Abstract
Objective
Sepsis poses a serious global health problem with an overall mortality rate of 30%, in which the vascular Injury is a major contributor of. The study is to determine the expression profile of microRNAs in endotoxic vascular walls and their potential roles in sepsis-related vascular injury.
Design
Prospective randomized study
Setting
Laboratory investigation
Subjects
Male C57BL/6 mice, average weight 26.5 ±1.8g.
Interventions
Endotoxemia was induced in mice via lipopolysaccharide (LPS) injection (20 mg/kg, ip) (Sigma) (25-27). The control mice were injected with the same amount of saline (500 μl, ip). In a sub-group of mice, a high dose of LPS (30mg/kg, ip) was applied to induce endotoxin-related death.
Measurements and Main Results
The miRNA expression profiles in aortas from lipopolysaccharide (LPS)-induced endotoxic mice were determined. The result demonstrated that some microRNAs were aberrantly expressed in endotoxic mouse arteries. Among them, the endothelial cell enriched/specific miR-126a-3p a-3p was significantly down-regulated in endotoxic mouse arteries, septic human vessels, as well as vascular endothelial cells isolated from endotoxic mice or treated with LPS. The down-regulation of miR-126a-3p occurred at transcriptional level via the decreased expression of krüppel-like factor 2 (KLF2), which could be inhibited by KLF2 over-expression via Ad-KLF2. The down-regulation of miR-126a-3p in endothelial cells resulted in the increased apoptosis, and decreased proliferation and migration, which were inhibited by miR-126a-3p mimics. In vivo, over-expression of miR-126a-3p via lentivirus (LV-miR-126a-3p) attenuated endotoxemia-induced injuries on endothelial function and vascular permeability. We found that SPRED1 and VCAM-1 were two direct target genes of miR-126a-3p related to miR-126a-3p-mediated effects in endotoxemia. Finally, the survival rate of endotoxic mice was significantly increased by the over-expression of miR-126a-3p.
Conclusions
The results suggest that vascular microRNAs such as miR-126a-3p may represent novel mechanisms and new therapeutic targets for endotoxemia-induced vascular injury and endotoxic mortality.
Keywords: MicroRNAs, miR-126, sepsis, endothelial dysfunction, vascular permeability, endothelial cells
INTRODUCTION
Sepsis poses a serious public health problem in the United States and globally with an overall mortality rate of 30% (1-3). A major contributor to sepsis mortality is the breakdown in the function of the blood/tissue barrier due to intravascular or extra-vascular infections (1-4). It is well known that the infections induce inflammatory events in blood and vascular systems that include innate and adaptive immune cells, red and white blood cells, platelets, and plasma proteins such as antibody, complement, coagulation and fibrinolysis networks. Finally, these microbial virulence factors and pro-inflammatory mediators during sepsis could result in severe endothelial dysfunction and injury which lead to systemic vascular leakage and irreversible multi-organ failure. To date, the molecular mechanisms responsible for sepsis-induced injures on vascular integrity are still unclear.
Until recently, recombinant human activated protein C, a coagulation protein, was the only FDA-approved adjunctive therapy for treating sepsis-related vascular injuries. However, in October 2011, it was withdrawn from the market after failure to demonstrate the improved patient survival in clinical applications (5). This has highlighted the importance and urgency of studying the novel mechanisms and novel therapeutic targets of sepsis-induced vascular damages.
MicroRNAs (miRNAs), with tremendous biological functions, are a class of endogenous, small, noncoding RNAs that directly regulate over 30% of genes in a cell (6-9). We are one of the first groups to explore the biological roles of miRNAs in vascular cell biology and vascular disease (10). The studies from us (10-14) and other groups (please see our recent review article, 15) have demonstrated that miRNAs play critical roles in vascular integrity, vascular inflammation and vascular disease. miRNAs in sepsis is a novel research field in the past 5 years (16-25). In this regard, the levels of many miRNAs in circulating blood and several organ tissues are changed in animals and patients with sepsis (16-25). miRNAs could be novel diagnostic biomarkers and new therapeutic targets for sepsis (16). However, to date, although the blood microRNAs are well studied in sepsis, the expression profiles of miRNAs in vascular walls in septic animals and septic patients, and their potential roles in sepsis-induced vascular injuries are still unknown.
To determine the potential roles of vascular miRNAs in sepsis-related vessel injuries and the potential mechanisms involved, we here use the miRNA microarray to determine the expression profile of miRNAs in the mouse aortas in a model of endotoxemia induced by lipopolysaccharide (LPS)-injection, and show that multiple miRNAs are aberrantly expressed in rat aortas after LPS injection. Among them, miR-126a-3p, an endothelial cell enriched/specific miRNA, is significantly downregulated. miR-126 is a miRNA family, which includes miR-126a-3p, miR-126a-5p, miR-126b-3p and miR-126a-5p. However, in normal vascular walls and vascular endothelial cells, only miR-126a-3p is expressed. The aberrant expression of miR-126a-3p is also demonstrated in human septic vessels. We further demonstrated that miR-126a-3p plays an important role in endotoxemia-induced injuries on vascular integrity via its target genes.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (8–10 weeks, 25–28 g) were used for this study. The animal protocol was approved by the Institutional Animal Care and Use Committee and was consistent with the Guide for the Care and Use of Laboratory Animals (NIH publication 85–23, revised 1985).
Endotoxemia model and endotoxin-induced death of mice
Endotoxemia was induced in mice via LPS injection (20 mg/kg, ip) (Sigma) (26-28). The control mice were injected with the same amount of saline (500 μl, ip). In a sub-group of mice, a high dose of LPS (30mg/kg, ip) was applied to induce endotoxin-related death. The survival rate was monitored up to 7 days after LPS injection.
miRNA microarray in mouse aortas
In this experiment, the endotoxemia was induced in 8 adult male C57BL/6 mice via LPS injection (20 mg/kg, ip). The control mice were injected with the same amount of saline (500 μl, ip). At 6 hours after LPS or vehicle injection, the aortas from mice were isolated for miRNA microarray analysis using the mouse miRNA array probes covering all the miRNAs in the latest version of the miRBase database as described previously (10).
Human skin biopsy samples and skin vessel isolation
Under a microscope, human vessels were isolated from skin biopsy samples of septic patients (n=5), and from non-septic control subjects (n=5). All data were de-identified before being provided to the investigators. This study was approved by the research ethics committee, complied with the declaration of Helsinki, and the informed consent was provided by all subjects.
Endothelial cell isolation and culture
Endothelial cells (ECs) were isolated from mouse aortas (macro-vascular ECs) and mouse lung micro-vessels (micro-vascular ECs) under sterile conditions by established techniques (29, 30). All the ECs were verified by the expression of endothelium-specific markers such as Ve-cadherin and CD31 and cultured with Cell Biologics’ complete growth medium for ECs.
Modulation of miR-126a-3p in cultured ECs
To determine the potential cellular effects of miR-126a-3p on ECs, gain-of-function and loss-of-function approaches were applied. The expression of miR-126a-3p was downregulated by its inhibitor, antagomiR-126a-3p (Integrated DNA Technologies, 30 nM), and was upregulated via its mimics (miR-126a-3p mimics) (Integrated DNA Technologies, 30 nM) as described in our previous studies (10-12). Vehicle and oligonucleotide control (scrambled control) (Integrated DNA Technologies) were used as controls.
