Abstract
The histone acetyltransferases CBP/p300 are involved in recurrent leukemia-associated chromosomal translocations and are key regulators of cell growth. Therefore, efforts to generate inhibitors of CBP/p300 are of clinical value. We developed a specific and potent acetyl-lysine competitive protein-protein interaction inhibitor, I-CBP112, that targets the CBP/p300 bromodomains. Exposure of human and mouse leukemic cell lines to I-CBP112 resulted in substantially impaired colony formation and induced cellular differentiation without significant cytotoxicity. I-CBP112 significantly reduced the leukemia-initiating potential of MLL-AF9+ AML cells in a dose-dependent manner in vitro and in vivo. Interestingly, I-CBP112 increased the cytotoxic activity of BET bromodomain inhibitor JQ1 as well as doxorubicin. Collectively we report the development and preclinical evaluation of a novel, potent inhibitor targeting CBP/p300 bromodomains that impairs aberrant self-renewal of leukemic cells. The synergistic effects of I-CBP112 and current standard therapy (doxorubicin) as well as emerging treatment strategies (BET inhibition) provide new opportunities for combinatorial treatment of leukemia and potentially other cancers.
Introduction
CBP (CREB (cAMP responsive element binding protein) binding protein (CREBBP) and p300 (adenovirus E1A-associated 300-kD protein) are two closely related and evolutionary conserved histone acetyl transferases (HATs) (1). CBP/p300 function as transcriptional regulators by acetylating histone tails and other nuclear proteins. They also act as scaffolds recruiting transcription factors to transcriptional active loci through a large diversity of protein interaction domains. The HAT activity of CBP is regulated by auto-acetylation of a basic surface loop region that competes with substrate binding and by the presence of a RING domain located adjacent to the catalytic HAT domain (2). Interestingly, CBP/p300 mediated acetylation creates binding sites for the acetyl-lysine specific CBP/p300 bromodomain which is required for proper substrate targeting resulting in a positive feedback loop and maintenance of CBP/p300 enzymatic activity (2).
Homozygous loss of either Cbp or p300 leads to embryonic lethality in utero in mice due to developmental defects including impaired hematopoiesis (3,4). Studies in heterozygous mice have characterized Cbp as an essential regulator of hematopoietic stem cell (HSC) self-renewal. Likewise, conditional ablation of Cbp in adult mice altered differentiation, quiescence, apoptosis and self-renewal of adult hematopoietic stem cells (HSC) (5).
CBP/p300 has been functionally linked to the development of multiple human cancers including solid tumors and hematological malignancies (6). CBP/p300 has been detected in several oncogenic fusions in leukemia involving either the MOZ acetyltransferase or the mixed linage leukemia (MLL) gene product promoting cell proliferation (7,8) . The most prevalent is the chromosomal translocation t(11;16)(q23;p13) associated with mostly therapy-related acute myeloid leukemia (AML) or myelodysplastic syndromes (MDS) resulting in a fusion protein that contains the bromdomains of CBP and parts of MLL (8,9). CBP/p300 was also proposed to act as transcriptional co-activator of other leukemogenic proteins such as the NUP98-HOXA9 fusion (10). Recently it has been shown that p300 interacts with the AML1-ETO fusion protein, present in over 20 % of human AML, and that it regulates transcription of multiple AML1-ETO target genes including Id1 (inhibitor of DNA binding 1), the cell cycle inhibitor p21 and Egr1 (early growth response 1) that are drivers of self-renewal of hematopoietic stem/progenitor cells (11). Furthermore, inhibition of p300 abrogates acetylation of AML1-ETO and impaired clonogenic growth in vitro and leukemic transformation in vivo (12).
The multi-domain organization of CBP/p300 has prompted several inhibitor development projects. The HAT activity has been targeted by natural compounds that mostly lacked specificity (13). The most potent HAT inhibitor developed so far is C646, which has low µM activity (14). C646 inhibits growth of tumour cell lines and promotes cellular senescence and reduced colony formation of AML1-ETO positive AML cell lines and primary blasts isolated from leukemic mice and AML patients (15). In addition, “ischemin” and a number of non-specific fragments with µM potency have been reported to target the CBP bromodomain (16,17).
Here we report a selective and highly potent chemical probe compound targeting the bromodomains of CBP/p300. Bromodomains are protein interaction domains with predicted good druggability (18), which has been demonstrated by potent and selective inhibitors developed for the BET (bromo and extracellular domain) family of transcriptional regulators (19–22). We developed the acetyl-lysine mimetic oxazepine inhibitor I-CBP112 that binds to CBP/p300 with nanomolar affinity and good selectivity. In leukemia we found that I-CBP112 impaired the disease-initiating self-renewal leukemic cells in vitro and in vivo without causing significant cytotoxicity.
Results
Discovery of I-CBP112
Based on the reported weak CBP/p300 bromodomain binding activity of BET inhibitors such as JQ1 and I-BET762 we analysed the CBP/p300 activity of a series of related compounds that all contained a benzo-oxazepine core structure (Fig. 1A). Hit expansion at several positions led to I-CBP112 with good activity against CBP/p300. The description of the structure activity relationship (SAR) of this compound class as well as the synthesis of I-CBP112 and its intermediates will be reported elsewhere.
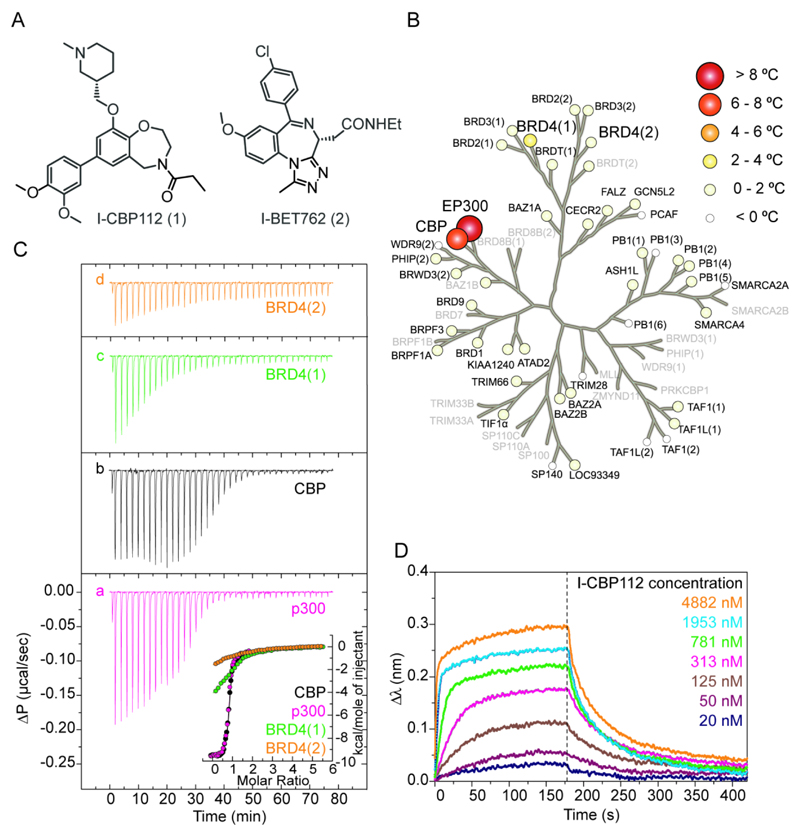
Figure 1. In vitro characterization of I-CBP112.
A: Chemical structure of I-CBP112 and the BET inhibitor I-BET762. B: Selectivity of I-CBP112 assessed by temperature shift data. Temperature shifts are shown as spheres as indicated in the figure and were mapped to the phylogenetic tree of the bromodomain family. Screened targets are labelled in black and targets that were not screened are shown in grey. C: Isothermal titration calorimetry titration for the bromodomain of CBP (a), p300 (b) and bromodomains BRD4(1) (c) and BRD4(2) (d), respectively. Shown are raw titration heats (upper panel) as well as binding isotherms shown as normalized binding heats (lower panel). Non-linear least squares fits to the data are shown as solid lines and fitted parameters are included in supplemental table 2. D: Concentration dependent sensograms (BLI) of I-CBP112 binding to CBP.
