Abstract
Matrix metalloproteinases (MMPs) are zinc- and calcium-dependent endoproteinases that have the ability to break down extracellular matrix. The large range of MMPs’ functions widens their spectrum of potential role as activators or inhibitors in tissue remodeling, cardiovascular diseases, and obesity. In particular, MMP-1, -2, and -9 may be associated with exercise and obesity. Thus, the current study reviewed the effects of different types of exercise (resistance and aerobic) on MMP-1, -2, and -9. Previous studies report that the response of MMP-2 and -9 to resistance exercise is dependent upon the length of exercise training, since long-term resistance exercise training increased both MMP-2 and -9, whereas acute bout of resistance exercise decreased these MMPs. Aerobic exercise produces an inconsistent result on MMPs, although some studies showed a decrease in MMP-1. Obesity is related to a relatively lower level of MMP-9, indicating that an exercise-induced increase in MMP-9 may positively influence obesity. A comprehensive understanding of the relationship between exercise, obesity, and MMPs does not exist yet. Future studies examining the acute and chronic responses of these MMPs using different subject models may provide a better understanding of the molecular mechanisms that are associated with exercise, obesity, and cardiovascular disease.
Keywords: cardiovascular disease, gelatinases, collagenases, TIMP
Introduction
The property of matrix metalloproteinases
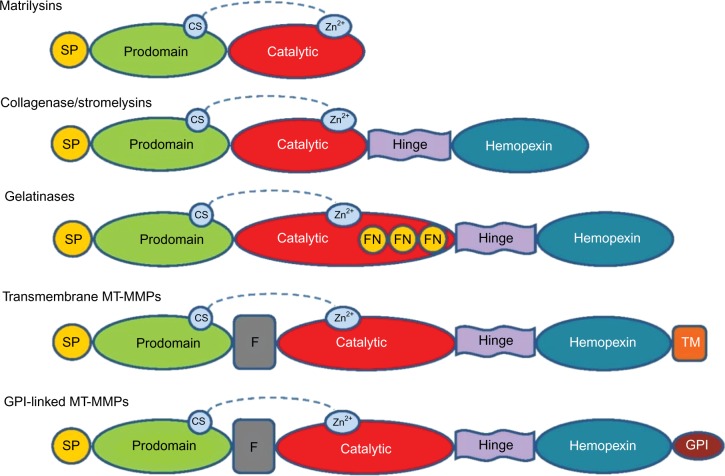
Matrix metalloproteinases (MMPs) were first observed in 1962 by Jerome Gross and Charles Lapiere in tadpole tissue that exhibited collagenolytic activity.1 Eisen et al2 were able to isolate human MMPs 6 years after its first discovery. MMPs are zinc-and calcium-dependent endoproteinases that play a crucial role in the remodeling of extracellular matrix (ECM) by breaking down its protein components.3 MMPs can be categorized, on the basis of substrate specificity and homology, into the following six family groups: collagenases, gelatinases, stromelysins, matrilysins, membrane-type MMPs, and other MMPs (Figure 1; Table 1). All MMPs share common domain structures that degrade various ECM and nonmatrix.4,5 Specific propeptide and catalytic domains exist (ie, MMP-7 and -26) along with a hemopexin-like, four-bladed, β-propeller domain located on the C-terminus, which is connected to a linker or hinge region (MMP-1, -3, -8, -11, -12, -13, -18, -19, -20, -21, -27, and -28).6 These are the domains and regions that are involved in substrate recognition and inhibitor binding.7–9 MMP-2 and -9 also have a fibronectin-like domain of three type II repeats.6
Figure 1.
General structure of MMP groups with SP, propeptide, catalytic, and hemopexin domains.
Notes: The active site of zinc bound to the catalytic domain and the CS can be found in all MMPs. Gelatinase FN repeats are found in the catalytic domain of gelatinases. A furin-cleavage site (F) between the prodomain and catalytic domain can be found in MT-MMPs. Some MMPs have a TM domain attached to the hemopexin domain (MT-MMP). Other MT-MMPs have GPI-anchor domain.
Abbreviations: CS, cysteine switch; FN, fibronectin; GPI, glycosylphosphatidylinositol; MMP, matrix metalloproteinase; MT-MMP, membrane type-matrix metalloproteinase; SP, signal peptide; TM, transmembrane.
Table 1.