Cell proliferation, migration and apoptosis
EC proliferation was determined by 3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide; thiazolyl blue (MTT) assay and bromodeoxyuridine (BrdU) incorporation assay (11). Endothelial cell migration was determined by a modified Boyden chamber assay as described previously (11). EC apoptosis in cultured cells was induced by treatment with H2O2 (300 μM) for 24 hours. Apoptosis was measured by Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis as described (11). Apoptotic cells were quantified by counting the percentage of TUNEL-positive cells against total nucleated cells stained by DAPI
Generation of recombinant lentivirus expressing miR-126a-3p and adenovirus expressing Krüppel-like Factor 2 (KLF2)
miR-126a-3p was constructed into the lentivirus expression vector using a lentivirus expressing system (Invitrogen corporation) (LV-miR-126a-3p ). Briefly, the oligonucleotides for miR-126a-3p were synthesized at Integrated DNA Technologies, annealed and ligated into pcDNATM6.2-GW/ EmGFP-miR. The viral particles were produced by third-generation packaging in 293FT cells and were concentrated using ultracentrifugation.Adenovirus expressing KLF2 (Ad-KLF2) and its control virus (Ad-GFP) were generated using the Adeno-X™ Expression Systems 2 kit (Clontech, CA) according to the manufacturer's protocols. The resulting adenoviruses were further amplified by infection of HEK293A cells and purified by cesium chloride gradient ultracentrifugation. The Ad-KLF2 and control Ad-GFP were titrated using a standard plaque assay.
Overexpression of miR-126a-3p and KLF2 in mice in vivo
To overexpress miR-126a-3p in mouse aorta, 100 μl of LV-miR-126a-3p (2×108 PFU) was injected into mice via external jugular vein. LV-Ctl (2×108 PFU) or vehicle (100 μl saline) was used as virus control and vehicle control. Seven days later, the overexpression of miR-126a-3p in mouse aortas was verified by real-time reverse transcription-PCR (qRT-PCR). To overexpress KLF2 in mouse aorta, 100 μl of Ad-KLF2 (2×108 PFU) was injected into mice via external jugular vein. Ad-GFP (2×108 PFU) was used as control.
Endothelial function assessment in isolated mouse aortas
Isometric tension was measured in isolated mouse aortic ring segments as described (31). The vessels were cut into individual ring segments (2–3 mm in width) and suspended from a force-displacement transducer in a tissue bath. Ring segments were bathed in Krebs-Henseleit (K-H) solution. The vessels were contracted to 50–60% of their maximal capacity (50–60% of KCl response) with phenylephrine (3×10–8-10–7 M). When tension development reached a plateau, ACh (10–9-3×10–6 M) was added cumulatively to the bath to stimulate endothelium-dependent relaxation.
Vascular permeability and vascular leakage
Vascular permeability and vascular leakage in vivo were determined based on the amounts of Evans blue extravasation into the peritoneal cavity (32, 33) and Evans blue leakage in lung (34). For the Evans blue extravasation into the peritoneal cavity, at 6 hours after LPS injection (20 mg/kg, ip) or vehicle (saline) injection, Evans blue dye (20 mg/kg) in saline was injected intravenously into each mouse immediately followed by an intraperitoneal injection of 0.7% acetic acid. Thirty minutes later, the mice were sacrificed and the peritoneal exudates were collected Evans blue measurement. For the Evans blue leakage in lung, at 6 hours after LPS injection (20 mg/kg, ip) or vehicle (saline) injection, Evans blue dye solution (20 mg/kg) in saline was injected intravenously into each mouse and was allowed to circulate for 1 hour. Then, mice were deeply anesthetized and perfused with saline plus 5 mM EDTA. Then, lungs were excised for Evans blue measurement.
RNA isolation and qRT-PCR
RNAs levels were isolated and determined by qRT-PCR as described previously (11). As an internal control, U6 was used for miRNA template normalization and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for other genes.
Western blot analysis
Proteins isolated from endothelial cells and vessels were determined by Western blot analysis. Equal amounts of protein were subjected to SDS-PAGE. A standard Western blot analysis was conducted using SPRED1 (Sprouty-related, EVH1 domain-containing protein 1) rabbit polyclonal antibody (1:100 dilution; Abcam), KLF2 rabbit polyclonal antibody (1:1000 dilution; Abcam) antibody and VCAM-1 (vascular cell adhesion molecule 1) rabbit polyclonal antibody (1:1000 dilution; Abcam). GAPDH antibody (1:5000 dilution; Cell Signalling) was used as a loading control.
Luciferase assay
The reporter plasmid, a firefly luciferase reporter construct psiCHECK-2 (Promega, WI) was inserted with a fragment of the 3′-UTR of mouse SPRED1 mRNA or VCAM-1 mRNA containing the putative miR-126a-3p binding sequence. The construct with mutated fragment of the 3′-UTR of SPRED1 mRNA or VCAM-1 mRNA without the putative miR-126a-3p binding sequences was used as the mutated control. HEK 293 cells were transfected with the construct or the mutated control construct. Then, these HEK 293 cells were treated with vehicle, pDNR-CMV (an empty plasmid, 0.2 μg/ml), or pmiR-126a-3p (a plasmid expressing miR-126a-3p , 0.2 μg/ml). Cell extract was isolated to measure the luciferase expression on a scintillation counter by using a dual luciferase reporter system.
Statistical analysis
All data are expressed as mean ± SEM (standard error of the mean). All the experiments were repeated independently at least three times. For relative gene expression, the mean value of control group is defined as 100%. SPSS was used to perform the statistical analysis. ANOVA repeated measures were used to assess changes within a group, and one-way ANOVA within groups were used to assess the significance of any change between groups. Comparisons between two groups were performed using the independent samples t-test. Statistical significance was accepted at P < 0.05.
RESULTS
Expression profile of miRNAs in arteries from endotoxic mice induced by LPS injection
Compared with these in control vessels, we found that multiple miRNAs were aberrantly expressed in endotoxic mouse aortas. Table 1 listed these miRNAs that are abundant and aberrantly expressed in endotoxic mouse arteries. Interestingly, the endothelial cell-specific/enriched miR-126a-3p was significantly down-regulated by about 50% in endotoxic aortas.
Table 1.
