Abstract
The NIH Chronic Graft-versus-Host Disease (cGVHD) Consensus Project Ancillary and Supportive Care Guidelines recommend annual assessment of bone mineral density (BMD) to monitor bone health. The study of osteoporosis in patients with cGVHD has been limited to small numbers of patients and the guidelines are based on experiences in other chronic diseases and expert opinion. We hypothesized that the prevalence of osteoporosis is high in a cohort of 258 patients with moderate to severe cGVHD due to prolonged exposure to risk factors for osteoporosis after allogeneic hematopoietic stem cell transplantation. We defined osteoporosis using BMD criteria (T-score ≤ -2.5) at three anatomical sites (femoral neck – FN, lumbar spine – LS, total hip – TH) and characterized risk factors through univariate and multivariate analyses. We found that low body weight (FN p<0.0001, LS p=0.0002, TH p<0.0001), malnutrition (FN p=0.0002, LS p=0.03, TH p=0.0076), higher platelet count (FN p=0.0065, TH p=0.0025), higher average NIH organ score (FN p=0.038), higher prednisone dose (LS p=0.032), lower complement component 3 (LS p=0.0073), and physical inactivity (FN p=0.01) were associated with osteoporosis in one or more site. T-scores were significantly lower in the FN than in the other two sites (p<0.0001 for both). The prevalence of osteoporosis and osteopenia was high (17% and 60%, respectively), supporting current recommendations for frequent monitoring of BMD. The association of higher platelet count in cGVHD patients with osteoporosis has not been previously reported and presents a new area of interest in the study of osteoporosis after allogeneic hematopoietic stem cell transplantation.
Keywords: Chronic Graft-versus-Host Disease, Allogeneic Hematopoietic Stem Cell Transplantation, Osteoporosis, Late Effects, Supportive Care, Platelets
Introduction
Improvements in the safety and efficacy of allogeneic hematopoietic stem cell transplantation (allo-HSCT) have resulted in an increased number of long-term survivors along with the need to identify and treat late complications that arise in this unique group of patients. Chronic Graft-versus-Host Disease (cGVHD) is a common cause of morbidity and non-relapse mortality in long-term survivors of allo-HSCT with an estimated incidence of 30-70% (1, 2). It is characterized by donor-derived lymphocyte infiltration and immune reaction against host tissues causing significant symptom burden and functional impairment among patients recovering from allo-HSCT (1-4).
Osteoporosis is another significant source of morbidity in patients after allo-HSCT (5). It is a disease process in which rapid resorption and subsequent loss of bone density takes place in the first 1-2 years after transplant with recovery occurring in some, but not all, affected anatomical sites (6-10). Contributing factors include myeloablative conditioning, secondary hypogonadism, abnormal metabolism of calcium and vitamin D, reduced mobility, and use of immunosuppressive medications such as glucocorticoids and calcineurin inhibitors (6, 11-14). These last two drug classes are associated with altered metabolism and absorption of calcium, phosphate, and vitamin D, and trabecular bone loss at the spine and femoral neck (6, 13-17). In addition, hyponatremia, an emerging contributor to osteoporosis, has been reported after allo-HSCT (18-20).
Bone loss is an immune-mediated process in which several cytokines create an imbalance between bone resorption and bone formation via a pathway of the receptor activator of nuclear factor-κB, its ligand, and osteoprotegerin (21). After allo-HSCT, patients experience a number of immunological processes including cytokine storm and GVHD which may contribute to the development of osteoporosis (22). It is of particular concern to patients and clinicians due to the painful and debilitating fractures that can lead to reduced mobility and quality of life (5). A recent retrospective analysis of over 3,500 allo-HSCT recipients found that 5% experienced a fracture during a median post-transplant follow-up period of 85 months (23).
The NIH Chronic GVHD Consensus Project Ancillary and Supportive Care Guidelines recommend annual monitoring of BMD, calcium, and vitamin D level (24). Anti-resorptive therapy is suggested for patients with BMD-derived T-score below -1.5 and referral to an endocrinologist is recommended for evaluation and treatment of secondary endocrine causes. These guidelines for osteoporosis are based on experiences in other diseases and expert opinion. Prior studies addressing osteoporosis in allo-HSCT have found the following associations of GVHD-related variables with osteoporosis: cumulative dose of glucocorticoids and calcineurin inhibitors, duration of therapy with glucocorticoids and calcineurin inhibitors, severe acute GVHD, any cGVHD, and cGVHD severity (7, 8, 11, 13, 14, 22, 25-27).
The aim of this study was to determine the prevalence of osteoporosis in a large, well-annotated cohort of patients with moderate and severe cGVHD as defined by NIH criteria, and to identify possible risk factors and correlates. We hypothesized that patients who are more severely affected by cGVHD are also predisposed to osteoporosis due to increased exposure to risk factors such as immune dysregulation, secondary hypogonadism, reduced mobility, and prolonged use of immunosuppressive therapy. Given this hypothesis, we expected a high prevalence of osteoporosis in this population.
Methods
Patients
Patients were enrolled on the National Institutes of Health protocol entitled “Factors Determining Outcomes in Patients with Graft-versus-Host Disease” (NCT00092235), a National Cancer Institute IRB-approved cross-sectional study in which patients provided written consent to undergo a 1-week comprehensive multidisciplinary evaluation. Patients were seen by subspecialists in dentistry, dermatology, gynecology, ophthalmology, pain and palliative care, rehabilitation medicine, and transplant clinicians and assessed using the NIH cGVHD diagnostic and staging system (28-30). In addition to collecting demographic, laboratory, and histopathology data, patients underwent dual-energy X-ray absorptiometry (DEXA) to determine BMD at the femoral neck, lumbar spine, and total hip.
337 patients were enrolled in this protocol from October 2004 to June 2014. For the purposes of this study, 79 patients were excluded for the following reasons: adult patients without DEXA (n=30), pediatric patients (n=27), patients who were not diagnosed with cGVHD at evaluation or who failed to complete the study (n=14), and patients whose DEXA yielded insufficient data (no T-score likely due to artifacts in scans; n=8), resulting in a study population of 258 patients.
Outcomes and Variables
DEXA was performed using Hologic scanners Delphi (n=174), Discovery C (n=74), and QDR4500 (n=10). BMD values at each anatomical site were converted to T-scores by comparison to a race and gender-matched reference population of healthy young adults using manufacturer databases. Osteoporosis was defined using World Health Organization criteria in which T-score of -2.5 or below indicates osteoporosis, T-score between -2.5 and -1.0 indicates osteopenia, and T-score of -1.0 or above is normal (31). Patients, regardless of age or gender, were divided into two groups, osteoporosis and non-osteoporosis, at each location based on their T-scores and potential risk factors for osteoporosis were compared between the two. Patients with osteopenia were placed in the non-osteoporosis group.