I-CBP112 is a potent and selective CBP/p300 inhibitor
We used temperature shift assays for the characterization of I-CBP112 potency and selectivity within the bromodomain family. This assay monitors binding of an inhibitor to a protein based on the magnitude of temperature stabilization (23). I-CBP112 showed significant stabilization of CBP/p300 melting temperatures (7.8 ºC and 8.6 ºC, respectively) while ΔTm values were low for 41 other members of the human bromodomain family suggesting excellent selectivity of the developed inhibitor (Fig. 1B, Supplemental Table 1). We investigated I-CBP112 selectivity further using biolayer interferometry (BLI) as an additional selectivity screening technology. Similar to the related surface plasmon resonance, this technology measures interactions of ligands with biomolecules immobilized on a surface monitoring changes in refractive index. To confirm the selectivity of I-CBP112 we screened a panel of 42 representative bromodomains which were homogenously biotinylated in vivo during recombinant expression. BLI confirmed the excellent selectivity measured by temperature shift experiments identifying only bromodomains of the BET family members as having weak off-target activity (Supplemental Fig. 1). Finally, we determined affinity of I-CBP112 for CBP/p300 in solution using isothermal titration calorimetry (ITC), which resulted in determination of a dissociation constant (KD) of 151 ± 6 nM and 167 ± 8 nM for CBP and p300, respectively (Figure 1C, Supplemental Table 2). Dose dependent BLI experiments resulted in a comparable KD value for CBP (KD: 142 nM; Figure 1D). The most significant off-target activity in our selectivity screen was against BRD4, which showed a ΔTm of 2.1 °C and 0.6 °C for the first (BRD4(1)) and second (BRD4(2)) bromodomain, respectively. ITC titrations resulted in the determination of KDs of 5.6 µM and 20 µM for BRD4(1) and BRD4(2), demonstrating 37 and 132 fold selectivity, respectively (Fig. 1C, Supplemental Table 2).
To assess selectivity of I-CBP112 outside the bromodomain family we screened both stereoisomers against a commercial selectivity panel including 104 binding assays monitoring binding to nuclear receptors, ion channels and 32 enzyme assays (including 10 kinases, 9 proteases and 5 phosphodiesterases). Both I-CBP112 stereoisomers showed similar behaviour when screened against this panel that revealed only weak interaction with two GPCRs (α1A and 5HT1) in the low µM region (Supplemental Table 3). The S-isomer was selected as chemical probe compound and was used in all subsequent cellular assays.
I-CBP112 is an acetyl-lysine competitive inhibitor
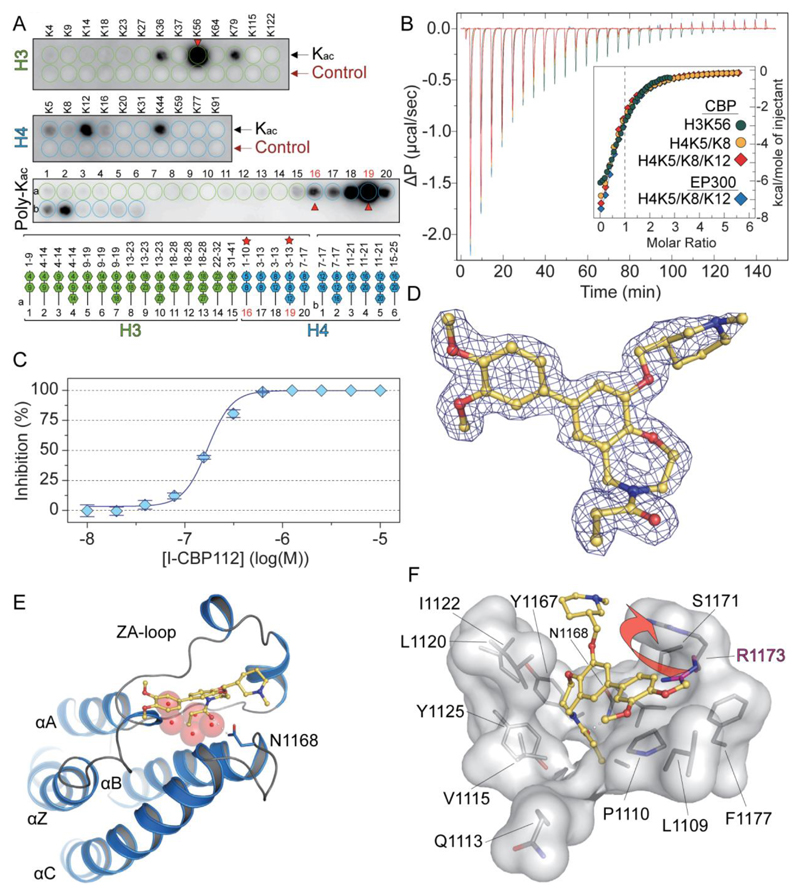
In order to establish a functional assay we studied the specificity of the CBP bromodomain towards histone peptide target sequences by synthesizing acetylated and non-acetylated histone peptides on a SPOT cellulose array (19). We found that the CBP bromodomain did not bind non-acetylated control peptides confirming high specificity for the acetyl-lysine modification (Fig. 2A). The peptide screening data revealed strong interaction of CBP with the histone 3 acetylated lysine 56 (H3K56ac), a CBP HAT substrate site, as well as interaction with H3K36ac and H3K79ac and H4K12ac and H4K44ac. Poly-acetylation seemed to increase binding for some of the histone H4 acetylation marks. However, ITC data showed that the CBP bromodomain had similar affinities for mono and di-acetylated peptides and the determined stoichiometries of 0.5 for polyacetylated peptides suggested that two bromodomains simultaneously bound one di-acetylated peptide supporting a canonical binding model in which one acetyl-lysine interacted with one CBP bromodomain (Fig. 2B, Supplemental Table 4). The strongest interaction was observed with H3K56ac (KD = 13.8 µM) which was used for the development of an Amplified Luminescence Proximity Homogeneous Assay (AlphaScreen) (24). However, we find it likely that additional interaction with acetylation sites other than H3K56ac may occur in the context of intact chromatin in cells. The developed assay demonstrated that I-CBP112 is an acetyl-lysine competitive inhibitor displacing H3K56ac from the CBP binding site with an IC50 value of 170 nM (Fig. 2C).
Figure 2. Recognition of acetylated peptide by CBP and structural features of I-CBP112 interaction with the CBP bromodomain.
A: SPOT analysis of the binding of CBP to a library of acetylated histone peptides. The row labelled “control” represents the corresponding non-acetylated peptides. The positions of the poly-acetylation sites are indicated in the lower panel. Peptides used for ITC experiments are highlighted by red stars and arrows on the plot. B: ITC experiments measuring binding affinities of identified mono and poly-acetylated peptides. C: Determination of in vitro IC50 value for the CBP/I-CBP112 interaction using AlphaScreen and the H3K56ac peptide. D: 2FoFc electron density map defining the conformation and binding mode of the I-CBP112 inhibitor in complex with CBP. E: Co-crystal structure of the bromodomain of CBP in complex with I-CBP112. Shown is a structural overview using a ribbon diagram. The main structural elements are labelled. The inhibitor is shown as a stick model. Hydrogen bonds are indicated by dotted lines and water molecules are show as red semi-transparent spheres. F: Details of the I-CBP112 interaction with the CBP bromodomain. The structural changed induced by the binding of the inhibitor involving and outward movement of R1173 is indicated by an arrow.
Co-crystallization of the R-isomer of I-CBP112 with the bromodomain of CBP confirmed the acetyl-lysine mimetic nature of this inhibitor. The structure of the complex was refined at 1.6 Å resolution (Supplemental Table 5). The inhibitor was well defined by electron density allowing assignment of its binding mode (Fig. 2D). As expected, the carbonyl group branching off the oxazepine ring acted as an acetyl-lysine mimetic moiety forming a hydrogen bond with the conserved asparagine (N1168 in CBP) as well as a water-mediated hydrogen bond with Y1125. The five water molecules that are typically present in bromodomain inhibitor and substrate complexes (25) were also conserved in the I-CBP112/CBP complex (Fig. 2E). Surprisingly, I-CBP112 bound with an induced fit binding mode leading to an outward movement of the side chain of R1173. This movement creates a deep pocket that accommodates the dimethoxyphenyl ring system forming an aromatic stacking interaction with the R1173 side chain (Fig. 2F).
