Abstract
Background
In the general population, compared wtih their White peers, African Americans suffer premature all-cause and cardiovascular (CV) deaths, attributed in part to reduced access to care and lower socioeconomic status. Prior reports indicated younger (aged 35 to 44 years) African Americans had a signficantly greater age-adjusted risk of death. Recent studies suggest that in a more egalitarian health care structure than typical United States (US) health care structures, African Americans may have similar or even better CV outcomes, but the impact of age is less well-known.
Methods
We examined age stratified all-cause mortality, and incident coronary heart disease (CHD) and ischemic stroke in 3,072,966 patients (547,441 African American and 2,525,525 White) with an estimated glomerular filtration rate (eGFR)>60 mL/min/1.73m2 receiving care from the US Veterans Health Administration. Outcomes were examined in Cox models adjusted for demographics, comorbidities, kidney function, blood pressure, socioeconomics and indicators of the quality of health care delivery.
Results
African Americans had an overall 30% lower all-cause mortality (P<.001) and 29% lower incidence of CHD (P<.001) and higher incidence of ischemic stroke (aHR, 95%CI: 1.16, 1.13-1.18, P<.001). The lower rates of mortality and CHD were strongest in younger African Americans and attenuated across patients aged ≥70 years. Stroke rates did not differ by race in persons aged <70 years.
Conclusions
Among patients with normal eGFR and receiving care in the Veterans Health Administration, younger African Americans had lower all-cause mortality and incidence of CHD and similar rates of stroke, independent of demographic, comorbidity and socioeconomic differences. The lower all-cause mortality persisted but attenuated with increasing age and the lower incidence of CHD ended at aged ≥80 years. The higher incidence of ischemic stroke in African Americans was driven by increasing risk in patients aged ≥70 years suggesting that the improved cardiovascular outcomes were most dramatic for younger African Americans.
Keywords: Race, African American, Age, Mortality, Coronary Heart Disease, Stroke, Incidence, Chronic Kidney Disease
Introduction
African Americans represent more than 13% of the US population, accounting for more than 41 million individuals.1 African Americans suffer from higher rates of infant mortality and low birth weight,2 higher incidence of maternal complications during pregnancy,3 higher prevalence of asthma,4 uncontrolled hypertension,5 and higher cardiovascular mortality rates.6 In fact, compared with Whites, African Americans have an approximately 20% higher age-adjusted all-cause mortality rate and 30% higher CVD mortality rate.7 These poor outcomes for African Americans have been attributed, in part, to the substantial socioeconomic disadvantage and residential segregation with resultant lower quality educational systems and lower health-literacy, decreased disease-awareness, suboptimal access to health care, and overt or latent discrimination in receiving recommended health care interventions.8
Notwithstanding the validity and importance of these factors, the underlying causes for differences in the health outcomes of African Americans are likely much more complex, and are affected not only by socioeconomic factors, but also by genetic differences between individuals of African and European ancestry.9 A notable example for this is chronic kidney disease (CKD) and end stage renal disease (ESRD), the incidence and prevalence of which are disproportionately higher in African Americans due in part to recently described genetic mutations in individuals of African ancestry,10,11 which may also impact CV disease.12 Paradoxically, African Americans with advanced CKD and ESRD have lower mortality rates than their White peers.13-15 This finding varies across age groups with younger African Americans on dialysis not having a survival advantage.16,17 The findings of age-related differences in mortality have been noted in the non-dialysis population as well with younger Black men (aged 35 to 44 years) in the Multiple Risk Factor Intervention Trial (MRFIT) who had worse outcomes (adjusted HR for death 1.36) than their White peers, compared with their older colleagues aged 45 to 57 years who only had a 14% higher adjusted HR for death than their White peers of similar age.18 We previously reported that African Americans without advanced CKD or ESRD in the US Veterans Health Administration (VHA), a more egalitarian health care system than other US health care systems, had similar or better all-cause mortality and CV event rates compared with Whites.19 We hypothesized that the improved all-cause mortality and CV event rates in the same cohort of African Americans without advanced CKD or ESRD in the VHA would exist regardless of age.