Potential risk factors for osteoporosis in this study were classic risk factors for osteoporosis such as age, sex, body weight, malnutrition (assessed using the Patient-Generated Subjective Global Assessment (PG-SGA) malnutrition screening tool recommended by the American Society of Parenteral and Enteral Nutrition (ASPEN)), physical inactivity (assessed using the PG-SGA activities and function evaluation), serum 25-hydroxyvitamin D deficiency, hypocalcemia, hyponatremia, hypogonadism (assessed using serum levels of estradiol, follicle stimulating hormone, luteinizing hormone, and testosterone), hyperparathyroidism, thyroid dysfunction, history of alcohol consumption (yes vs. no; self-reported current or prior alcohol consumption of any frequency or duration), history of cigarette smoking (yes vs. no; self-reported current or prior smoking of any frequency or duration), current use of selective serotonin reuptake inhibitors (SSRIs; yes vs. no), and current use of proton-pump inhibitors (PPIs; yes vs. no) (32-34).
In the risk factor analysis we also considered transplant characteristics including total body irradiation (yes vs. no), intensity of conditioning (myeloablative vs. non-myeloablative/reduced intensity conditioning), HLA match (match vs. mismatch), donor relationship (related vs. unrelated), indication for allo-HSCT, and time since transplant.
Finally, we considered a number of variables that reflected cGVHD activity and severity including NIH global score, individual NIH organ scores, average NIH organ score (sum of all NIH organ scores divided by number of organs assessed; 7 for males, 8 for females) (29), time since diagnosis of cGVHD, body surface area of deep and superficial sclerotic skin involvement, serum markers of inflammation (platelet count, complement component 3 (C3), complement component 4, C-reactive protein (CRP), and albumin), number of prior systemic immunosuppressive therapies for cGVHD, intensity of current immunosuppression (defined per Mitchell et al (4) as none, mild = single-agent prednisone <0.5mg/kg/day, moderate = prednisone ≥ 0.5mg/kg/day and/or any single agent/modality, and high = 2 or more agents/modalities ± prednisone ≥ 0.5mg/kg/day), and current systemic glucocorticoid dose converted to equivalent prednisone dose.
Statistical Analysis
Separate statistical analyses were conducted for each of the three anatomical sites at which BMD was assessed. For evaluation of factors associated with osteoporosis, continuous parameters were compared between the two groups using an exact Wilcoxon rank sum test. Fisher's exact test was used to compare dichotomous parameters, Mehta's modification to Fisher's exact test was used to compare categorical parameters, and a Cochran-Armitage test for trend was used to compare ordered categorical parameters (35, 36). Once factors were identified as being potentially associated with osteoporosis in a given site, multiple logistic regression analysis using backward selection was used to identify which factors may potentially be jointly predictive of osteoporosis.
To determine factors associated with continuous raw T-scores in the three sites, Spearman correlation analysis was used to find the correlation between raw T-scores and continuous parameters. The magnitude of the correlation coefficient was used to gauge the strength of the correlation as follows: |r|>0.70 is strong correlation, 0.50<|r|<0.70 is moderately strong correlation, 0.30<|r|<0.50 is weak to moderately strong correlation, and |r|<0.30 is weak correlation. The association between raw T-scores and ordered categorical parameters was evaluated using a Johnkheere-Terpstra trend test (37). The Kruskal-Wallis test was used to find the association between categorical parameters and continuous T-scores, while an exact Wilcoxon rank sum test was used if two groups were compared with respect to T-scores. Once factors were identified as being potentially associated with raw T-scores at a given site, the parameters were evaluated using linear regression with backward selection to try to determine factors which may jointly be associated with raw T-scores.
All p-values are two-tailed and presented without any formal adjustment for multiple comparisons. For the univariate screening analyses, in view of the large number of parameters explored, only tests for which p<0.005 were considered to be statistically significant while those for which 0.005<p<0.05 were considered to exhibit strong trends towards statistical significance.
Results
Patient Characteristics
The study population consisted of 258 patients with a relatively even gender split (n=145 males, n=113 females) and a median age of 48 years (range, 20-71). The most frequent indications for transplant were acute leukemia and myelodysplastic syndrome (n=117, 45%) followed by lymphoma (n=58, 23%). Myeloablative conditioning was slightly more common (n=138, 53%) than reduced intensity conditioning and just over a third of patients received total body irradiation (n=92, 36%). Patients predominantly received peripheral blood stem cells (n=216, 84%), usually from HLA-matched donors (n=221, 86%). 161 patients (62%) were related to their respective donors (Table 1).
Table 1. Patient Demographics.
| N (% or range) | |
|---|---|
| All | 258 |
| Median age in years | 48 (20-71) |
| Sex | |
| Male | 145 (56) |
| Female | 113 (44) |
| Disease | |
| ALL, AML, MDS | 117 (45) |
| CML, IMF, MPD | 34 (13) |
| CLL | 21 (8) |
| HL, NHL | 58 (23) |
| MM | 15 (6) |
| AA, PNH | 8 (3) |
| Othera | 5 (2) |
| Conditioning Regimen | |
| Myeloablative | 138 (53) |
| Non-Myeloablative | 118 (46) |
| Unknown | 2 (1) |
| Total Body Irradiation | |
| Yes | 90 (35) |
| No | 166 (64) |
| Unknown | 2 (1) |
| Stem Cell Source | |
| BM | 39 (15) |
| PBSC | 216 (84) |
| Cord | 3 (1) |
| Donor Relationship | |
| Related | 161 (62) |
| Unrelated | 96 (37) |
| Unknown | 1 (<1) |
| HLA Match | |
| Yes | 221 (86) |
| No | 32 (12) |
| Unknown | 5 (2) |
Other includes Ewing's sarcoma (n=2), essential thrombocythemia (n=1), sickle cell anemia (n=1), and Wiskott-Aldrich syndrome (n=1).
Abbreviations: ALL–acute lymphocytic leukemia, AML–acute myeloid leukemia, MDS–myelodysplastic syndrome, CML–chronic myeloid leukemia, IMF–idiopathic myelofibrosis, MPD–myeloproliferative disorder, CLL–chronic lymphocytic leukemia, HL–Hodgkin's lymphoma, NHL–non-Hodgkin's lymphoma, MM–multiple myeloma, AA–aplastic anemia, PNH–paroxysmal nocturnal hemoglobinuria, BM–bone marrow, PBSC–peripheral blood stem cells, HLA–human leukocyte antigen
Patients were diagnosed with cGVHD a median of 7 months (range, 2-267) after transplant and were enrolled at a median of 23 months (range, 0-222) and 36 months (range, 4-297) after cGVHD diagnosis and transplant, respectively. Most patients had severe (71%) or moderate (28%) cGVHD per NIH global scoring. The median average NIH organ score was 1.13 (range, 0.14-2.14) with a median of 5 affected organs (range, 1-8). Patients were most frequently on high (39%) or moderate (38%) intensity immunosuppression and had received a median of 4 prior systemic immunosuppressive therapies for cGVHD (range, 0-9) (Table 2).