Different expression profiles of miRNAs in aortas from septic mice and normal control mice
| MicroRNA | P-value | Control | LPS | Log2 (G2/G1) | ||
|---|---|---|---|---|---|---|
| Mean signal | SD | Mean signal | SD | |||
| mmu-miR-378d | 3.47E-03 | 2,778 | 15 | 322 | 5 | −3.11 |
| mmu-miR-378c | 2.03E-02 | 5,488 | 231 | 1,140 | 65 | −2.27 |
| mmu-miR-378b | 1.86E-02 | 5,375 | 219 | 1,134 | 56 | −2.24 |
| mmu-miR-378a | 1.10E-02 | 6,733 | 150 | 2,050 | 38 | −1.72 |
| mmu-miR-26b-5p | 3.92E-02 | 3,812 | 345 | 1,318 | 23 | −1.53 |
| mmu-let-7j | 5.18E-02 | 1,501 | 120 | 703 | 24 | −1.09 |
| mmu-miR-126a-3p | 6.52E-02 | 6,868 | 41 | 3,568 | 80 | −0.94 |
| mmu-miR-16-5p | 6.85E-02 | 6,106 | 186 | 3,946 | 234 | −0.63 |
| mmu-miR-466i-5p | 5.24E-03 | 1,903 | 7 | 1,403 | 11 | −0.44 |
| mmu-miR-151-5p | 7.88E-02 | 1,151 | 20 | 981 | 22 | −0.23 |
| mmu-miR-2137 | 1.68E-02 | 2,054 | 27 | 2,953 | 11 | 0.52 |
| mmu-miR-214-3p | 3.23E-02 | 4,732 | 23 | 7,014 | 195 | 0.57 |
| mmu-miR-31-5p | 1.82E-02 | 1,035 | 4 | 1,574 | 26 | 0.61 |
| mmu-miR-133a-3p | 2.49E-02 | 1,419 | 33 | 2,224 | 19 | 0.65 |
| mmu-let-7i-5p | 9.00E-02 | 4,355 | 444 | 7,494 | 301 | 0.78 |
Verify the down-regulation of miR-126a-3p in endotoxic mouse arteries and in vascular ECs isolated from endotoxic aortas and lungs by qRT-PCR
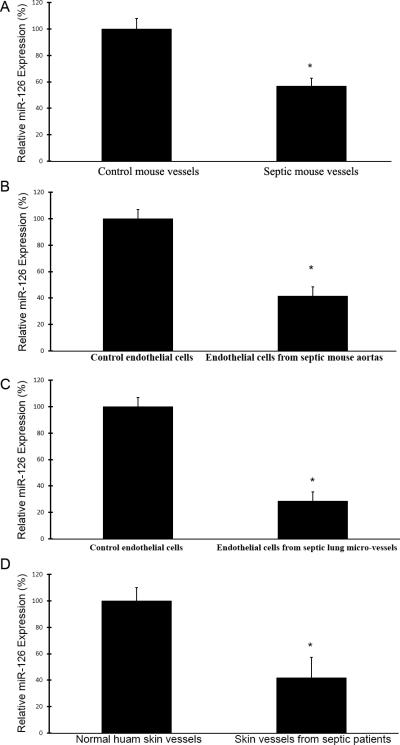
In this experiment, the expression of miR-126a-3p in mouse arteries and ECs isolated from mouse aortas and lungs was determined by qRT-PCR. Compared with that from control mice, the expression of miR-126a-3p in endotoxic mouse arteries was significantly down-regulated, which was consistent with the result determined by miRNA microarray analysis (Fig. 1A). Moreover, the miR-126a-3p expression in ECs isolated from these endotoxic mouse aortas and lungs was also strongly down-regulated compared with that in cells from normal control mice (Fig. 1B and 1C).
Fig.1. Down-regulation of miR-126a-3p in endotoxic mouse arteries, in vascular ECs isolated from endotoxic aortas and lungs, and in septic human vessels.
A. miR-126a-3p levels in endotoxic mouse aortas at 6 hours after LPS injection (20 mg/kg, ip) (n=8) and in control aortas isolated from vehicle-treated mice (n=8) (500 μl, ip) determined by qRT-PCR. Note: *p<0.05 compared with that in control aortas. B. miR-126a-3p levels in vascular ECs isolated from endotoxic mouse aortas (macro-vascular ECs) (n=6) and from normal control mouse aortas (n=6) determined by qRT-PCR. Note: *p<0.05 compared with that in ECs from control aortas. C. miR-126a-3p levels in vascular ECs isolated from endotoxic lungs (micro-vascular ECs) (n=6) and from normal control mouse lungs (n=6) determined by qRT-PCR. Note: *p<0.05 compared with that in ECs from control lungs. D. miR-126a-3p levels in vessels of skin biopsy samples from patients with sepsis (n=5) and from control subjects (n=5) determined by qRT-PCR. Note: *p<0.05 compared with that in vessels from control subjects.
The expression change of miR-126a-3p in human vessels from patients with sepsis
MiRNAs were isolated from these vessels and miR-126a-3p levels were determined by qRT-PCR. We found that the expression of miR-126a-3p was significantly decreased in skin vessels from patients with sepsis (Fig. 1D).
miR-126a-3p has strong effects on the proliferation, migration, and apoptosis of cultured ECs
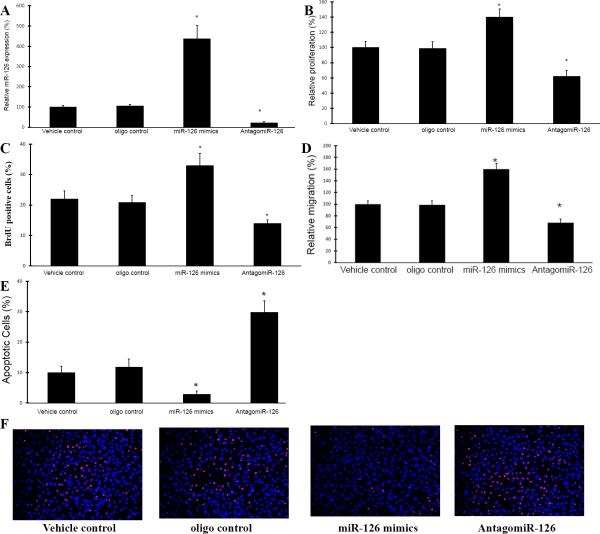
In this experiment, mouse ECs at passage 3 to 5 were used. The miR-126a-3p levels in these cells were modulated by miR-126a-3p mimics (30 nM) and AntagomiR-126a-3p (30 nM) as demonstrated in Fig. 2A. Vehicle control and oligo control were applied in this experiment. As shown in Fig. 2B and 2D, cell proliferation and migration was increased by miR-126a-3p overexpression, but was inhibited by miR-126a-3p knockdown. In contrast, cell apoptosis was inhibited by miR-126a-3p overexpression, but was increased by miR-126a-3p knockdown (Fig. 2E and 2F).
Fig. 2. The effects of miR-126a-3p on the proliferation, migration, and apoptosis of cultured mouse aortic ECs.
A. Modulation of miR-126a-3p expression in ECs by miR-126a-3p mimics (miR-126 mimics) (30 nM) and AntagomiR-126a-3p (AntagomiR-126a) (30 nM). Note: n=6; *p<0.05 compared with that in oligo control-treated cells. B. Cell proliferation determined by MTT assay. Note: n=6; *p<0.05 compared with that in oligo control-treated cells. C. Cell proliferation determined by BrdU assay. Note: n=6; *p<0.05 compared with that in oligo control-treated cells. D. Cell migration was determined by a modified Boyden chamber assay. Note: n=6; *p<0.05 compared with that in oligo control-treated cells. E. Cell apoptosis determined by TUNEL analysis. Note: n=6; *p<0.05 compared with that in oligo control-treated cells. F. Representative cell images of TUNEL showing the apoptotic ECs with different treatments.
