Abstract
Schizophrenia is characterized by profound cognitive deficits that are not alleviated by currently available medications. Many of these cognitive deficits involve dysfunction of the newly evolved, dorsolateral prefrontal cortex (dlPFC). The brains of patients with schizophrenia show evidence of dlPFC pyramidal cell dendritic atrophy, likely reductions in cortical dopamine (DA), and possible changes in DA D1 receptors (D1R). It has been appreciated for decades that optimal levels of DA are essential for dlPFC working memory function, with many beneficial actions arising from D1R stimulation. D1R are concentrated on dendritic spines in the primate dlPFC, where their stimulation produces an inverted U dose-response on dlPFC neuronal firing and cognitive performance during working memory tasks. Research in both academia and the pharmaceutical industry has led to the development of selective D1 agonists, e.g., the first full D1 agonist, dihydrexidine, which at low doses improved working memory in monkeys. Dihydrexidine has begun to be tested in patients with schizophrenia or schizotypal disorder. Initial results are encouraging, but studies are limited by the pharmacokinetics of the drug. These data have, however, spurred efforts towards the discovery and development of improved or novel new compounds, including D1 agonists with better pharmacokinetics, functionally selective D1 ligands, and D1R positive allosteric modulators. One or several of these approaches should allow optimization of the beneficial effects of D1R stimulation in the dlPFC that can be translated into clinical practice.
Keywords: prefrontal cortex, D1 agonist, working memory, schizophrenia, executive function, D2 receptors
General Introduction
Drugs targeting dopamine (DA) receptors have been a cornerstone of schizophrenia pharmacotherapy for more than a half-century. To date, all approved antipsychotic drugs target DA D2 receptors (D2R), and although these agents can reduce positive symptoms, improvement of cognitive function, if any, is modest and difficult to dissociate from other drug effects (1, 2). Schizophrenia involves profound dysfunction of the newly evolved, dorsolateral prefrontal cortex (dlPFC). The pioneering research of Patricia Goldman-Rakic uncovered the neuronal circuits in dlPFC that generate the mental representations needed for working memory that are the foundation of abstract thought. She discovered that the dlPFC is greatly influenced by DA, as DA depletion causes profound impairment in working memory (3). Thus, enhancing DA’s beneficial actions is a logical approach to ameliorating cognitive deficits.
Five DA receptor genes encode six receptors in humans (D1, D2L, D2S, D3, D4, D5). Two of these, the D1 and D5, are very homologous such that no current drugs are adequately selective for them. Because of this, when we refer to D1 agonists or antagonists we mean compounds that bind to both D1 and D5 receptors. The members of the D2-like family include the D2L and D2S (splice variants), D3, and D4 receptors. Although there are some selective ligands for these subtypes, except where noted, our references to D2 drugs will indicate compounds with modest or no selectivity among D2L, D2S, D3 or D4 receptors. We shall focus on the rationale for DA therapeutics as cognitive-enhancers, specifically the role of the D1 receptor (D1R).
Dopamine influences on the dlPFC microcircuits afflicted in schizophrenia
Schizophrenia is characterized by striking deficits in cognitive function, especially the higher cognitive operations that depend on the dlPFC. In contrast to rodents, DA axons project throughout most of the primate cortex, with greatest innervation of the motor cortices and least in the primary visual cortex. The DA neurons that project to the dlPFC are likely “salience” cells that fire in response to both rewarding and aversive events, e.g. during stress exposure. Analyses of post-mortem brain tissue have identified grave insults to the layer III microcircuits that subserve working memory, including spine loss and underactivity of layer III pyramidal cells (4). In monkeys, these pyramidal cells generate the persistent firing needed for working memory, whereas GABAergic interneurons refine the tuning of the circuits. Together, they generate the mental representations, the foundation of abstract thought, that keep information “in mind” in the absence of sensory stimulation. These neurons are termed “Delay cells,” and their activity is greatly influenced by D1R, but not D2R stimulation.
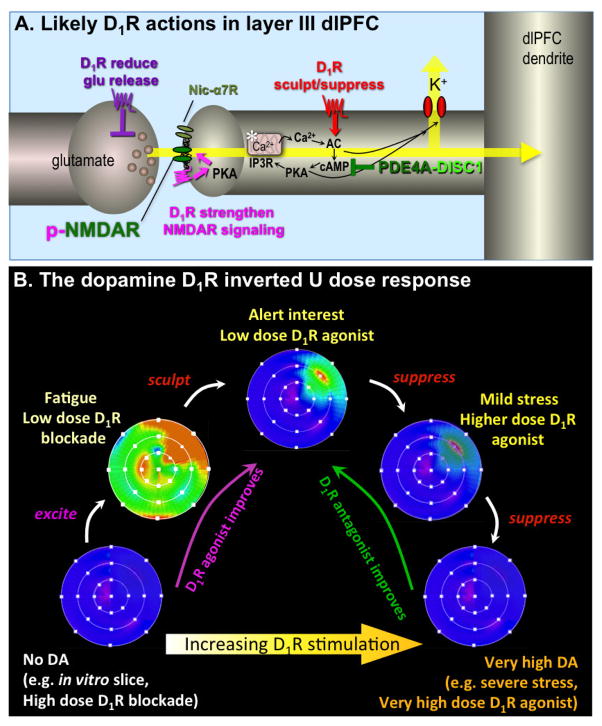
Thus, studies of the actions of DA receptors in the primate dlPFC (reviewed in 5) have shown that optimal levels of D1R stimulation are essential for working memory function. Layer III pyramidal cells excite each other through NMDA receptor (NMDAR) synapses on spines; NMDAR channel opening depends on cholinergic stimulation of nicotinic α7 receptors (α7nChR) within the glutamate synapse (6). Defects in both NMDAR and α7nChR have been linked to schizophrenia. D1Rs are concentrated on layer III spines, and can even be found within the synapse (Figure 1A). D1Rs are often co-localized with HCN (Hyperpolarization-activated cyclic nucleotide gated) channels on spines; D1R mediated increases in cAMP open HCN channels and reduce synaptic efficacy (Figure 1A). The effects of D1R stimulation on Delay cell firing are schematically summarized in Figure 1B. Evidence suggests that low levels of D1R stimulation increase firing by maintaining NMDAR within the synapse, whereas slightly greater D1R activity sculpts away “noise” by gating out non-preferred inputs via HCN channel opening (7). Conversely, high levels of stimulation (e.g., as occur during stress) suppress all firing. This results in an inverted U dose response to D1 agonists seen at the behavioral level. As described below, there is evidence of reduced DA levels in the dlPFC in schizophrenia, and thus therapeutic strategies have focused on enhancing D1R stimulation. Of particular relevance to treatment development for schizophrenia, Castner et al. (8) found that intermittent, trace doses (0.00001–0.0001 mg/kg) of a D1 agonist could ameliorate working memory deficits induced in monkeys by haloperidol, effects possibly due to indirect down-regulation of D1 receptors caused by haloperidol. These data spurred efforts to determine if low levels of D1R stimulation could improve cognitive function in patients.