CBP remains bound to chromatin in the presence of I-CBP112
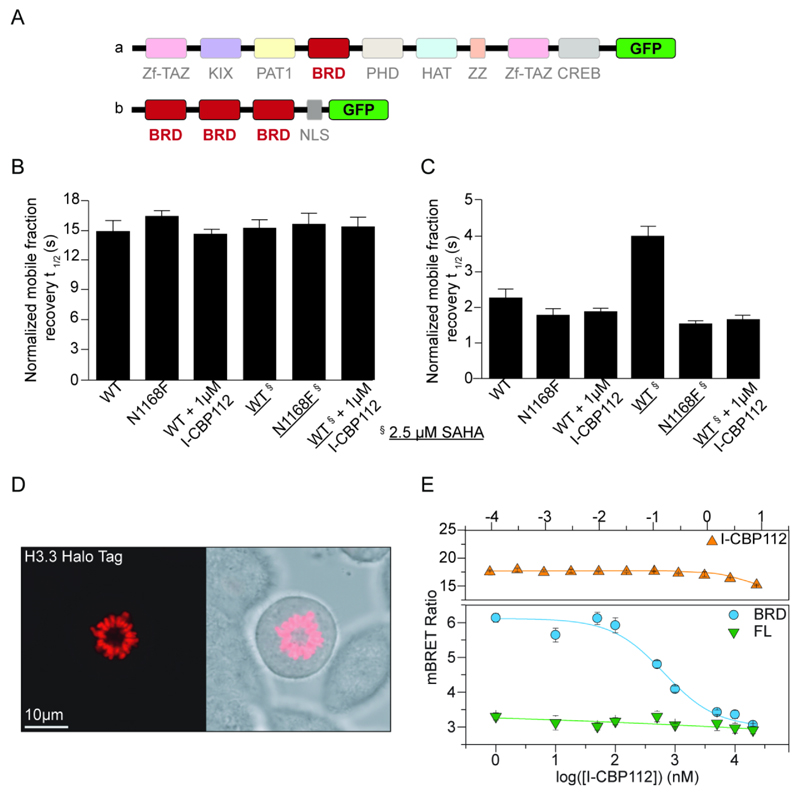
We next investigated whether I-CBP112 could dissociate CBP from chromatin using a fluorescent recovery after photo bleaching (FRAP) assay (26). Full-length CBP contains a large number of protein and DNA interaction domains (Fig. 3A). FRAP measurements using a GFP-tagged full-length CBP construct did not lead to decreased recovery times when treated with I-CBP112, or when a conserved key residue for acetyl-lysine interaction (N1168) was mutated to phenylalanine (N1168F), indicating that full-length CBP cannot be displaced from chromatin by bromodomain inhibitors (Fig. 3B). We used the pan-HDAC inhibitor SAHA to increase chromatin acetylation levels. In agreement with FRAP data using I-CBP112 and site directed mutants, SAHA treatment did not significantly influence fluorescent recovery times of full-length CBP, suggesting that tight chromatin association of CBP is not exclusively acetylation-dependent and is primarily mediated by interaction domains other than the bromodomain. To verify that I-CBP112 bound to the CBP bromodomain in nuclei of cells, we cloned the CBP bromodomain fused to a nuclear localization signal and a GFP tag. The assay window obtained for this construct was small, which was expected considering the low affinity of isolated bromodomain with acetylated histone tails. However, expression of three bromodomains (3xCBPBRD/GFP) fused in frame by a linker region resulted in a GFP-fusion protein that showed significant increase in FRAP signal after SAHA treatment and provided a sufficient assay window when compared to the N1168F mutant construct. Exposure to 1 µM I-CBP112 significantly reduced FRAP signal after SAHA treatment demonstrating that I-CBP112 can indeed target the CBP bromodomain in the nucleus and is capable of competing with acetyl-lysine mediated interactions of the CBP bromodomain in cellular environments (Fig. 3C). Representative data of beached nuclei as well as normalized fluorescent recovery data are shown in Supplemental Fig. 2. We confirmed these observations by a bioluminescence resonance energy transfer (BRET) assay using NanoLuc Luciferase fusions of full-length CBP and the isolated CBP bromodomain as well as Halo-tagged Histone H3.3, which was readily incorporated into chromatin (Fig 3D), as BRET pairs. In agreement with our FRAP experiments, dose response experiments revealed that I-CBP112 had no significant effect on the recruitment of full-length CBP to chromatin whereas a construct containing the bromodomain was readily replaced with a cellular IC50 value of 600 ± 50 nM (Fig. 3E) confirming cellular activity of I-CBP112 inhibiting acetylation dependent CBP bromodomain mediated interactions.
Figure 3. I-CBP112 displaces the CBP bromodomain from chromatin.
A: Domain organization of full length CBP (upper panel) and of the 3xCBPBRD/GFP construct used (lower panel). B: FRAP experiment showing half-recovery times for full length CBP/GFP and its inactivating mutant N1168F. C: FRAP experiment showing half-recovery times of the fluorescent signal of 3xCBPBRD/GFP (WT) and its inactivating mutant . Experiments with SAHA treated cells (2.5 µM) are indicated by underlined constructs. D: Fluorescent image (left panel) and phase contrast image (right panel) of metaphase chromosomes of Halo-tagged histone H3.3 transfected HEK293T cells showing incorporation of the tagged histone into chromatin. E: NanoBRET experiments showing the dose response of NanoLuc Luciferase fusion full-length CBP and the isolated CBP bromodomain. The construct containing only the bromodomain was readily displaced by I-CBP112 with a cellular IC50 value of 600 ± 50 nM. No displacement was observed using full-length GFP-BRD4 in agreement with the in vitro selectivity of I-CBP112 (upper panel).
Phenotypic responses of CBP/p300 bromodomain inhibition
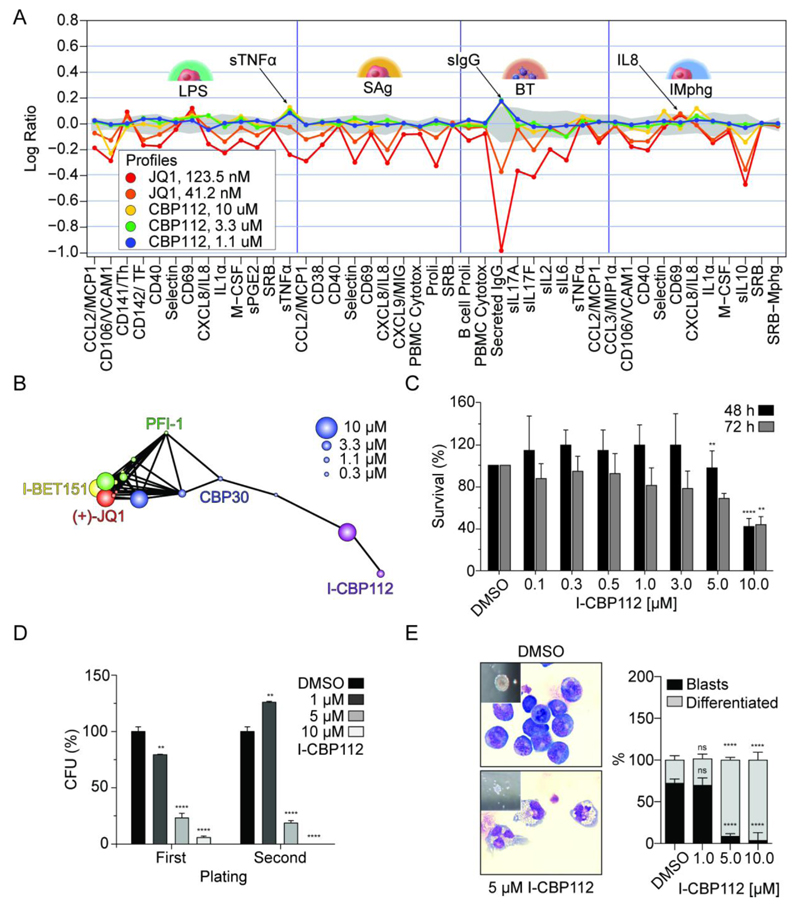
In order to get insight on the wider consequences of CBP/p300 bromodomain inhibition in cellular systems we exposed a panel of 12 stimulated primary human co-cultured cell types (BioMAP) with I-CBP112 and monitored a broad array of protein biomarkers. This unbiased approach has been well established to assess target specific phenotypic responses by proving a phenotypic fingerprint of an inhibitor based on a large number of marker proteins (27). For I-CBP112, the analysis of BioMAP data revealed a modest but robust response on the expression of some of the measured protein markers. Comparison with fingerprints generated by well-established BET bromodomain inhibitors, I-CBP112 revealed a very distinct phenotypic response (Fig 4A, Supplemental Fig. 3). Some effects of cytokine expression were shared between BET and CBP/p300 inhibitors. Expression of the anti-inflammatory cytokine IL10 or the vascular cell adhesion protein 1 (VCAM1/CD106) for instance was down-regulated by both I-CBP112 as well as the BET inhibitor JQ1. In contrast to BET inhibition, I-CBP112 had no effect on expression levels of a number of marker proteins including for instance the chemokine (C-C motif) ligand 2 (CCL2) or the cell adhesion molecule Selectin (CD62). In addition, we observed a number of differential responses comparing both inhibitors. Secreted IgG was for instance strongly down-regulated by the BET inhibitor JQ1, but it was up-regulated by I-CBP112. Using statistical methods comparing profile similarity we found that the BET bromodomain inhibitors JQ1, PFI-1 and I-BET151 clustered closely together (Pearson ≥0.6) in agreement with their reported selectivity for BET bromodomains. I-CBP112 in contrast showed a distinct phenotypic response (Fig 4B). Interestingly, another bromodomain inhibitor that we developed recently, CBP30, that has potent in vitro activity for the CBP/p300 bromodomain but also significant BET activity (28) clustered with I-CBP112 at low concentration but showed a clear BET bromodomain signature at 10 µM as expected from its dual activity. Thus, exposure of the BioMAP primary cell panel to I-CBP112 resulted in a unique response on cytokine and marker protein expression.