Methods
Study Design and Participants
We used data from a historic cohort study (Racial and Cardiovascular Risk Factor Anomalies in CKD, RCAV) examining risk factors in patients with serum creatinine measurements performed during October 1, 2004-September 30, 2006.20,21 The RCAV cohort included 3,582,478 patients with eGFR >60 mL/min/1.73m2, calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Equation.22 We excluded 509,512 patients with race other than African American or White. Race was determined from VA demographic data from Medicare.19 Our final analytic sample consisted of 3,072,966 patients (547,441 African American and 2,525,525 White) as previously reported.19
Sociodemographic Characteristics, Comorbidities, Medication Use and Laboratory Variables
Sociodemographics, comorbid conditions, medication use and laboratory characteristics were obtained and reported as previously described.19 Briefly, socio-demographics were obtained from various national VA research data files, and comorbidities and clinical events from the VA Inpatient and Outpatient Medical SAS Datasets using International Classification of Diseases, Ninth Revision (ICD-9) diagnostic and procedure codes and Current Procedural Terminology (CPT) codes.19
Outcomes
Major outcomes included all-cause mortality, incident CHD and incident ischemic stroke, stratified by age. Deaths were ascertained from the VA Vital Status Files, and incident CHD was the composite of a first occurrence of an ICD9 or CPT code for acute myocardial infarction (MI), percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) following October 1, 2006 in patients without such diagnoses prior to this date.19 Incident ischemic stroke was defined as the first occurrence of an ICD9 code for ischemic stroke following October 1, 2006 in patients without such diagnosis prior to this date.19
Statistical Analyses
Data were expressed as means (standard deviations), medians (interquartile ranges) and proportions. Baseline characteristics were compared using t-tests, non-parametric tests and chi-square tests, as appropriate. Due to the large sample size, most variables differed at P<.001; hence, the significance of differences was based on clinically meaningful differences. Patients were followed from date of cohort entry, which was defined as the date of the first eGFR >60 mL/min/1.73m2 during October 1, 2004-September 30, 2006, until death or were censored at the date of the last health care or administrative VA encounter.19
The association of African American race with the outcomes of interest was examined in Cox models with incremental adjustment for various effect mediators: Model 1: unadjusted, Model 2: adjusted for age, sex and baseline eGFR; Model 3: adjusted for Model 2 variables plus prevalent comorbidities (diabetes mellitus, hypertension, CHD, congestive heart failure, cerebro-vascular disease, peripheral vascular disease, chronic lung disease, peptic ulcer disease, hemiplegia, liver disease, dementia, rheumatic disease, malignancy, HIV/AIDS and depression); Model 4: adjusted for Model 3 variables plus baseline BMI, SBP and DBP; Model 5: adjusted for Model 4 variables plus mean per capita income, marital status, service connectedness, and use of ACEI and statins, and receipt of influenza vaccination(s) throughout the follow-up period. For age stratifed analyses, we used the fully adjusted model 5 as well as housing stress, low education, low employment, and persistent poverty using 2004 county typology codes based on the patient’s residential address (Area Health Resources Files, http://ahrf.hrsa.gov/).19
Statistical analyses were performed using STATA MP version 12 (STATA Corporation, College Station, TX). The study protocol was approved by the Research and Development Committees at the Memphis VA Medical Center and Long Beach VA Medical Center.
Results
The mean ± SD age of the cohort at baseline was 59.9 ± 13.4 years, 93.6% were men, and the mean baseline eGFR was 84.0 ± 15.7 mL/min/1.73m2. Baseline characteristics are shown in Table 1. African American patients were younger, more likely to be female, to be service connected, and had higher systolic and diastolic blood pressure, and a lower mean income. They were less likely to be married (data not shown). The use of ACEI/ARB (53%) and measurement of blood cholesterol (93%) did not differ by race. African Americans were slightly less likely to use statins or receive an influenza vaccination.