Table 2. Chronic GVHD Characteristics.
| N (% or range) | |
|---|---|
| All | 258 |
| Median months from transplant to cGVHD Dx | 7 (2-267a) |
| Median months from cGVHD Dx to consent | 23 (0-222) |
| Median months from transplant to consent | 36 (4-297) |
| cGVHD Organ Involvementb | |
| Skin | 204 (79) |
| Joints and Fascia | 164 (64) |
| Eyes | 206 (80) |
| Mouth | 176 (68) |
| Lungs | 198 (77) |
| Liver | 132 (51) |
| GI | 113 (44) |
| Genital (females only, N=113) | 64 (57) |
| Average NIH Organ Score | 1.13 (0.14-2.14) |
| Number of organs affected by cGVHD | Median 5 (1-8) |
| 1-2 | 12 (4) |
| 3-4 | 92 (36) |
| 5-6 | 111 (43) |
| 7-8 | 43 (17) |
| NIH Global Scorec | |
| Mild | 3 (1) |
| Moderate | 71 (28) |
| Severe | 184 (71) |
| Prior cGVHD Systemic Treatment Regimens | |
| <2 | 26 (10) |
| 2-3 | 95 (37) |
| 4-5 | 89 (34) |
| >5 | 46 (18) |
| Unknown | 2 (1) |
| Intensity of Current Immunosuppressiond | |
| None/Mild | 60 (23) |
| Moderate | 97 (38) |
| High | 100 (39) |
| Unknown | 1 (<1) |
| Current Use of Calcineurin Inhibitor | |
| Yes | 120 (47) |
| No | 138 (53) |
| Prednisone-Equivalent Corticosteroid Dose | |
| None | 114 (44) |
| <0.25mg/kg/day | 69 (26) |
| 0.25mg/kg/day to 0.5mg/kg/day | 35 (14) |
| >0.5mg/kg/day | 40 (16) |
cGVHD diagnosed in patient after donor lymphocyte infusion; longest period from transplant to cGVHD diagnosis without intermediate donor lymphocyte infusion was 103 months
cGVHD organ involvement per NIH organ staging (28).
Definition for NIH Global score is as follows: mild (1 to 2 organs affected by chronic GVHD with scores of 1), moderate (more than 2 organs with score of 1, any score of 2, or lung score of 1), or severe (any score of 3 or lung score of 2) (28).
Definition of intensity of current immunosuppression is as follows: mild (single-agent prednisone 0.5mg/kg/day), moderate (single-agent prednisone 0.5mg/kg/day and/or any single agent/modality), high (2 or more agents/modalities +/- prednisone 0.5mg/kg/day) (4).
Abbreviations: cGVHD–Chronic GVHD, Dx–Diagnosis, GI–Gastrointestinal
Prevalence of Osteoporosis and Osteopenia
The median T-scores were -1.3 (range, -4.5 to 3.4) at the femoral neck (FN), -0.9 (range, -4.8 to 3.2) at the lumbar spine (LS), and -0.95 (range, -3.6 to 2.5) at the total hip (TH). By paired Wilcoxon signed rank test, T-scores at the FN were significantly lower than those at the LS and TH (p<0.0001 for both) while T-scores in the latter two sites were not statistically different from each other (p=0.12), suggesting a pathophysiology particularly harmful to the FN (Figure 1). 43 (17%) patients were found to have osteoporosis in at least one site. The femoral neck was the site most frequently affected by osteoporosis (n=33; 13%) followed by the LS (n=24; 9%) and TH (n=15; 6%). 10 (4%) patients were diagnosed with osteoporosis in all three sites. Osteopenia was most common at the FN (n=133; 51%) followed by the TH (n=100; 39%) and LS (n=91; 35%) (Figure 2). 154 (60%) patients with no osteoporosis were found to have osteopenia in at least one site.
Figure 1. Distribution of Bone Mineral Density (T-Scores) at Each Anatomical Site.
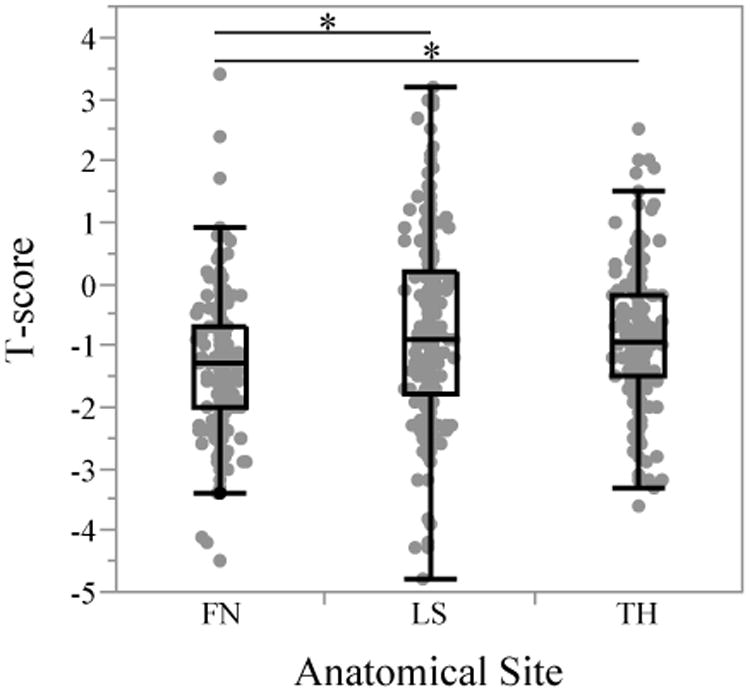
T-scores at the femoral neck were significantly lower than both lumbar spine and total hip T-scores (p<0.0001 for both; indicated by asterisk) whereas lumbar spine and total hip T-scores were not statistically different from each other (p=0.55). Abbreviations: FN–femoral neck, LS–lumbar spine, TH–total hip
Figure 2. Prevalence of Osteoporosis and Osteopenia.