Figure 4. BioMAP data for I-CBP112 and effects on mouse MLL-CBP-immortalized bone marrow cells.
A: BioMAP data of four hematopoietic co-culture systems (Sag and LPS: Peripheral blood mononuclear cells cultured with venular endothelial cells; BT: B-cells and peripheral blood mononuclear cells; IMphg: venular endothelial cells co-cultured with M1 macrophages) treated with JQ1 or I-CBP112. The full BioMAP and explanation of the monitored marker proteins is shown in supplemental figure 2. B: Correlation of the phenotypic response of the panBET inhibitors JQ1, PFI-1, IBET-151, CBP30 and I-CBP112. Responses to compound inhibition above the selected threshold (Pearson ≥ 0.6) are represented by connected dots. C: Cytotoxicity (WST1-assay) of I-CBP112 on murine bone marrow cells immortalized by retroviral expression of the MLL-CBP fusion oncogene. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values were calculated by using ANOVA and Dunnett multiple comparison, ** p<0.01 **** p<0.0001, n=4. D: Clonogenic growth and replating assay of mouse MLL-CBP immortalized bone marrow-derived murine progenitors in methylcellulose, treated with I-CBP112. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values were calculated by using ANOVA and Dunnett multiple comparison, ** p<0.01 **** p<0.0001, n=2. E: Morphological changes of MLL-CBP immortalized progenitors on cellular and colony level upon exposure to increasing concentrations of CBP112 (left panel). Percentage of blasts and differentiated cells scored on cytospots. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values were calculated by using ANOVA and Dunnett multiple comparison, **** p<0.0001, n=20 scored high-magnification (x60) fields (right panel).
I-CBP112 impairs clonogenic growth of immortalized murine bone marrow cells
Next we aimed to explore the anti-cancer potential of I-CBP112 in a CBP/p300 dependent transformation model. Chromosomal translocations have been observed in patients after therapy with topoisomerase inhibitors leading to MLL-CBP fusion proteins that include the bromodomain. Expression of MLL-CBP has been shown to immortalize mouse bone marrow cells in vitro but it has a limited capacity of inducing leukemia in vivo (29). To address the effect of I-CBP112 on MLL-CBP-driven transformation we therefore established an immortalized hematopoietic cell line by retroviral expression of the MLL-CBP fusion in lineage marker depleted mouse bone marrow cells and expansion by two rounds of replating in methylcellulose (MC). Interestingly, exposure of these cells with I-CBP112 did not affect cell survival and significant cytotoxicity was only observed at high inhibitor concentrations and after prolonged exposure (Fig. 4C). However, exposure to the inhibitor significantly reduced clonogenic growth of MLL-CBP immortalized cells in MC which became even more apparent upon re-plating of the cells (Fig. 4D). I-CBP112 exposure resulted in morphologic changes from large round compact, so called class I type, to smaller and more loosely shaped colonies. Wright-Giemsa stained cytospots of treated cells harvested from MC, revealed morphological changes indicating differentiation of MLL-CBP immortalized cells (Fig. 4E).
I-CBP112 pre-treatment of MLL-AF9+ murine AML blasts reduced the number of LICs and delayed induction of the disease upon transplantation into irradiated recipients
We next studied the efficacy of I-CBP112 on leukemic cells isolated from mice transplanted with bone marrow retrovirally expressing fusion oncogenes (Fig. 5A) (30). Treatment of primary murine MLL-AF9+ leukemic blasts with I-CBP112 significantly impaired self-renewal in clonogenic re-plating assays in MC without significant cytotoxicity and induced significant morphological cellular differentiation (Fig. 5B-D) Similar results were obtained in bone marrow cells in vitro immortalized by expression of the NUP98-HOXA9 or the MLL-AF9 fusion (Supplemental Fig. 4). The observation of reduced in vitro clonogenic growth (Fig. 5C) and absence of general toxicity (Fig. 5D) suggested that I-CBP112 might selectively reduce the number of leukemia-initiating cells (LICs) often also referred as leukemic stem cells. We therefore addressed the stem frequency of MLL-AF9 expressing blasts in MC by extreme limiting dilution analysis (ELDA) (31) and found that treatment of the cells with 5µM I-CBP112 for 3 days resulted in a significant decrease in the estimated stem cell frequency (Fig. 5E).
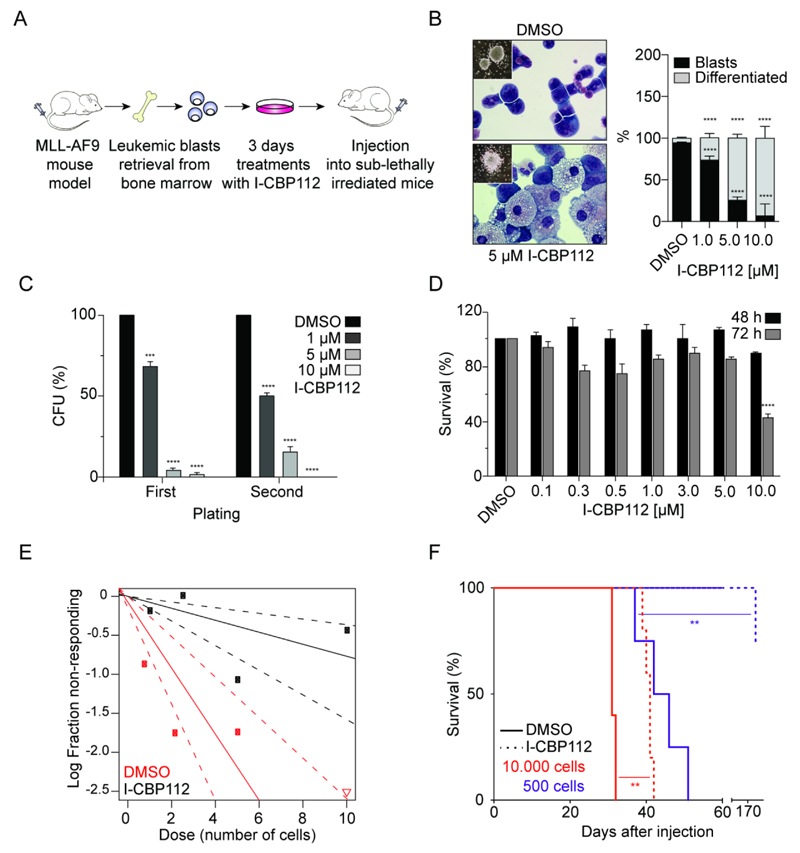
Figure 5. I-CBP112 impairs self-renewal and disease induction of murine MLL-AF9-driven acute leukemia.
A: Schematic outline of the used mouse model. Transplanting bone marrow cells retrovirally expressing the MLL-AF9 fusion oncogene leads to AML. Leukemic blasts from diseased mice were ex vivo treated with 5µM of I-CBP112 or DMSO vehicle for 3 days and transplanted into syngeneic secondary recipients. B: Changes on cell morphology and colony formation of murine MLL-AF9 leukemic blasts treated with I-CBP112 at different concentrations as indicated in the figure (left panel). Percentage of blasts and differentiated cells scored on cytospots. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values were calculated by using ANOVA and Dunnett multiple comparison, **** p<0.0001, n=20 scored high-magnification (x60) fields (right panel). C: Clonogenic growth and re-plating assay of leukemic MLL-AF9 blasts treated with I-CBP112. Concentration as indicated in the figure. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values were calculated by using ANOVA and Dunnett multiple comparison, *** p<0.001 **** p<0.0001, n=2. D: Cytotoxicity (WST1 assay) of leukemic MLL-AF9 blasts treated with I-CBP112. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values were calculated by using ANOVA and Dunnett multiple comparison, **** p<0.0001, n=4. E: Extreme limiting dilution analysis (ELDA). Shown is a log-fraction plot of the limiting dilution model fitted to the data. The slope of the line represents the log-active cell fraction. The dotted lines indicate the 95% confidence interval. The data value with zero negative response at highest dose (10 plated cells) is represented by a down-pointing triangle. The estimated stem cell frequency decreased from 1/2.3 to 1/13. Statistical significance was assessed by an overall test for differences with Chi-square 18.3 and DF 1 showing p<0.00001. F: Kaplan-Meier diagram showing significantly extended survival of mice injected with different number of I-CBP112 treated cells (dotted lines) compared to vehicle-treated controls (solid lines). The number of injected cells were 50 000 cells (blue), 10 000 cells (red), 5 000 cells (green) and 500 cells (magenta). P- values were calculated using log- rank (Mantel-Cox) Test, n=4/5 per group.