Figure 1. Schematic illustration of DA D1R influences on Delay cell firing in layer III of the primate dlPFC.
A. Localization of D1R in layer III dlPFC pyramidal cell networks. Pyramidal cells interconnect via NMDAR synapses on spines, with permissive actions from nicotinic α7 receptors (nic- α7R). Immunoelectron microscopy has shown that D1R are concentrated on dendritic spines, where they can be seen directly within the synapse (magenta), and near the synapse where they often co-localize with HCN or KCNQ potassium channels (red). The open state of both of these channels is increased by cAMP-PKA signaling. Physiological recordings from monkeys indicate that D1R activates feedforward cAMP-PKA-calcium signaling, which opens K+ channels and weakens nearby synaptic inputs (red). At optimal doses this sculpts away noise from irrelevant inputs, but at higher doses, e.g. as occurs during stress, it causes nonspecific suppression of Delay cell firing and loss of working memory. Feedforward cAMP-calcium signaling is held in check by the phosphodiesterase, PDE4A, which is anchored in place by DISC1 (Disrupted In Schizophrenia). Studies in nonprimate species suggest that D1R within the synapse phosphorylate NMDAR via activation of cAMP-PKA and PKC signaling; this maintains NMDAR in the synaptic membrane and strengthens connections (magenta). There are also D1R on glutamate axon terminals that may reduce glutamate release (purple). For detailed description, see Arnsten et al, 2015. The asterisk indicates the spine apparatus, the extension of the smooth endoplasmic reticulum into the spine.
B. A schematic illustration of the DA D1R inverted U influence on the “memory fields” of dlPFC Delay cells. For details, see (5). Under optimal arousal conditions, Delay cells generate persistent representations of visual space, displaying high rates of firing (orange-red) to the memory of one spatial location, and low rates of firing (blue) to the memory of all other spatial locations. When there is no D1R stimulation, Delay cells have little firing. Low levels of D1R stimulation appear to be excitatory, e.g. phosphorylating NMDAR to increase their trafficking into the synapse (7). This can produce noisy firing for all directions, as represented by the generalized green-orange coloring of the memory field. With optimal levels of D1R stimulation, there are additional sculpting actions, gating out “noise”, e.g. by opening a subset of HCN channels. At still higher levels of D1R stimulation (e.g. as occurs during stress), there is excessive HCN channel opening and Delay cell firing is generally suppressed. Under these conditions the neuron is not able to generate persistent representations of visual space.
Approaches to modulating dopamine function
D1 antagonists
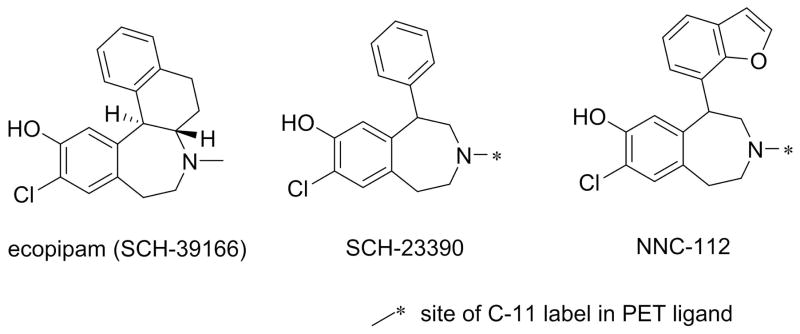
As the understanding of D1R microcircuitry was evolving, and the first selective antagonist SCH23390 (Figure 2) was reported (9), one prevalent hypothesis was that D1R antagonists might be novel treatments for schizophrenia (10). SCH-23390 has pharmacokinetic limitations, but its analog SCH39166 (ecopipam, Figure 2) had improved properties (11). Ecopipam was studied clinically for a variety of indications, including schizophrenia (12–15), drug abuse (16), and obesity (17), but in each case undesirable effects were observed. In addition to the basic neurobiology discussed above, it was “back-translation” of the observations with D1R antagonists [e.g., induction of preclinical amotivational states and cognitive deficits (18)] that increased the interest in therapeutic utility of D1 agonists. These antagonists have, however, continued to impact neuroscience because 11C-SCH-23390 and its closely related analog, 11C-NNC-112 (Figure 2), have become the mainstay PET ligands for clinical imaging of D1Rs (19, 20), including studies which demonstrate direct relationships between D1R binding potential and cognitive function (21, 22) (see below).
Figure 2. Structures of selective D1 antagonists and PET ligands.