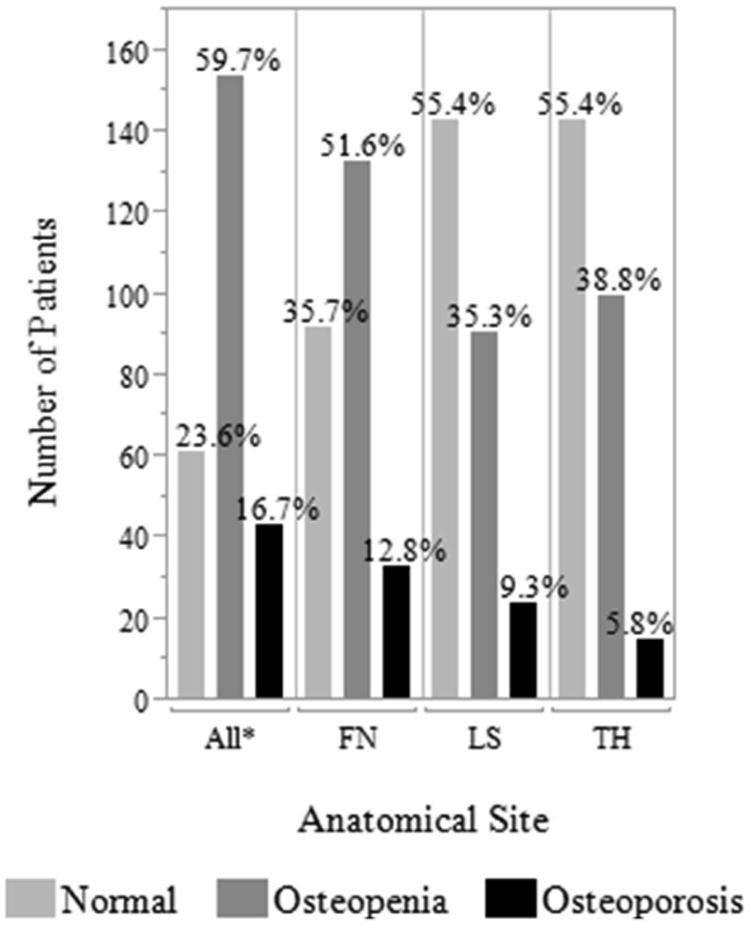
The definition for osteoporosis was based on the World Health Organization criteria: T-score ≤ -2.5 indicates osteoporosis, -2.5 < T-score < -1.0 indicates osteopenia, and T-score ≥ -1.0 is normal (29). n=258 patients. *For All, patients defined using lowest T-score, Abbreviations: FN–femoral neck, LS–lumbar spine, TH–total hip
Univariate Analysis
Lower body weight (FN: p<0.0001, LS: p=0.0002, TH: p<0.0001) was strongly associated with osteoporosis in all three anatomical sites. Higher platelet count was associated at the TH (p=0.0025) and the FN (p=0.0065). In addition, higher average NIH organ score (p=0.038) and lower CRP (p=0.023) were associated with osteoporosis at the FN while higher current glucocorticoid dose (p=0.032) and lower C3 (p=0.0073) were associated with osteoporosis at the LS. Lastly, patients with osteoporosis in the TH were farther out from transplant than those without osteoporosis at this site (p=0.036) (Table 3).
Table 3. Results of Univariate Analysis of Potential Risk Factors for Osteoporosis.
| Femoral Neck | Lumbar Spine | Total Hip | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No OP N=225 | OP N=33 | p-value | No OP N=234 | OP N=24 | p-value | No OP N=243 | OP N=15 | p-value | |
|
| |||||||||
| Age (years) | 48 | 50 | 0.74 | 48.7 | 41.9 | 0.074 | 48.5 | 44.1 | 0.10 |
|
| |||||||||
| Body Weight (kg) | 72.6 | 58.4 | <0.0001 | 72.5 | 55.5 | 0.0002 | 72.6 | 53.6 | <0.0001 |
|
| |||||||||
| BMI (kg/m2) | 24.7 | 20.5 | 0.0004 | 24.8 | 19.7 | <0.0001 | 24.6 | 19.4 | 0.0003 |
|
| |||||||||
| Average NIH Organ Scorea | 1.1 | 1.4 | 0.038 | 1.1 | 1 | 0.71 | 1.1 | 1 | 0.45 |
|
| |||||||||
| 25-OH Vitamin D (ng/mL) | 30 | 32 | 0.70 | 30 | 30 | 0.62 | 30 | 32 | 0.44 |
|
| |||||||||
| Sodium (mmol/L) | 138 | 140 | 0.11 | 139 | 139.5 | 0.63 | 139 | 140 | 0.47 |
|
| |||||||||
| Prednisone Dose (mg/kg/day) | 0.08 | 0.1 | 0.79 | 0.07 | 0.19 | 0.032 | 0.07 | 0.16 | 0.71 |
|
| |||||||||
| Platelet Count (K/μL) | 235 | 307 | 0.0065 | 239 | 241 | 0.76 | 239 | 336 | 0.0025 |
|
| |||||||||
| CRP (mg/L) | 1.88 | 0.7 | 0.023 | 1.77 | 0.81 | 0.17 | 1.7 | 0.76 | 0.41 |
|
| |||||||||
| C3 (mg/dL) | 134.3 | 131.5 | 0.28 | 135 | 116 | 0.0073 | 133.4 | 128 | 0.67 |
|
| |||||||||
| Sex | |||||||||
| Male | 132 | 13 | 0.041 | 130 | 15 | 0.6665 | 140 | 5 | 0.11 |
| Female | 93 | 20 | 104 | 9 | 103 | 10 | |||
|
| |||||||||
| Smoking History | |||||||||
| Yes | 83 | 14 | 88 | 9 | 92 | 5 | |||
| No | 135 | 16 | 0.43 | 138 | 13 | 1.00 | 143 | 8 | 1.00 |
| Unknown | 7 | 3 | 8 | 2 | 8 | 2 | |||
|
| |||||||||
| Alcohol History | |||||||||
| Yes | 152 | 13 | 152 | 13 | 159 | 6 | |||
| No | 64 | 17 | 0.0061 | 72 | 9 | 0.48 | 74 | 7 | 0.13 |
| Unknown | 9 | 3 | 10 | 2 | 10 | 2 | |||
|
| |||||||||
| Physical Activity | |||||||||
| Normal | 51 | 4 | 0.01 | 51 | 4 | 0.52 | 54 | 1 | 0.15 |
| Fairly Normal | 110 | 16 | 114 | 12 | 117 | 9 | |||
| Fairly Inactive | 30 | 1 | 30 | 1 | 30 | 1 | |||
| Inactive | 25 | 11 | 31 | 5 | 32 | 4 | |||
|
| |||||||||
| Normal or Fairly Normal | 161 | 20 | 0.20 | 165 | 16 | 1.00 | 171 | 10 | 0.56 |
| Inactive or Fairly Inactive | 55 | 12 | 61 | 6 | 62 | 5 | |||
|
| |||||||||
| Nutrition | |||||||||
| Well-Nourished | 155 | 12 | 157 | 10 | 162 | 5 | |||
| Suspected Malnutrition | 52 | 14 | <0.0001 | 59 | 7 | 0.0011 | 60 | 6 | 0.0002 |
| Severe Malnutrition | 8 | 6 | 9 | 5 | 10 | 4 | |||
|
| |||||||||
| Well-Nourished | 155 | 12 | 0.0002 | 157 | 10 | 0.030 | 162 | 5 | 0.0076 |
| Malnutrition | 60 | 20 | 68 | 12 | 70 | 10 | |||
|
| |||||||||
| Anti-Depressant | |||||||||
| Yes | 65 | 12 | 0.42 | 67 | 10 | 0.24 | 70 | 7 | 0.15 |
| No | 160 | 21 | 167 | 14 | 173 | 8 | |||
|
| |||||||||
| Yesb | |||||||||
| SSRI | 42 | 10 | 0.32 | 44 | 8 | 0.48 | 46 | 6 | 0.42 |
| Other | 23 | 2 | 23 | 2 | 24 | 1 | |||
|
| |||||||||
| SSRIc | |||||||||
| Yes | 42 | 10 | 0.16 | 44 | 8 | 0.11 | 46 | 6 | 0.088 |
| No | 183 | 23 | 190 | 16 | 197 | 9 | |||
|
| |||||||||
| Proton Pump Inhibitor | |||||||||
| Yes | 118 | 17 | 1.00 | 125 | 10 | 0.29 | 125 | 10 | 0.30 |
| No | 107 | 16 | 109 | 14 | 118 | 5 | |||
|
| |||||||||
| Anti-Resorptive | |||||||||
| Yes | 34 | 15 | 0.0002 | 45 | 4 | 1.00 | 44 | 5 | 0.17 |
| No | 191 | 18 | 189 | 20 | 199 | 10 | |||
All comparisons for continuous variables were performed using the Wilcoxon rank sum test. All comparisons for dichotomous variables were performed using Fisher's Exact Test. All comparisons for ordered categorical variables were performed using the Cochrane-Armitage Test. All p-values are two-tailed and are presented without adjustment for multiple comparisons. Tests for which p<0.005 (bold) should be considered statistically significant while tests for which 0.005<p<0.05 (italics) may be considered as exhibiting strong trends toward statistical significance.
Abbreviations: OP–osteoporosis, CRP–C-reactive protein, C3–complement component 3, SSRI–selective serotonin reuptake inhibitor
Definition of average NIH organ score is as follows: sum of all NIH organ scores (per NIH organ staging; 28) divided by number of organs assessed (7 for men, 8 for women).
