Abstract
Anorectal emergencies refer to anorectal disorders presenting with some alarming symptoms such as acute anal pain and bleeding which might require an immediate management. This article deals with the diagnosis and management of common anorectal emergencies such as acutely thrombosed external hemorrhoid, thrombosed or strangulated internal hemorrhoid, bleeding hemorrhoid, bleeding anorectal varices, anal fissure, irreducible or strangulated rectal prolapse, anorectal abscess, perineal necrotizing fasciitis (Fournier gangrene), retained anorectal foreign bodies and obstructing rectal cancer. Sexually transmitted diseases as anorectal non-surgical emergencies and some anorectal emergencies in neonates are also discussed. The last part of this review dedicates to the management of early complications following common anorectal procedures that may present as an emergency including acute urinary retention, bleeding, fecal impaction and anorectal sepsis. Although many of anorectal disorders presenting in an emergency setting are not life-threatening and may be successfully treated in an outpatient clinic, an accurate diagnosis and proper management remains a challenging problem for clinicians. A detailed history taking and a careful physical examination, including digital rectal examination and anoscopy, is essential for correct diagnosis and plan of treatment. In some cases, some imaging examinations, such as endoanal ultrasonography and computerized tomography scan of whole abdomen, are required. If in doubt, the attending physicians should not hesitate to consult an expert e.g., colorectal surgeon about the diagnosis, proper management and appropriate follow-up.
Keywords: Anorectal, Emergencies, Hemorrhoid, Fissure, Abscess, Rectal prolapse, Sepsis, Complication, Sexually transmitted disease, Imperforate anus, Rectal cancer
Core tip: Anorectal emergencies refer to anorectal disorders presenting with acute symptoms and signs which might require an immediate management. Anorectal emergencies usually include acutely thrombosed external hemorrhoid, complicated internal hemorrhoid, anal fissure, irreducible rectal prolapse, anorectal sepsis, sexually transmitted proctitis, obstructing rectal cancer and early complications after anorectal procedures. A detailed history taking, careful physical examination including digital rectal examination and anoscopy, and some radiological imaging are essential for correct diagnosis and plan of treatment. Clinicians should be familiar with these conditions especially anorectal sepsis which could be potentially lethal if delay in diagnosis and management.
INTRODUCTION
Anorectal emergencies refer to anorectal disorders presenting with some alarming symptoms such as anorectal pain and bleeding which might require an immediate management. Anorectal emergencies include acutely thrombosed external hemorrhoid, complicated internal hemorrhoid, anal fissure, anorectal sepsis, irreducible rectal prolapse, sexually transmitted proctitis and obstructing rectal cancer. Although most of these conditions are not life-threatening and may be successfully treated in an outpatient setting, an accurate diagnosis remains a challenging problem for physicians and surgeons[1]. It should be noted that patients with acute anorectal problem should be handled with a careful clinical assessment since many of them are suffering from pain, discomfort and embarrassment. If necessary, rectal examination may be performed under anesthesia. In some cases, some imaging examinations, such as endoanal ultrasonography and computerized tomography (CT) scan, are required to confirm diagnosis and plan for treatment. A delay to diagnosis or appropriate treatment of these anorectal disorders was associated with poor outcomes[2]. A referral or consultation should be made to surgeon if an operation is, or may be, needed. This paper summarizes the diagnosis and treatment of common anorectal emergencies excluding anorectal trauma. The last part of this review dedicates to the management of early complications following common anorectal procedures that may present as an emergency.
ACUTELY THROMBOSED EXTERNAL HEMORRHOID
Diagnosis
Classic symptoms of this condition are acute anal pain with a newly enlarged or tender bluish lump at the anal verge. Some patients may give a history of recent constipation or prolonged straining. Acutely thrombosed external hemorrhoid usually causes severe pain in the first couple of days and the pain will gradually subside thereafter. High pressure within the thrombus may cause the erosion of overlying skin and thus resulting in bleeding. Acutely thrombosed external hemorrhoid must be differentiated from complicated internal hemorrhoids and, sometimes, from anal pigmented melanoma. A practical point is that the former is covered by anoderm and the clot formation is lying beneath the skin (Figure 1A and B). In contrary, internal hemorrhoid is covered by anal mucosa and anal pigmented melanoma presents with a longer history of dark pigmented skin lesion (Figure 1C and D).
Figure 1.
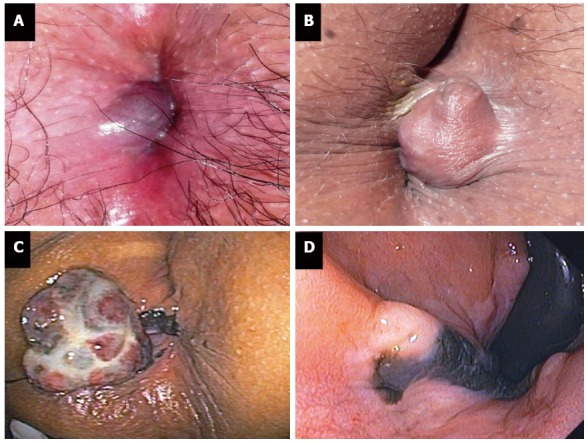
Acutely thrombosed external hemorrhoid (A, B) and anal melanoma (C, D).
Management
Acutely thrombosed external hemorrhoid can be treated conservatively or surgically depending on patient’s symptoms (mainly the intensity of present pain). Basically, excision of thrombosed external hemorrhoid or surgical removal of clot is reserved in patients experiencing severe pain - usually within 48-72 h of onset. Otherwise, conservative management would be offered including anti-inflammatory analgesics, warm sitz bath, reducing activity and avoiding constipation. Education and reassurance about this condition and its benign nature would be beneficial to the patient.