Classification of MMPs
| MMP | Category | Enzyme | Target(s) | Inhibitor(s) |
|---|---|---|---|---|
| MMP-1 | Collagenases | Collagenase-1 | Collagens (I–III, VII, VIII, and X), gelatin, aggrecan, L-selectin, IL-1β, proteoglycans, entactin, ovostatin, MMP-2, MMP-9 | Batimastat (BB-94), BB-1101, MMI270B, Metastat (CMT-3), Doxycycline, FN-439, Ilomastat, Marimastat (BB-2516), Minocycline |
| MMP-2 | Gelatinases | Gelatinase-A | Gelatin, collagen IV–VI, X, elastin, fibronectin | TIMP-4, Batimastat (BB-94), BB-1101, MMI270B, Doxycycline, Ilomastat, Marimastat (BB-2516), Minocycline |
| MMP-3 | Stromelysins | Stromelysin-1 | Collagens (III–V, and IX), gelatin, aggrecan, perlecan, decorin, laminin, elastin, casein, osteonectin, ovostatin, entactin, plasminogen, MBP, IL-1β, MMP-2/TIMP-2, MMP-7, MMP-8, MMP-9, MMP-13 | Batimastat (BB-94), BB-1101, MMI270B, Doxycycline, FN-439, Ilomastat, Marimastat (BB-2516), Minocycline |
| MMP-7 | Matrilysins | Matrilysin (PUMP) | Collagens (IV, X), gelatin, aggrecan, decorin, fibronectin, laminin, elastin, casein, transferrin, plasminogen, β4 -integrin, MMP-1, MMP-2, MMP-9, MMP-9/TIMP-1 | Batimastat (BB-94), BB-1101, Doxycycline, Marimastat (BB-2516), Minocycline |
| MMP-8 | Collagenases | Collagenase-2/neutrophil | Collagens (I–III, V, VII, VIII, and X), gelatin, aggrecan, fibronectin | TIMP-1, Batimastat (BB-94), BB-1101, MMI270B, Metastat (CMT-3), Doxycycline, FN-439, Ilomastat, Marimastat (BB-2516) |
| MMP-9 | Gelatinases | Gelatinase-A | Collagens (IV, V, VII, X, and XIV), gelatin, entactin, aggrecan, elastin, fibronectin, osteonectin, plasminogen, MBP, IL-1β | TIMP-1, Batimastat (BB-94), BB-1101, MMI270B, FN-439, Ilomastat, Marimastat (BB-2516), Minocycline |
| MMP-10 | Stromelysins | Stromelysin-2 | Collagens (III–V), gelatin, casein, aggrecan, elastin, MMP-1, MMP-8 | |
| MMP-11 | Stromelysins | Stromelysin-3 | Unknown (casein) | |
| MMP-12 | Other enzymes | Macrophage metalloelastease | Collagen IV, gelatin, elastin, casein, fibronectin, vitronectin, laminin, entactin, fibrinogen, fibrin, plasminogen | BB-1101 |
| MMP-13 | Collagenases | Collagenase-3 | Collagens (I–IV, IX, X, and XIV), gelatin, plasminogen, aggrecan, perlecan, fibronectin, osteonectin, MMP-9 | BB-1101, Metastat (CMT-3), MMI270B, Doxycycline |
| MMP-14 | MT-MMP | MT1-MMP | Collagens (I–III), gelatin, casein, fibronectin, laminin, vitronectin, entactin, proteoglycans, MMP-2, MMP-13 | TIMP-1, TIMP-2, BB-1101, Ilomastat, Marimastat (BB-2516) |
| MMP-15 | MT-MMP | MT2-MMP | Fibronectin, entactin, laminin, aggrecan, perlecan; MMP-2 | |
| MMP-16 | MT-MMP | MT3-MMP | Collagen III, gelatin, casein, fibronectin, MMP-2 | |
| MMP-17 | Stromelysins | Homology tostromelysin-2 (51.6%) | ||
| MMP-17 | MT-MMP | MT4-MMP | TIMP-1, TIMP-2 | |
| MMP-18 | Collagenases | Collagnease-4 | Type I collagen | |
| MMP-19 | Other enzymes | RASI 1 | Type I collagen | |
| MMP-20 | Other enzymes | Enamelysin | Amelogenin, aggrecan | |
| MMP-21 | Other enzymes | MMP identified on chromosome 1 | ||
| MMP-22 | Other enzymes | MMP identified on chromosome 1 | ||
| MMP-23 | Other enzymes | From human ovary cDNA | ||
| MMP-24 | MT-MMP | MT5-MMP | Fibronectin, but not collagen type I or laminin | |
| MMP-25 | MT-MMP | MT6-MMP | Progelatinase A | TIMP-1, TIMP-4 |
| MMP-26 | Matrilysins | Matrilysin-2 | Collagen IV, fibronectin, fibrinogen, gelatin, α (1)-proteinase inhibitor | |
| MMP-28 | Other enzymes | Epilysin | ||
| MMP-29 | Other enzymes | Unnamed |
Abbreviations: MMP, matrix metalloproteinase; IL, interleukin; MT-MMP, membrane type-matrix metalloproteinase; RASI, rice-amylase/subtilisin inhibitor; cDNA, complementary DNA; CMT, chemically modified tetracycline; MMI, matrix metalloproteinase inhibitor; TIMP, tissue inhibitor of metalloproteinase.
Activation of MMPs
There are three general activation mechanisms of MMPs, including the pro-MMP cleavage, phosphorylation, and oxidative stressors. The cleavage of pro-MMP is known as “cysteine switch” that exists in all known MMPs.10 The cleavage targets an intramolecular complex between a single cysteine residue in the prodomain and the zinc ion in the catalytic domain (Figure 1). This cleavage of MMPs is a required activation before any other forms of catalytic activation may occur. MMPs can be activated by phosphorylation. One study examined ubiquitous MMP-2 in human connective tissue and found that five of 29 potential phosphorylation sites are targets for protein kinase C to alter MMP-2 activity. Enzymatic property changes were confirmed through zymograph, gelatin dequenching assays, and analysis of kinetic parameters.11 MMPs can also be activated by oxidative stressors such as homocysteine (Hcy), nitric oxide (NO), and hydrogen sulfide (H2S). Hcy is a metabolite of the amino acids cysteine and methionine that activates MMPs by the extracellular signal-regulated kinase pathway.12,13 In mice, high levels of Hcy showed increased aortic MMP-2 and -9 along with increased aortic blood pressure, resistance, pulse rate, wall thickness, and extracellular collagen accumulation.14 On the other hand, H2S, a metabolite of Hcy, has been clinically used to treat atherosclerosis, since it deactivates MMP-9 and activates MMP-2 in heart tissue. Thus, H2S may promote angiogenesis and inhibit antiangiogenic factors.15 H2S is an important factor for an anaerobic pathway conversion process of Hcy, and plays a major role in vasodilation and antioxidant health by normalizing the levels of redox stress and MMPs, as seen in vascular remodeling of damaged carotid artery.16 Injected H2S into the carotid artery showed increased levels of MMP-9 and decreased levels of MMP-2.17 One study reported that H2S-knockout mice showed more outgrowth in the vessels, as compared to donor H2S that increased the levels of neointima formation in the carotid artery. This result supports evidence on how H2S can be seen as a clinical route to treat atherosclerosis.17 Although NO plays an important role in MMP regulation, its exact mechanism has not been completely understood. Moreover, the results of previous studies investigating the MMP regulation by NO or NO-dependent pathways are equivocal. One study reported that NO inhibited MMP-9 expression in activated astrocytes,18 while another study showed that NO inhibitor rather decreased MMP-9 in rat cardiac allografts.19 The three MMP regulators mentioned above can give more insight on how MMP levels can be regulated in ways that can benefit the human body against diseases. However, more knowledge of the exact mechanisms related to each regulator is necessary for a full understanding of the MMP processes.