The site of radiolabeling is shown by the asterisk. The most pharmacologically active isomer is shown, although these compounds are sometimes used as racemates.
Indirect activation of dopamine receptors
A variety of strategies have been employed to try to enhance DA actions in the dlPFC. Mechanisms such as DA transporter (DAT) inhibition, increased DA synthesis or release, or inhibition of degradation by monoamine oxidases (MAO) or catechol-O-methyl-transferase (COMT) can augment the amount of synaptic catecholamines, including DA. Yet therapeutically, generalized increases in catecholamine actions (e.g., levodopa, methylphenidate, amphetamines) will tend to cause undesired side effects, especially in many target populations who are susceptible to exacerbation of psychoses, to substance abuse, etc., that can be affected by activation of D2R. As DAT expression is low in the dlPFC, it has been hypothesized that COMT might be an excellent target for more selective regional activation, albeit of all catecholamine receptors (23, 24). Unfortunately, the three approved COMT inhibitors (tolcapone, entacapone, ocicapone) (25, 26), while useful for slowing the peripheral degradation of levodopa in treatment of Parkinson’s disease, have little brain penetration (27, 28). Rigorous testing of this hypothesis must await a non-toxic brain penetrant COMT inhibitor. This suggested that targeting specific dopamine receptors, especially the D1R, might be a useful strategy.
Concepts relevant to dopamine receptor ligands
Clinical and basic neuroscientists are generally aware of the classical issues in the use of receptor-targeting drugs, specifically the affinity of the drug for the desired versus off-target receptors, and the property of the drug (e.g., agonist or antagonist) at the primary targeted receptor. There are two directly important subtleties that can often impact interpretation, but are less widely appreciated. The classical one is partial agonism, reflecting that a particular drug even at near-saturating concentrations will only activate the target receptor to some fraction of the natural ligand of the receptor. (The degree to which a drug activates a receptor relative to the natural ligand for the receptor is called intrinsic activity.) Classically, partial agonists are also known to be partial antagonists, and may inhibit the actions of the natural ligand. The second and now generally accepted concept is called “functional selectivity” or “ligand bias” (29–31). Essentially, this concept reflects the fact that some drugs can have very diverse functional effects on different signaling pathways mediated by a single receptor. In the extreme, this can even be full activation (full agonism) of one signaling pathway of a receptor, and pure antagonism at another pathway of the same receptor even in the same cell (32, 33). This differential activation may also be expressed as large differences in potency at two different signaling pathways (29, 34).
Finally, the concept of allosteric drugs (i.e., ligands acting at sites on the receptor distant from the binding site of endogenous ligands) is well known in biological psychiatry as the mechanism of action at ionotropic receptors (e.g., benzodiazepines at GABAA receptors). Recently, there has been a surge of activity in the discovery of allosteric ligands for G protein-coupled receptors, and this is beginning to impact DA receptors. These three concepts will be relevant to specific compounds discussed below.
Non-selective drugs with D1 agonist properties
There is sometimes confusion when there is discussion of approved “DA agonists,” and a brief discussion may be useful All of the currently approved “dopamine agonists” are selective to a greater or lesser extent for the D2-like versus the D1-like receptors. Indeed, many (e.g., ropinirole, pramipexole, bromocriptine, cabergoline) are devoid of D1 agonist activity, though there are three exceptions. Apomorphine has D1R high intrinsic activity, but is selective for D2R; its activity at D1R may be why it is effective in advanced Parkinson’s Disease (PD) (35). Unfortunately, its poor pharmaceutical properties and D2R side effects make it unsuitable for treating cognition (36–41). Rotigotine has similar properties. Pergolide, which will soon be unavailable, has modest D1 partial agonist activity (42, 43), and has been used for cognitive experiments as discussed below. Lastly, CY-208,243 had modest selectivity for D1 versus D2 receptors (44), and D1-dependent antiparkinsonian actions in monkeys (45–47), (but not in humans (48)), but its development ceased. Ergolines are generally promiscuous for monoamine receptors, and many activate serotonin 5-HT2B receptors (49) making them unsuitable for human studies.
Selective D1 agonists used in laboratory animal studies
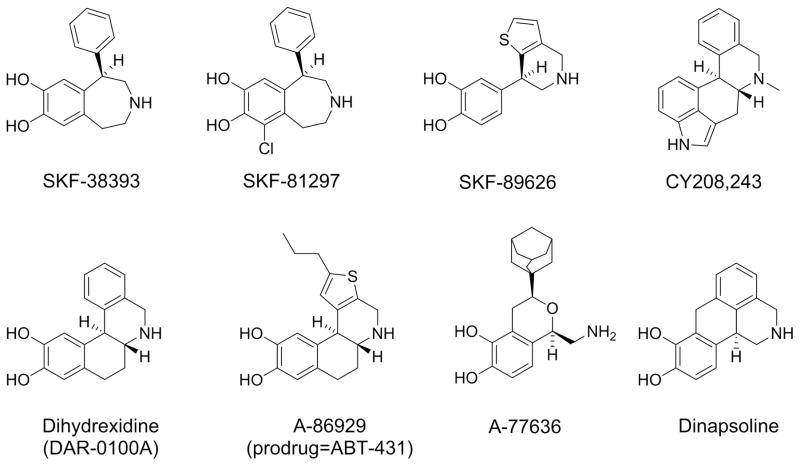
The prototype of selective D1 agonists is SKF-38393 (Figure 3), first reported nearly forty years ago (50, 51), which is a member of the 1-phenyl-tetrahydro-benzazepine chemotype that has yielded dozens of experimental compounds (52–54). SKF-38393 probably has been the most widely used D1 agonist, but although it is quite selective, it is only a partial agonist with modest intrinsic activity in canonical signaling pathways (51, 55–57). When this is ignored in interpreting pharmacological studies, it may lead to erroneous conclusions. SKF-81297 (Figure 3) is another commonly used member of the benzazepine chemotype that has much higher intrinsic activity than SKF-38393 (57), and has been a useful tool for in vitro and animal experimentation.