THROMBOSED OR STRANGULATED INTERNAL HEMORRHOID
Diagnosis
Internal hemorrhoid may become strangulated and thrombosed when prolapsed part is left protruded until vascular compromise or venous stasis occurs. Patients with acute thrombosis and strangulation of internal hemorrhoids usually present with acute irreducible and painful hemorrhoid. Foul-smelling discharge may be seen in those with mucosal necrosis.
Management
This condition is difficult to manage especially in case of extensive thrombosis and strangulation. Manual reduction of the hemorrhoid masses might help in reducing pain and tissue congestion. Urgent hemorrhoidectomy is usually required[3] (Figure 2). Some technical notes of hemorrhoidectomy in this situation are listed in Table 1.
Figure 2.
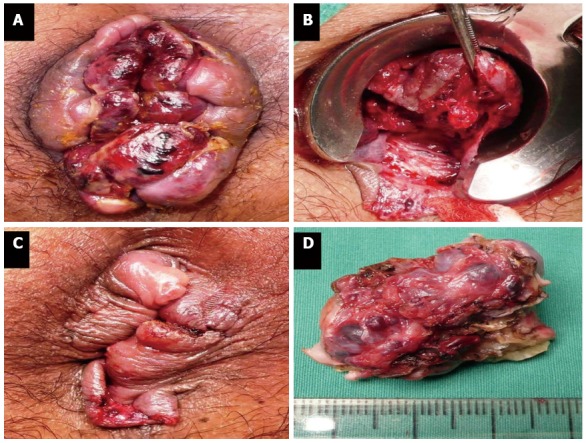
Urgent hemorrhoidectomy for thrombosed internal hemorrhoids (A-D).
Table 1.
Technical notes for urgent hemorrhoidectomy
| Preoperative intravenous antibiotics |
| Surgery under general anesthesia, regional anesthesia, or intravenous sedation plus perianal infiltration of local anesthetic agent(s) |
| Prone jackknife position |
| Manual reduction of prolapsing hemorrhoids |
| Compression of hemorrhoids to reduce edema |
| During an operation, use of large-diameter anoscope e.g., Fansler anoscope |
| Anoderm or mucosa-sparing hemorrhoidectomy (preferably semi-closed technique) |
| Allowance of at least 1-cm mucosal bridge between surgical wounds and at least 50% of good circumferential mucosa |
| Use of long-lasting absorbable sutures e.g., polyglactin 910 for mucosal approximation |
| If applicable, instead of hemorrhoidectomy, plication of hemorrhoid may be applied to small lesions |
| Oral postoperative antibiotics against anaerobes for 1 wk |
BLEEDING HEMORRHOID
Diagnosis
Bleeding from hemorrhoid is characterized by a painless passage of bright-red blood during bowel movements, with or without prolapsed hemorrhoid. The blood may be spotted on toilet paper after cleansing or drip into toilet bowel. Bleeding tends to be mild except individuals having antiplatelet or anticoagulant therapy. Differential diagnoses include anal fissure (which is associated with painful defecation) and bleeding rectal neoplasm. The diagnosis can be confirmed by a typical history, digital rectal examination and anoscopy - which usually reveal some stigmata of recent bleeding on hemorrhoid tissue.
Management
Choices of treatment depend on the degree of bleeding, grade of hemorrhoid, patient’s comorbidity and patient’s preference[4]. For low-graded hemorrhoid, management includes dietary and lifestyle modification, avoidance of constipation or diarrhea, topical medication, oral venotonic drug, and some office-based procedures e.g., rubber band ligation and injection sclerotherapy. For high-graded hemorrhoid, surgical management may be offered including hemorrhoidectomy, dopper-guided hemorrhoidal artery ligation and stapled hemorrhoidopexy. The management of bleeding hemorrhoids in complicated situations (such as pregnancy, immunocompromised host and patients having antiplatelet or anticoagulant therapy) has been recently reviewed in this journal[5].
BLEEDING ANORECTAL VARICES
Diagnosis
Anorectal bleeding in patients with a history of long-standing or uncontrolled portal hypertension would give a clinician clues about this condition. However, hemorrhoid is more prevalent in such patients[6]. It is important to differentiate bleeding hemorrhoids from bleeding anorectal varices because the choices of treatment are different. Practically, diagnosis and differentiation between the two conditions is best achieved with anoscopy or flexible sigmoidoscopy. Since hemorrhoid is an abnormal anal cushion with dilatation of hemorrhoid venous plexus, it is located within the anal canal[4]. On the other hand, anorectal varices - dilated submucosal veins of portosystemic collateral circulation[7] - can be visualized as enlarged and tortuous submucosal veins extended from the anal canal up to the middle rectum.
Management
The management of bleeding anorectal varices can be very challenging. In mild cases, intravenous fluid replacement, blood transfusion, correction of coagulopathy and optimal medication for portal hypertension is usually effective. In active variceal bleeding, per anal suture ligation along the course of varices, endoscopic ligation of the varices and injection sclerotherapy are helpful[3]. In severe or recurrent cases, a decrease in portal pressure is an ultimate goal of treatment which can be achieved by means of surgical portosystemic shunt or preferably transjugular intrahepatic portosystemic shunt (TIPS)[8].
ANAL FISSURE
Diagnosis
Painful defecation with a passage of red blood is a typical symptom of this condition. Pain is usually excruciating and may last from minutes to several hours. Although patients are relatively pain-free between bowel movement, experiencing severely painful defecation may preclude patients to have another bowel movement resulting in even harder stool. A vicious cycle of pain, anal spasm and passage of hard stool would exacerbate further traumatic and ischemic injury to anoderm and prevent the fissure from healing.
For those with a short history of painful defecation, a small shallow linear laceration of the anoderm in the midline (acute anal fissure) is normally evident without the need of digital rectal examination. Meanwhile, a chronic linear laceration of anoderm exposure to the underlying internal anal sphincter, with or without hypertrophic anal papilla and enlarged perianal skin tag, is a paramount finding of chronic anal fissure.