Table 1. Baseline characteristics.
| All, N=3,072,966 | Whites, n=2,525,525 (82%) | African Americans, n=547,441 (18%) | |
| Age, years | 59.9 ± 13.4 | 61.0 ± 13.9 | 54.5 ± 13.2 |
| Estimated GFRa | 84.0 ± 15.7 | 82.3 ± 14.4 | 91.9 ± 18.8 |
| Sex, males | 2,876,626 (94) | 2,383,874 (94) | 492,752 (90) |
| Hypertension | 1,842,120 (60) | 1,503,404 (60) | 338,716 (62) |
| DM | 735,372 (24) | 598,022 (24) | 137,350 (25) |
| CHD | 359,848 (12) | 321,545 (13) | 38,303 (7) |
| CHF | 143,230 (5) | 118,970 (5) | 24,260 (4) |
| CVD | 194,493 (6) | 163,514 (6) | 30,979 (6) |
| Mean annual income | 22,496 (11,643-35,000) | 24,100 (12,284-37,533) | 16,732 (10,044-29,416) |
| Service-connectedness | 1,273,171 (41) | 1,009,039 (40) | 264,132 (48) |
| BMI (kg/m2) | 29.2 ± 5.8 | 29.2 ± 5.7 | 29.0 ± 6.0 |
| SBP (mm Hg) | 135.4 ± 19.2 | 135.2 ± 18.9 | 136.8 ± 20.5 |
| DBP (mm Hg) | 77.2 ± 11.9 | 76.6 ± 11.6 | 79.9 ± 12.8 |
| ACEI/ARB use | 1,636,622 (53) | 1,342,705 (53) | 293,917 (54) |
| Statin use | 1,688,623 (55) | 1,417,215 (56) | 271,408 (50) |
| Influenza vaccination | 2,006,550 (65) | 1,672,423 (66) | 334,127 (61) |
| Cholesterol measured | 2,866,616 (93) | 2,355,044 (93) | 511,572 (93) |
Data are presented as means ± SD, medians (interquartile ranges) or number (% of total).
ACEI/ARB, angiotensin converting enzyme inhibitors/angiotensin receptop blockers; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; CHD, coronary heart disease; CHF, chronic heart failure; CVD, cerebrovascular disease.
a. GFR=mL/min/1.73m2.
Mortality
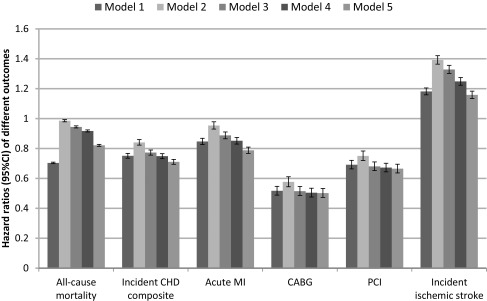
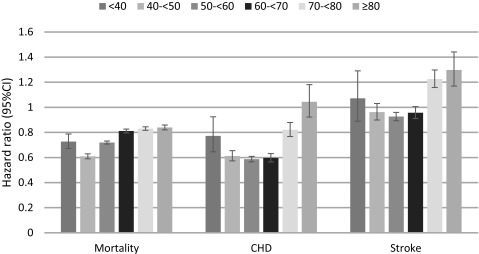
A total of 638,536 patients died overall (mortality rate: 30.16/1000 patient-years [PY], 95% CI: 30.09-30.24) during a median follow-up of 7.9 years. There were 551,208 deaths in White patients (mortality rate: 31.87/1000PY, 95%CI: 31.79-31.96) and 87,328 deaths in African American patients (mortality rate: 22.53/1000PY, 95%CI: 22.38-22.68). Compared with Whites, African American patients had an overall crude all-cause mortality hazard ratio (95%CI) of .70 (.69-.71, P<.001) (Figure 1). Adjustment for age, sex and baseline eGFR resulted in the attenuation of the African American mortality advantage (hazard ratio, 95%CI: .99, .98-.99, P<.001), but further adjustment for additional covariates resulted in a gradual decrease in the mortality risk associated with African American race (fully adjusted HR, 95%CI: .82, .81-.83, P<.001). When stratified by age using the fully adjusted model including community level factors, African American patients had a lower all-cause adjusted mortality hazard ratio at all ages but at aged ≥60 years was attenauted slightly, yet was still only 80% of Whites (Table 2 and Figure 2).
Figure 1. Hazard ratios for association of African American race with all-cause mortality, incident coronary heart disease (CHD) composite (acute MI, CABG and PCI), acute myocardial infarction (acute MI), coronary artery bypass graft (CABG), percutaneous coronary intervention (PCI), and incident ischemic stroke in the overall cohort of 3,072,966 veterans.