Figure 3. Examples of important experimental D1 agonists.
[Top Row] SKF-38393 (partial agonist) and SKF-82958 (full agonist) are phenylbenzazepines. SKF-89626 had higher intrinsic activity than SKF-38393, but lacked BBB permeability. CY208243 is a high D1 intrinsic activity ergoline. [Bottom row] Four full D1 agonists from four different chemotypes: A-77636, A-86829 (the active compound of the diacetyl prodrug ABT-431), dihydrexidine (DAR-0100A), and dinapsoline. The most pharmacologically active isomer is shown in all cases, although these compounds are sometimes used as racemates.
Two D1 agonists from this class, SPD-451 and SKF-83959, have been particularly provocative because both were proposed to be functionally selective D1R ligands that had high intrinsic activity for D1R-activation of phospholipase C and low intrinsic activation for the canonical cAMP-mediated signaling. Extensive studies with SKF-83959 also led to the hypothesis that its novel functionally selective signaling was mediated by a D1D2 receptor heterodimer (58). The development of SPD-451, originally advanced by CeNeS Pharmaceuticals plc and later Shire, has apparently ceased. SKF-83959 has never been advanced to humans, but has been widely used experimentally because of these purported novel properties (59–62). Unfortunately, recent data suggest that this compound is actually similar to other benzazepine partial agonists, and is neither highly functionally selective, nor works through a D1/D2 heterodimer (63–65). As a class, the benzazepines also have poor oral bioavailability and short duration of action due to the inherent catechol group (66), and in addition, many of the members of this group have a propensity to cause seizures (67).
Non-benzazepine centrally available full D1 agonists
The first full D1 agonists were fenoldopam and SKF-89626 (Figure 3), but neither compound was brain penetrable (57, 68, 69). The first selective centrally available D1R full agonist was dihydrexidine (Figure 3) (55–57, 70–73), and it has been a very useful tool in testing hypotheses about the roles of D1R receptors, such as for antiparkinsonian therapy (74) or effects on cognition (75). Although dihydrexidine is only ten-fold D1:D2 selective (72), it has profound D2R functional selectivity (32, 33), and its behavioral effects generally lack D2R properties (76). Dihydrexidine, however, has two major limitations for human experimentation: it has very little oral bioavailability, and is metabolized very rapidly.
Chronologically, the next compounds of importance were A-68930 and A-77636 (Figure 3), two selective D1 agonists from the novel isochroman chemotype (77, 78). A-68930 caused seizures (79), but A-77636 has been widely used experimentally because it appeared to have overcome the bioavailability problems of dihydrexidine and had a long duration of action. In murine and primate species, both compounds caused profound antiparkinsonian effects like dihydrexidine (80, 81), but both also caused a profound and rapid tolerance (78, 82–84). Both the tolerance and seizures are potential developmental liabilities that are discussed below.
Because of the tolerance caused by A-77636, Abbott laboratories next reported A-86929 (Figure 3) and its diacetyl prodrug ABT-431 (adrogolide) (Abbott Laboratories). A86929 is similar in structure and pharmacological properties to dihydrexidine (85, 86). Like dihydrexidine, ABT-431 caused dramatic antiparkinsonian effects (74, 86, 87), but like dihydrexidine, even the prodrug ABT-431 had poor oral bioavailability. ABT-431 was out-licensed to Drug Abuse Sciences as a potential anti-cocaine therapy, but development ceased for reasons that are not public. Another compound that failed development was dinapsoline, a D1:D2 agonist with high D1R intrinsic activity and significant functional selectivity at D2 receptors. Like dihydrexidine, its behavioral actions in animal models of Parkinson’s models were D1R, not D2R, dependent (88–90). Unfortunately, there have not been newer compounds with marked advantages reported recently.
Challenges and opportunities for clinical development
A major problem in development of selective full D1 agonists has been oral bioavailability. All reported chemotypes for selective full D1 agonists contain a catechol moiety (as in dopamine) that, at least to date, seems necessary for full D1R agonism but which causes predictable problems in bioavailability. In the few cases where drugs have reasonable bioavailability (e.g., A-77636 (78) or dinapsoline (89)), the compounds had high plasma protein binding and low free fraction, and unbound clearance remains high. A-77636 also has near irreversible binding to the D1R in vivo (91). Addressing these pharmacokinetic issues is essential for any major advance.
There are two primary safety concerns with this class. Some D1 agonists have been reported to induce seizures or lower seizure thresholds, and D1 activation seems to be critical as they are prevented by D1R antagonists and not seen in D1R knockout mice (67). This is drug specific, however, as the full D1 agonist A-77636 does not induce seizures, unlike its analog A-68930 (79). Similarly, monotherapy with dihydrexidine or ABT-431 has not been associated with epileptogenic potential in rats, mice, or primates. Thus, D1R activation alone is not sufficient to cause seizures, and the involved mechanism(s) and the relative safety margin of D1 agonists as a class are unclear.
The other D1R-related safety concern is hypotension, the reason for the premature termination of the first pilot human trial of dihydrexidine (92). These effects are probably mediated by peripheral D1R in the kidney and elsewhere in the cardiovascular system (93). Because the cardiovascular system can respond rapidly to such challenges, this may not be limiting if blood concentrations of the drug do not rise too rapidly as suggested by recent clinical studies (58, 94, 95).