Management
Acute anal fissure usually heal within a few week by means of conservative treatment - which includes adequate pain control, stool softeners, laxative and warm sitz bath. Patient education is also essential to prevent or minimize disease recurrence. Medication that reduce anal sphincter tome may be prescribed in patients with acute or chronic anal fissure such as topical nitrate and topical calcium channel blocker[9]. Although lateral internal anal sphincterotomy remains a standard treatment for chronic anal fissure[10], botulinum toxin injection is an effective alternative to surgery especially in those with coexisting anal incontinence or anal sphincter hypotonia.
IRREDUCIBLE OR STRANGULATED RECTAL PROLAPSE
Diagnosis
First, clinicians should differentiate prolapsed rectum from circumferentially prolapsed internal hemorrhoid. Classic signs of rectal prolapse are protruding full-thickness rectal wall with concentric rings of mucosa (Figure 3A), while hemorrhoid contains only mucosa and there are radial sulci between hemorrhoid bundles. Irreducible rectal prolapse may occur but acute strangulation of rectal prolapse is quite rare (Figure 3B). Nevertheless, both conditions require prompt intervention.
Figure 3.
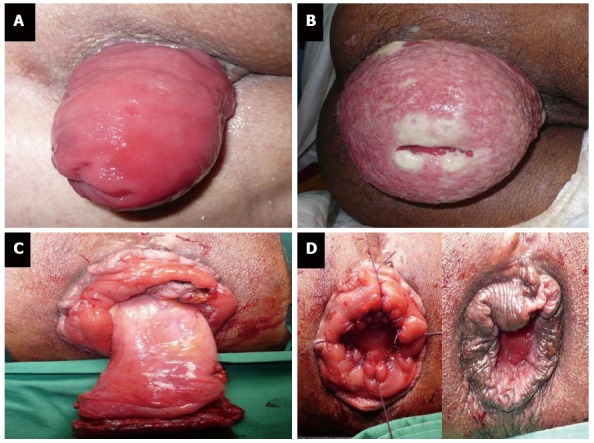
Rectal prolapse (A), strangulated rectal prolapse (B), and perineal rectosigmoidectomy or Altemeier’s procedure (C, D).
Management
Although a range of techniques and approaches have been described to treat reducible rectal prolapse[11], perineal rectosigmoidectomy (Altemeier’s procedure) is the treatment of choice in strangulated rectal prolapse (Figure 3C and D). For irreducible non-strangulated rectal prolapse, gentle reduction under intravenous sedation and analgesia is helpful and definitive surgery can be deferred[12].
ANORECTAL ABSCESS
Diagnosis
An abscess forming in the anorectal region usually originates from an infected anal gland which is located in the anal mucosa and its opening is at the level of dentate line. Once the anal gland is infected, an abscess may form within an intersphincteric area or it could spread to an adjacent area such as perianal region, deep postanal space, ischiorectal fossa or, rarely, a supralevator space. Acute anorectal abscess may be an initial manifest of anal fistula.
Most anorectal abscesses can be readily diagnosed by a careful history and physical examination. The leading symptom of anorectal abscess is acute and throbbing anal pain, which may be aggravated by coughing and sitting. Tender lump or swelling of affected area, with or without fever, is commonly seen. Fluctuation of the abscess is usually minimal or not evident in the anorectal abscess because of loose fatty connective tissue in this area. In case of an intersphincteric abscess or a suprasphincteric abscess, perianal inspection could appear normal but digital rectal examination often reveals a painful bulging area of the abscess. Three-dimensional endoanal ultrasonography (Figure 4) or CT scan and MRI of the pelvis may give some additional information on the location and extension of the abscess[13,14].
Figure 4.
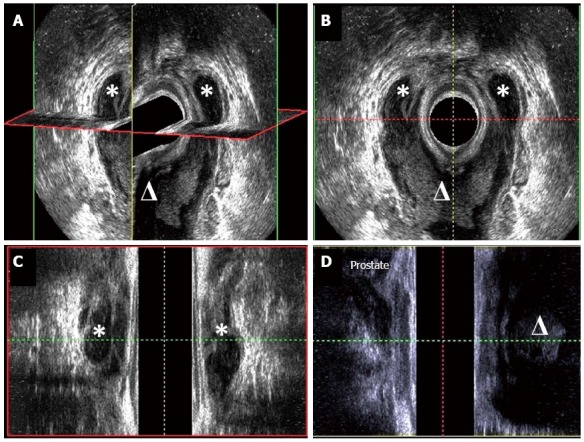
Three-dimensional endoanal ultrasonography of horseshoe abscess (A), cross-sectional view (B), coronal view (C) and sagittal view (D). The asterisk means abscess in ischiorectal space and the triangle means abscess in deep postanal space.
Management
The goal of treatment is to provide an adequate and dependent drainage of the abscess. For perianal abscess and ischioanal (ischiorectal) abscess, an elliptical or crucial incision will be performed over the abscess to provide an adequate drainage and to prevent a premature closure of the incision. If possible, the drainage should be performed close to the anal verge because it will shorten the length of potential subsequent fistula tract[15]. An intersphincteric approach is used for surgical drainage of intersphincteric abscesses - with or without the division of internal anal sphincter. With the guidance of diagnostic imaging modalities, drainage of supralevator abscess can be performed by transrectal approach, intersphincteric approach, and trans-ischioanal approach depending on the origin of infection. Transrectal drainage is used for supralevator abscess with intact pelvic floor muscle. Intersphincteric drainage and trans-ischioanal drainage are used for supralevator abscess originated from intersphincteric space and ischioanal fossa, respectively.