Patients with White race served as referent. Model 1: unadjusted, Model 2: adjusted for age, sex, baseline estimated glomerular filtration rate; Model 3: adjusted for Model 2 variables plus comorbidities; Model 4: adjusted for Model 3 variables plus baseline body mass index, systolic and diastolic blood pressure; Model 5: adjusted for Model 4 variables plus mean income, marital status, service connectedness, use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers and statins, and receipt of influenza vaccination(s).
Table 2. Multivariable adjusted hazard ratios and 95% confidence intervals of all-cause mortality, incident coronary heart disease and incident ischemic stroke for Black vs White race in four different age groups, adapted from 44.
| Age, years | All-cause mortality | Incident coronary heart disease | Incident ischemic stroke |
| <40 | .70 (.65-.76) | .70 (.59-.84) | .99 (.82-1.20) |
| 40-59 | .70 (.69-.71) | .60 (.58-.62) | .95 (.92-.98) |
| 60-79 | .81 (.80-.82) | .68 (.65-.71) | 1.06 (1.02-1.10) |
| ≥80 | .80 (.78-.82) | 1.05 (.93-1.19) | 1.30 (1.17-1.44) |
Hazard ratios were adjusted for age, sex, baseline estimated glomerular filtration rate (eGFR), comorbidities, baseline body mass index, systolic and diastolic blood pressures, mean income, marital status, service connectedness, area-level housing stress, low education, low employment, persistent poverty, frequency of Veterans Affairs (VA) health care encounters, use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and statins, receipt of influenza vaccination(s) [Modle 5], each patient’s VA health care center as well as housing stress, low education, low employment, and persistent poverty.
Figure 2. Hazard ratios for association of African American race with all-cause mortality, incident coronary heart disease (CHD) composite (acute myocardial infarction, coronary artery bypass graft, percutaneous coronary intervention) and incident stroke stratified by age.
Patients with White race served as referent. Adjusted for age, sex, baseline estimated glomerular filtration rate; comorbidities; baseline body mass index, systolic and diastolic blood pressure; mean income, marital status, service connectedness, use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers and statins, receipt of influenza vaccination(s) [Model 5] as well as each patient’s VA healthcare center as well as housing stress, low education, low employment, and persistent poverty.
Incident CHD
During the observed cohort period 63,808 patients experienced an incident CHD event (event rate: 3.43/1000PY, 95%CI: 3.40-3.46), with 53,988 events in White patients (event rate: 3.60/1000PY, 95%CI: 3.57-3.63) and 9,820 events in African American patients (event rate: 2.73/1000PY, 95%CI: 2.68-2.79). Both crude and adjusted risks of incident CHD composite events, and of the individual components (acute MI, CABG and PCI) were lower in African American patients (Figure 1). When stratified by age using the fully adjusted model including community level factors, African American patients had a lower all-cause adjusted risks of incident CHD composite events up to age 79 (Table 2). From age 40-69 the event rate was 40% lower than Whites, at aged <40 years and between aged 70-79 years, the event rate was 20% lower than Whites and for aged ≥80 years, the rates did not differ (Figure 2).
Incident Stroke
During the same period 59,734 patients experienced an incident stroke (event rate: 3.02/1000PY, 95%CI: 2.99-3.04), with 46,984 events in White patients (event rate: 2.91/1000PY, 95%CI: 2.88- 2.93) and 12,750 events in African American patients (event rate: 3.49/1000PY, 95%CI: 3.43- 3.55). Overall both crude and adjusted risks of incident stroke were higher in African American patients (Figure 1). When stratified by age using the fully adjusted model including community level factors, African American patients up to aged 69 years had a similar adjusted risk of stroke and those aged ≥70 years had a 20-30% greater adjusted risk of stroke (Figure 2).