In contrast to MLL-CBP, the MLL-AF9-driven mouse AML can be efficiently propagated by transplantation into irradiated recipient mice (30) making it an ideal model to study the antileukemic potential of CBP/p300 bromdomain inhibition in vivo. We first carried out a pharmacokinetic study in mice to assess suitability of I-CBP112 for progression into xenograft models. Intraperitoneal (IP) administration of the compound at 3 mg/kg yielded moderate systemic exposure of the compound with a mean Cmax of 590 ng/mL (1.26 ± 0.74 µM (n=3)), an AUC of 384 ng/h/mL and a half-life of 0.6 ± 0.1 h. Intravenous (IV) administration (1.2 mg/kg) revealed high blood clearance, moderate volume distribution and comparison with intraperitoneal dosing (IP) suggested that I-CBP112 was bioavailable F%: 114 ± 63 (AUCt/Dip)/(AUC∞/Div).
Due to the modest pharmacokinetic properties of I-CBP112 we used a pre-treatment scheme to obtain a preliminary evaluation of I-CBP112 in vivo activity (Fig. 5A). I-CBP112 exposure of the cells for 3 days (5 µM) in liquid cultures led to minor changes of expression of cell surface markers, only c-Kit/CD117 and granulocyte marker Gr-1 were slightly decreased (Supplemental Fig. 5).
We then compared disease initiation in vivo by transplanting I-CBP112 or DMSO pre-treated MLL-AF9+ AML cells in limited dilutions into secondary recipients. We observed that in doses from 5-50 x 103 cells, mice receiving treated cells developed the disease significantly later. Transplantation of as little as 500 control cells induced AML in all recipients after 50 days. In contrast, only 1 mouse transplanted with 500 I-CBP112 treated cells developed the disease only after a long latency (Fig. 5F, Supplemental Fig. 6). Collectively these data demonstrate that exposure of murine MLL-AF9+ leukemic blasts to the I-CBP112 CBP/p300 bromodomain inhibitor significantly reduced colony formation in vitro, as measured in the ELDA assay, and delayed induction of AML upon transplantation into irradiated recipients.
I-CBP112 impairs the clonogenic growth of human leukemic cells
To corroborate the observations made in the mouse model in the human disease, we screened a panel of 18 human leukemic cell lines for their sensitivity to I-CBP112. Based on the cancer cell line encyclopedia (CCLE), none of these cell lines harbour any mutations in the coding region of CBP or EP300 (Supplemental Table 4). Similar to our observation on primary mouse leukemic blasts, we found that I-CBP112 did not cause immediate cytotoxic effects but significantly impaired self-renewal (Supplemental Fig. 7-12). However, I-CBP112 significantly reduced the clonogenic growth in all 12 human cell lines that form visible colonies in methylcellulose (Supplemental Fig. 8). We selected three leukemic cell lines with distinct genetic lesions: KASUMI-1 (AML1-ETO+), SEM (MLL-AF4+) and MOLM13 (MLL-AF9+/FLT3-ITD+) for more detailed studies. I-CBP112 significantly impaired clonogenic growth of all three lines in a dose dependent manner (Fig. 6A). I-CBP112 treatment resulted in dose-dependent G1 arrest of the cell cycle. Time-dependent analysis of KASUMI-1 cells showed a prominent G1 arrest at day 2 and 3 that was even more pronounced at day 6 (Fig. 6B). Prolonged exposure of cells to increasing concentrations of the compound in liquid culture resulted in signs of apoptosis (as measured by 7-AAD/AnnexinV staining) in KASUMI-1 cells at day 5, whereas MOLM13 and SEM were less sensitive (Supplemental Fig. 9-11).
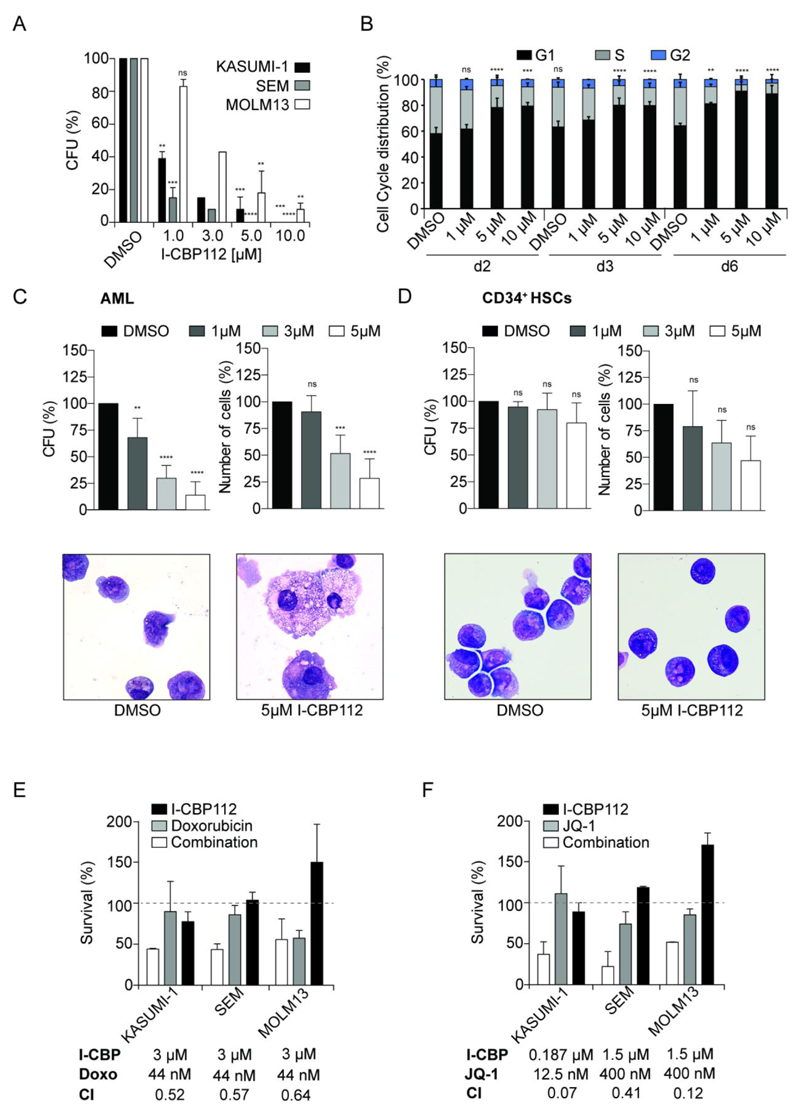
Figure 6. I-CBP112 impairs the clonogenic growth of human leukemic cells and sensitizes them to BET inhibition and doxorubicin.
A: Clonogenic activity of three human leukemic cell lines in methylcellulose, exposed to different doses of I-CBP112. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values were calculated by using ANOVA and Dunnett multiple comparison, ** p<0.01 *** p<0.001 **** p<0.0001, n=3. B: Cell cycle phase distribution of KASUMI-1 cells analysed by flow cytometry upon treatment with increasing doses of I-CBP112 at different time points. Medium and compound were renewed after 3 days. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values are shown for G1 phases and were calculated by using two-way ANOVA and Turkey multiple comparison, ** p<0.01 *** p<0.001 **** p<0.0001, n=2-4. C: Clonogenic growth and cell numbers of primary blast cells from 5 AML patients. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values were calculated by using ANOVA and Dunnett multiple comparison, ** p<0.01 *** p<0.001 **** p<0.0001, n=5. Morphology (Giemsa-Wright stain) of primary blast cells from AML patients treated with DMSO or 5 µM I-CBP112. D: Clonogenic growth and cell numbers of CD34+ cells from healthy donors. Shown is the mean percentage normalized to vehicle- treated, error bars represent ±SD, P values were calculated by using ANOVA and Dunnett multiple comparison, n=3. Morphology (Giemsa-Wright stain) of CD34+ cells from a healthy donor treated with DMSO or 5 µM I-CBP112. E: Synergy study of KASUMI-1, SEM and MOLM13 cells treated either with indicated non-cytotoxic doses of I-CBP112 (black bars), doxorubicin (grey bars) or both compounds (white bars). Concentrations used and combination index (CI) are given in the figure. CI<1 indicates a synergistic effect. F: Synergy study of KASUMI-1, SEM and MOLM13 cells treated either with indicated non-cytotoxic doses of I-CBP112 (black bars), JQ1 (grey bars) or both compounds (white bars). Concentrations used and combination index (CI) are given in the figure. CI<1 indicates a synergistic effect.