Comparison of patients currently taking SSRI vs. patients taking non-SSRI anti-depressant.
Comparison of patients currently taking SSRI vs. patients taking non-SSRI or no anti-depressant.
Malnutrition, as assessed by the PG-SGA, was associated with osteoporosis in all three sites (FN: p<0.0001, LS: p<0.0001, TH: p=0.0002 when assessed as three categories; FN: p=0.0002, LS: p=0.03, TH: p=0.0076 when assessed as two categories). Physical inactivity, also assessed by the PG-SGA, was associated with osteoporosis in the femoral neck (p=0.01). Female patients, compared to males, were more likely to have osteoporosis in the femoral neck (17.7% vs. 9%; p=0.041). Patients who reported no history of alcohol use were found to have osteoporosis in the femoral neck more frequently than patients who reported alcohol use (21% vs. 7.9%, p=0.0061). Moderate alcohol consumption has previously been shown to be positively associated with BMD in a nationally representative population (38). Not surprisingly, patients found to have osteoporosis at the femoral neck were taking anti-resorptive therapy more frequently than those without osteoporosis (30.6% vs. 8.6%, p=0.0002) (Table 3).
Several classic risk factors were not found to be associated with osteoporosis in our patient population. Serum 25-hydroxyvitamin D was not associated, which may be due to use of supplements (n=90, 35%) or multivitamins (n=57; 22%). Serum calcium and serum sodium were not associated, most likely due to the narrow distribution of values which were mostly within the normal range for both variables in our population. Other classic risk factors not associated with osteoporosis included age, hormone levels used to assess hypogonadism and thyroid dysfunction (data not shown), smoking history, current use of SSRIs, and current use of PPIs. Finally, several factors reflecting inflammation and cGVHD involvement were not associated, including complement component 4, albumin, body surface area of cGVHD skin involvement, time since cGVHD diagnosis, and number of prior systemic immunosuppressive therapies for cGVHD (data not shown).
Only body weight showed any correlation with continuous raw T-scores. Body weight showed weak to moderately strong correlation at the femoral neck (r=0.448) and LS (r=0.303) and moderately strong correlation at the total hip (r=0.514).
Multivariable Analysis
Multiple logistic regression modeling produced models predictive of osteoporosis at each anatomical site. Parameters with p≤0.10 in the univariate analyses were considered for inclusion in the multiple logistic regression analyses. Predictors were identified using backward selection and then refined to exclude parameters with p>0.05. The resulting models were then tested using the data that was used to generate the classification rules in order to determine their specificities and sensitivities. The model for the total hip used body weight and platelet count while the model for the femoral neck used these two measures in addition to the PG-SGA nutrition score. The model for the lumbar spine used body weight, current prednisone dose, and number of prior systemic immunosuppressive therapies (Table 4). Linear regression modeling was unable to generate a suitable model for predicting osteoporosis in the lumbar spine and generated only weak models based on body weight alone for the femoral neck (adjusted R2=0.16) and total hip (adjusted R2=0.28).
Table 4. Classification Rules for Osteoporosis at Each Site and Odds Ratios for Jointly Associated Factors.
| Femoral Neck | Lumbar Spine | Total Hip | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Measures | Body Weight (kg) | p=0.0024 | Body Weight (kg) | p=0.0002 | Body Weight (kg) | p<0.0001 |
| OR: 0.971 (95% CI 0.952-0.989) | OR: 0.938 (95% CI 0.908-0.970) | OR: 0.929 (95% CI 0.907-0.952) | ||||
|
| ||||||
| PG-SGA Nutrition | p=0.027 | Current Prednisone Dose (mg/kg/d) | p=0.067 | |||
| OR: 0.467 (95% CI 0.238-0.916) | OR: 2.478 (95% CI 1.081-5.680) | |||||
|
| ||||||
| Platelet Count (K/μL) | p=0.0033 | Number of Prior Systemic Therapies | p=0.0093 | Platelet Count (K/μL) | p=0.002 | |
| OR: 1.005 (95% CI 1.002-1.008) | OR: 0.763 (95% CI 0.596-0.976) | OR: 1.007 (95% CI 1.002-1.011) | ||||
|
| ||||||
| Classification Rule | X=0.0298(body weight) – 0.00453(platelet count) + 0.7619(PG-SGA nutrition) | X=0.0636(body weight) – 0.9076(current pred. dose) + 0.2710(num. prior therapies) | X=0.0733(body weight) – 0.00666(platelet count) | |||
|
| ||||||
| If x≤2.15355, then osteoporosis is present | If x≤5.0543, then osteoporosis is present | If x≤2.54149, then osteoporosis is present | ||||
|
| ||||||
| Specificity | 77.4% | 75% | 73.3% | |||
|
| ||||||
| Sensitivity | 64.5% | 63.4% | 81.5% | |||
Multiple logistic regression modeling was used to generate models predictive for osteoporosis at each anatomical site. Classification rules were tested using the data used to generate the models, yielding specificities and sensitivities for each.
