructive Pulmonary Disease study. Am J Respir Crit Care Med. 2013;188:60–68. doi: 10.1164/rccm.201209-1697OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crothers K, Thompson BW, Burkhardt K, Morris A, Flores SC, Diaz PT, et al. HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc. 2011;8:275–281. doi: 10.1513/pats.201009-059WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Detels R, Jacobson L, Margolick J, Martinez-Maza O, Munoz A, Phair J, et al. The multicenter AIDS Cohort Study, 1983 to. Public Health. 2012;126:196–198. doi: 10.1016/j.puhe.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 50.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 51.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 52.Neas LM, Schwartz J. The determinants of pulmonary diffusing capacity in a national sample of U.S. adults. Am J Respir Crit Care Med. 1996;153:656–664. doi: 10.1164/ajrccm.153.2.8564114. [DOI] [PubMed] [Google Scholar]
- 53.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 54.Attia EF, Akgun KM, Wongtrakool C, Goetz MB, Rodriguez-Barradas MC, Rimland D, et al. Increased risk of radiographic emphysema in HIV is associated with elevated soluble CD14 and nadir CD4. Chest. 2014;146:1543–1553. doi: 10.1378/chest.14-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Browning KK, Wewers ME, Ferketich AK, Diaz P. Tobacco use and cessation in HIV-infected individuals. Clin Chest Med. 2013;34:181–190. doi: 10.1016/j.ccm.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162:335–344. doi: 10.7326/M14-0954. [DOI] [PubMed] [Google Scholar]
- 57.Zietkowski Z, Skiepko R, Tomasiak-Lozowska MM, Bodzenta-Lukaszyk A. Anti-IgE therapy with omalizumab decreases endothelin-1 in exhaled breath condensate of patients with severe persistent allergic asthma. Respiration. 2010;80:534–542. doi: 10.1159/000317137. [DOI] [PubMed] [Google Scholar]
- 58.Salama M, Jaksch P, Andrukhova O, Taghavi S, Klepetko W, Aharinejad S. Endothelin-1 is a useful biomarker for early detection of bronchiolitis obliterans in lung transplant recipients. J Thorac Cardiovasc Surg. 2010;140:1422–1427. doi: 10.1016/j.jtcvs.2010.08.046. [DOI] [PubMed] [Google Scholar]
- 59.Barjaktarevic I, Springmeyer S, Gonzalez X, Sirokman W, Coxson HO, Cooper CB. Diffusing Capacity for Carbon Monoxide Correlates Best with Tissue Volume from Quantitative Ct Analysis. Chest. 2014 doi: 10.1378/chest.14-1693. [DOI] [PubMed] [Google Scholar]
- 60.Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J Biol Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- 61.Fagan KA, McMurtry IF, Rodman DM. Role of endothelin-1 in lung disease. Respir Res. 2001;2:90–101. doi: 10.1186/rr44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldie RG, Henry PJ, Knott PG, Self GJ, Luttmann MA, Hay DW. Endothelin-1 receptor density, distribution, and function in human isolated asthmatic airways. Am J Respir Crit Care Med. 1995;152:1653–1658. doi: 10.1164/ajrccm.152.5.7582310. [DOI] [PubMed] [Google Scholar]
- 63.Simonson MS, Ismail-Beigi F. Endothelin-1 increases collagen accumulation in renal mesangial cells by stimulating a chemokine and cytokine autocrine signaling loop. J Biol Chem. 2011;286:11003–11008. doi: 10.1074/jbc.M110.190793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Browatzki M, Schmidt J, Kubler W, Kranzhofer R. Endothelin-1 induces interleukin-6 release via activation of the transcription factor NF-kappaB in human vascular smooth muscle cells. Basic Res Cardiol. 2000;95:98–105. doi: 10.1007/s003950050170. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, Yang W, Hu B, Wu W, Fallon MB. Endothelin-1 activation of the endothelin B receptor modulates pulmonary endothelial CX3CL1 and contributes to pulmonary angiogenesis in experimental hepatopulmonary syndrome. Am J Pathol. 2014;184:1706–1714. doi: 10.1016/j.ajpath.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Finsnes F, Lyberg T, Christensen G, Skjonsberg OH. Effect of endothelin antagonism on the production of cytokines in eosinophilic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2001;280:L659–L665. doi: 10.1152/ajplung.2001.280.4.L659. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Hanaoka M, Droma Y, Chen P, Voelkel NF, Kubo K. Endothelin-1 receptor antagonists prevent the development of pulmonary emphysema in rats. Eur Respir J. 2010;35:904–912. doi: 10.1183/09031936.00003909. [DOI] [PubMed] [Google Scholar]
- 68.Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, Khatib AM, et al. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin Sci (Lond) 2006;110:645–654. doi: 10.1042/CS20050286. [DOI] [PubMed] [Google Scholar]
- 69.Hsieh WT, Yeh WL, Cheng RY, Lin C, Tsai CF, Huang BR, et al. Exogenous endothelin-1 induces cell migration and matrix metalloproteinase expression in U251 human glioblastoma multiforme. J Neurooncol. 2014;118:257–269. doi: 10.1007/s11060-014-1442-1. [DOI] [PubMed] [Google Scholar]
- 70.Giordano RJ, Lahdenranta J, Zhen L, Chukwueke U, Petrache I, Langley RR, et al. Targeted induction of lung endothelial cell apoptosis causes emphysema-like changes in the mouse. J Biol Chem. 2008;283:29447–29460. doi: 10.1074/jbc.M804595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bellien J, Iacob M, Remy-Jouet I, Lucas D, Monteil C, Gutierrez L, et al. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation. 2012;125:1266–1275. doi: 10.1161/CIRCULATIONAHA.111.070680. [DOI] [PubMed] [Google Scholar]