We also tested the effects of I-CBP112 on clonogenic growth of primary human cells. Exposure of cells from five different AML patients to I-CBP112 showed a dose dependent reduction in the number of colonies and cells in MC. Similar to the murine and human leukemic cell lines, I-CBP112 treated primary AML cells showed morphologic signs of differentiation (Fig. 6C). In contrast, I-CBP112 exposure of peripheral CD34+ cells from three healthy donors did not affect the colony number in MC. However, the size of the colonies was reduced, thus resulting in a statistically not significant tendency of lower overall cell numbers (Fig. 6D and Supplemental Fig. 12). These observations suggest that inhibition of the CBP/p300 bromodomain may have therapeutic potential in AML by suppressing self-renewal.
I-CBP112 sensitizes human leukemic cells to BET inhibition and doxorubicin
CBP/p300 and the BRD4 BET protein often co-localize on active promoters (32). However, in contrast to inhibition of BET bromodomains, blocking CBP/p300 BRDs by I-CBP112 resulted only in limited cytotoxicity. We therefore wondered whether exposure of combinations of both BRD inhibitors might show synergistic effects. In parallel we also tested whether I-CBP112 might enhance cytotoxicity of doxorubicin, a clinically widely used genotoxic agent. We tested 8 different concentrations of I-CBP112 ranging from 0.1875 µM to 24 µM in combination with 15 different concentrations of JQ1 and doxorubicin respectively in ratios ranging from 8:1 to 1:16 in WST1 proliferation/survival assays for KASUMI-1, MOLM13 and SEM cells (Supplement Fig. 12A-B). .As expected JQ1 and doxorubicin alone showed low nM activity that was different for each of the three cell lines. We analyzed the potential synergistic activity using the Chou-Talalay method (33). We observed strong synergy combining either JQ1 or doxorubicin with I-CBP112 except for MOLM13 cells in which synergism between doxorubicin and I-CBP112 was less pronounced (Fig. 6E-F, Supplement Fig. 12C-D). We also compared the impact of these drug combinations on DNA damage by measuring γH2Ax foci formation and H2Ax Ser139 phosphorylation In SEM and MOLM13 but not KASUM1 cells increased γH2Ax foci and H2Ax Ser139 phosphorylation was seen after treatment with the I-CBP112/JQ1 combination. Exposure to doxorubicin increased H2Ax phosphorylation and foci that were not further increased by the combination with I-CBP112 (Supplement Fig. 14). The combination of BET and CBP/p300 BRD inhibitors is of particular interest as both target sub-families could be simultaneously targeted using a single agent with dual activity.
I-CBP112 modulates transcription of key inflammatory genes
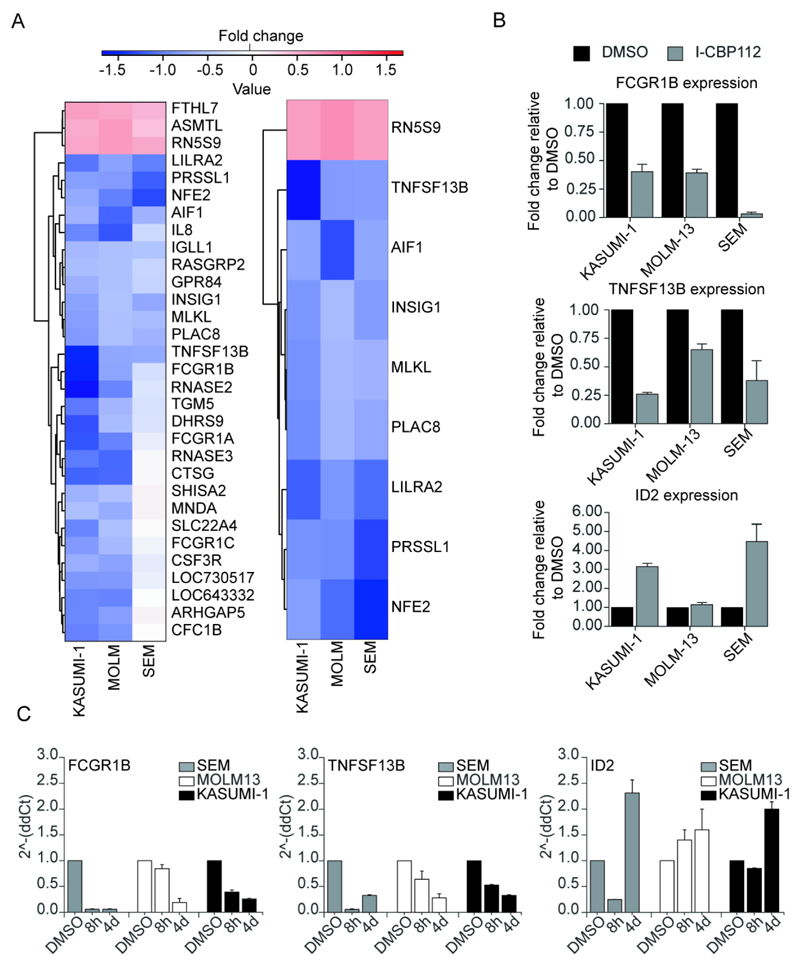
To address the transcriptional consequences of exposure to I-CBP112, we performed microarray gene expression studies. We compared the gene expression signatures from KASUMI-1, MOLM13 and SEM leukemic cell lines that have been exposed to I-CBP112 for 4 days in liquid culture, a time point where cell cycle alterations become detectable without any pronounced effects on cellular viability (Supplemental Fig. 7). Exposure of the three cell lines to I-CBP112 (3 µM) resulted in transcriptional repression rather than activation. Unsupervised hierarchical clustering analysis identified significantly regulated genes. Heat maps were generated for all genes identified as significantly altered (>1.5 FC, p<0.01) in both KASUMI-1 and MOLM13 (Fig. 7A, left panel), or in all three of KASUMI-1, MOLM13 and SEM (Fig. 7A, right panel). Interestingly, many of the repressed genes were key regulators of immune response including the Fc fragment of IgG, high affinity 1b, receptor (FCGR1B; CD64) playing an important role in humoral immune response (34), or TNFSF13B (B-cell-activating factor; B-lymphocyte stimulator; Delta4 BAFF) a member of the tumour necrosis factor (TNF) ligand family (35). The expression changes of selected genes were confirmed by q-RTPCR (Fig. 7B) and found to be consistent with the array data. A list of all deregulated genes is compiled in Supplemental Tables 7-9. To differentiate between early and late transcriptional changes we measured mRNA levels at 8h and 4 days on a set of three representative deregulated genes identified in the array study (Fig. 7C). For the genes tested stronger deregulation was observed after 4 days exposure to I-CBP112.
Figure 7. I-CBP112 selectively modulates transcription.
A: Heat map of selected genes. Shown are differences in gene transcription (log2(fold change)) colour coded as indicated in the figure. B: Confirmation of expression changes if selected genes that are regulated by I-CBP112 by q-RTPCR. C: Time dependence of gene expression of selected genes monitored by q-RTPCR. Shown are average values of three biological replicates.
We used chromatin immunoprecipitation (ChIP) in combination with PCR to detect p300 levels in KASUMI-1 cells at promoter, enhancer and exonic region of two significantly regulated genes: TNFSF13B and ID2. In agreement with our FRAP data we did not see significant displacement of p300 after 8h exposure with 3 µM I-CBP112. At longer exposure however (4 days), we observed increased p300 occupancy at most regions investigated when compared with DMSO treated cells, whereas control genes (IgG) were unaffected (Supplemental Fig.16). Acetylation levels at p300/CBP substrate sites (H3K56ac, H3K56ac) , which are also P300/CBP bromodomain binding sites, were significantly reduced in particular after 4 day exposure to I-CBP112 suggesting that bromodomain inhibition affects also p300/CBP HAT activity at regulated gene promoters.
Discussion
High-resolution crystal structures allowed to rationally design several small molecules that selectively interact with high affinity to bromodomains of the BET family (19–22). BET inhibitors of the benzodiazepine scaffold gave rise to the inhibitor I-CBP112, the first selective and potent inhibitor developed for CBP/p300 bromodomains. Together with the HAT inhibitor C646 and inhibitors targeting other protein interaction domains the developed chemical probe extends the set of tool molecules targeting this key chromatin protein. Several studies demonstrated that BET inhibitors potently impaired the growth of cells originating from hematologic and solid cancers (22,27,36,37). Analysis of a large number of different cell lines and primary murine and human cells revealed that I-CBP112 selectively impaired aberrant leukemic self-renewal. In contrast, BET inhibitors, such as JQ1 or PFI-1 potently impaired proliferation, survival and self-renewal not only of cancer cells but also activated immune cells. This wide window of cellular activity might result from blocking interaction of BRD4 with a large number of super enhancer elements regulating multiple transcription factors including the Myc oncogene (38,39). Not surprisingly, conditional ablation of BRD4 in vivo resulted in a severe phenotype due to stem cell depletion e.g. in the small intestine (40). Conditional ablation of Cbp in the hematopoietic system of adult mice led to an increase in differentiation, quiescence, apoptosis and defects in adult HSC maintenance and self-renewal (5). Comparison with the observed cellular phenotypes induced by I-CBP112 observed in this study suggests that chemical inhibition of the CBP/p300 bromodomain phenocopies some but not all of the cellular effects observed in knockout studies including most remarkably defects in self-renewal and increased differentiation of immortalized hematopoietic cells as well as primary leukemic blasts. Reduction of colony formation by MLL-AF9 immortalized cells as shown by the ELDA assay in vitro and impaired disease induction by I-CBP112 treated leukemic blasts after transplantation strongly suggest significant activity of the compound on aberrant self-renewal of leukemic cells.