Discussion
The prevalence of osteoporosis or osteopenia in this cGVHD patient population was high (77%) which supports current recommendations for regular monitoring of BMD (24). Osteoporosis was found in 17% of patients with the most frequently affected site being the femoral neck (13%). We also found that, across our entire cohort, T-scores were significantly lower at the femoral neck than in the other two sites (p<0.0001 for both) with 153 (59%) patients with their lowest T-score at this site. This is in contrast to the majority of patients with osteoporosis where the most frequently affected site is the lumbar spine (39). We may speculate that post-allo-HSCT patients have a different mechanism for the development of osteoporosis than the general population.
This study observed a previously not reported association between higher platelet count and osteoporosis after allo-HSCT. Prior studies of platelets in postmenopausal osteoporosis have assessed platelet count and mean platelet volume (MPV), a marker of platelet activation usually inversely related to platelet count, for association with osteoporosis (40). Li et al found that higher MPV was associated with low BMD whereas Akbal et al found that lower MPV and lower platelet distribution width, another marker of platelet activation, were associated with osteoporosis (representing low BMD) (41, 42). Neither of these studies found any association between platelet count and osteoporosis. In our own patient cohort, we were unable to adequately evaluate MPV because we obtained MPV for only 100 patients, amounting to 39% of the study population. It is worth noting that MPV showed no correlation with platelet count (R2=0.04) among these 100 patients.
The role of platelets is not yet fully understood in cGVHD with a number of studies producing contradictory results. Higher platelet count and more active thrombopoiesis have been associated with both cGVHD activity and severity in two studies that analyzed patients drawn from the NIH cGVHD study population analyzed here (43, 44). In contrast, lower platelet count has been shown to be among the most consistent and strongest negative prognostic factors for survival across a number of cGVHD studies. (45-49). We have previously reported an association between higher platelet counts and cGVHD activity and severity in the same cGVHD patient cohort reported here, suggesting higher platelet count could be interpreted as a surrogate for cGVHD activity and severity in our cohort.
The association of higher average NIH organ score, indicating more severe disease, with osteoporosis at the femoral neck is also the first association of a validated assessment of cGVHD activity and severity with osteoporosis. The guidelines used to prospectively grade cGVHD in our patients were first published in 2005 and have since been validated. This finding, coupled with the association of higher platelets with osteoporosis of the femoral neck, suggests that cGVHD-related inflammation or immune dysregulation may be involved in either the development of osteoporosis or the lack of BMD recovery at this site. Given the majority of patients in this cGVHD patient cohort were severely affected (per NIH criteria: 71% severe, 28% moderate), this should be studied in more detail in a population of patients with a broader spectrum of cGVHD severity.
Lower body weight, malnutrition, physical inactivity, higher glucocorticoid dose, and female gender represent classic risk factors which were associated with osteoporosis in at least one site. On the other hand, several classic risk factors were not associated for a number of potential reasons. Age was not associated possibly due to the fact that this was a relatively young population (median age of 48) where age-related effects on BMD may have been introduced prematurely as a result of chemotherapy, radiation, transplant, and steroid use (50-52). Furthermore, we believe that effects of hypogonadism and other endocrine dysfunction were not associated because they may also have been attenuated by the strength of the treatment-related effects. Smoking history, similar to history of alcohol consumption, was not quantified and patients sometimes reported current use while neglecting to mention a history of prior use. Finally, current use of SSRIs and PPIs were assessed using a binary variable (yes/no), and dosages and lengths of exposure were not quantified.
Osteoporosis is a disease in which bone demineralization takes place over an extended period of time and to varying degrees across the bony areas. Given the design of the study, we were unable to track changes in BMD over time or observe which factors may coincide with these changes. We were also unable to quantify cumulative doses and durations of immunosuppressive therapies, prior therapies for osteoporosis, and relevant supplements, such as vitamin D. Nevertheless, we were able to effectively analyze and document BMD in a large and well-annotated population of patients over a broad spectrum of times after cGVHD diagnosis, thus representing the largest study of osteoporosis in this patient population to date (7, 8, 11, 13, 14, 22, 25-27). Many potential risk factors assessed could have been influenced by short-term events, such as infections or cGVHD flares, resulting in skewing of clinical and laboratory assessments. However, we believe that any such events or anomalies did not influence our results due to the large size of the study population.
One limitation of this analysis is that it did not include any non-GVHD controls. While the cGVHD natural history study from which we drew our cohort has enrolled post-allo-HSCT non-GVHD controls, none underwent a DEXA scan and were thus unavailable for analysis. We were also unable to use the WHO Fracture Risk Assessment Tool (FRAX) due to a lack of systematic capture of necessary data, such as bone fracture history. The FRAX would have provided additional information on the clinical impact of osteoporosis or potential risk factors in our study population. Finally, this study was prone to referral center bias. The NIH/NCI natural history of cGVHD study primarily enrolls patients who have failed several lines of therapy and are seeking expert opinion and additional options. As a result, the population analyzed in this study, 71% of whom had severe cGVHD, does not represent patients less severely affected by cGVHD and thus any conclusions from this study should not be applied to this group.
Despite the extensive study of osteoporosis in patients after allogeneic transplant, there are still questions that remain to be addressed. Immunosuppressive treatments for cGVHD have been shown to contribute to the development of osteoporosis (6, 13-17); however, it would also be of interest to determine whether control of cGVHD can prevent osteoporosis or improve recovery in affected sites. In addition, further study is needed to improve our understanding of the differential pathogeneses of osteoporosis at separate sites and how these events and subsequent recoveries are influenced by various events over the course of the post-transplant period. In order to address these questions, future studies of osteoporosis in the setting of allo-HSCT and cGVHD should be longitudinal in design, including baseline and serial assessments of BMD at multiple anatomical sites and any bone fractures. Given the current standard of practice in allo-HSCT, assessments of bone metabolism and health should be made contemporaneously with regular transplant follow-up.
In conclusion, this study found that the prevalence of osteoporosis or osteopenia is high in this population of patients severely affected by cGVHD and warrants continued monitoring as recommended by the NIH Chronic GVHD Consensus Project Ancillary and Supportive Care Guidelines (18). This study also identified several parameters that were strongly associated with osteoporosis, most notably higher platelet count, which may identify a subset of patients that are at higher risk of developing osteoporosis and may benefit from early screening, intervention, and additional research.