Overexpression of miR-126a-3p attenuates LPS-induced cellular effects on ECs
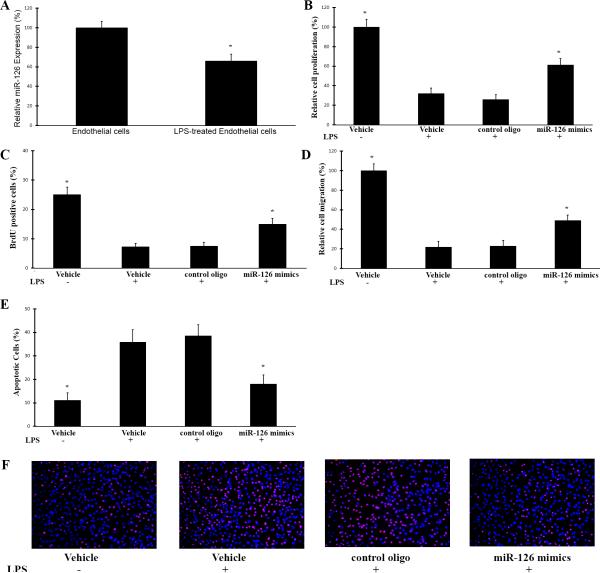
As shown in Fig. 3A, compared with in vehicle-treated ECs, the expression of miR-126a-3p in LPS-treatedECs (100 ng/ml for 6 hours) was significantly decreased. At 24 hours after LPS-treatment, cell proliferation, migration, and apoptosis were determined. As shown Fig. 3B-3F, LPS damaged the functions of ECs as shown by the decreased proliferation and migration, and increased apoptosis. Interestingly, the impaired EC functions by LPS were partially inhibited by overexpression of miR-126a-3p via its mimics (30 nM) (Fig. 3B-3F).
Fig. 3. The effects of miR-126a-3p overexpression on proliferation, migration, and apoptosis of LPS-treated ECs.
In this experiment, the mouse ECs were pre-treated with vehicle, control oligo (30 nM) or miR-126a-3p mimics (miR-126 mimics) (30 nM). Twenty-four hours later, the cells were treated with LPS (LPS (1 μg/ml) for 24h. Then, proliferation, migration, and apoptosis of these ECs were determined. One group of vehicle-treated cells without LPS-treatment was used as normal control cells. The levels of miR-126a-3p were determined by qRT-PCR. A. LPS decreased the expression of miR-126a-3p in ECs. Note: n=6; *p<0.05 compared with that in control oligo-treated cells. B. Improved cell proliferation by miR-126a-3p overexpression determined by MTT assay. Note: n=6; *p<0.05 compared with that in control oligo-treated cells. C. Improved cell proliferation by miR-126a-3p overexpression determined by BrdU assay. Note: n=6; *p<0.05 compared with that in control oligo-treated cells. D. Improved cell migration by miR-126a-3p overexpression determined by Boyden chamber assay. Note: n=6; *p<0.05 compared with that in control oligo-treated cells. E. Decreased cell apoptosis by miR-126a-3p overexpression determined by TUNEL assay. Note: n=6; *p<0.05 compared with that in control oligo-treated cells. F. Representative cell images of TUNEL showing the apoptotic ECs with different treatments.
Endothelial function and vascular permeability are impaired in LPS-induced endotoxic mice, which are partially inhibited by miR-126a-3p overexpression
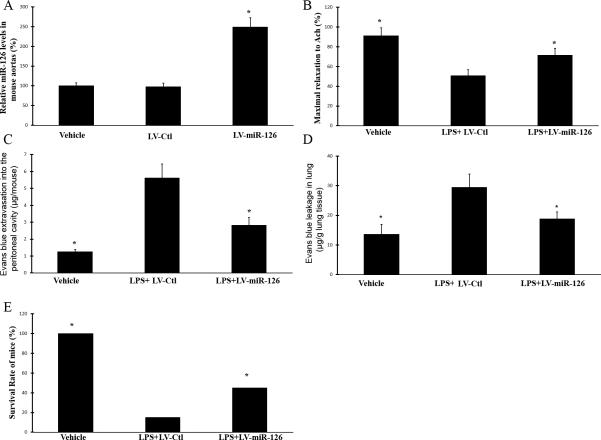
As shown in Fig. 4A, compared with that in aortas from vehicle or LV-Ctl injected mice, the expression of miR-126a-3p in aortas from LV-miR-126a-3p injected was increased by about 2.5 folds. We found that LPS injection impaired endothelial function (endothelium-dependent relaxation) (Fig. 4B). Moreover, LPS injection damaged the vascular barrier as shown by the increased Evans blue extravasation into the peritoneal cavity (Fig. 4C) and Evans blue leakage in lung (Fig. 4D). Clearly, endotoxemia could significantly damage the vascular integrity via the impaired endothelial function and the increased vascular permeability. Interestingly, endotoxemia-induced injuries on vascular integrity were partially inhibited by miR-126a-3p overexpression (Fig. 4B-4D).
Fig. 4. The effects of miR-126a-3p over-expression on LPS-induced injuries on vascular endothelial function and vascular permeability, and on LPS-induced mortality.
Mice were treated with vehicle (100 μl of saline), lenti-virus vector control (LV-Ctl, 2 × 108 PFU) or LV-miR-126a-3p (LV-miR-126a) (2 × 108 PFU) via external jugular vein. Seven days later, miR-126a-3p expression in aortas was determined. Then, the animals will be treated with vehicle (500 μl of saline, ip) or LPS (20 mg/kg, ip). Six hours later, the vascular permeability in vivo and endothelial function in isolated aortas was determined. A. miR-126a-3p expression in mouse aortas was increased by LV-miR-126a-3p. Note: n=6; *p<0.05 compared with that in aortas from LV-Ctl-treated mice. B. miR-126a-3p overexpression inhibited LPS-induced endothelial dysfunction in endotoxic mice. Note: n=8; *p<0.05 compared with that in LV-Ctl-treated mice. C. miR-126a-3p overexpression inhibited LPS-induced abnormal Evans blue extravasation into the peritoneal cavity in mice. Note: n=8; *p<0.05 compared with that in LV-Ctl-treated mice. C. miR-126a-3p overexpression inhibited LPS-induced abnormal Evans blue leakage in lung in mice. Note: n=6; *p<0.05 compared with that in LV-Ctl-treated mice. For LPS-induced mortality, C57BL/6 mice were pre-treated with vehicle, LV-Ctl or LV-miR-126a-3p. Seven days later, the animals were treated with high dose of LPS (30mg/kg, ip) to induce animal death. The survival rate of these mice was recorded up to 7 days after LPS-injection. Note: n=20; *p<0.05 compared with that in LV-Ctl-treated mice.
Overexpression of miR-126a-3p via LV-miR-126a-3p reduces the endotoxin-induced mortality in mice
To test the role of miR-126a-3p in LPS-induced mortality, C57BL/6 mice were pre-treated with vehicle, LV-Ctl or LV-miR-126a-3p. Seven days later, the animals were treated with high dose of LPS (30mg/kg, ip). Each group had 20 mice. The survival of these mice was recorded up to 7 days after LPS-injection. As shown in Fig. 4E, miR-126a-3p overexpression significantly reduces the LPS-induced mortality in mice as shown by the increased survival rate.