Inhibition of MMPs
Two types of MMP inhibitors exist: endogenous and exogenous inhibitors. Tissue inhibitors of metalloproteinase (TIMPs) are endogenous inhibitors that can be secreted (TIMP-1, TIMP-2, TIMP-4) or bound to ECM components (TIMP-3).20 They inactivate MMPs by forming bonds with catalytic zinc in 1:1 ratios within the MMP structure.21 They do so by creating noncovalent interactions between the N-terminal domain of the TIMP and the active site of MMPs. Recent studies have shown TIMPs’ therapeutic potential in cardiovascular diseases (CVDs). The MMP-inhibitory effects of TIMP-1 involve binding of C-terminal domain to pro-MMP-2 and pro-MMP-9. TIMP-1 may specifically help ECM manipulation in ischemia in such a way that it may be a surrogate marker for increased ECM turnover.22 TIMP-2 reverses ECM remodeling in human cardiac tissue in a dose-dependent manner.23 TIMP-3 prevented degradation of cardiac tissue matrix after a myocardial infarction by reducing MMPs in vascular smooth muscle cells.24 Changes in the ECM of atrial fibroblast in rheumatic heart disease have been associated with TIMP-4 expression.25
Exogenous inhibitors include hydroxamic acid derivative and thiirane gelatinase inhibitor SB-3CT on several MMPs.26–29 Batimastat (BB-94), a hydroxamic acid derivative, has been recently found to treat aneurysms on using nanoparticle technology to directly administer anti-MMP target to abdominal aorta aneurysm.30 In rat aorta cell culture, administration of BB-94 decreased 90% of MMP-9 activity and 10% of MMP-2 activity, while showing no effect on TIMP-2 activity. The effect of change in MMP activity was tested by injecting BB-94 directly into the abdominal aorta. The blank control showed a 269% increase in the aneurysm, while the treatment group showed only a 40% increase in expansion. Another derivative, marimastat, has shown success as a therapeutic drug in repressing non-small-cell lung cancer in a Phase I trial.31 Oral administration of marimastat was added to the accepted treatment with carboplatin and paclitaxel in order to test whether or not marimastat would affect the kinetics of the treatment. TIMPs and hydroxamic acid derivatives play significant roles in general physiology and pathology, and thus can develop as a therapeutic target in the future.
The roles of MMPs in CVD and obesity
Breakdown of ECM by MMPs includes physiological processes such as embryonic development, reproduction, and tissue remodeling, as well as disease processes. MMPs are involved in the remodeling process of cell membrane and in cell behaviors like proliferation, migration, and apoptosis.32 CVD is the number one leading cause of death in the world and includes any disease associated with the heart and blood vessels, such as myocardial infarction, heart failure, atherosclerosis, stroke, aortic aneurysms, and so on.33 MMPs are expressed and activated in many different types of CVDs including atherosclerosis, myocardial infarction, and cardiac dysfunction.34,35 In particular, MMP-2 and -9 degrade a major element in the basement membrane, collagen IV, which helps in cellular arrangement of skeletal muscle.36 MMPs also play a role in disease diagnoses. For instance, MMP-2 and -9 are found to be independent predictors for kidney disease progression and its associated mortality.37 Obesity is strongly associated with CVDs and occurs when pre-existing, fully differentiated adipocytes are enlarged by excess energy input and accumulate to a point where the pathological expansion becomes a concern.38 Higher levels of MMPs are associated with obesity and CVDs. For instance, MMP-1, -2, -3, -7, -9, -10, -11, and -12 are found at higher levels in atherosclerotic arteries.39 Among these MMPs, the current review focused on MMP-1, -2, and -9 due to their strong association with obesity and CVDs.
MMP-1
MMP-1 may be involved in plaque burden, although plaque morphology was not tested.40 A strong correlation between MMP-1 mRNA and lupus erythematic atherosclerosis has been reported.41 Elevated MMP-1 was also associated with myocardial infarction and angiographic coronary artery disease, although the mechanistic pathway was not examined.42 One study promoted inflammation and atherosclerosis using C-reactive protein to examine the effects of MMP-1 in human mammary arteries and carotid arteries. Both MMP-1 and its mRNA expression had increased significantly, suggesting an association with inflammation and plaque vulnerability.43 Higher levels of MMP-1 have also been observed in several types of human carotid atherosclerosis, and also, histological associations with plaque instability have been found.44 However, controversial results were obtained on the association of MMP-1 with obesity, since the expression of MMP-1 may be different between obese and nonobese people. According to a recent study, MMP-1 may stimulate tissue remodeling during adipose tissue expansion in obesity. Certain MMP-1 alleles showed increased frequency with high body mass index, potentially suggesting a defensive role.45 In contrast, MMP-1 was reported to have a strong association with nonobese individuals.46 In spite of the contradictory effect of MMP-1 on obesity, the majority of evidence leans toward MMP-1 increasing with obesity.