The addition of intravenous antibiotics to surgical drainage is recommended in patients with extensive overlying cellulitis, immunocompromised hosts, those with concomitant systemic illness and those with prosthetic heart valves[16]. The management of anal fistula-associated anorectal abscess remains controversial. An experienced surgeon may provide a definite treatment of anal fistula in this situation. However, it is widely accepted that the abscess can be drained first and then a schedule is made for fistula management in another setting[17]. Treatment following drainage of abscess usually includes warm sitz bath, adequate pain control and the prevention of constipation. The patients should be advised about an approximately 30% chance of anal fistula formation following incision and drainage[15].
PERINEAL NECROTIZING FASCIITIS (FOURNIER GANGRENE)
Diagnosis
Perineal necrotizing fasciitis is a severe and life-threatening form of skin and soft tissue infection in the anal and perineal region. It is usually polymicrobial infection that develops secondary to untreated anorectal abscess, genitourinary infection or cutaneous infection[18]. It is more likely to occur in diabetic individuals and immunocompromised hosts. Patient with perineal necrotizing fasciitis is characterized by severe perineal pain and high-graded fever. Septic shock and acute urinary retention may develop. On a physical examination, markedly swelling of buttock and perineum, with or without purple bullae and necrosis of overlying skin, is a hallmark feature. The most important tool for early diagnose this condition is to have a high index of suspicion. CT scan of lower abdomen may be useful in the diagnosis and delineation of the extension of perineal necrotizing fasciitis[19].
Management
Intravenous fluid resuscitation and broad spectrum intravenous antibiotics must be given. Prompt and adequate surgical debridement of infected tissue is the mainstay of treatment (Figure 5). A delay in treatment would have a negative impact on patient’s survival[20]. Diverting colostomy may be performed to reduce fecal contamination and to facilitate perineal wound healing. Several reconstructive procedures, such as vacuum-assisted closure, skin graft and myocutaneous flap, can be used to correct the tissue defect.
Figure 5.
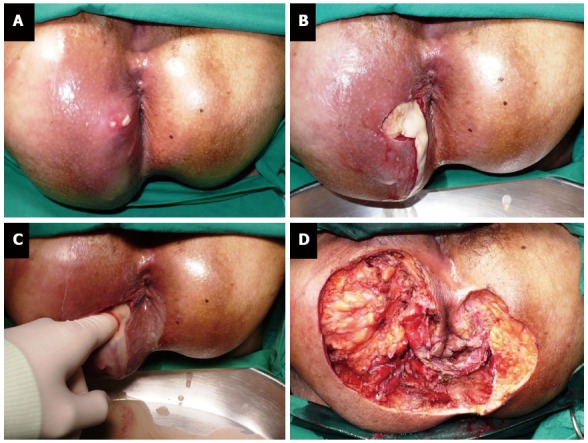
Perineal necrotizing fasciitis (Fournier gangrene) (A-D).
RETAINED ANORECTAL FOREIGN BODIES
Diagnosis
Diagnosis of retained foreign bodies in the rectum could pose a challenge on a clinician because patients may not give an accurate history of object insertion due to their fear and embarrassment. Many patients make some efforts to remove the object before seeking medical attention. Sexual pleasure is the most common reason for introducing foreign bodies into the rectum[21]. However, it could be a result from an accident, trafficking of illegal drugs (body packing) or criminal assault. Therefore, attending physician must treat these patients with appropriate respect and emotional support. Foreign bodies could be sharp or blunt objects with a variety of sizes and shapes. Digital rectal examination may reveal some part of the retained foreign body. More importantly, physician must evaluate whether there is an evidence of rectal perforation or an injury to the anal sphincter. Severe pelvic pain, abdominal pain, fever, tachycardia and peritonitis are suggestive of rectal perforation. Plain abdominal radiographs may reveal the number, shape and location of retained objects as well as the presence of free air (if any). Meanwhile, ultrasonography and CT scan may help detecting non-opaque objects[22].
Management
The goal is to successfully remove the retained foreign body without causing a further injury to bowel wall and anal sphincter complex. This could be achieved by transanal extraction, endoscopic removal and surgical intervention (object removal via a colotomy by laparoscopy or laparotomy). For non-operative approach, it is wise to perform a maneuver when patients receive adequate pain control, with or without conscious sedation, in the lithotomy position[23]. Peritonitis and failure to remove the object by means of transanal and endoscopic extraction are indications for surgery. After the removal of an object, the rectum should be assessed by endoscopic examination to detect any damage to the rectal wall - and to plan treatment accordingly. The details of various extraction techniques have been recently reviewed elsewhere[24,25].
OBSTRUCTING RECTAL CANCER
Diagnosis
Up to 15% of patients with rectal cancer present with acute distal colonic obstruction[26]. Marked abdominal distension, obstipation and abdominal pain are among cardinal symptoms of this situation. Vomitus with appearance and odor of feces is suggestive of long-standing obstruction. Localized peritonitis and generalized peritonitis may be seen in case of perforated rectal cancer or cecal perforation as a result of closed-loop obstruction. Obstructing rectal cancer is very likely to be a locally advanced disease but distant metastasis may be evident at the time of diagnosis[27]. Digital rectal examination usually reveals rectal mass causing luminal obstruction. Plain abdominal radiography can quickly confirm mechanical colonic obstruction and determine whether there is intestinal perforation or not (Figure 6A). CT scan of chest and abdomen is very useful imaging modality for the diagnosis and evaluation of the extension of rectal cancer and its complication (Figure 6B). Differential diagnosis of obstructing rectal cancer includes rectal endometriosis, colitis cystica profunda and Crohn’s disease.
Figure 6.