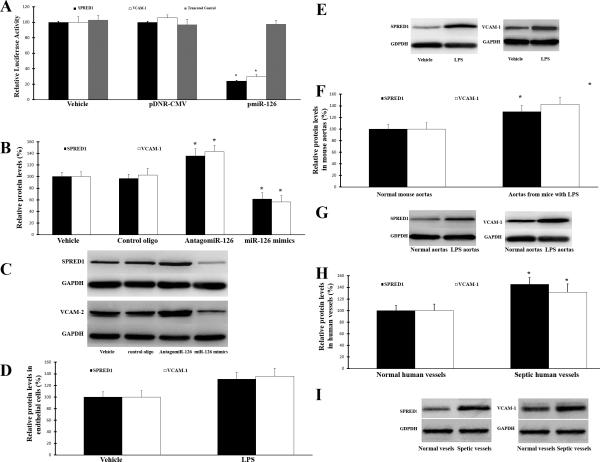
SPRED1 and VCAM-1 are two direct target genes of miR-126a-3p that are involved in LPS-induced injures on ECs and vascular walls
Computational analysis via online software such as TargetScan 5.1 predicts that SPRED1 and VCAM-1 have miR-126a-3p binding sites in their 3’-untranslated region (3’-UTR). Thus, there are potential direct target genes of miR-126a-3p. Bioinformatics analysis also suggests that these genes are related to cell proliferation, apoptosis, migration, senescence and inflammation (oxidative stress). To identify that miR-126a-3p is able to directly bind to SPRED1 and VCAM-1 and inhibit their expression, a firefly luciferase reporter construct containing a fragment of the 3’-UTR of SPRED1 or VCAM-1 mRNA with the putative miR-126a-3p binding sequence or its truncated control was transfected into HEK 293 cells. As shown in Fig. 5A, pmiR-miR-126a-3p, but not pDNR-CMV, inhibited luciferase activity. The results suggest that miR-126a-3p could directly bind to and inhibit the expression of SPRED1 and VCAM-1. In cultured ECs, the expression of SPRED1 and VCAM-1 proteins were increased by miR-126a-3p inhibition. In contrast, their expression was downregulated by miR-126a-3p overexpression (Fig. 5B and 3C). Moreover, the expression of SPRED1 and VCAM-1 proteins was significantly increased in LPA-treated ECs in vitro (Fig. 5D and 5E), and in aortas from LPS-injected mice (Fig. 5F and 5G) and in vessels from patients with sepsis (Fig. 5H and 5I) in vivo.
Fig. 5. SPRED1 and VCAM-1 are two direct target genes of miR-126a-3p.
A. a luciferase reporter construct, containing the putative miR-126a-3p binding sequence from 3′-UTR of the SPRED1 or VCAM-1 mRNA was transfected into HEK293 with vehicle, pDNR-CMV, pmiR-126a-3p . The constructs without the miR-126a-3p binding sequence were used as the truncated controls. pmiR-miR-126a-3p inhibited luciferase activity. In the truncated control group, the inhibitory effect of pmiR-126a-3p disappeared. Note: n=6; *p < 0.05 compared with pDNR-CMV group. B. The effects of miR-126a-3p inhibition or miR-126a-3p overexpression on the protein levels of SPRED1 and VCAM-1 in cultured ECs. Note: n=6; *p < 0.05 compared with oligo control group. C. Representative western blots of SPRED1 and VCAM-1 in ECs with different treatments. D. Increased protein levels of SPRED1 and VCAM-1 in LPS-treated ECs. Note: n=6; *p < 0.05 compared with control group. E. Representative western blots of SPRED1 and VCAM-1 of D. F. Increased protein levels of SPRED1 and VCAM-1 in aortas of LPS-treated mice.Note: n=5; *p < 0.05 compared with control group. G. Representative western blots of SPRED1 and VCAM-1 of F. H. Increased protein levels of SPRED1 and VCAM-1 in skin vessels of patients with sepsis Note: n=5; *p < 0.05 compared with control group. I. Representative western blots of SPRED1 and VCAM-1 of H.
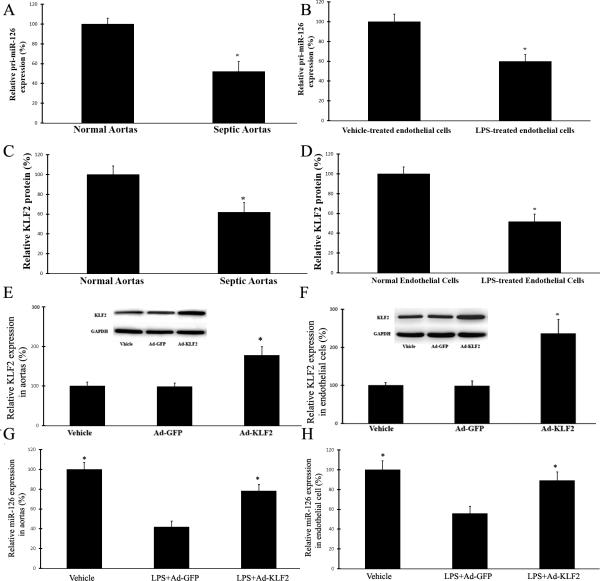
KLF2 is a key upstream transcription factor involved in the down-regulation of miR-126a-3p in vascular ECs and arteries in endotoxemia
miRNAs are subjected to transcriptional and posttranscriptional expressional regulation. To test which regulatory mechanism is responsible for the down-regulation of miR-126a-3p in endotoxemia, we first determined the levels of pri-miR-126a-3p in aortas from LPS-injected mice and in endothelial cells treated with LPS was determined by qRT-PCR. As shown in Fig. 6A and 6B, the expression of pri-miR-126a-3p is remarkably decreased in endotoxic vessels and in endothelial cells treated with LPS (100 ng/ml), which is consistent with the expression changes of mature miR-126a-3p in these cells and vessels. The results suggest that the down-regulation of mature miR-126a-3p in response to sepsis is mainly transcriptionally regulated. We next performed the experiments to uncover which transcription factor was involved in the transcriptional regulation of miR-126a-3p in sepsis. Putative regulatory elements flanking the pri-miR-126a-3p, by integrating publicly available Encyclopedia of non-coding element datasets (ENCODE), was analyzed. By the results, we identified that the promoter of miR-126a-3p has binding sites for transcription factor KLF2. We then determined the levels of KLF2 in aortas from LPS-injected mice and in endothelial cells treated with LPS. As shown in Fig. 6C and 6D, its expression was remarkably decreased in endotoxic vessels and in endothelial cells treated with LPS (100 ng/ml). To provide a direct link between KLF2 and miR-126a-3p expression, we created Adenovirus expressing KLF2. As shown, Fig. 6E and 6F, the expression of KLF2 in mouse aortas and in ECs was significantly increased by Ad-KLF2 in mice. Interesting, LPS-induced down-regulation of miR-126a-3p in mouse aortas and ECs could be efficiently inhibited by KLF2 over-expression via Ad-KLF2 (Fig. 6G and 6H).
Fig. 6. KLF2 is a key upstream transcription factor involved in the down-regulation of miR-126a-3p in vascular ECs and arteries in endotoxemia.