MMP-2
MMP-2 has been reported to promote atherosclerosis. Elevated MMP-2 may negatively affect vascular permeability and play an important role in the progression of heart failure.47 One study reported that MMP-2 knockout mice showed decrease in atherogenesis.48 Increased duration of ischemia and delayed functional recovery have been linked to elevated MMP-2 levels,49 while decreased MMP-2 levels provided protection from cardiac dysfunction.50 The role of MMP-2 in voluntary exercise and its effects on infarct size have been investigated in rats.51 A 6-week voluntary wheel-running exercise, where the rats self-selected the time, duration, and intensity in a nonstressful environment, was used as the exercise protocol. Induced ischemia by left anterior descending coronary artery occlusion ex vivo and angina provoked by epinephrine plus phentolamine protocols were used on the rats. Serum MMP-2, coronary effluent MMP-2 activity, and infarct size all showed a significant decrease after exercise. These results show that MMP-2 may be soon viewed as a cardioprotective molecule for myocardial infarctions and perfusions.
MMP-9
It has been reported that MMP-9 may be involved in elastase action related to aortic stiffening and development of isolated systolic hypertension in healthy and younger individuals.52 However, MMP-9 may attenuate atherosclerotic development and prevent plaque development, since MMP-9 knockout mice showed plaque development.53 Both MMP-2 and -9 have been linked to increased inflammation under high coronary risk events and high plaque instability.54,55 An early study showed that genetically obese rats had high levels of MMP-2 and low levels of MMP-9,56 suggesting that MMP-2 may be involved in adipose ECM degradation. In addition, high-fat diet-induced obese (HFDIO) mice had lower MMP-9 mRNA, and an antigrowth myostatin (MSTN), which is known to be suppressed in HFDIO condition, showed resistance to HFDIO.57 This indicates that decreased levels of MMP-2 and increased MMP-9 levels can prevent or regulate obesity development.
The effects of exercise on MMPs
It is evident that exercise favorably affects CVDs and obesity. According to the recent studies examining the effects of exercise on the regulation of MMPs, the responses of MMPs to exercise may be more dependent upon the mode and length of exercise performed in a variety of subject models (Table 2). Thus, understanding the relationship between MMPs and exercise is particularly important in obese population, since obesity is strongly associated with CVDs and other types of metabolic diseases.58 In the current review, we examined how different types of exercise (resistance and aerobic) influence MMP-1, -2, and -9.
Table 2.
Effects of different types of exercise on MMPs
| Study | Samples | Training type | Length of study | Tissue | Outcome | P-value |
|---|---|---|---|---|---|---|
| Souza et al62 | Wistar rats (N=32; C=8, C-OB=8, EG=8, EG-OB=8) | RT | 12 weeks | Skeletal muscle | MMP-2 ↑ | P<0.05 |
| Leite et al59 | Wistar rats (N=32; C=8, C-OB=8, EG=8, EG-OB=8) | RT | 12 weeks | Left ventricle | MMP-2 ↑ | P<0.01 |
| Nascimento et al63 | Obese elderly women (N=10) | RT | Acute | Plasma | MMP-2 ↓ MMP-9 ↓ |
P<0.05 |
| Rullman et al60 | Healthy males (N=10) | RT | 5 weeks | Skeletal muscle | MMP-2 ↑ MMP-9 ↑ |
P<0.001 P<0.05 |
| Scheede-Bergdahl et al61 | Type 2 diabetic males (N=22; C=10, T2D=12) | RT | 8 weeks | Skeletal muscle | MMP-2 ↑ | P<0.05 |
| Donley et al69 | MetS females (C=22, MetS=22) | AT | 8 weeks | Plasma | MMP-1 ↓ | P<0.05 |
| Rullman et al68 | Healthy males (N=10) | AT | Acute | Skeletal muscle | MMP-2 ↔ MMP-9 ↑ |
P<0.05 P<0.05 |
| Shon et al65 | ApoE-/- mice (N=68; C=15, C-OB=26, EG=9, EG-OB=18) | AT | 10 weeks | Atheroma | MMP-2 ↓ MMP-9 ↓ |
P<0.05 P<0.05 |
| Posa et al51 | Wistar rats (N=70; C=35, EG=35) | AT | 6 weeks | Plasma | MMP-2 ↓ | P<0.001 |
| Kwak et al64 | Aging rat model (N=40; C=10, EG=10, O=10, O-EG=10) | AT | 12 weeks | Left ventricle | MMP-1 ↓ MMP-2 ↓ MMP-9 ↔ |
P<0.05 P<0.05 NS |
| De Aro et al66 | Wistar rats (N=77; C=11, 1d1h=11, 1d3h=11, 3d1h=11, 3d3h=11, 6d1h=11, 6d3h=11) | AT | 1, 3, 6 days | Calcaneal tendon | MMP-2 ↔, ↔, ↑ MMP-9 ↔, ↔, ↔ |
P<0.05 NS |
| Nishijima et al67 | Wistar rats (N=4, EG=16) | AT | 1 week | Hippocampus | MMP-2 ↔ MMP-9 |
NS P<0.05 |
Note: “↑” indicates an increased level; “↓” indicates a decreased level; and “↔” indicates no change.