Finally, a non-safety issue, rapid tolerance, may limit use of D1 agonists. During antiparkinsonian studies in both murine and primate species, the dramatic effectiveness of A77636 and A68930 was rapidly (within 24–48 h) lost (82, 96). Indeed, a single dose of A77636 results in almost complete tolerance (Blanchet et al., 1996), hypothesized to be due to rapid desensitization probably resulting from irreversible binding to the D1R (91). On the other hand, the fact that dinapsoline (a drug that does not bind irreversibly) did not cause tolerance with once or twice daily subcutaneous injection, but did with constant minipump infusion (89), suggests more complicated mechanisms. When a clinical candidate is identified, it will be important to determine the conditions under which it may cause this rapid tolerance.
Dopamine changes in schizophrenia
The initial hypotheses about the mechanism of positive symptoms of schizophrenia were based in large measure on pharmacological data in which the clinical effectiveness of antipsychotic drugs correlated well with their affinity for D2-like receptors (97). Even today, all approved antipsychotics require as part of their pharmacology interactions with the D2R. The antipsychotic drugs are generally effective against positive symptoms (e.g., delusions, hallucinations, etc.) (98, 99). A variety of clinical experimental studies showing that sensitivity to stimulant medications (100–103), increases in DA synthesis (104–106), and predictive utility of baseline occupancy of striatal D2Rs (107) suggest that increased activity in striatal DA neurotransmission is associated with positive symptoms (108), explaining the efficacy of D2R antagonists.
Conversely, effects on negative symptoms are limited (109–112). Effects on neurocognitive deficits (e.g., working memory, verbal memory, attention, executive functioning) have also been reported (1, 2, 113–118), but have been small (99). The relative lack of effects of antipsychotic medications on cognition are particularly important given that approximately 75% of patients with schizophrenia have clinically meaningful deficits in at least two cognitive domains, and 90% have deficits in at least one domain (119). In addition, cognitive deficits are present at illness onset (120), persist over time (121), and are more predictive of overall psychosocial functioning than are positive symptoms (122, 123).
Negative symptoms and cognitive deficits are thought to be related, at least in part, to a cortical DA deficit (124–126). An abundance of preclinical and other indirect data suggest that hypodopaminergic function in the dlPFC may be associated with these symptom domains. As described above, either DA depletion in the dlPFC, or DA antagonists, impair cognitive function (3, 127–129). D1 agonists can reverse these deficits (75), although higher doses of D1 agonists impair working memory function, leading to inverted-U dose-response curves (75, 130–133).
Evidence for DA receptor hypofunction in PFC in schizophrenia comes from several lines of research. There are decreased levels of DA metabolites in the cerebrospinal fluid of schizophrenic individuals, and these are correlated with poor working memory performance (134, 135). DA agonists may be able to ameliorate the patterns of activation in dlPFC during these tasks (136). Genetic variations of the COMT gene that cause increased metabolism of DA are also associated with impaired PFC function, and a greater risk of schizophrenia (137). One post-mortem study of tyrosine hydroxylase immunolabeling showed differences in DA innervation of the dlPFC in patients (138), whereas postmortem studies of D1Rs have not shown differences (139, 140). PET labeling studies of cortical D1R density have not yielded consistent results (21, 141–143). More recently, Slifstein et al. (144) reported decreased amphetamine-induced DA release in the dlPFC in patients with schizophrenia, further supporting the hypothesis of cortical hypodopaminergia in this disorder. Thus, there is a major unmet need for the development of new psychopharmacologic agents for schizophrenia, in particular for cognitive deficits.
Clinical studies of D1 agonists
Single doses of the full D1 agonist dihydrexidine have been given both to individuals with PD (92) and with schizophrenia (58, 94). In the schizophrenia study, patients were randomized in a cross-over design between dihydrexidine and placebo (58, 94). Side effects were minimal, no orthostatic changes were observed, and increased perfusion (i.e., via fMRI) in bilateral PFC was noted (58, 94), but with only a trend for improvements on the Controlled Oral Word Association Test or the Hopkins Verbal Learning Test. Another study used the mixed D2:D1 agonist pergolide in 25 subjects with schizotypal personality disorder and compared placebo and drug on neuropsychological performance (145). The pergolide group showed improvements in visual-spatial working memory, executive function, and verbal learning and memory, and suggested that drugs with D1R properties may provide benefit for the cognitive abnormalities of schizophrenia spectrum disorders.
Because of the data on dlPFC hypofunction and D1Rs, the effects of the active (+)-enantiomer (146) of dihydrexidine (numbered DAR-0100A) was studied as an add-on treatment for cognitive enhancement in schizophrenia. A PET receptor occupancy study in non-human primates using [11C]NNC112 and [11C]raclopride to examine D1R and D2R/D3R, respectively, showed dose-dependent selective binding of DAR-0100A to the D1R (147). A trial of 49 clinically stable individuals with schizophrenia involved three weeks of intermittent treatment with either placebo (normal saline), or a high (15 mg, the MTD) or low dose (0.5 mg) of DAR-0100A. These doses were chosen to avoid drug-induced hypotension (92). Because of the rapid metabolism in man (92), blood levels of DAR-0100A were below limits of quantification, suggesting that these doses of DAR-0100A achieved only minimal engagement of the D1R, No treatment effects were found on BOLD fMRI signals during working memory tasks, on working memory domains of the MATRICS battery, or on clinical measures. Conversely, some improvement on the CogState Schizophrenia Battery was seen in the high dose group, as well as improvement in attention on the MATRICS battery in both treatment groups. Differences in patient population (especially medication status) may explain the more positive findings of Rosell et al. (95) who administered three 15 mg doses of DAR-0100A or placebo to 16 individuals with schizotypal personality disorder. Improvement was observed in working memory on the Paced Auditory Serial Audition Task (PASAT) and on the 2-Back to 0-back ratio, although this ratio was improved in part due to worse performance on the 0-back condition. Although these results are not totally conclusive, they suggest a pro-cognitive effect of a full D1 agonist in schizophrenia. Importantly, that unquantifiable blood levels of DAR-0100A (although nonspecific metabolites were qualitatively observed at levels commensurate with the two doses) were observed suggests that these doses of DAR-0100A achieved only minimal engagement of the D1R, likely explaining these mixed results. These results certainly suggest studies of better D1 agonists (i.e., that achieve more target engagement) are needed to more fully test the efficacy of this mechanism for cognitive enhancement in schizophrenia and reconcile the mixed findings from these trials.