A. Expression of pri-miR-126a-3p in aortas from LPS-injected mice is decreased. n=6; *p<0.05 vs control group. B. Expression of pri-miR-126a-3p in LPS-treated endothelial cells is decreased. n=6; *p<0.05 vs control group. C. Expression of KLF2 protein in aortas from LPS-injected mice is decreased. n=6; *p<0.05 vs control group. D. Expression of KLF2 protein in LPS-treated endothelial cells is decreased. n=6; *p<0.05 vs control group. E. Overexpression of KLF2 via Ad-KLF2 in mouse aortas in vivo. n=6; *p<0.05 vs control group. F. Overexpression of KLF2 via Ad-KLF2 in cultured mouse endothelial cells in vitro. n=6; *p<0.05 vs control group. G. LPS-induced down-regulation of miR-126a-3p in mouse aortas could be efficiently inhibited by KLF2 over-expression via Ad-KLF2 (Fig. 6G and 6H). n=6; *p<0.05 vs Ad-GFP control group. H. LPS-induced down-regulation of miR-126a-3p in mouse ECs could be efficiently inhibited by KLF2 over-expression via Ad-KLF2. =6; *p<0.05 vs Ad-GFP control group.
DISCUSSION
miRNAs are able to directly regulate over 30% of genes in a cell. In addition, the remaining 70% might also be regulated indirectly by these noncoding RNAs. It is therefore not surprising that miRNAs are involved in the regulation of approximately all major cellular functions, such as cell proliferation, migration, and apoptosis. Accordingly, miRNAs could be critical regulators for physiology and diseases.
More recently, the potential involvements of miRNAs in sepsis have been intensively studied both in endotoxic animal models and in patients with sepsis. In this regard, the expression of multiple miRNAs in inflammatory cells is found to be strongly regulated by LPS both in vitro and in vivo (18,35,36). Tili E et al identified, for the first time, that the expression of miR-155 and miR-125b in mouse Raw 264.7 macrophages was strongly regulated by LPS (34). More excitingly, the levels of many plasma/serum miRNAs and whole blood miRNAs are remarkably changed in endotoxic animals and in patients with sepsis (22, 37, 38). These blood miRNAs might be used as novel diagnostic biomarkers for sepsis (17, 23, 39). In addition, the aberrant expression of miRNAs is also identified in some organs such as lung, liver and heart from endotoxic animals (39-41). These discoveries indicate that miRNAs might play important roles in sepsis. Indeed, modulation of one miRNA, microRNA-142-3p in dendritic cells could remarkably affect the endotoxin-induced mortality (18).
Recent studies from us and other groups suggest that miRNAs in vascular cells play very important roles in vascular injuries, endothelial function, vascular integrity and almost all types of vascular diseases (15). For example, miR-145, miR-221/222, miR-21, miR-31 are critical regulators for vascular smooth muscle cell function and vascular neointimal formation, whereas miR-126a-3p, miR-124, miR-34a, miR-146a have strong effects on EC function and endothelial function (9). However, to date, the expression profiles of miRNAs in vascular walls in endotoxic animals and septic patients and their potential roles in sepsis-related vascular barrier injuries are still unknown.
In this study, we thus determined the expression profile of miRNAs in aortas from LPS-induced endotoxic mice. Clearly, multiple vascular miRNAs were aberrantly expressed in endotoxic mouse arteries. Among them, miR-126a-3p is significantly down-regulated by about 50% in septic mouse aortas and in septic human vessels. miR-126a-3p is an EC-enriched and EC-specific miRNA. Recent studies have revealed that miR-126a-3p plays important roles in proliferation, migration and apoptosis of vascular ECs, in angiogenesis as well as in vascular diseases such as atherosclerosis (42-46). We are focused on miR-126a-3p in this study to test its potential involvement in endotoxemia-induced vascular damages. However, other miRNAs in Table 1 such as miR-378, miR-16, miR-26, which were aberrantly expressed in endotoxic vessels, are also significant enough for investigation in future studies.
We hypothesized that miR-126a-3p might be a critical participant in sepsis-related injuries on vascular ECs and vascular integrity. To test it, we first determined the levels of miR-126a-3p in aortic ECs from endotoxic mice. The result demonstrated that the expression of miR-126a-3p was significantly downregulated in ECs from septic mice. In addition, miR-126a-3p was also downregulated in ECs after treatment with LPS. By both gain-of-function and loss-of-function approaches, we found that miR-126a-3p had strong effects on apoptosis, proliferation and migration of ECs. The results are consistent with recent studies from other groups (42-46). Thus, downregulation of miR-126a-3p could be a novel mechanism in sepsis-mediated injuries on vascular ECs. To further test our hypothesis, miR-126a-3p mimics was applied in cultured ECs before they were damaged by LPS. We found that LPS-induced injuries on ECs were attenuated via miR-126a-3p overexpression, as shown by the improved proliferation, migration, and the reduced apoptosis.
Sepsis induces by LPS injection elicits endothelial dysfunction and the increased vascular permeability. To test the potential roles of miR-126a-3p in sepsis-induced injuries on vascular integrity, we generated lenti-virus expressing miR-126a-3p and applied it to mice. We found that LPS-induced endothelial dysfunction and vascular leakage were partially inhibited by miR-126a-3p treatment. Vascular damages such as endothelial dysfunction and vascular leakage in sepsis are critical contributors in sepsis-induced mortality. As we found that miR-126a-3p had strong effects on LPS-induced vascular damages, we finally determined the effect of miR-126a-3p on mortality of endotoxemia. Our result demonstrated that the survival rate of endotoxic mice was significantly improved by the overexpression of miR-126a-3p.
SPRED1 and VCAM-1 are two genes that are related to cell proliferation, apoptosis, migration, senescence and inflammation. In addition, both SPRED1 and VCAM-1 are key molecules in EC activation and vascular permeability (47-49). Recent studies have identified that they are direct target genes of miR-126a-3p (43, 44). Our luciferase assay and both gain-of-function and loss-of-function approaches verified that these two genes are indeed the direct target genes in vascular ECs. To further confirm that SPRED1 and VCAM-1 may be involved in sepsis, we determined the protein levels of SPRED1 and VCAM-1 and found that they are indeed increased in LPS-treated ECs and in vessels from LPS-injected mice and from patients with sepsis. Moreover, we found that the increased expression of SPRED1 and VCAM-1 was significantly inhibited by overexpression of miR-126a-3p both in vitro and in vitro.
It is well established that miRNAs are subjected to transcriptional and posttranscriptional expressional regulation. One recent study has reported that KLF2 is strong regulator for the expression of miRNAs in ECs (5). In this study, we identified that the down-regulation of miR-126a-3p in sepsis was mainly induced by transcriptional inhibition due to the deceased expression of its binding transcription factor KLF2. Indeed, the expression of KLF2 was decreased in ECs and vascular walls in endotoxemia. In addition, LPS-induced down-regulation both in ECs and mouse vessels could be efficiently inhibited by KLF2 over-expression via Ad-KLF2.