Discussion
In this large cohort of more than 3 million contemporary US veterans with normal renal function (eGFR>60 mL/min/1.73m2), we found that, contrary to findings in the general population, African Americans receiving care in the VHA system, experienced a lower incidence of CHD and lower all-cause mortality in comparison with White veterans. When we analyzed the data by age, using a fully adjusted model, the advantage was even greater for younger African American veterans. Consistent with previous literature, we found a higher incidence of stroke among African American veterans, but this was driven by those aged ≥70 years, while those aged <70 years had rates similar to their White veteran peers. Our findings raise several key questions.23 One question is: Might this cohort systematically differ from the general population? The VHA cohort likely has a selection bias based on people who desire to join the military creating a different set of sociodemographic, clinical and other characteristics than the general population. To mitigate this possibility in a prior analysis, we performed a propensity-matched analysis to examine differences in the VHA cohort by race, and mortality and incident events in a National Health and Nutrition Examination Survey (NHANES) and it did not change our findings.19 However, unmeasured confounding variables such as physical activity, diet and others could still play a role.23 A second question is what characteristics of the VHA system or care delivery within the system explain this paradox? We did not define the cohort as individuals who solely received their care in the VHA system and this may have led to incomplete ascertainment of all cardiovascular events. While this could affect our findings related to incident CHD and stroke, it should not affect findings related to all-cause mortality.19,23
A third question is not only why did African Americans exhibit such good outcomes, but why younger African Americans did especially well. Traditionally younger African Americans have an increased risk for premature all-cause mortality due to factors such as: 1) higher death rates from homicide, motor vehicle accident, suicide, and drug overdose; 2) greater likelihood to be uninsured or underinsured than their White peers; 3) an even greater distrust of institutions, including medical establishments, leading to delayed/reduced visits and/or lesser adherence even if access to care is available; and 4) possible permissive or effect-modifying role of genetic factors that impact on survival.13 By contrast, being a young veteran may provide certain advantages to African Americans by bringing them a level of greater equity with their White peers in contrast to not being a veteran, such as a more extensive social network, a greater sense of the ability to persevere, better habits for diet and physical activity. It should also be noted that African American veterans have higher income and higher levels of educational attainment than non-veteran African Americans.24-26 Being enrolled in the VHA eliminates the likelihood to be uninsured or underinsured, and being a veteran and connected to the VHA may attenuate the traditional level of distrust that African Americans have for the medical establishment thereby enhancing their likelihood to seek preventive care or treatment.
A fourth question is how can these findings be extrapolated and applied within the VHA and beyond? Consideration of key VHA system structures may provide clues. For instance, universal veteran access to care and focused quality improvement are important VHA system characteristics that may have contributed to improved CHD and mortality outcomes for African Americans veterans.27-29 Multiple factors influence access to high quality care, including both individual level (eg, sociodemographics, geography, and health literacy, health beliefs and behaviors) and health care system level (eg, referral patterns, quality of care) factors.30 In addition to facilitating veterans’ access to services addressing their social and behavioral needs, the VHA maintains an open-access appointment system for veterans, which enables ready access to care. The VHA intense focus on quality improvement may also influence measures of care and outcomes for cardiovascular disease.31-33 While all elements of VHA care may not be replicable in alternatively structured health care systems, a focus on these important aspects of care could influence outcomes in other systems.