Abbreviations: ApoE−/−, apolipoprotein E knockout; AT, aerobic exercise; C, control; C-OB, control obese; d, days, EG, exercise group; EG-OB, obese exercise group; h, hours; MetS, metabolic syndrome; MMP, matrix metalloproteinase; NS, not shown; O, old; O-EG, old exercise group; RT, resistance training; T2D, type 2 diabetes.
Resistance training and MMPs
Based on the previously published studies, the responses of MMPs to resistance training are more likely related to duration of exercise training. Resistance training lasting from 5 to 12 weeks may increase MMP-2 and -9 in both animal and human subjects,59–62 whereas acute bout of resistance training may decrease these MMPs.63 In an animal study, HFDIO rats that performed resistance training had significantly increased MMP-2 levels in bicep and gastrocnemius muscles. A comparable difference in MMP-2 levels between obese and nonobese rats indicated that it was related to the levels of obesity and also suggested that this exercise-induced increase in MMP-2 in rats may prevent obesity. In this regard, MMP-2 could potentially be a negative regulator of obesity.62 In another study, rats fed with high-fat diet performed a 12-week strength training program that consisted of vertical ladder exercise three times a week with weights attached to their tails. Following 12 weeks of training, rats showed increase in muscle MMP-2, suggesting that high levels of MMP-2 may be inversely related to obesity.59 One study examined the effects of oxidative stress on MMP-2 and -9 in skeletal muscle using the exercise protocol composed of leg presses for 45 minutes, four times a week for 5 weeks. The levels of MMP-2 increased by day 10, and MMP-2 mRNA in myofibril increased with training as well. The responses of MMP-9 and its mRNA activity at day 10 were relatively lower as compared to those of MMP-2, but held the same level of activity after the first exercise.60 One study examining the relationship between diabetes and MMPs reported that patients with type 2 diabetes who performed a rowing exercise at 65%–70% of VO2 (volume of oxygen intake) peak significantly increased MMP-2 mRNA in skeletal muscle, suggesting that exercise-induced changes in MMP-2 may benefit type 2 diabetes.61 In contrast, obese elderly women who performed acute eccentric resistance training (ten repetitions at 110% of ten-repetition maximum) had decreased levels of both MMP-2 and -9 in the plasma after 48 hours of the training session. The authors concluded that this exercise-induced reduction in MMP-2 and -9 may be a positive sign in the transient defense of inflammatory MMPs associated with obesity and atherosclerosis.63
Aerobic exercise and MMPs
The impact of aerobic exercise training on MMPs may be related to duration of exercise. In general, the long-term aerobic exercise training lasting up to 12 weeks may decrease both MMP-2 and -9,51,64,65 while these MMPs increase following acute bouts of exercise.66–68 Moreover, only limited information regarding the effects of aerobic exercise on MMP-1 is currently available. MMP-1 in serum and heart tissue decreased following aerobic exercise training (up to 12 weeks) in female subjects with metabolic syndrome69 and in aged mice.64 MMP-9 and its mRNA activity in skeletal muscle significantly increased after a 65-minute aerobic cycling exercise, while MMP-2 mRNA did not change.68 In an animal study, apoE−/− mice were given a Western diet to develop plaque while participating in a 30-minute treadmill exercise program (5 days/week) for 10 weeks. Exercise intervention did not attenuate aortic plaque, but MMP-2 and -9 significantly decreased.65 Another study examining the effects of voluntary wheel-running exercise for 6 weeks reported a reduction in serum MMP-2 as part of a cardio-protective mechanism against cardiac injury.51 Aged rats that performed a 45-minute aerobic exercise on a treadmill for up to 12 weeks (5 days/week) showed decrease in both MMP-1 and -2 in the heart tissue, while MMP-9 was not altered.64 To examine the influence of exercise with various durations and resting periods, Wistar rats were separated into several groups that performed either 1, 3, or 6 days of exercise training (three sessions per day) with an 1-, 3-, or 6-hour resting period between each session. No significant difference was seen in either protein MMP-2 or -9 in the calcaneal tendon in any of the groups, except the group that performed the 6-day with 3-hour rest exercise protocol which showed an increase in MMP-2.66 One study examined the responses of MMP-2 and -9 levels of hippocampi samples in rats following treadmill exercise. The rats exercised at moderate intensity on the treadmill for 30 minutes for 7 days, and hippocampi samples were analyzed by gel zymography to examine the changes in the proteolytic activity of MMP-2 and -9 at 0, 6, 12, and 24 hours postexercise. Results showed that MMP-2 did not change significantly, whereas the 12-hour samples exhibited a significant increase in MMP-9.67
Conclusion
The previous studies we reviewed show that the response of MMPs following resistance exercise is more related to the length of resistance exercise training. In general, long-term resistance exercise training may increase both MMP-2 and -9, while acute bouts of resistance exercise may decrease these MMPs. Furthermore, aerobic exercise training leads to an inconsistent result in MMP-2 and -9, although some studies showed a reduction in MMP-1. Also, a relatively lower level of MMP-9 has been observed in obese subjects, indicating that an exercise-induced increase in MMP-9 may play a positive role in obesity.