There are some limitations in this study. First, the murine model was used in this project, which might be different in humans. Thus, more human studies should be performed. Second, the LPS-injected mice are model of endotoxemia. Although the vascular damages share many aspects with bacterial sepsis-induced vascular injuries, they are still different. Third, this study is the first to explore the role of miR-126a-3p in an animal model of vascular dysfunction due to systemic inflammation. However, the roles of miR-126a-3p in endothelial proliferation and apoptosis are well documented. Fourth, the cellular functions of miR-126a-3p are mainly determined in cultured ECs in vitro. These findings should be translated into the in vivo vascular response and sepsis both in animal and in human in future studies. Fifth, the LV-miR-126 we used in this study are not specific for ECs. Thus, is it possible the secondary effect of other tissues infected with LV-miR-126 may also lead to endothelial injuries.
In summary, as shown in Fig. 7, in this study, we have identified that microbial virulence factors and numerous pro-inflammatory mediators in blood under septic condition are able to down-regulate the expression of miR-126a-3p in vascular ECs and in vascular walls. The downregulated miR-126a-3p could result in the increase of its target genes such as SPRED1, VCAM-1, etc, and induce vascular injuries such as increased EC apoptosis; decreased EC proliferation; increased EC permeability, impaired vascular functions including endothelial dysfunction, and increased vascular leakage. The breakdown of vascular integrity could induce irreversible multi-organ failure and sepsis-related death. Thus, miR-126a-3p may represent a novel mechanism and a new therapeutic target for sepsis and its related vascular complications.
Fig. 7. Schematic representation shows a miR-126a-3p mechanism of sepsis-related injuries on vascular ECs and vascular integrity.
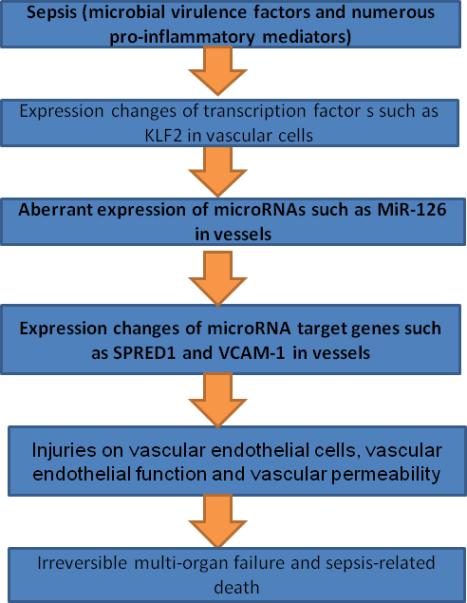
Endotoxemia in sepsis is able to downregulate the expression of EC-enriched miR-126a-3p in the vascular walls via KLF2. The downregulation of miR-126a-3p could induce endothelial dysfunction, and vascular leakage via their target genes such as SPRED1 and VCAM-1 that could induce vascular injury, irreversible multi-organ failure and sepsis-related death.
ACKNOWLEDGMENT
None
Financial support: This work was supported by the National Institutes of Health (HL095707, HL109656, and NR013876) to C. Zhang. This work was also supported by Zhejiang Natural Science Foundation (LY12H02003), Zhejiang Medical and Health Science and Technology plan (2010KYA138, 2012ZDA035), Zhejiang TCM Science and Technology plan (2010ZA089), Scientific Research Foundation of Wenzhou (Y20140051), Project of Science and Technology Department of Zhejiang Province (2015C33163) to M. Chu.
Copyright form disclosures:
The authors received support for article research from the National Institutes of Health (NIH) and disclosed work for hire. Dr. Zhang's institution received support for article research from the NIH.
REFERENCES
- 1.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. Jan. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schorr CA, Zanotti S, Dellinger RP. Severe sepsis and septic shock: Management and performance improvement. Virulence. 2014;5:190–9. doi: 10.4161/viru.27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cinel I, Dellinger RP. Advances in pathogenesis and management of sepsis. Curr Opin Infect Dis. 2007;20:345–52. doi: 10.1097/QCO.0b013e32818be70a. [DOI] [PubMed] [Google Scholar]
- 5.Martí-Carvajal AJ, Solà I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;12:CD004388. doi: 10.1002/14651858.CD004388.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman JM, Jones PA. MicroRNAs: critical mediators of differentiation, development and disease. Swiss Med Wkly. 2009;139:466–72. doi: 10.4414/smw.2009.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–21. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 8.Pasquinelli AE, Hunter S, Bracht J. MicroRNAs: a developing story. Curr Opin Genet Dev. 2005;15:200–5. doi: 10.1016/j.gde.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C. Novel functions for small RNA molecules. Curr Opin Mol Ther. 2009;11:641–51. [PMC free article] [PubMed] [Google Scholar]
- 10.Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of microRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Cheng Y, Yang J, Xu L, Zhang C. Cell-specific effects of miR-221/222 in vessels: Molecular mechanism and therapeutic application. J Mol Cell Cardiol. 2012;52:245–55. doi: 10.1016/j.yjmcc.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res. 2009;105:158–166. doi: 10.1161/CIRCRESAHA.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Cheng Y, Chen X, Yang J, Xu L, Zhang C. MicroRNA-31 regulated by the extracellular regulated kinase is involved in vascular smooth muscle cell growth via large tumor suppressor homolog 2. J Biol Chem. 2011;286:42371–80. doi: 10.1074/jbc.M111.261065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y, Liu X, Cheng Y, Yang J, Huo Y, Zhang C. Involvement of microRNAs in hydrogen peroxide-mediated gene regulation and cellular injury response in vascular smooth muscle cells. J Biol Chem. 2009;284:7903–7913. doi: 10.1074/jbc.M806920200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin S, Zhang C. MicroRNAs in vascular disease. J Cardiovasc Pharmacol. 2011;57:8–12. doi: 10.1097/FJC.0b013e318203759b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Essandoh K, Fan GC. Role of extracellular and intracellular microRNAs in sepsis. Biochim Biophys Acta. 2014;1842:2155–2162. doi: 10.1016/j.bbadis.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vasilescu C, Rossi S, Shimizu M, et al. MicroRNA fingerprints identify miR-150 as a plasma prognostic marker in patients with sepsis. PLoS One. 2009;4:e7405. doi: 10.1371/journal.pone.0007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang JF, Yu ML, Yu G, Bian JJ, Deng XM, Wan XJ, Zhu KM. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun. 2010;394:184–8. doi: 10.1016/j.bbrc.2010.02.145. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Varambally S, Maher CA, Cao Q, Chockley P, Toubai T, Malter C, Nieves E, Tawara I, Wang Y, Ward PA, Chinnaiyan A, Reddy P. Targeting of microRNA-142-3p in dendritic cells regulates endotoxin-induced mortality. Blood. 2011;117:6172–83. doi: 10.1182/blood-2010-12-325647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risøe PK, Ryg U, Wang YY, Rutkovskiy A, Smedsrød B, Valen G, Dahle MK. Cecal ligation and puncture sepsis is associated with attenuated expression of adenylyl cyclase 9 and increased miR142-3p. Shock. 2011;36:390–5. doi: 10.1097/SHK.0b013e318228ec6f. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Meng K, Chen Wj, Feng D, Jia Y, Xie L. Serum miR-574-5p: a prognostic predictor of sepsis patients. Shock. 2012;37:263–7. doi: 10.1097/SHK.0b013e318241baf8. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh CH, Rau CS, Jeng JC, Chen YC, Lu TH, Wu CJ, Wu YC, Tzeng SL, Yang JC. Whole blood-derived microRNA signatures in mice exposed to lipopolysaccharides. J Biomed Sci. 2012;19:69. doi: 10.1186/1423-0127-19-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HJ, Zhang PJ, Chen WJ, Jie D, Dan F, Jia YH, Xie LX. Characterization and Identification of novel serum microRNAs in sepsis patients with different outcomes. Shock. 2013;39:480–7. doi: 10.1097/SHK.0b013e3182940cb8. [DOI] [PubMed] [Google Scholar]