- 72.Odoux C, Crestani B, Lebrun G, Rolland C, Aubin P, Seta N, et al. Endothelin-1 secretion by alveolar macrophages in systemic sclerosis. Am J Respir Crit Care Med. 1997;156:1429–1435. doi: 10.1164/ajrccm.156.5.96-11004. [DOI] [PubMed] [Google Scholar]
- 73.Sikkeland LI, Dahl CP, Ueland T, Andreassen AK, Gude E, Edvardsen T, et al. Increased levels of inflammatory cytokines and endothelin-1 in alveolar macrophages from patients with chronic heart failure. PLoS One. 2012;7:e36815. doi: 10.1371/journal.pone.0036815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moller HJ. Soluble CD163. Scand J Clin Lab Invest. 2012;72:1–13. doi: 10.3109/00365513.2011.626868. [DOI] [PubMed] [Google Scholar]
- 75.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Jr, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219–1228. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28:969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaked I, Hanna DB, Gleissner C, Marsh B, Plants J, Tracy D, et al. Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol. 2014;34:1085–1092. doi: 10.1161/ATVBAHA.113.303153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anderson AM, Fennema-Notestine C, Umlauf A, Taylor MJ, Clifford DB, Marra CM, et al. CSF biomarkers of monocyte activation and chemotaxis correlate with magnetic resonance spectroscopy metabolites during chronic HIV disease. J Neurovirol. 2015 doi: 10.1007/s13365-015-0359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barak V, Selmi C, Schlesinger M, Blank M, Agmon-Levin N, Kalickman I, et al. Serum inflammatory cytokines, complement components, and soluble interleukin 2 receptor in primary biliary cirrhosis. J Autoimmun. 2009;33:178–182. doi: 10.1016/j.jaut.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 81.Seidler S, Zimmermann HW, Weiskirchen R, Trautwein C, Tacke F. Elevated circulating soluble interleukin-2 receptor in patients with chronic liver diseases is associated with non-classical monocytes. BMC Gastroenterol. 2012;12:38. doi: 10.1186/1471-230X-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Semenzato G, Bambara LM, Biasi D, Frigo A, Vinante F, Zuppini B, et al. Increased serum levels of soluble interleukin-2 receptor in patients with systemic lupus erythematosus and rheumatoid arthritis. J Clin Immunol. 1988;8:447–452. doi: 10.1007/BF00916949. [DOI] [PubMed] [Google Scholar]
- 83.Alcaide ML, Parmigiani A, Pallikkuth S, Roach M, Freguja R, Della Negra M, et al. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One. 2013;8:e63804. doi: 10.1371/journal.pone.0063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;60:359–368. doi: 10.1097/QAI.0b013e31825b03be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hiemstra PS. Altered macrophage function in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(Suppl):S180–S185. doi: 10.1513/AnnalsATS.201305-123AW. [DOI] [PubMed] [Google Scholar]
- 86.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59:574–580. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agusti A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One. 2012;7:e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:1065–1072. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 90.Romero-Sanchez M, Gonzalez-Serna A, Pacheco YM, Ferrando-Martinez S, Machmach K, Garcia-Garcia M, et al. Different biological significance of sCD14 and LPS in HIV-infection: importance of the immunovirology stage and association with HIV-disease progression markers. J Infect. 2012;65:431–438. doi: 10.1016/j.jinf.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 91.Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS. 2015;29:1263–1265. doi: 10.1097/QAD.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drummond MB, Merlo CA, Astemborski J, Kalmin MM, Kisalu A, McDyer JF, et al. The effect of HIV infection on longitudinal lung function decline among IDUs: a prospective cohort. AIDS. 2013;27:1303–1311. doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gan WQ, Man SF, Postma DS, Camp P, Sin DD. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2006;7:52. doi: 10.1186/1465-9921-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han MK, Postma D, Mannino DM, Giardino ND, Buist S, Curtis JL, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med. 2007;176:1179–1184. doi: 10.1164/rccm.200704-553CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tostes RC, Fortes ZB, Callera GE, Montezano AC, Touyz RM, Webb RC, et al. Endothelin, sex and hypertension. Clin Sci (Lond) 2008;114:85–97. doi: 10.1042/CS20070169. [DOI] [PubMed] [Google Scholar]
- 96.Kittikulsuth W, Looney SW, Pollock DM. Endothelin ET(B) receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet. Clin Exp Pharmacol Physiol. 2013;40:362–370. doi: 10.1111/1440-1681.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Periyalil HA, Wood LG, Scott HA, Jensen ME, Gibson PG. Macrophage activation, age and sex effects of immunometabolism in obese asthma. Eur Respir J. 2015;45:388–395. doi: 10.1183/09031936.00080514. [DOI] [PubMed] [Google Scholar]
- 98.Rojo-Martinez G, Maymo-Masip E, Rodriguez MM, Solano E, Goday A, Soriguer F, et al. Serum sCD163 levels are associated with type 2 diabetes mellitus and are influenced by coffee and wine consumption: results of the Di@bet.es study. PLoS One. 2014;9:e101250. doi: 10.1371/journal.pone.0101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thomsen HH, Moller HJ, Trolle C, Groth KA, Skakkebaek A, Bojesen A, et al. The macrophage low-grade inflammation marker sCD163 is modulated by exogenous sex steroids. Endocr Connect. 2013;2:216–224. doi: 10.1530/EC-13-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