Future opportunities
As can be gleaned from the material above, one major goal should be the discovery of safe and highly bioavailable (probably non-catechol containing), full D1 agonists. The accumulating evidence for a potential therapeutic role of D1 agonists in several areas will hopefully spur both pharmaceutical companies and academic groups to jump existing hurdles. Patent activity suggests progress toward structurally novel agents, but none have been disclosed in the peer-reviewed literature at this time. There are, however, some alternate approaches that may be useful.
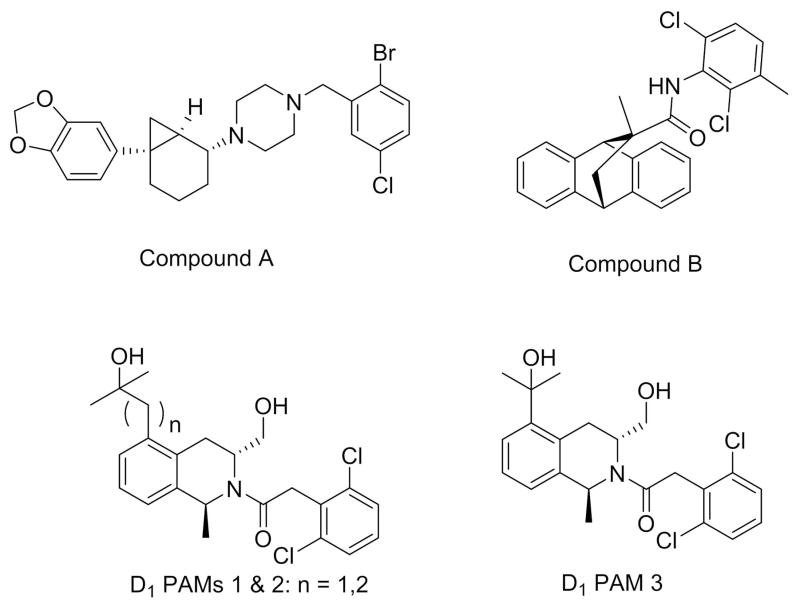
A D1 positive allosteric modulator (PAM) would enhance the endogenous actions of DA at D1R, and recently, the first published preclinical breakthrough in this area was reported for two series of PAMs (see Figure 4) that are active in vitro (148). One of the compounds was selective for D1R, but had much lower activity at the murine vs. the human D1R. A patent from Eli-Lilly also reports an additional chemotype for D1 PAMs that potentiate relevant in vivo signals including locomotor activity and release of acetylcholine in PFC (149).
Figure 4. Structures of D1 positve allosteric modulators (PAMs).
PAMs may offer an advantage over orthosteric (direct) agonists by interacting with endogenous dopamine tone, and as such, may possibly avoid biphasic effects currently seen with direct D1 agonists.
Another intriguing possibility is functionally selective D1 ligands. As an example, in most assays, SKF-38393 is a partial agonist because it has much less intrinsic activity than DA or dihydrexidine. Consistent with classical pharmacology, this would explain why SKF-38393 is a much less effective antiparkinsonian drug than dihydrexidine. Yet in some systems, the two compounds are equally effective physiologically (75, 150). These data suggest that SKF-38393, while a partial agonist at most commonly tested D1R mediated signaling pathways, may have higher intrinsic activity in others. There is significant functional selectivity even among experimental compounds considered “full agonists” (90, 91). Thus, discovery of new highly-biased D1R ligands may be an important route to more selective actions in vivo, that also avoid problems like seizures and rapid tolerance.
Finally, to maximize the beneficial effects of D1R stimulation on dlPFC function in schizophrenia will require addressing the “inverted U” dose-response curves seen in vivo. As noted earlier, one D1R mechanism involves increased cAMP opening of HCN channels that can suppress all firing if doses are too high. Optimal effects on working memory can be achieved by careful dose titration, but this will be challenging because of inter-individual differences in drug metabolism and distribution. Besides a D1R PAM or functionally selective D1R orthosteric ligand, it may be that a D1R selective compound with more “dopamine-like” affinity would be useful, as this might attenuate excessive cAMP-mediated opening of HCN channels. The potential for large effect sizes shown by existing data makes these challenges worthy of tackling.
Acknowledgments
This work was supported by PHS grants DP1AG047744 to AFTA; and MH040537, MH082441, and NS039036 to RBM.
Footnotes
Disclosures AFTA previously received funding from Pfizer Pharmaceuticals relevant to the topic of this review. RRG receives funding from Otsuka, Genentech, and PharmaNac. DLG is a Pfizer employee. RBM previously received funding from Pfizer Pharmaceuticals relevant to the topic of this review. RBM also is an inventor on patents related to D1 agonists and their use. His potential conflicts-of-interest are managed by the Penn State University Conflict of Interest system.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ragy R. Girgis, Email: ragygir@nyspi.columbia.edu.
David I. Gray, Email: David.L.Gray@pfizer.com.
Richard B. Mailman, Email: rmailman@psu.edu.