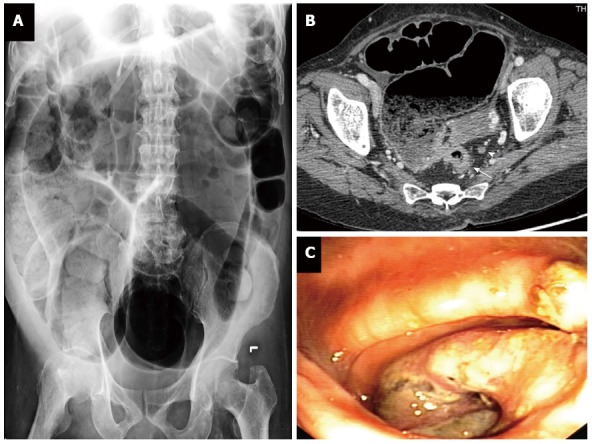
Obstructing rectal cancer: plain abdominal radiography (A), computerized tomography (B), and endoscopic view (C). T3 rectal cancer (white arrow) and perirectal lymph node (arrow head).
Management
Initial therapy comprises intravenous fluid resuscitation, correction of metabolic derangement and intestinal decompression via nasogastric tube. The decision to perform surgery or endoscopic decompression for obstructing rectal cancer depended on patient’s status, presence of intestinal ischemia or perforation, location and stage of rectal cancer, and surgeon or endoscopist’s experience. Surgical options range from diverting colostomy to total protocolectomy with or without restoration of bowel continuity.
For those with sign of peritonitis, intestinal ischemia or perforation, immediate surgical exploration is mandatory. Otherwise, diverting colostomy (either transverse loop colostomy or sigmoid loop colostomy) following by neoadjuvant long-course chemoradiation is recommended in patients with obstructing rectal cancer because the disease tends to be locally advanced and difficult to achieve adequate oncological clearance in an index operation. Diverting colostomy is also an effective operation for palliation of obstructive symptoms in patients with unresectable rectal cancer or those with unresectable metastatic disease. Relieving rectal obstruction by stenting may be possible for some patients with upper or middle rectal cancer[28].
SEXUALLY TRANSMITTED DISEASE AS ANORECTAL NON-SURGICAL EMERGENCIES
Some sexually transmitted diseases (STDs) may present in an emergency department manifesting as proctitis which could mimic other infectious proctitis and Crohn’s disease. Unprotected anal receptive intercourse is the greatest risk factor for sexually transmitted proctitis. Leading symptoms of such proctitis include urgency and frequency of bowel movement, anal pain and anal discharge (mucous, purulent or bloodstained). Gonorrhea, chlamydia, herpes simplex virus, syphilis and lymphogranuloma venereum (LGV) are common STDs causing proctitis or proctocolitis[29]. It is worth noting that Herpes simplex virus and Treponema pallidum (syphilis) breach both stratified squamous epithelium and columnar epithelium, but Neisseria gonorrhoeae and Chlamydia trachomatis infect only columnar epithelium[30]. C. trachomatis serovars D-K is less virulent than C. trachomatis serovars L1, L2 and L3, a causative agent for LGV. As a result, LGV may present with severe proctitis, deep rectal ulcer and inguinal lymphadenopathy. Sexually transmitted proctitis in immunocompromised host e.g., HIV-infected individuals could be severe and be coinfected with several pathogens. Table 2 summarizes the diagnosis and treatment of common infectious organisms causing sexually transmitted proctitis[31].
Table 2.
Diagnosis and treatment of common infectious organisms causing sexually transmitted proctitis (by the frequency of occurrence)
| Disease (causative organism) | Common symptoms and signs | Suggested investigations | Recommended first line treatment |
| Chlamydia (Chlamydia trachomatis serovars D-K) | Commonly asymptomatic, mild proctitis, cervicitis, vaginitis, urethritis | Nucleic acid amplification test (NAAT) from rectal, endocervical or urethral swab specimens | Azithromycin 1 g orally in a single dose |
| OR | |||
| Doxycycline 100 mg orally twice a day for 7 d | |||
| Gonorrhea (Neisseria gonorrhoeae) | Lower abdominal pain, diarrhea, rectal bleeding, tenesmus, purulent rectal discharge, urethral discharge and/or pharyngeal infection | Gram stain (Gram-negative diplococci) and bacterial culture from anogential and pharyngeal swab | Ceftriaxone 250 mg IM in a single dose |
| PLUS | |||
| Azithromycin 1 g orally in a single dose | |||
| Herpes simplex virus (Herpes simplex virus) | Painful multiple vesicular or ulcerative lesions at perianal skin and anal canal, painful defecation, fever | Viral culture or polymerase chain reaction (PCR) from vesicular lesions | Acyclovir 400 mg orally three times a day for 7-10 d |
| OR | |||
| Acyclovir 200 mg orally five times a day for 7-10 d | |||
| Syphilis (Treponema pallidum) | Depending on the stage of infection - Primary syphilis: painless ulcers or chancre in the anorectal region | Darkfield examination and test to detect T. pallidum from lesion exudate or tissue | Benzathine penicillin G 2.4 million unit IM in a single dose |
| Secondary syphilis: maculopapular rash, condyloma lata, snail-track ulcer and mucous patch at the rectum, lymphadenopathy | OR | ||
| Ceftriaxone 1-2 g either IV or IM for 10-14 d | |||
| OR | |||
| Doxycycline 100 mg orally twice a day for 14 d | |||
| Lymphogranuloma venereum (Chlamydia trachomatis serovars L1, L2 and L3) | Anal pain, mucous or bloody rectal discharge, anorectal ulcer, fever, inguinal or femoral lymphadenopathy | Culture, direct immunofluorescence or nucleic acid detection form rectal lesion and lymph node specimen | Doxycycline 100 mg orally twice a day for 21 d |
| OR | |||
| Erythromycin base 500 mg oral four times a day for 21 d |
ANORECTAL EMERGENCIES IN THE NEONATES
Pediatrician and pediatric surgeon should be familiar with this anorectal disorder which usually presents with delay or failure to pass meconium. In general, 99% of healthy full-term newborns will pass meconium within the first 24 h of birth[32]. Delayed first passage of meconium raises a concern about colonic obstruction which may be related to meconium plug syndrome, Hirschsprung’s disease and anorectal malformations[33]. Notably, anorectal malformations could be associated with other congenital anomalies or as a part of combined anomalies e.g., VACTERL anomalies (Vertebral anomalies, Anorectal malformations or Anal atresia, Cardiac anomalies, TracheoEsophageal fistula or esophageal atresia, Renal and urinary anomalies, Limb lesions)[34] or a rare complete tubular colonic duplication[35]. Clinical examination, including anal inspection for the presence of imperforate anus or perineal fistula, combined with plain or contrast enema abdominal radiographs would help determine the diagnosis. Some common disorders presenting as anorectal emergencies in the neonates are listed in Table 3.