Elevated MMP-1 has been shown to be strongly associated with CVDs and obesity. Thus, a treatment targeting on lowering MMP-1 may benefit the most in patients with CVDs or obesity. In this regard, an exercise intervention should include a long-term aerobic training as it can reduce the MMP-1 levels and prevent obesity. Reducing MMP-2 would improve prognosis of CVDs, and long-term aerobic training would help reduce MMP-2 levels as well. However, the potential drawback of long-term aerobic training would be decreased level of MMP-9, which may hinder some beneficial effects of exercise on obesity and plaque development. Therefore, it is important to balance aerobic exercise with long-term resistance training, which may increase MMP-9, thereby offering the most positive effects of exercise training on improvement in MMPs. Future studies examining the effects of different types of aerobic and resistance exercise training or a combination of these two forms of training would give more insight on how exercise influences MMPs. Also, exercise intensity may play a role in changes in MMPs, although it has not been fully examined. Therefore, findings on how different types, intensities, and duration of exercises and when to measure MMPs will open a new spectrum of molecular mechanism that is mostly unknown. Finding which MMPs are associated with obesity, exercise, and CVDs is the first step toward understanding the mechanism of this potentially powerful group of proteins. More knowledge of MMPs could lead to more effective treatment or approach to weight loss or new drugs that safely help patients with obesity, CVDs, or other types of diseases. Not all major MMPs have been fully tested with different types and duration of exercise regimens. This calls for research on different MMPs in exercise to get a better understanding on how the human body can naturally manipulate MMP metabolism to benefit the body as a whole.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisen AZ, Jeffrey JJ, Gross J. Human skin collagenase. Isolation and mechanism of attack on the collagen molecule. Biochim Biophys Acta. 1968;151(3):637–645. doi: 10.1016/0005-2744(68)90010-7. [DOI] [PubMed] [Google Scholar]
- 3.Renaud S, Leppert D. Matrix metalloproteinases in neuromuscular disease. Muscle Nerve. 2007;36(1):1–13. doi: 10.1002/mus.20772. [DOI] [PubMed] [Google Scholar]
- 4.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 5.Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4(7):544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 6.Sekhon B. Matrix metalloproteinase – an overview. Res Rep Biol. 2010;1:1–20. [Google Scholar]
- 7.Chaudhary AK, Singh M, Bharti AC, Asotra K, Sundaram S, Mehrotra R. Genetic polymorphisms of matrix metalloproteinases and their inhibitors in potentially malignant and malignant lesions of the head and neck. J Biomed Sci. 2010;17:10. doi: 10.1186/1423-0127-17-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadoglou NP, Liapis CD. Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr Med Res Opin. 2004;20(4):419–432. doi: 10.1185/030079904125003143. [DOI] [PubMed] [Google Scholar]
- 9.Cho C, Bunch DO, Faure JE, et al. Fertilization defects in sperm from mice lacking fertilin beta. Science. 1998;281(5384):1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- 10.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sariahmetoglu M, Crawford BD, Leon H, et al. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21(10):2486–2495. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- 12.Moshal KS, Sen U, Tyagi N, et al. Regulation of homocysteine-induced MMP-9 by ERK1/2 pathway. Am J Physiol Cell Physiol. 2006;290(3):C883–891. doi: 10.1152/ajpcell.00359.2005. [DOI] [PubMed] [Google Scholar]
- 13.Bescond A, Augier T, Chareyre C, Garcon D, Hornebeck W, Charpiot P. Influence of homocysteine on matrix metalloproteinase-2: activation and activity. Biochem Biophys Res Commun. 1999;263(2):498–503. doi: 10.1006/bbrc.1999.1391. [DOI] [PubMed] [Google Scholar]
- 14.Ovechkin AV, Tyagi N, Sen U, et al. 3-Deazaadenosine mitigates arterial remodeling and hypertension in hyperhomocysteinemic mice. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L905–911. doi: 10.1152/ajplung.00543.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Givvimani S, Munjal C, Gargoum R, et al. Hydrogen sulfide mitigates transition from compensatory hypertrophy to heart failure. J Appl Physiol (1985) 2011;110(4):1093–1100. doi: 10.1152/japplphysiol.01064.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids. 2010;39(5):1161–1169. doi: 10.1007/s00726-010-0550-2. [DOI] [PubMed] [Google Scholar]
- 17.Yang G, Li H, Tang G, et al. Increased neointimal formation in cystathionine gammalyase deficient mice: role of hydrogen sulfide in alpha5beta1-integrin and matrix metalloproteinase-2 expression in smooth muscle cells. J Mol Cell Cardiol. 2012;52(3):677–688. doi: 10.1016/j.yjmcc.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Shin CY, Lee WJ, Choi JW, et al. Down-regulation of matrix metallo-proteinase-9 expression by nitric oxide in lipopolysaccharide-stimulated rat primary astrocytes. Nitric Oxide. 2007;16(4):425–432. doi: 10.1016/j.niox.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Egi K, Conrad NE, Kwan J, Schulze C, Schulz R, Wildhirt SM. Inhibition of inducible nitric oxide synthase and superoxide production reduces matrix metalloproteinase-9 activity and restores coronary vasomotor function in rat cardiac allografts. Eur J Cardiothorac Surg. 2004;26(2):262–269. doi: 10.1016/j.ejcts.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 20.Melendez-Zajgla J, Del Pozo L, Ceballos G, Maldonado V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol Cancer. 2008;7:85. doi: 10.1186/1476-4598-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3(3):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 22.Dinh W, Futh R, Scheffold T, et al. Increased serum levels of tissue inhibitor of metalloproteinase-1 in patients with acute myocardial infarction. Int Heart J. 2009;50(4):421–431. doi: 10.1536/ihj.50.421. [DOI] [PubMed] [Google Scholar]