The race differences between the outcomes in veterans persisted and were even magnified after multiple adjustment for demographics, socioeconomic characteristics, and comorbidities. This highlights the potentially important role of social determinants or possible race-based biological differences in common disease processes that could explain race differences in outcomes. In regard to key social determinants that can influence differences in common disease processes and health outcomes, major unique characteristics of African American veterans include a lower uninsured rate, a lower poverty rate, and higher levels of income and educational attainment than Black non-Veterans. Furthermore, there is a lesser racial disparity in these major social factors among veterans.24-26 In regard to education, while the percent of African American veterans and non-veterans with a bachelor’s degree does not differ, African American veterans are much more likely to have some college education or an advancced degree than their non-veteran peers.26 They still have lower levels of bachelor and advanced degrees compared with their White veteran peers, but the overall racial educational gap is less for veterans than for non-veterans.26 Given the powerful contribution of education-related differences to disparities in cardiovascular mortality, the relatively higher level of educational attainment (as well as income/lower level of poverty) among African American veterans could be an important contributor to our finding of racial differences in all-cause mortality and CHD.34,35
In regard to biological differences, there is now mounting evidence that some African Americans have distinctly unique genetic characteristics linked to their African ancestry such as sickle cell trait/disease (linked to CKD, CVD, and Stroke)36-38 and APOL1 high risk alleles (linked to CKD and possibly CVD) that help to protect against malaria and trypanosomiasis respectively.9-12 These genetic traits, which are thought to have evolved to protect against acute infections, have maladaptive consequences for CVD and related disorders in environments where these infections do not exist; they may have a direct impact on health outcomes, independent of socioeconomic factors. There may also be African ancestry genetic traits that enhance CV health. The longevity associated G allele of FOXO3 (rs2802292) has been associated with an all-cause mortality risk reduction of 11% in Japanese American men in the Honolulu Heart Program (P=.004), and 9% in Whites (P=.06) and 13% in Blacks (P=.29) in the Health ABC cohort.39 However, for CHD mortality, the risk reduction associated with the longevity associated G allele of FOXO3 in Japanese American men was 25% (P=.001), 24% for Whites (P=.036) and even larger for Blacks (39%; P=.068).39 What the prevalence of the longevity associated G allele of FOXO3 is in our cohort is unknown as well as ways in which veteran status may potentiate its effect. The frequency of the FOXO3 G allele was reported to be 72.3% in Blacks, more than twice that for Whites and Japanese American men.39 The higher prevalence of FOXO3 in Blacks may be due to its role to regulate stress resistance and protect against ultraviolet damage,40 which would have been a major biological stressor in African populations due to their equatorial location and sun exposure. However, genetic differences should be randomly distributed across veterans and non-veterans and therefore less likely to account for a reversal in findings.
Our study had several limitations, including a predominance of men in our cohort. However, despite the low percentage of women in our study limiting many sub-analyses, we previously reported our overall findings not stratified by age were similar in female compared with male veterans.19 In addition the absolute number of women (>150 000 patients) in our study was greater than in most previous VHA studies. Race was determined by self-identification and as such captures social constructs more so than ancestral biology. However, this method has consistently characterized racial disparities in health outcomes. Enrollment in the US armed services and subsequently into the VHA may include distinct populations of both Blacks and Whites. Although we cannot discount this possibility, the basic characteristics of our cohort suggest that differences between Blacks and Whites seen in the general population were indeed present in our cohort (eg, differences in income, education, marital status, and certain comorbidities), albeit to a lesser degree. While we attempted to minimize selection bias and control for socio-demographics, factors such as income and education are estimated at a ZIP code level and analyses at smaller geographic areas such as Census track may be needed to better delineate the effects of key community level social determinants on health outcomes.35 Of note, higher stroke rates41 and lower incidence of CHD42,43 in populations of African ancestry have also been previously reported in non-veterans, suggesting that our findings are not limited to US veterans alone. Clinical events were captured using diagnostic codes, which are not as accurate as adjudication procedures used in clinical trials. However, this limitation does not apply to all-cause mortality. We only examined all-cause mortality because we did not have detailed information on the causes of death. Finally, despite the large number of demographic, social, economic, and quality of care indexes that we adjusted for, we cannot exclude the possibility that unmeasured confounders may contribute to the observed differences.
Conclusion
In summary, among patients with normal kidney function (eGFR>60 mL/min/1.73m2) and receiving care in the VHA, improved cardiovascular outcomes were most dramatic for younger African Americans, independent of demographic, comorbidity and socioeconomic differences. We hypothesized that the improved all-cause mortality and CV event rates in the same cohort of African Americans without CKD or ESRD in the VHA would exist regardless of age. We found age had an important influence on racial differences in mortality and CV events among veterans, and that younger African Americans had even lower risk of all-cause mortality and CHD, and did not suffer from increased stroke risk unlike older African Americans who suffer from a 20%-30% increased risk of stroke.
Our findings likely reflect important socio-cultural and access to quality care mediated differences. While race-specific biologic moderators of cardiovascular outcomes may or may not differ between veteran and non-veteran African Americans, health system-specific factors and other variables such as education, income, and other social determinants of health that vary systematically between White and Black veterans and between veteran and non-veteran African Americans deserve further research.
Acknowledgments
This study was supported by grant R01DK096920 to CPK and KKZ and is the result of work supported with resources and the use of facilities at the Memphis VA Medical Center and the Long Beach VA Medical Center. Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). Dr Norris is supported by National Institutes of Health grants TR000124, MD000182 and AG021684.