Table 3.
Common anorectal disorders presenting with delay or failure to pass meconium in the neonates
| Diagnosis | Rate | Common physical findings | Suggested investigation: expected findings | Initial management |
| Meconium plug syndrome | 1/500-1000 | Abdominal distension, normal anus and anal sphincter complex | Contrast enema radiologic examination: meconium plug in colon | Rectal stimulation with finger or saline enema |
| Hirschsprung’s disease | 1/4000 | Abdominal distension, tight anal sphincter, empty rectum, sudden evacuation of stool on digital rectal examination if “transitional zone” is reached | Contrast enema radiologic examination without colonic preparation: transitional zone separating aganglionic segment and dilated proximal colon | Intravenous hydration, gastric decompression, rectal washout with warm saline, and consider colostomy in high-grade obstruction and intravenous board-spectrum antibiotics in those with suspected diagnosis of Hirschprung-associated enterocolitis |
| Imperforate anus (IA) | 1/5000 | Absence or stenosis of anus, perineal fistula (low IA), meconium in urine (rectourinary fistula: low or high IA), flat or not well formed median raphe (high IA), cloaca (high IA), VACTERL anomalies1 | Inverted lateral radiography (invertography) or transperineal ultrasonography: differentiation between low IA and high IA | Anal or fistula dilatation for temporary relief of obstruction and plan for elective posterior sagittal anorectoplasty (low IA), loop sigmoid colostomy (high IA or some low IA) |
| - Low IA = distal rectal pouch lining below or at the puborectalis muscle | ||||
| - High IA = distal rectal pouch lining above the puborectalis muscle |
VACTERL anomalies include vertebral anomalies (V), anorectal malformations (A), congenital cardiac anomalies (C), tracheoesophageal fistula or esophageal atresia (TE), renal and urinary anomalies (R), limb lesions (L).
EARLY POSTOPERATIVE COMPLICATIONS OF ANORECTAL SURGERY
Since many anorectal procedures can be performed safely and effectively in an ambulatory setting (day-surgery)[36] or an overnight stay, some patients may develop complications sooner or later after hospital discharge. Common early postoperative complications that bring patients back to the hospital include acute urinary retention, bleeding, fecal impaction and anorectal sepsis.
The incidence of acute urinary retention following benign anorectal surgery ranges from 0.5%[36] to 17%[37] depending on the extent of surgery, anesthetic technique, analgesic method, amount of intravenous fluid given and patient’s underlying disease[38]. Bladder catheterization is the standard treatment of acute postoperative urinary retention. Should the volume of urine is less than 600 mL in low-risk individuals, the patients may be sent home without voiding[39]. But if the catheterized urine volume exceeds 600 mL especially in high-risk patients (e.g., severe anal pain, benign prostatic hypertrophy and immobilized patients), a self-retaining Foley catheterization may be required before discharge.
Postoperative bleeding is another common reason that brings patients to an emergency unit - with the overall incidence of 2%-4% after hemorrhoidectomy[40] and stapled hemorrhoidopexy[41]. A small to moderate amount of blood may be seen after an anorectal procedure especially during bowel movement and usually subsides with a conservative approach. Any major hemorrhage within the first 48 h after an operation is often caused by incomplete hemostasis. Meanwhile, delayed bleeding could be linked with a surgical site infection. Examination under anesthesia, identification and management of bleeder including re-suturing and rectal packing, correction of coagulopathy (if any) and re-hospitalization are recommended in this group of patient.
Fecal impaction after anorectal surgery is uncommon nowadays, but it could be a difficult situation to deal with especially in the early postoperative period. Postoperative fecal impaction could be a result of anal spasm, severe pain, patient’s fear of defecation, inadequate intake of water and laxative, opioid-induced constipation and postsurgical edematous anorectal tissue. Mild fecal impaction may be relieved with gentle rectal enema and administration of laxatives. However, many impactions require manual disimpaction under anesthesia[42]. The cause(s) of fecal impaction should be identified and treated accordingly.
Sepsis following an anorectal procedure is a rare but serious and potentially fatal complication. The infection could confine in anorectal region or extend into the pelvis and retroperitoneal area[43]. It has been described after lateral internal sphincterotomy for chronic anal fissure and after various treatment of hemorrhoid (sclerosing injection, rubber band ligation, hemorrhoidectomy and stapled hemorrhoidopexy)[43,44]. This complication could occur in otherwise healthy patients. Unexpectedly severe and persistent pain, urinary difficulties and fever are alarming symptoms and signs of perirectal sepsis. The management of this condition is in line with that of perineal necrotizing fasciitis (Fournier gangrene) as previously discussed.
CONCLUSION
Some anorectal disorders may present as an emergency. A detailed history taking and a careful physical examination, including digital rectal examination and anoscopy, is essential for correct diagnosis and plan of treatment. Clinicians should maintain a high index of suspicion for anorectal sepsis and anorectal neoplasms. If in doubt, the attending physicians should not hesitate to consult an expert e.g., colorectal surgeon about the diagnosis, proper management and appropriate follow-up.