- 24.Wu SC, Yang JC, Rau CS, Chen YC, Lu TH, Lin MW, Tzeng SL, Wu YC, Wu CJ, Hsieh CH. Profiling Circulating MicroRNA Expression in Experimental Sepsis Using Cecal Ligation and Puncture. PLoS One. 2013;8:e77936. doi: 10.1371/journal.pone.0077936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Vilanova D, Atalar K, Delfour O, Edgeworth J, Ostermann M, Hernandez-Fuentes M, Razafimahatratra S, Michot B, Persing DH, Ziegler I, Törös B, Mölling P, Olcén P, Beale R, Lord GM. Genome-wide sequencing of cellular microRNAs identifies a combinatorial expression signature diagnostic of sepsis. PLoS One. 2013;8:e75918. doi: 10.1371/journal.pone.0075918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alves JN, Pires KM, Lanzetti M, Barroso MV, Benjamim CF, Costa CA, Resende AC, Santos JC, Ribeiro ML, Porto LC, Valença SS. Critical role for CCR2 and HMGB1 in induction of experimental endotoxic shock. Arch Biochem Biophys. 2013;537:72–81. doi: 10.1016/j.abb.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Tiwari MM, Messer KJ, Holthoff JH, Gokden N, Brock RW, Mayeux PR. Peritubular capillary dysfunction and renal tubular epithelial cell stress following lipopolysaccharide administration in mice. Am J Physiol Renal Physiol. 2007;292:F261–8. doi: 10.1152/ajprenal.00263.2006. [DOI] [PubMed] [Google Scholar]
- 28.Kim YH, Yoon DW, Kim JH, Lee JH, Lim CH. Effect of remote ischemic postconditioning on systemic inflammatory response and survival rate in lipopolysaccharide-induced systemic inflammation model. J Inflamm (Lond) 2014;11:16. doi: 10.1186/1476-9255-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivasan S, Yeh M, Danziger EC, Hatley ME, Riggan AE, Leitinger N, Berliner JA, Hedrick CC. Glucose regulates monocyte adhesion through endothelial production of interleukin-8. Circ. Res. 2003;92:371–377. doi: 10.1161/01.RES.0000061714.74668.5C. [DOI] [PubMed] [Google Scholar]
- 30.White LE, Cui Y, Shelak CM, Lie ML, Hassoun HT. Lung EC apoptosis during ischemic acute kidney injury. Shock. 2012;38:320–7. doi: 10.1097/SHK.0b013e31826359d0. [DOI] [PubMed] [Google Scholar]
- 31.Guo Y, Zhang C, Du X, Nair U, Yoo TJ. The morphological and functional alterations of the cochlea in apolipoprotein E deficient mice. Hear Res. 2005;208:54–67. doi: 10.1016/j.heares.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Lee W, Yang EJ, Ku SK, Song KS, Bae JS. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation. 2013;36:94–102. doi: 10.1007/s10753-012-9523-9. [DOI] [PubMed] [Google Scholar]
- 33.Bae JS, Lee W, Rezaie AR. Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models. J Thromb Haemost. 2012;10:1145–51. doi: 10.1111/j.1538-7836.2012.04671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Drygalski A, Furlan-Freguia C, Ruf W, Griffin JH, Mosnier LO. Organ-specific protection against lipopolysaccharide-induced vascular leak is dependent on the endothelial protein C receptor. Arterioscler Thromb Vasc Biol. 2013;33:769–76. doi: 10.1161/ATVBAHA.112.301082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–9. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt WM, Spiel AO, Jilma B, Wolzt M, Müller M. In vivo profile of the human leukocyte microRNA response to endotoxemia. Biochem Biophys Res Commun. 2009;380:437–41. doi: 10.1016/j.bbrc.2008.12.190. [DOI] [PubMed] [Google Scholar]
- 37.Tacke F, Roderburg C, Benz F, Cardenas DV, Luedde M, Hippe HJ, Frey N, Vucur M, Gautheron J, Koch A, Trautwein C, Luedde T. Levels of circulating miR-133a are elevated in sepsis and predict mortality in critically ill patients. Crit Care Med. 2014;42:1096–104. doi: 10.1097/CCM.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 38.Shankar-Hari M, Lord GM. How might a diagnostic microRNA signature be used to speed up the diagnosis of sepsis? Expert Rev Mol Diagn. 2014;14:249–51. doi: 10.1586/14737159.2014.899151. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y, Yin N, Jiang L. miR-27a is up regulated and promotes inflammatory response in sepsis. Cell Immunol. 2014;290:190–195. doi: 10.1016/j.cellimm.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Moore CC, McKillop IH, Huynh T. MicroRNA expression following activated protein C treatment during septic shock. J Surg Res. 2013;182:116–26. doi: 10.1016/j.jss.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Huang W, Yang Y, Wang Y, Peng T, Chang J, Caldwell CC, Zingarelli B, Fan GC. Loss of duplexmiR-223 (5p and 3p) aggravates myocardial depression and mortality in polymicrobial sepsis. Biochim Biophys Acta. 2014;1842:701–11. doi: 10.1016/j.bbadis.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S, Wenzel D, Vosen S, Franklin BS, Fleischmann BK, Nickenig G, Werner N. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular EC repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–38. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 43.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126a-3p governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–71. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci U S A. 2008;105:1516–21. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J Mol Cell Cardiol. 2012;53:64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 46.Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J, Megens RT, Heyll K, Noels H, Hristov M, Wang S, Kiessling F, Olson EN, Weber C. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368–76. doi: 10.1038/nm.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pepini T, Gorbunova EE, Gavrilovskaya IN, Mackow JE, Mackow ER. Andes virus regulation of cellular microRNAs contributes to hantavirus-induced endothelial cell permeability. J Virol. 2010;84:11929–36. doi: 10.1128/JVI.01658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho YS, Kim CH, Kim HN, Ha TS, Ahn HY. Ginsenoside Rg3 inhibits lipopolysaccharide-induced adhesion molecule expression in human umbilical vein endothelial cell and C57BL/6 mice. Pharmazie. 2014;69:818–22. [PubMed] [Google Scholar]
- 49.Yang JC, Huang F, Wu CJ, Chen YC, Lu TH, Hsieh CH. Simvastatin reduces VCAM-1 expression in human umbilical vein endothelial cells exposed to lipopolysaccharide. Inflamm Res. 2012;61:485–91. doi: 10.1007/s00011-012-0435-9. [DOI] [PubMed] [Google Scholar]
- 50.Hergenreider E, Heydt S, Tréguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]