Domain specific functions have recently been addressed in a systematic p300 deletion study in which loss of the p300 KIX or CH1 domains have been reported to cause profound defects in hematopoiesis, whereas deletion of other domains including the p300 bromodomain lead to lineage restricted effects (41). Deletion of the bromodomain only modestly impaired p300 hematopoietic function in B-cells but the bromodomain was required during T-cell development. Thus, the bromodomain interaction module may only be required for a subset of albeit critical fraction of CBP/p300 functions. Indeed, our FRAP and BRET experiments demonstrated that wild-type full-length CBP/p300 or mutants that lack the bromodomain interaction with acetylated histones remained bound to chromatin in the presence of I-CBP112. The lack of full displacement of CBP/p300 from chromatin may explain the modest effects of I-CBP112 on gene transcription in our microarray study that showed few genes up-regulated greater than 2-fold after treatment with I-CBP112. The observed modest changes in gene transcription are however consistent with expression studies carried out using genetic deletion of CBP (5,42,43). The small number of commonly regulated genes in all three cell lines tested was surprising, suggesting a strong context dependence of CBP/p300 regulated transcriptional events.
The role of CBP/p300 in human leukemogenesis is complex. Conditional knock-in of MLL-CBP in mice revealed that the fusion protein selectively expanded granulocyte/macrophage progenitors and enhanced their self-renewal/proliferation (44). In the MLL-CBP fusion the bromo/HAT domain is maintained and required for the transforming activity of this oncogene (29). CBP/p300 was also found to regulate the transcriptional activity of the AML1-ETO fusion driving AML-M2 by posttranslational modification (12). CBP/p300 seem also to cooperate with the c-MYB proto-oncogene in cellular transformation by different leukemia-associated fusions including AML1-ETO, AML1-ETO9a, MLL-ENL, and MLL-AF9 (45). These observations suggest that the block of aberrant self-renewal in leukemic cells mediated by I-CBP112 might be the consequence of multiple activities influenced by the driver fusion oncogenes and multiple protein-protein interactions of CBP/p300. It has been estimated that CBP/p300 physically or functionally interact with close to 400 proteins acting as a co-activator of many transcription factors including NF-κB, HIF-lα, p53 or β-catenin that are known key regulators of leukemogenesis (46). It is likely that some of these interactions are influenced by the bromodomains. The p300 bromodomain for instance, has been reported to be required for recognition of specific acetylated lysine residues in p53 (47–49). We have recently observed that p53 dependent induction of the cell cycle inhibitor p21 by genotoxic stress might be dependent on the CBP/p300 BRD. Interestingly we also found regulation of some p53 target genes at early time points in I-CBP112 treated KASUMI-1 leukemic cells (Supplemental Fig. 15). Hence we found that the combination of I-CBP112 with doxorubicin resulted in significantly cytotoxicity in leukemic cells. Interestingly, we also saw also synergy between the BET inhibitor JQ1 and I-CBP112. Previous studies have shown synergistic cytotoxic activity of BET inhibitors with some HDAC inhibitors such as SAHA or LBH-589 in Myc induced murine lymphoma (50). Thus, small molecules CBP/p300 bromodomain inhibitors may have not only the potential to improve current therapies by selectively targeting aberrant self-renewal linked to disease relapse but also to enhance protocols combining BET inhibitors and traditional chemotherapy and suggest to further explore dual inhibitors targeting CBP/p300 and BET family bromodomains for their anti-cancer potential. Several compounds with dual BET and CBP/p300 activity have been developed in our laboratory demonstrating that selective targeting bromodomains of both bromodomain subfamilies is feasible using a single agent.
Supplementary Material
Acknowledgements
The SGC is a registered charity (number 1097737) that receives funds from the Canadian Institutes for Health Research, the Canada Foundation for Innovation, Genome Canada, GlaxoSmithKline, Pfizer, Eli Lilly, the Novartis Research Foundation, Takeda, Bayer, the Ontario Ministry of Research and Innovation and the Wellcome Trust [092809/Z/10/Z]. P.F. and S.P. are supported by a Wellcome Trust Career Development Fellowship (095751/Z/11/Z). The work of the laboratory of J.S. is supported by the Swiss National Science Foundation (SNF-31003A-130661/1), the Swiss Cancer League (KFS-3019-08-2012) and the Gertrude Von Meissner Foundation (Basel, Switzerland). We also thank S. Juge and U. Schneider for providing technical support.
Footnotes
Conflict of interest statement:
None of the authors have a conflict of interest to declare.
References
- 1.Chen J, Li Q. Life and death of transcriptional co-activator p300. Epigenetics. 2011;6(8):957–61. doi: 10.4161/epi.6.8.16065. [DOI] [PubMed] [Google Scholar]
- 2.Delvecchio M, Gaucher J, Aguilar-Gurrieri C, Ortega E, Panne D. Structure of the p300 catalytic core and implications for chromatin targeting and HAT regulation. Nat Struct Mol Biol. 2013;20(9):1040–6. doi: 10.1038/nsmb.2642. [DOI] [PubMed] [Google Scholar]
- 3.Kung AL, Rebel VI, Bronson RT, Ch’ng LE, Sieff CA, Livingston DM, et al. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 2000;14(3):272–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Yao TP, Oh SP, Fuchs M, Zhou ND, Ch’ng LE, Newsome D, et al. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell. 1998;93(3):361–72. doi: 10.1016/s0092-8674(00)81165-4. [DOI] [PubMed] [Google Scholar]
- 5.Chan WI, Hannah RL, Dawson MA, Pridans C, Foster D, Joshi A, et al. The transcriptional coactivator Cbp regulates self-renewal and differentiation in adult hematopoietic stem cells. Mol Cell Biol. 2011;31(24):5046–60. doi: 10.1128/MCB.05830-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23(24):4225–31. doi: 10.1038/sj.onc.1207118. [DOI] [PubMed] [Google Scholar]
- 7.Katsumoto T, Yoshida N, Kitabayashi I. Roles of the histone acetyltransferase monocytic leukemia zinc finger protein in normal and malignant hematopoiesis. Cancer Sci. 2008;99(8):1523–7. doi: 10.1111/j.1349-7006.2008.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Zeleznik-Le N, Emmanuel N, Jayathilaka N, Chen J, Strissel P, et al. Characterization of genomic breakpoints in MLL and CBP in leukemia patients with t(11;16) Genes Chromosomes Cancer. 2004;41(3):257–65. doi: 10.1002/gcc.20077. [DOI] [PubMed] [Google Scholar]
- 9.Rowley JD, Reshmi S, Sobulo O, Musvee T, Anastasi J, Raimondi S, et al. All patients with the T(11;16)(q23;p13.3) that involves MLL and CBP have treatment-related hematologic disorders. Blood. 1997;90(2):535–41. [PubMed] [Google Scholar]
- 10.Kasper LH, Brindle PK, Schnabel CA, Pritchard CE, Cleary ML, van Deursen JM. CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol Cell Biol. 1999;19(1):764–76. doi: 10.1128/mcb.19.1.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Lu S, Wu L, Chai G, Wang H, Chen Y, et al. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21(Waf1/Cip1) Mol Cell Biol. 2006;26(7):2782–90. doi: 10.1128/MCB.26.7.2782-2790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Gural A, Sun XJ, Zhao X, Perna F, Huang G, et al. The leukemogenicity of AML1-ETO is dependent on site-specific lysine acetylation. Science. 2011;333(6043):765–9. doi: 10.1126/science.1201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arif M, Pradhan SK, Thanuja GR, Vedamurthy BM, Agrawal S, Dasgupta D, et al. Mechanism of p300 specific histone acetyltransferase inhibition by small molecules. J Med Chem. 2009;52(2):267–77. doi: 10.1021/jm800657z. [DOI] [PubMed] [Google Scholar]
- 14.Santer FR, Hoschele PP, Oh SJ, Erb HH, Bouchal J, Cavarretta IT, et al. Inhibition of the acetyltransferases p300 and CBP reveals a targetable function for p300 in the survival and invasion pathways of prostate cancer cell lines. Mol Cancer Ther. 2011;10(9):1644–55. doi: 10.1158/1535-7163.MCT-11-0182. [DOI] [PubMed] [Google Scholar]