References
- 1. .US Census Bureau State and County QuickFacts. http://quickfacts.census.gov/qfd/states/00000.html. Accessed May 5, 2016.
- 2.Centers for Disease Control and Prevention (CDC) . Infant mortality and low birth weight among black and white infants--United States, 1980-2000. MMWR Morb Mortal Wkly Rep. 2002;51(27):589-592. [PubMed] [Google Scholar]
- 3.Tucker MJ, Berg CJ, Callaghan WM, Hsia J. The Black-White disparity in pregnancy-related mortality from 5 conditions: differences in prevalence and case-fatality rates. Am J Public Health. 2007;97(2):247-251. 10.2105/AJPH.2005.072975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26(1):89-113. 10.1146/annurev.publhealth.26.021304.144528 [DOI] [PubMed] [Google Scholar]
- 5.Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345(7):479-486. 10.1056/NEJMoa010273 [DOI] [PubMed] [Google Scholar]
- 6.Clark LT, Ferdinand KC, Flack JM, et al. Coronary heart disease in African Americans. Heart Dis. 2001;3(2):97-108. 10.1097/00132580-200103000-00007 [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: Final Data for 2013. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2016;64(2):1-119. [PubMed] [Google Scholar]
- 8.Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annu Rev Psychol. 2007;58(1):201-225. 10.1146/annurev.psych.57.102904.190212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman BI, Register TC. Effect of race and genetics on vitamin D metabolism, bone and vascular health. Nat Rev Nephrol. 2012;8(8):459-466. 10.1038/nrneph.2012.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841-845. 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491. 10.1681/ASN.2013010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito K, Bick AG, Flannick J, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res. 2014;114(5):845-850. 10.1161/CIRCRESAHA.114.302347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Kovesdy CP, Norris KC. Racial survival paradox of dialysis patients: robust and resilient. Am J Kidney Dis. 2012;60(2):182-185. 10.1053/j.ajkd.2012.02.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306(6):620-626. 10.1001/jama.2011.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3(9):493-506. 10.1038/ncpneph0570 [DOI] [PubMed] [Google Scholar]
- 16.Rhee CM, Lertdumrongluk P, Streja E, et al. Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol. 2014;39(3):183-194. 10.1159/000358497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan G, Norris KC, Yu AJ, et al. The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol. 2013;8(6):953-961. 10.2215/CJN.09180912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuller LH, Neaton JD. Letter by Kuller and Neaton Regarding Article, “Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans”. Circulation. 2016;133(12):e452. 10.1161/CIRCULATIONAHA.115.020379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132(16):1538-1548. 10.1161/CIRCULATIONAHA.114.015124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosmanova EO, Lu JL, Streja E, Cushman WC, Kalantar-Zadeh K, Kovesdy CP. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension. 2014;64(5):951-957. 10.1161/HYPERTENSIONAHA.114.03805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VA Information Resource Center VIReC Resource Guide: VA Corporate Data Warehouse. Hines, IL: US Department of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2012. [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook NL, Mensah GA. Eliminating Health Disparities: What Can We Learn From the Veterans Health Administration? Circulation. 2015;132(16):1519-1521. 10.1161/CIRCULATIONAHA.115.018953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Census Bureau Current Population Surveys. Prepared by the National Center for Veterans Analysis and Statistics; 2009. [Google Scholar]
- 25. .Minority Veterans : 2011. Prepared by the National Center for Veterans Analysis and Statistics May 2013. accessed at <http://www.va.gov/vetdata/docs/SpecialReports/Minority_Veterans_2013.pdf> on May 25, 2016.
- 26. .Educational Attainment of Veterans : 2000 to 2009. Prepared by the National Center for Veterans Analysis and Statistics January 2011. accessed at <http://www.va.gov/VETDATA/docs/SpecialReports/education_FINAL.pdf> on May 25, 2016.