Footnotes
Conflict-of-interest statement: The author has no conflict of interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: March 24, 2016
First decision: May 12, 2016
Article in press: June 15, 2016
P- Reviewer: Casadesus D, Ruffolo C, Sergi CM S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
References
- 1.Grucela A, Salinas H, Khaitov S, Steinhagen RM, Gorfine SR, Chessin DB. Prospective analysis of clinician accuracy in the diagnosis of benign anal pathology: comparison across specialties and years of experience. Dis Colon Rectum. 2010;53:47–52. doi: 10.1007/DCR.0b013e3181bbfc89. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein ET. Outcomes of anorectal disease in a health maintenance organization setting. The need for colorectal surgeons. Dis Colon Rectum. 1996;39:1193–1198. doi: 10.1007/BF02055107. [DOI] [PubMed] [Google Scholar]
- 3.Lohsiriwat V. Approach to hemorrhoids. Curr Gastroenterol Rep. 2013;15:332. doi: 10.1007/s11894-013-0332-6. [DOI] [PubMed] [Google Scholar]
- 4.Lohsiriwat V. Hemorrhoids: from basic pathophysiology to clinical management. World J Gastroenterol. 2012;18:2009–2017. doi: 10.3748/wjg.v18.i17.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohsiriwat V. Treatment of hemorrhoids: A coloproctologist’s view. World J Gastroenterol. 2015;21:9245–9252. doi: 10.3748/wjg.v21.i31.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TF, Lee FY, Tsai YT, Lee SD, Wang SS, Hsia HC, Lin WJ, Lin HC, Lai KH, Chan CY. Relationship of portal pressure, anorectal varices and hemorrhoids in cirrhotic patients. J Hepatol. 1992;15:170–173. doi: 10.1016/0168-8278(92)90031-j. [DOI] [PubMed] [Google Scholar]
- 7.Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (1991). Japanese Society for Portal Hypertension. World J Surg. 1995;19:420–422; discussion 423. doi: 10.1007/BF00299178. [DOI] [PubMed] [Google Scholar]
- 8.Maslekar S, Toh EW, Adair R, Bate JP, Botterill I. Systematic review of anorectal varices. Colorectal Dis. 2013;15:e702–e710. doi: 10.1111/codi.12417. [DOI] [PubMed] [Google Scholar]
- 9.Altomare DF, Binda GA, Canuti S, Landolfi V, Trompetto M, Villani RD. The management of patients with primary chronic anal fissure: a position paper. Tech Coloproctol. 2011;15:135–141. doi: 10.1007/s10151-011-0683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry WB, Dykes SL, Buie WD, Rafferty JF. Practice parameters for the management of anal fissures (3rd revision) Dis Colon Rectum. 2010;53:1110–1115. doi: 10.1007/DCR.0b013e3181e23dfe. [DOI] [PubMed] [Google Scholar]
- 11.Tou S, Brown SR, Nelson RL. Surgery for complete (full-thickness) rectal prolapse in adults. Cochrane Database Syst Rev. 2015;(11):CD001758. doi: 10.1002/14651858.CD001758.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seenivasagam T, Gerald H, Ghassan N, Vivek T, Bedi AS, Suneet S. Irreducible rectal prolapse: emergency surgical management of eight cases and a review of the literature. Med J Malaysia. 2011;66:105–107. [PubMed] [Google Scholar]
- 13.Khati NJ, Sondel Lewis N, Frazier AA, Obias V, Zeman RK, Hill MC. CT of acute perianal abscesses and infected fistulae: a pictorial essay. Emerg Radiol. 2015;22:329–335. doi: 10.1007/s10140-014-1284-3. [DOI] [PubMed] [Google Scholar]
- 14.Brillantino A, Iacobellis F, Di Sarno G, D’Aniello F, Izzo D, Paladino F, De Palma M, Castriconi M, Grassi R, Di Martino N, et al. Role of tridimensional endoanal ultrasound (3D-EAUS) in the preoperative assessment of perianal sepsis. Int J Colorectal Dis. 2015;30:535–542. doi: 10.1007/s00384-015-2167-0. [DOI] [PubMed] [Google Scholar]
- 15.Lohsiriwat V, Yodying H, Lohsiriwat D. Incidence and factors influencing the development of fistula-in-ano after incision and drainage of perianal abscesses. J Med Assoc Thai. 2010;93:61–65. [PubMed] [Google Scholar]
- 16.Steele SR, Kumar R, Feingold DL, Rafferty JL, Buie WD. Practice parameters for the management of perianal abscess and fistula-in-ano. Dis Colon Rectum. 2011;54:1465–1474. doi: 10.1097/DCR.0b013e31823122b3. [DOI] [PubMed] [Google Scholar]
- 17.Malik AI, Nelson RL, Tou S. Incision and drainage of perianal abscess with or without treatment of anal fistula. Cochrane Database Syst Rev. 2010;(7):CD006827. doi: 10.1002/14651858.CD006827.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Shyam DC, Rapsang AG. Fournier’s gangrene. Surgeon. 2013;11:222–232. doi: 10.1016/j.surge.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Levenson RB, Singh AK, Novelline RA. Fournier gangrene: role of imaging. Radiographics. 2008;28:519–528. doi: 10.1148/rg.282075048. [DOI] [PubMed] [Google Scholar]
- 20.Thwaini A, Khan A, Malik A, Cherian J, Barua J, Shergill I, Mammen K. Fournier’s gangrene and its emergency management. Postgrad Med J. 2006;82:516–519. doi: 10.1136/pgmj.2005.042069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurer MA, Davey C, Khan S, Chintapatla S. Colorectal foreign bodies: a systematic review. Colorectal Dis. 2010;12:851–861. doi: 10.1111/j.1463-1318.2009.02109.x. [DOI] [PubMed] [Google Scholar]