- 15.Gao XN, Lin J, Ning QY, Gao L, Yao YS, Zhou JH, et al. A histone acetyltransferase p300 inhibitor C646 induces cell cycle arrest and apoptosis selectively in AML1-ETO-positive AML cells. PLoS One. 2013;8(2):e55481. doi: 10.1371/journal.pone.0055481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borah JC, Mujtaba S, Karakikes I, Zeng L, Muller M, Patel J, et al. A small molecule binding to the coactivator CREB-binding protein blocks apoptosis in cardiomyocytes. Chem Biol. 2011;18(4):531–41. doi: 10.1016/j.chembiol.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewings DS, Wang M, Philpott M, Fedorov O, Uttarkar S, Filippakopoulos P, et al. 3,5-dimethylisoxazoles act as acetyl-lysine-mimetic bromodomain ligands. J Med Chem. 2011;54(19):6761–70. doi: 10.1021/jm200640v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidler LR, Brown N, Knapp S, Hoelder S. Druggability analysis and structural classification of bromodomain acetyl-lysine binding sites. J Med Chem. 2012;55(17):7346–59. doi: 10.1021/jm300346w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–73. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468(7327):1119–23. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picaud S, Da Costa D, Thanasopoulou A, Filippakopoulos P, Fish PV, Philpott M, et al. PFI-1, a highly selective protein interaction inhibitor, targeting BET Bromodomains. Cancer research. 2013;73(11):3336–46. doi: 10.1158/0008-5472.CAN-12-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478(7370):529–33. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fedorov O, Niesen FH, Knapp S. Kinase inhibitor selectivity profiling using differential scanning fluorimetry. Methods Mol Biol. 2012;795:109–18. doi: 10.1007/978-1-61779-337-0_7. [DOI] [PubMed] [Google Scholar]
- 24.Philpott M, Yang J, Tumber T, Fedorov O, Uttarkar S, Filippakopoulos P, et al. Bromodomain-peptide displacement assays for interactome mapping and inhibitor discovery. Mol Biosyst. 2011;7(10):2899–908. doi: 10.1039/c1mb05099k. [DOI] [PubMed] [Google Scholar]
- 25.Filippakopoulos P, Picaud S, Fedorov O, Keller M, Wrobel M, Morgenstern O, et al. Benzodiazepines and benzotriazepines as protein interaction inhibitors targeting bromodomains of the BET family. Bioorg Med Chem. 2012;20(6):1878–86. doi: 10.1016/j.bmc.2011.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philpott M, Rogers CM, Yapp C, Wells C, Lambert JP, Strain-Damerell C, et al. Assessing cellular efficacy of bromodomain inhibitors using fluorescence recovery after photobleaching. Epigenetics Chromatin. 2014;7:14. doi: 10.1186/1756-8935-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciceri P, Muller S, O’Mahony A, Fedorov O, Filippakopoulos P, Hunt JP, et al. Dual kinase-bromodomain inhibitors for rationally designed polypharmacology. Nat Chem Biol. 2014;10(4):305–12. doi: 10.1038/nchembio.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hay DA, Fedorov O, Martin S, Singleton DC, Tallant C, Wells C, et al. Discovery and Optimization of Small-Molecule Ligands for the CBP/p300 Bromodomains. J Am Chem Soc. 2014;136(26):9308–19. doi: 10.1021/ja412434f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavau C, Du C, Thirman M, Zeleznik-Le N. Chromatin-related properties of CBP fused to MLL generate a myelodysplastic-like syndrome that evolves into myeloid leukemia. EMBO J. 2000;19(17):4655–64. doi: 10.1093/emboj/19.17.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mereau H, De Rijck J, Cermakova K, Kutz A, Juge S, Demeulemeester J, et al. Impairing MLL-fusion gene-mediated transformation by dissecting critical interactions with the lens epithelium-derived growth factor (LEDGF/p75) Leukemia. 2013;27(6):1245–53. doi: 10.1038/leu.2013.10. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1–2):70–8. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Reynoird N, Schwartz BE, Delvecchio M, Sadoul K, Meyers D, Mukherjee C, et al. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J. 2010;29(17):2943–52. doi: 10.1038/emboj.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010;70(2):440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland JS, Loxton AG, Haks MC, Kassa D, Ambrose L, Lee JS, et al. Differential gene expression of activating Fcgamma receptor classifies active tuberculosis regardless of human immunodeficiency virus status or ethnicity. Clin Microbiol Infect. 2013 doi: 10.1111/1469-0691.12383. [DOI] [PubMed] [Google Scholar]
- 35.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293(5537):2108–11. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 36.Filippakopoulos P, Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014;13(5):337–56. doi: 10.1038/nrd4286. [DOI] [PubMed] [Google Scholar]
- 37.Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011 doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi J, Vakoc CR. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol Cell. 2014;54(5):728–36. doi: 10.1016/j.molcel.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM, et al. BET Bromodomain Inhibition as a Therapeutic Strategy to Target c-Myc. Cell. 2011;146(6):904–17. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolden JE, Tasdemir N, Dow LE, van Es JH, Wilkinson JE, Zhao Z, et al. Inducible in vivo silencing of Brd4 identifies potential toxicities of sustained BET protein inhibition. Cell Rep. 2014;8(6):1919–29. doi: 10.1016/j.celrep.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimbrel EA, Lemieux ME, Xia X, Davis TN, Rebel VI, Kung AL. Systematic in vivo structure-function analysis of p300 in hematopoiesis. Blood. 2009;114(23):4804–12. doi: 10.1182/blood-2009-04-217794. [DOI] [PubMed] [Google Scholar]
- 42.Kang-Decker N, Tong C, Boussouar F, Baker DJ, Xu W, Leontovich AA, et al. Loss of CBP causes T cell lymphomagenesis in synergy with p27Kip1 insufficiency. Cancer Cell. 2004;5(2):177–89. doi: 10.1016/s1535-6108(04)00022-4. [DOI] [PubMed] [Google Scholar]
- 43.Xu W, Fukuyama T, Ney PA, Wang D, Rehg J, Boyd K, et al. Global transcriptional coactivators CREB-binding protein and p300 are highly essential collectively but not individually in peripheral B cells. Blood. 2006;107(11):4407–16. doi: 10.1182/blood-2005-08-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Iwasaki H, Krivtsov A, Febbo PG, Thorner AR, Ernst P, et al. Conditional MLL-CBP targets GMP and models therapy-related myeloproliferative disease. EMBO J. 2005;24(2):368–81. doi: 10.1038/sj.emboj.7600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pattabiraman DR, McGirr C, Shakhbazov K, Barbier V, Krishnan K, Mukhopadhyay P, et al. Interaction of c-Myb with p300 is required for the induction of acute myeloid leukemia (AML) by human AML oncogenes. Blood. 2014;123(17):2682–90. doi: 10.1182/blood-2012-02-413187. [DOI] [PubMed] [Google Scholar]
- 46.Mendonca DB, Mendonca G, Cooper LF. Mammalian two-hybrid assays for studies of interaction of p300 with transcription factors. Methods Mol Biol. 2013;977:323–38. doi: 10.1007/978-1-62703-284-1_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei L, Jamonnak N, Choy J, Wang Z, Zheng W. Differential binding modes of the bromodomains of CREB-binding protein (CBP) and p300 with acetylated MyoD. Biochem Biophys Res Commun. 2008;368(2):279–84. doi: 10.1016/j.bbrc.2008.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou T, Ray S, Lee C, Brasier AR. The STAT3 NH2-terminal domain stabilizes enhanceosome assembly by interacting with the p300 bromodomain. J Biol Chem. 2008;283(45):30725–34. doi: 10.1074/jbc.M805941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mujtaba S, He Y, Zeng L, Yan S, Plotnikova O, Sachchidanand, et al. Structural mechanism of the bromodomain of the coactivator CBP in p53 transcriptional activation. Mol Cell. 2004;13(2):251–63. doi: 10.1016/s1097-2765(03)00528-8. [DOI] [PubMed] [Google Scholar]
- 50.Bhadury J, Nilsson LM, Muralidharan SV, Green LC, Li Z, Gesner EM, et al. BET and HDAC inhibitors induce similar genes and biological effects and synergize to kill in Myc-induced murine lymphoma. Proc Natl Acad Sci U S A. 2014;111(26):E2721–30. doi: 10.1073/pnas.1406722111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.