- 27.Jha AK, Shlipak MG, Hosmer W, Frances CD, Browner WS. Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. JAMA. 2001;285(3):297-303. 10.1001/jama.285.3.297 [DOI] [PubMed] [Google Scholar]
- 28.Gao SW, Oliver DK, Das N, et al. Assessment of racial disparities in chronic kidney disease stage 3 and 4 care in the department of defense health system. Clin J Am Soc Nephrol. 2008;3(2):442-449. 10.2215/CJN.03940907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deswal A, Petersen NJ, Souchek J, Ashton CM, Wray NP. Impact of race on health care utilization and outcomes in veterans with congestive heart failure. J Am Coll Cardiol. 2004;43(5):778-784. 10.1016/j.jacc.2003.10.033 [DOI] [PubMed] [Google Scholar]
- 30.Mensah GA. Eliminating disparities in cardiovascular health: six strategic imperatives and a framework for action. Circulation. 2005;111(10):1332-1336. 10.1161/01.CIR.0000158134.24860.91 [DOI] [PubMed] [Google Scholar]
- 31.Cohen MG, Fonarow GC, Peterson ED, et al. Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines-Coronary Artery Disease program. Circulation. 2010;121(21):2294-2301. 10.1161/CIRCULATIONAHA.109.922286 [DOI] [PubMed] [Google Scholar]
- 32.Cook NL, Ayanian JZ, Orav EJ, Hicks LS. Differences in specialist consultations for cardiovascular disease by race, ethnicity, gender, insurance status, and site of primary care. Circulation. 2009;119(18):2463-2470. 10.1161/CIRCULATIONAHA.108.825133 [DOI] [PubMed] [Google Scholar]
- 33.Shaw KM, Handler J, Wall HK, Kanter MH. Improving blood pressure control in a large multiethnic California population through changes in health care delivery, 2004-2012. Prev Chronic Dis. 2014;11:E191. 10.5888/pcd11.140173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585-1592. 10.1056/NEJMsa012979 [DOI] [PubMed] [Google Scholar]
- 35.Mode NA, Evans MK, Zonderman AB. Race, Neighborhood Economic Status, Income Inequality and Mortality. PLoS One. 2016;11(5):e0154535. 10.1371/journal.pone.0154535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ribera MC, Ribera RB, Koifman RJ, Koifman S. Echocardiography in sickle cell anaemia patients under 20 years of age: a descriptive study in the Brazilian Western Amazon. Cardiol Young. 2015;25(1):63-69. 10.1017/S104795111300156X [DOI] [PubMed] [Google Scholar]
- 37.Naik RP, Derebail VK, Grams ME, et al. Association of sickle cell trait with chronic kidney disease and albuminuria in African Americans. JAMA. 2014;312(20):2115-2125. 10.1001/jama.2014.15063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neumayr L, Vichinsky E. Stroke recurrence in adult sickle cell patients: it is time for action! Transfusion. 2016;56(5):1001-1004. 10.1111/trf.13614 [DOI] [PubMed] [Google Scholar]
- 39.Willcox BJ, Tranah GJ, Chen R, et al. The FoxO3 gene and cause-specific mortality. Aging Cell. 2016. 10.1111/acel.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JY, Xia W, Hu MC. Ionizing radiation activates expression of FOXO3a, Fas ligand, and Bim, and induces cell apoptosis. Int J Oncol. 2006;29(3):643-648. [PMC free article] [PubMed] [Google Scholar]
- 41.Howard VJ, Kleindorfer DO, Judd SE, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69(4):619-627. 10.1002/ana.22385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. .Davis TM, Coleman RL, Holman RR Ethnicity and long-term vascular outcomes in Type 2 diabetes: a prospective observational study (UKPDS 83). Diabetic medicine : a journal of the British Diabetic Association. 2014;31(2):200-207. [DOI] [PubMed]
- 43.Gillum RF, Mussolino ME, Madans JH. Coronary heart disease incidence and survival in African-American women and men. The NHANES I Epidemiologic Follow-up Study. Ann Intern Med. 1997;127(2):111-118. 10.7326/0003-4819-127-2-199707150-00003 [DOI] [PubMed] [Google Scholar]
- 44.Kovesdy CP, Norris KC, Boulware LE, et al. Response to Letter Regarding Article, “Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans.” Circulation. 2016;133(12):e453. 10.1161/CIRCULATIONAHA.116.021164 [DOI] [PMC free article] [PubMed] [Google Scholar]