- 23.Ngu JM, Teng G, Meijndert HC, et al. Human cardiac fibroblast extracellular matrix remodeling: dual effects of tissue inhibitor of metal-loproteinase-2. Cardiovasc Pathol. 2014;23(6):335–343. doi: 10.1016/j.carpath.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jia ZB, Tian H, Kang K, et al. Expression of the tissue inhibitor of metalloproteinase-3 by transplanted VSMCs modifies heart structure and function after myocardial infarction. Transpl Immunol. 2014;30(4):149–158. doi: 10.1016/j.trim.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y, Huang ZY, Wang ZH, et al. TGF-beta1 and TIMP-4 regulate atrial fibrosis in atrial fibrillation secondary to rheumatic heart disease. Mol Cell Biochem. 2015;406(1–2):131–138. doi: 10.1007/s11010-015-2431-1. [DOI] [PubMed] [Google Scholar]
- 26.Kruger A, Soeltl R, Sopov I, et al. Hydroxamate-type matrix metallo-proteinase inhibitor batimastat promotes liver metastasis. Cancer Res. 2001;61(4):1272–1275. [PubMed] [Google Scholar]
- 27.Kohno T, Hochigai H, Yamashita E, Tsukihara T, Kanaoka M. Crystal structures of the catalytic domain of human stromelysin-1 (MMP-3) and collagenase-3 (MMP-13) with a hydroxamic acid inhibitor SM-25453. Biochem Biophys Res Commun. 2006;344(1):315–322. doi: 10.1016/j.bbrc.2006.03.098. [DOI] [PubMed] [Google Scholar]
- 28.Skarja GA, Brown AL, Ho RK, May MH, Sefton MV. The effect of a hydroxamic acid-containing polymer on active matrix metalloproteinases. Biomaterials. 2009;30(10):1890–1897. doi: 10.1016/j.biomaterials.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Gu Z, Cui J, Brown S, et al. A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci. 2005;25(27):6401–6408. doi: 10.1523/JNEUROSCI.1563-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosoudi N, Nahar-Gohad P, Sinha A, et al. Prevention of abdominal aortic aneurysm progression by targeted inhibition of matrix metallo-proteinase activity with batimastat-loaded nanoparticles. Circ Res. 2015;117(11):e80–89. doi: 10.1161/CIRCRESAHA.115.307207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goffin JR, Anderson IC, Supko JG, et al. Phase I trial of the matrix metalloproteinase inhibitor marimastat combined with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2005;11(9):3417–3424. doi: 10.1158/1078-0432.CCR-04-2144. [DOI] [PubMed] [Google Scholar]
- 32.Murphy G, Nagase H. Localizing matrix metalloproteinase activities in the pericellular environment. FEBS J. 2011;278(1):2–15. doi: 10.1111/j.1742-4658.2010.07918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Benjamin EJ, Go AS, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 34.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thorp EB. Contrasting inflammation resolution during atherosclerosis and post myocardial infarction at the level of monocyte/macrophage phagocytic clearance. Front Immunol. 2012;3:39. doi: 10.3389/fimmu.2012.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hopps E, Caimi G. Matrix metalloproteinases in metabolic syndrome. Eur J Intern Med. 2012;23(2):99–104. doi: 10.1016/j.ejim.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Hsu TW, Kuo KL, Hung SC, Huang PH, Chen JW, Tarng DC. Progression of kidney disease in non-diabetic patients with coronary artery disease: predictive role of circulating matrix metalloproteinase-2, -3, and -9. PLoS One. 2013;8(7):e70132. doi: 10.1371/journal.pone.0070132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 39.Back M, Ketelhuth DF, Agewall S. Matrix metalloproteinases in atherothrombosis. Prog Cardiovasc Dis. 2010;52(5):410–428. doi: 10.1016/j.pcad.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 40.Lehrke M, Greif M, Broedl UC, et al. MMP-1 serum levels predict coronary atherosclerosis in humans. Cardiovasc Diabetol. 2009;8:50. doi: 10.1186/1475-2840-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang HY, Bao SM, Shou WL, et al. Expression of matrix metallo-proteinase-1 mRNA in peripheral blood mononuclear cells of systemic lupus erythematosus patients and its relationship with atherosclerosis. Chin Med J (Engl) 2009;122(21):2593–2597. [PubMed] [Google Scholar]
- 42.Horne BD, Camp NJ, Carlquist JF, et al. Multiple-polymorphism associations of 7 matrix metalloproteinase and tissue inhibitor metallo-proteinase genes with myocardial infarction and angiographic coronary artery disease. Am Heart J. 2007;154(4):751–758. doi: 10.1016/j.ahj.2007.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montero I, Orbe J, Varo N, et al. C-reactive protein induces matrix metalloproteinase-1 and -10 in human endothelial cells: implications for clinical and subclinical atherosclerosis. J Am Coll Cardiol. 2006;47(7):1369–1378. doi: 10.1016/j.jacc.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 44.Nikkari ST, O’Brien KD, Ferguson M, et al. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis. Circulation. 1995;92(6):1393–1398. doi: 10.1161/01.cir.92.6.1393. [DOI] [PubMed] [Google Scholar]