References
- 1.Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker WW, Keefe RS, et al. Cognitive Effects of Antipsychotic Drugs in First-Episode Schizophrenia and Schizophreniform Disorder: A Randomized, Open-Label Clinical Trial (EUFEST) Am J Psychiat. 2009;166:675–682. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- 2.Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- 3.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 4.Arion D, Corradi JP, Tang S, Datta D, Boothe F, He A, et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry. 2015 Jan 6; doi: 10.1038/mp.2014.171. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnsten AFT, Wang M, Paspalas CD. Dopamine’s actions in primate prefrontal cortex: Challenges for treating cognitive disorders. Pharmacological Rev. 2015;67:681–696. doi: 10.1124/pr.115.010512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AFT, Wang M. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Nat Acad Sci USA. 2013;110:12078–12083. doi: 10.1073/pnas.1307849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li YC, Liu G, Hu JL, Gao WJ, Huang YQ. Dopamine D(1) receptor-mediated enhancement of NMDA receptor trafficking requires rapid PKC-dependent synaptic insertion in the prefrontal neurons. J Neurochem. 2010;114:62–73. doi: 10.1111/j.1471-4159.2010.06720.x. [DOI] [PubMed] [Google Scholar]
- 8.Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- 9.Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba CA. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. Journal of Pharmacology and Experimental Therapeutics. 1983;226:462–468. [PubMed] [Google Scholar]
- 10.Bourne JA. SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS drug reviews. 2001;7:399–414. doi: 10.1111/j.1527-3458.2001.tb00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger JG, Chang WK, Clader JW, Hou D, Chipkin RE, McPhail AT. Synthesis and receptor affinities of some conformationally restricted analogs of the dopamine D1 selective ligand (5R)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepin-7-ol. Journal of Medicinal Chemistry. 1989;32:1913–1921. doi: 10.1021/jm00128a038. [DOI] [PubMed] [Google Scholar]
- 12.Karlsson P, Smith L, Farde L, Harnryd C, Sedvall G, Wiesel FA. Lack of apparent antipsychotic effect of the D1-dopamine receptor antagonist SCH39166 in acutely ill schizophrenic patients. Psychopharmacology (Berl) 1995;121:309–316. doi: 10.1007/BF02246068. [DOI] [PubMed] [Google Scholar]
- 13.Labelle A, de Beaurepaire R, Boulay LJ, Naber D, Jones BD, Barnes TR. A pilot study of the safety and tolerance of SCH 39166 in patients with schizophrenia. Journal of Psychiatry and Neuroscience. 1998;23:93–94. [PMC free article] [PubMed] [Google Scholar]
- 14.Den Boer JA, van Megen HJ, Fleischhacker WW, Louwerens JW, Slaap BR, Westenberg HG, et al. Differential effects of the D1-DA receptor antagonist SCH39166 on positive and negative symptoms of schizophrenia. Psychopharmacology (Berl) 1995;121:317–322. doi: 10.1007/BF02246069. [DOI] [PubMed] [Google Scholar]
- 15.de Beaurepaire R, Labelle A, Naber D, Jones BD, Barnes TR. An open trial of the D1 antagonist SCH 39166 in six cases of acute psychotic states. Psychopharmacology (Berl) 1995;121:323–327. doi: 10.1007/BF02246070. [DOI] [PubMed] [Google Scholar]
- 16.Haney M, Ward AS, Foltin RW, Fischman MW. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 2001;155:330–337. doi: 10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- 17.Astrup A, Greenway FL, Ling W, Pedicone L, Lachowicz J, Strader CD, et al. Randomized Controlled Trials of the D1/D5 Antagonist Ecopipam for Weight Loss in Obese Subjects. Obesity. 2007;15:1717–1731. doi: 10.1038/oby.2007.205. [DOI] [PubMed] [Google Scholar]
- 18.Stuchlik A, Vales K. Effect of dopamine D1 receptor antagonist SCH23390 and D1 agonist A77636 on active allothetic place avoidance, a spatial cognition task. Behavioural Brain Research. 2006;172:250–255. doi: 10.1016/j.bbr.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Halldin C, Foged C, Chou YH, Karlsson P, Swahn CG, Sandell J, et al. Carbon-11-NNC 112: a radioligand for PET examination of striatal and neocortical D1-dopamine receptors. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1998;39:2061–2068. [PubMed] [Google Scholar]
- 20.Abi-Dargham A, Thompson J, Urban N, Xu X, Kegeles L, Narendran R, et al. PET imaging of D1 receptors in striatal subdivisions in schizophrenia. J NUCL MED MEETING ABSTRACTS. 2009;50:81. [Google Scholar]
- 21.Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, et al. Changes in Cortical Dopamine D1 Receptor Binding Associated with Cognitive Training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- 23.Roussos P, Giakoumaki SG, Bitsios P. Tolcapone effects on gating, working memory, and mood interact with the synonymous catechol-O-methyltransferase rs4818c/g polymorphism. Biol Psychiatry. 2009;66:997–1004. doi: 10.1016/j.biopsych.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Forsberg M, Lehtonen M, Heikkinen M, Savolainen J, Jarvinen T, Mannisto PT. Pharmacokinetics and pharmacodynamics of entacapone and tolcapone after acute and repeated administration: a comparative study in the rat. The Journal of pharmacology and experimental therapeutics. 2003;304:498–506. doi: 10.1124/jpet.102.042846. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira JJ, Rocha JF, Falcao A, Santos A, Pinto R, Nunes T, et al. Effect of opicapone on levodopa pharmacokinetics, catechol-O-methyltransferase activity and motor fluctuations in patients with Parkinson’s disease. Eur J Neurol. 2015;22:815–825. e856. doi: 10.1111/ene.12666. [DOI] [PubMed] [Google Scholar]