Highlights.
Prevalence of osteoporosis and osteopenia in patients with severe cGVHD is high
Higher platelet count, higher average NIH organ score associated with osteoporosis
Femoral neck most frequently and most severely affected among three sites
Findings suggest possible independent role of cGVHD in post-allo HSCT osteoporosis
Acknowledgments
We would like to thank all the patients and their families for their participation in the NIH cGVHD natural history study. This study was supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, and National Cancer Institute. The views expressed in this work do not necessarily represent the views of the National Institutes of Health or the United State Government.
Footnotes
Presented in part as an oral presentation at the 2015 ASBMT/CIBMTR Bone Marrow Transplantation Tandem Meetings
Financial Disclosure Statement: We have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai S, Arora M, Wang T, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015;21:266–274. doi: 10.1016/j.bbmt.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015;125:606–615. doi: 10.1182/blood-2014-08-551994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell SA, Leidy NK, Mooney KH, et al. Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD) Bone Marrow Transplant. 2010;45:762–769. doi: 10.1038/bmt.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebeling PR. Approach to the patient with transplantation-related bone loss. J Clin Endocrinology Metab. 2009;94:1483–1490. doi: 10.1210/jc.2009-0205. [DOI] [PubMed] [Google Scholar]
- 6.Tauchmanova L, Serio B, Del Puente A, et al. Long-lasting bone damage detected by dual-energy x-ray absorptiometry, phalangeal osteosonogrammetry, and in vitro growth of marrow stromal cells after allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2002;87:5058–5065. doi: 10.1210/jc.2002-020800. [DOI] [PubMed] [Google Scholar]
- 7.Stern JM, Sullivan KM, Ott SM, et al. Bone Density Loss After Allogeneic Hematopoietic Stem Cell Transplantation: A Prospective Study. Biol Blood Marrow Transplant. 2001;7:257–264. doi: 10.1053/bbmt.2001.v7.pm11400947. [DOI] [PubMed] [Google Scholar]
- 8.Schulte C, Beelen DW, Schaefer UW, Mann K. Bone Loss in Long-Term Survivors after Transplantation of Hematopoietic Stem Cells: A Prospective Study. Osteoporos Int. 2000;11:344–353. doi: 10.1007/s001980070124. [DOI] [PubMed] [Google Scholar]
- 9.Tauchmanova L, Colao A, Lombardi G, Rotoli B, Selleri C. Bone Loss and Its Management in Long-Term Survivors from Allogeneic Stem Cell Transplantation. J Clin Endocrinol Metab. 2007;92:4536–4545. doi: 10.1210/jc.2006-2870. [DOI] [PubMed] [Google Scholar]
- 10.Yao S, McCarthy PL, Dunford LM, et al. High prevalence of early-onset osteopenia/osteoporosis after allogeneic stem cell transplantation and improvement after bisphosphonate therapy. Bone Marrow Transplant. 2008;41:393–398. doi: 10.1038/sj.bmt.1705918. [DOI] [PubMed] [Google Scholar]
- 11.Valimaki MJ, Kinnunen K, Volin L, et al. A prospective study of bone loss and turnover after allogeneic bone marrow transplantation: effect of calcium supplementation with or without calcitonin. Bone Marrow Transplant. 1999;23:355–361. doi: 10.1038/sj.bmt.1701586. [DOI] [PubMed] [Google Scholar]
- 12.Kananen K, Volin L, Tahtela R, et al. Recovery of bone mass and normalization of bone turnover in long-term survivors of allogeneic bone marrow transplantation. Bone Marrow Transplant. 2002;29:33–39. doi: 10.1038/sj.bmt.1703317. [DOI] [PubMed] [Google Scholar]
- 13.Ebeling PR, Thomas DM, Erbas B, Hopper JL, Szer J, Grigg AP. Mechanisms of Bone Loss Following Allogeneic and Autologous Hematopoietic Stem Cell Transplantation. J Bone Miner Res. 1999;14:342–350. doi: 10.1359/jbmr.1999.14.3.342. [DOI] [PubMed] [Google Scholar]
- 14.Stern JM, Chesnut CH, III, Bruemmer B, et al. Bone density loss during treatment of chronic GVHD. Bone Marrow Transplant. 1996;17:395–400. [PubMed] [Google Scholar]
- 15.Canalis E, Mazziotti G, Guistina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007;18:1319–1328. doi: 10.1007/s00198-007-0394-0. [DOI] [PubMed] [Google Scholar]
- 16.Maalouf NM, Shane E. Osteoporosis after Solid Organ Transplantation. J Clin Endocrinol Metab. 2005;90:2456–2465. doi: 10.1210/jc.2004-1978. [DOI] [PubMed] [Google Scholar]
- 17.Early C, Stuckey L, Tischer S. Osteoporosis in the adult solid organ transplant population: underlying mechanisms and available treatment options. Osteoporos Int. 2015 doi: 10.1007/s00198-015-3367-8. Published online. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi R, Iguchi A, Nakajima M, et al. Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion complication stem cell transplantation. Bone Marrow Transplant. 2004;34:975–979. doi: 10.1038/sj.bmt.1704688. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Choi SJ, Lee JH, et al. Severe metabolic abnormalities after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:63–69. doi: 10.1038/sj.bmt.1704708. [DOI] [PubMed] [Google Scholar]
- 20.Usala RL, Fernandez SJ, Mete M, et al. Hyponatremia is Associated with Increased Osteoporosis and Bone Fractures in a Large US Health System Population. J Clin Endocrinol Metab. 2015;100:3021–3031. doi: 10.1210/jc.2015-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207–227. doi: 10.1111/j.0105-2896.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee WY, Baek KH, Rhee RJ, et al. Impact of circulating bone-resorbing cytokines on the subsequent bone loss following bone marrow transplantation. Bone Marrow Transplant. 2004;34:89–94. doi: 10.1038/sj.bmt.1704535. [DOI] [PubMed] [Google Scholar]
- 23.Pundole XN, Barbo AG, Lin H, Champlin RE, Lu H. Increased incidence of fractures in recipients of hematopoietic stem-cell transplantation. J Clin Oncol. 2015;33:1364–1370. doi: 10.1200/JCO.2014.57.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter PA, Kitko CL, Elad S, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015;21:1167–1187. doi: 10.1016/j.bbmt.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campos DJ, Boguszewski CL, Funke VAM, et al. Bone mineral density, vitamin D, and nutritional status of children submitted to hematopoietic stem cell transplantation. Nutrition. 2014;30:654–659. doi: 10.1016/j.nut.2013.10.014. 2014. [DOI] [PubMed] [Google Scholar]