- 45.Nho YK, Ha E, Yu KI, et al. Matrix metalloproteinase-1 promoter is associated with body mass index in Korean population with aged greater or equal to 50 years. Clin Chim Acta. 2008;396(1–2):14–17. doi: 10.1016/j.cca.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Huang HL, Wu S, Hsu LA, et al. Genetic variants associated with circulating MMP1 levels near matrix metalloproteinase genes on chromosome 11q21-22 in Taiwanese: interaction with obesity. BMC Med Genet. 2013;14:30. doi: 10.1186/1471-2350-14-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122(12):1221–1238. doi: 10.1161/CIRCULATIONAHA.110.939959. [DOI] [PubMed] [Google Scholar]
- 48.Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26(5):1120–1125. doi: 10.1161/01.ATV.0000218496.60097.e0. [DOI] [PubMed] [Google Scholar]
- 49.Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metallopro-teinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85(3):413–423. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- 50.Fert-Bober J, Leon H, Sawicka J, et al. Inhibiting matrix metallopro-teinase-2 reduces protein release into coronary effluent from isolated rat hearts during ischemia-reperfusion. Basic Res Cardiol. 2008;103(5):431–443. doi: 10.1007/s00395-008-0727-y. [DOI] [PubMed] [Google Scholar]
- 51.Posa A, Szabo R, Kupai K, et al. Cardioprotective effects of voluntary exercise in a rat model: role of matrix metalloproteinase-2. Oxid Med Cell Longev. 2015;2015:876805. doi: 10.1155/2015/876805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yasmin, McEniery CM, Wallace S, et al. Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(2):372. doi: 10.1161/01.ATV.0000151373.33830.41. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JL, George SJ, Newby AC, Jackson CL. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci U S A. 2005;102(43):15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nascimento Dda C, Durigan Rde C, Tibana RA, Durigan JL, Navalta JW, Prestes J. The response of matrix metalloproteinase-9 and -2 to exercise. Sports Med. 2015;45(2):269–278. doi: 10.1007/s40279-014-0265-8. [DOI] [PubMed] [Google Scholar]
- 55.Heo SH, Cho CH, Kim HO, et al. Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: involvement of matrix metalloproteinases 2 and 9. J Clin Neurol. 2011;7(2):69–76. doi: 10.3988/jcn.2011.7.2.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chavey C, Mari B, Monthouel MN, et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003;278(14):11888–11896. doi: 10.1074/jbc.M209196200. [DOI] [PubMed] [Google Scholar]
- 57.Biga PR, Froehlich JM, Greenlee KJ, Galt NJ, Meyer BM, Christensen DJ. Gelatinases impart susceptibility to high-fat diet-induced obesity in mice. J Nutr Biochem. 2013;24(8):1462–1468. doi: 10.1016/j.jnutbio.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 59.Leite RD, Durigan Rde C, de Souza Lino AD, et al. Resistance training may concomitantly benefit body composition, blood pressure and muscle MMP-2 activity on the left ventricle of high-fat fed diet rats. Metabolism. 2013;62(10):1477–1484. doi: 10.1016/j.metabol.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Rullman E, Norrbom J, Stromberg A, et al. Endurance exercise activates matrix metalloproteinases in human skeletal muscle. J Appl Physiol (1985) 2009;106(3):804–812. doi: 10.1152/japplphysiol.90872.2008. [DOI] [PubMed] [Google Scholar]
- 61.Scheede-Bergdahl C, Bergdahl A, Schjerling P, Qvortrup K, Koskinen SO, Dela F. Exercise-induced regulation of matrix metalloproteinases in the skeletal muscle of subjects with type 2 diabetes. Diab Vasc Dis Res. 2014;11(5):324–334. doi: 10.1177/1479164114535943. [DOI] [PubMed] [Google Scholar]
- 62.Souza MV, Leite RD, Souza Lino AD, et al. Resistance training improves body composition and increases matrix metalloproteinase 2 activity in biceps and gastrocnemius muscles of diet-induced obese rats. Clinics (Sao Paulo) 2014;69(4):265–270. doi: 10.6061/clinics/2014(04)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nascimento DD, Navalta JW, Durigan JL, et al. Acute eccentric resistance exercise decreases matrix metalloproteinase activity in obese elderly women. Clin Physiol Funct Imaging. 2016;36:039–145. doi: 10.1111/cpf.12207. [DOI] [PubMed] [Google Scholar]
- 64.Kwak HB, Kim JH, Joshi K, Yeh A, Martinez DA, Lawler JM. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 2011;25(3):1106–1117. doi: 10.1096/fj.10-172924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shon SM, Park JH, Nahrendorf M, et al. Exercise attenuates matrix metalloproteinase activity in preexisting atherosclerotic plaque. Atherosclerosis. 2011;216(1):67–73. doi: 10.1016/j.atherosclerosis.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 66.De Aro AA, Ferrucci DL, Borges FP, Stach-Machado DR, Macedo DV, Pimentel ER. Exhaustive exercise with different rest periods changes the collagen content and MMP-2 activation on the calcaneal tendon. Anat Rec (Hoboken) 2014;297(2):281–288. doi: 10.1002/ar.22842. [DOI] [PubMed] [Google Scholar]
- 67.Nishijima T, Kawakami M, Kita I. A bout of treadmill exercise increases matrix metalloproteinase-9 activity in the rat hippocampus. Neurosci Lett. 2015;594:144–149. doi: 10.1016/j.neulet.2015.03.063. [DOI] [PubMed] [Google Scholar]
- 68.Rullman E, Rundqvist H, Wagsater D, et al. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol (1985) 2007;102(6):2346–2351. doi: 10.1152/japplphysiol.00822.2006. [DOI] [PubMed] [Google Scholar]
- 69.Donley DA, Fournier SB, Reger BL, et al. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol (1985) 2014;116(11):1396–1404. doi: 10.1152/japplphysiol.00151.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]