- 22.Aras MH, Miloglu O, Barutcugil C, Kantarci M, Ozcan E, Harorli A. Comparison of the sensitivity for detecting foreign bodies among conventional plain radiography, computed tomography and ultrasonography. Dentomaxillofac Radiol. 2010;39:72–78. doi: 10.1259/dmfr/68589458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayantunde AA. Approach to the diagnosis and management of retained rectal foreign bodies: clinical update. Tech Coloproctol. 2013;17:13–20. doi: 10.1007/s10151-012-0899-1. [DOI] [PubMed] [Google Scholar]
- 24.Cologne KG, Ault GT. Rectal foreign bodies: what is the current standard? Clin Colon Rectal Surg. 2012;25:214–218. doi: 10.1055/s-0032-1329392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson KL, Dean AJ. Foreign bodies in the gastrointestinal tract and anorectal emergencies. Emerg Med Clin North Am. 2011;29:369–400, ix. doi: 10.1016/j.emc.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Moolla Z, Madiba TE. Trends in demographics and management of obstructing colorectal cancer. World J Surg. 2014;38:2466–2470. doi: 10.1007/s00268-014-2595-y. [DOI] [PubMed] [Google Scholar]
- 27.Lohsiriwat V. Enhanced recovery after surgery vs conventional care in emergency colorectal surgery. World J Gastroenterol. 2014;20:13950–13955. doi: 10.3748/wjg.v20.i38.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chinswangwatanakul V, Angkurawaranon C, Phalanusitthepha C, Methasate A, Trakarnsagna A, Swangsri J, Akaraviputh T. Outcomes of self-expandable metallic stent insertion on acute colorectal obstruction: a single endoscopist experience. J Tumor. 2014;2:229–232. [Google Scholar]
- 29.Klausner JD, Kohn R, Kent C. Etiology of clinical proctitis among men who have sex with men. Clin Infect Dis. 2004;38:300–302. doi: 10.1086/380838. [DOI] [PubMed] [Google Scholar]
- 30.Hamlyn E, Taylor C. Sexually transmitted proctitis. Postgrad Med J. 2006;82:733–736. doi: 10.1136/pmj.2006.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 32.Clark DA. Times of first void and first stool in 500 newborns. Pediatrics. 1977;60:457–459. [PubMed] [Google Scholar]
- 33.Loening-Baucke V, Kimura K. Failure to pass meconium: diagnosing neonatal intestinal obstruction. Am Fam Physician. 1999;60:2043–2050. [PubMed] [Google Scholar]
- 34.Totonelli G, Catania VD, Morini F, Fusaro F, Mosiello G, Iacobelli BD, Bagolan P. VACTERL association in anorectal malformation: effect on the outcome. Pediatr Surg Int. 2015;31:805–808. doi: 10.1007/s00383-015-3745-5. [DOI] [PubMed] [Google Scholar]
- 35.Jellali MA, Mekki M, Saad J, Zrig A, Elanes I, Mnari W, Maatouk M, Harzallah W, Toumi S, Krichène I, et al. Perinatally discovered complete tubular colonic duplication associated with anal atresia. J Pediatr Surg. 2012;47:e19–e23. doi: 10.1016/j.jpedsurg.2012.01.082. [DOI] [PubMed] [Google Scholar]
- 36.Lohsiriwat V, Lohsiriwat D. Ambulatory anorectal surgery under perianal anesthetics infiltration: analysis of 222 cases. J Med Assoc Thai. 2007;90:278–281. [PubMed] [Google Scholar]
- 37.Toyonaga T, Matsushima M, Sogawa N, Jiang SF, Matsumura N, Shimojima Y, Tanaka Y, Suzuki K, Masuda J, Tanaka M. Postoperative urinary retention after surgery for benign anorectal disease: potential risk factors and strategy for prevention. Int J Colorectal Dis. 2006;21:676–682. doi: 10.1007/s00384-005-0077-2. [DOI] [PubMed] [Google Scholar]
- 38.Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110:1139–1157. doi: 10.1097/ALN.0b013e31819f7aea. [DOI] [PubMed] [Google Scholar]
- 39.Pavlin DJ, Pavlin EG, Fitzgibbon DR, Koerschgen ME, Plitt TM. Management of bladder function after outpatient surgery. Anesthesiology. 1999;91:42–50. doi: 10.1097/00000542-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Crystal RF, Hopping RA. Early postoperative complications of anorectal surgery. Dis Colon Rectum. 1974;17:336–341. doi: 10.1007/BF02586977. [DOI] [PubMed] [Google Scholar]
- 41.Ravo B, Amato A, Bianco V, Boccasanta P, Bottini C, Carriero A, Milito G, Dodi G, Mascagni D, Orsini S, et al. Complications after stapled hemorrhoidectomy: can they be prevented? Tech Coloproctol. 2002;6:83–88. doi: 10.1007/s101510200018. [DOI] [PubMed] [Google Scholar]
- 42.Obokhare I. Fecal impaction: a cause for concern? Clin Colon Rectal Surg. 2012;25:53–58. doi: 10.1055/s-0032-1301760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldouri AQ, Alexander DJ. Presentation and management of perirectal sepsis. Ann R Coll Surg Engl. 2008;90:W4–W7. doi: 10.1308/147870808X303047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCloud JM, Doucas H, Scott AD, Jameson JS. Delayed presentation of life-threatening perineal sepsis following stapled haemorrhoidectomy: a case report. Ann R Coll Surg Engl. 2007;89:301–302. doi: 10.1308/003588407X179134. [DOI] [PMC free article] [PubMed] [Google Scholar]