- 26.Kang MI, Lee WY, Oh KW, et al. The Short Term Changes of Bone Mineral Metabolism Following Bone Marrow Transplantation. Bone. 2000;26:275–279. doi: 10.1016/S8756-3282(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 27.Savani BN, Donohue T, Kozanas E, et al. Increased risk of bone loss without fracture risk in long-term survivors after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:517–520. doi: 10.1016/j.bbmt.2007.01.085. [DOI] [PubMed] [Google Scholar]
- 28.Filipovich AH, Wiesdorf D, Pavletic SZ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Baird K, Steinberg SM, Grkovic L, et al. National Institutes of Health chronic graft-versus-host disease staging in severely affected patients: organ and global scoring correlate with established indicators of disease severity and prognosis. Biol Blood Marrow Transplant. 19:632–639. doi: 10.1016/j.bbmt.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis LM, Grkovic L, Mitchell SA, et al. NIH response criteria measures are associated with important parameters of disease severity in patients with chronic GVHD. Bone Marrow Transplant. 49:1513–1520. doi: 10.1038/bmt.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanis JA, Gluer CC. An Update on the Diagnosis and Assessment of Osteoporosis with Densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11:192–2002. doi: 10.1007/s00198-005-028-1. [DOI] [PubMed] [Google Scholar]
- 32.Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutritional tool in patients with cancer. Eur J Clin Nutr. 2002;56:779–85. doi: 10.1038/sj.ejcn.1601412. [DOI] [PubMed] [Google Scholar]
- 33.Wu Q, Bencaz AF, Hentz JG, Crowell MD. Selective serotonin reuptake inhibitor treatment and risk of fractures: a meta-analysis of cohort and case-control studies. Osteoporos Int. 2012;23:365–375. doi: 10.1007/s00198-011-1778-8. [DOI] [PubMed] [Google Scholar]
- 34.Van der Hoorn MM, Tett SE, de Vries OJ, Dobson AJ, Peeters GM. The effect of dose and type of proton pump inhibitor use on risk of fractures and osteoporosis treatment in older Australian women: A prospective cohort study. Bone. 2015;81:675–682. doi: 10.1016/j.bone.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Mehta CR, Patel NR. A Network Algorithm for Performing Fisher's Exact Test in r × c Contingency Tables. J Am Stat Assoc. 1983;78:427–434. doi: 10.1080/01621459.1983.10477989. [DOI] [Google Scholar]
- 36.Agresti A. Categorical Data Analysis. New York, NY: John Wiley and Sons, Inc.; 1990. pp. 79–129. [Google Scholar]
- 37.Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York, NY: John Wiley and Sons, Inc.; 1999. pp. 189–269. [Google Scholar]
- 38.Wosje KS, Kalkwarf HJ. Bone density in relation to alcohol intake among men and women in the United States. Osteoporos Int. 2007;18:391–400. doi: 10.1007/s00198-006-0249-0. [DOI] [PubMed] [Google Scholar]
- 39.Faulkner KG, von Stetten E, Miller P. Discordance in Patient Classification Using T-Scores. Journal of Clinical Densitometry. 1999;2:343–350. doi: 10.1385/JCD:2:3:343. [DOI] [PubMed] [Google Scholar]
- 40.Thompson CB, Jakubowski JA. The Pathophysiology and Clinical Relevance of Platelet Heterogeneity. Blood. 1988;72:1–8. [PubMed] [Google Scholar]
- 41.Li XS, Zhang JR, Meng SY, Li Y, Wang RT. Mean platelet volume is negatively associated with bone mineral density in postmenopausal women. J Bone Miner Metab. 2012;30:660–665. doi: 10.1007/s00774-012-0362-4. [DOI] [PubMed] [Google Scholar]
- 42.Akbal A, Gokmen F, Gencer M, Inceer BS, Komurcu E. Mean platelet volume and platelet distribution width can be related to bone mineralization. Osteoporos Int. 2014;25:2291–2295. doi: 10.1007/s00198-014-2764-8. [DOI] [PubMed] [Google Scholar]
- 43.Grkovic L, Baird K, Steinberg SM, et al. Clinical laboratory markers of inflammation as determinants of chronic graft-versus-host disease activity and NIH global severity. Leukemia. 2012;26:633–643. doi: 10.1038/leu.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bat T, Steinberg SM, Childs R, et al. Active thrombopoiesis is associated with worse severity and activity of chronic GVHD. Bone Marrow Transplant. 2013;48:1569–1573. doi: 10.1038/bmt.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anasetti C, Rybka W, Sullivan KM, Banaji M, Slichter SJ. Graft-v-Host Disease is Associated With Autoimmune-like Thrombocytopenia. Blood. 1989;73:1054–1058. [PubMed] [Google Scholar]
- 46.Akpek G, Zahurak ML, Piantadosi S, et al. Development of a prognostic model for grading chronic graft-versus-host disease. Blood. 2001;97:1219–1226. doi: 10.118/blood.V97.5.1219. [DOI] [PubMed] [Google Scholar]
- 47.Pavletic SZ, Smith LM, Bishop MR, et al. Prognostic factors of chronic graft-versus-host disease after allogeneic blood stem-cell transplantation. Am J Hematol. 2005;78:265–74. doi: 10.1002/ajh.20275. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsohn DA, Arora M, Klein JP, et al. Risk factors associated with increased nonrelapse mortality and with poor overall survival in children with chronic graft-versus-host disease. Blood. 2011;118:4472–4479. doi: 10.1182/blood-2011-04-349068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pulanic D, Lozier JN, Pavletic SZ. Thrombocytopenia and hemostatic disorders in chronic graft versus host disease. Bone Marrow Transplant. 2009;44:393–403. doi: 10.1038/bmt.2009.196. [DOI] [PubMed] [Google Scholar]
- 50.Assouline E, Crocchiolo R, Prebet T, et al. Impact of reduced-intensity conditioning allogeneic stem cell transplantation on women's fertility. Clin Lymphoma Myeloma Leuk. 2013;13:704–710. doi: 10.1016/j.clml.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Naessen S, Bergstrom I, Ljungman P, Landgren B. Long-term follow-up of bone density, general and reproductive health in female survivors after treatment for haematological malignancies. Eur J Haematol. 2014;93:137–142. doi: 10.1111/ejh.12317. [DOI] [PubMed] [Google Scholar]
- 52.Trudgen K, Ayensu-Coker L. Fertility preservation and reproductive health in the pediatric, adolescent, and young adult female cancer patient. Curr Opin Obstet Gynecol. 2014;26:372–380. doi: 10.1097/GCO.0000000000000107. doi:10.1097.GCO.0000000000000107. [DOI] [PubMed] [Google Scholar]