- 27.Borges N. Tolcapone in Parkinson’s disease: liver toxicity and clinical efficacy. Expert opinion on drug safety. 2005;4:69–73. doi: 10.1517/14740338.4.1.69. [DOI] [PubMed] [Google Scholar]
- 28.Apud JA, Weinberger DR. Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS drugs. 2007;21:535–557. doi: 10.2165/00023210-200721070-00002. [DOI] [PubMed] [Google Scholar]
- 29.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, et al. Functional selectivity and classical concepts of quantitative pharmacology. The Journal of pharmacology and experimental therapeutics. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 30.Mailman RB, Nichols DE, Lewis MM, Blake BL, Lawler CP. Functional Effects of Novel Dopamine Ligands: Dihydrexidine and Parkinson’s Disease as a First Step. In: Jenner P, Demirdemar R, editors. Dopamine Receptor Subtypes: From Basic Science to Clinical Application. IOS Stockton Press; 1998. pp. 64–83. [Google Scholar]
- 31.Mailman RB, Murthy V. Ligand functional selectivity advances our understanding of drug mechanisms and drug discovery. Neuropsychopharmacology. 2010;35:345–346. doi: 10.1038/npp.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, et al. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. Journal of Pharmacology and Experimental Therapeutics. 2002;301:1166–1178. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- 33.Kilts JD, Connery HS, Arrington EG, Lewis MM, Lawler CP, Oxford GS, et al. Functional selectivity of dopamine receptor agonists. II. Actions of dihydrexidine in D2L receptor-transfected MN9D cells and pituitary lactotrophs. Journal of Pharmacology and Experimental Therapeutics. 2002;301:1179–1189. doi: 10.1124/jpet.301.3.1179. [DOI] [PubMed] [Google Scholar]
- 34.Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB. Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol. 2004;66:97–105. doi: 10.1124/mol.66.1.97. [DOI] [PubMed] [Google Scholar]
- 35.Poewe W, Kleedorfer B, Gerstenbrand F, Oertel W. Subcutaneous apomorphine in Parkinson’s disease. Lancet. 1988;943 doi: 10.1016/s0140-6736(88)91755-2. [DOI] [PubMed] [Google Scholar]
- 36.Poewe W, Kleedorfer B, Wagner M, Bosch S, Schelosky L. Continuous subcutaneous apomorphine infusions for fluctuating Parkinson’s disease. Long-term follow-up in 18 patients. Adv Neurol. 1993;60:656–659. [PubMed] [Google Scholar]
- 37.Colzi A, Turner K, Lees AJ. Continuous subcutaneous waking day apomorphine in the long term treatment of levodopa induced interdose dyskinesias in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:573–576. doi: 10.1136/jnnp.64.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes AJ, Bishop S, Kleedorfer B, Turjanski N, Fernandez W, Lees AJ, et al. Subcutaneous apomorphine in Parkinson’s disease: response to chronic administration for up to five years. Mov Disord. 1993;8:165–170. doi: 10.1002/mds.870080208. [DOI] [PubMed] [Google Scholar]
- 39.Kapoor R, Turjanski N, Frankel J, Kleedorfer B, Lees A, Stern G, et al. Intranasal apomorphine: a new treatment in Parkinson’s disease [letter] J Neurol Neurosurg Psychiatry. 1990;53:1015. doi: 10.1136/jnnp.53.11.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Laar T, Jansen EN, Essink AW, Neef C. Intranasal apomorphine in parkinsonian on-off fluctuations. Arch Neurol. 1992;49:482–484. doi: 10.1001/archneur.1992.00530290064013. [DOI] [PubMed] [Google Scholar]
- 41.Van Laar T, Neef C, Danhof M, Roon KI, Roos RA. A new sublingual formulation of apomorphine in the treatment of patients with Parkinson’s disease. Mov Disord. 1996;11:633–638. doi: 10.1002/mds.870110607. [DOI] [PubMed] [Google Scholar]
- 42.Goldstein M, Lew JY, Sauter A, Lieberman A. The affinities of ergot compounds for dopamine agonist and dopamine antagonist receptor sites. Adv Biochem Psychopharmacol. 1980;23:75–82. [PubMed] [Google Scholar]
- 43.Fuller RW, Clemens JA. Pergolide: a dopamine agonist at both D 1 and D 2 receptors. Life Sciences. 1991;49:925–930. doi: 10.1016/0024-3205(91)90074-l. [DOI] [PubMed] [Google Scholar]
- 44.Andersen PH, Gingrich JA, Bates MD, Dearry A, Falardeau P, Senogles SE, et al. Dopamine receptor subtypes: beyond the D1/D2 classification. Trends in Pharmacological Sciences. 1990;11:231–236. doi: 10.1016/0165-6147(90)90249-8. [DOI] [PubMed] [Google Scholar]
- 45.Temlett JA, Chong PN, Oertel WH, Jenner P, Marsden CD. The D-1 dopamine receptor partial agonist, CY 208-243, exhibits antiparkinsonian activity in the MPTP-treated marmoset. European Journal of Pharmacology. 1988;156:197–206. doi: 10.1016/0014-2999(88)90322-6. [DOI] [PubMed] [Google Scholar]
- 46.Nomoto M, Fukuda T. The effects of D1 and D2 dopamine receptor agonist and antagonist on parkinsonism in chronic MPTP-treated monkeys. Adv Neurol. 1993;60:119–122. [PubMed] [Google Scholar]
- 47.Gomez-Mancilla B, Boucher R, Gagnon C, Di Paolo T, Markstein R, Bedard PJ. Effect of adding the D1 agonist CY 208-243 to chronic bromocriptine treatment. I: Evaluation of motor parameters in relation to striatal catecholamine content and dopamine receptors. Mov Disord. 1993;8:144–150. doi: 10.1002/mds.870080205. [DOI] [PubMed] [Google Scholar]
- 48.Tsui JK, Wolters EC, Peppard RF, Calne DB. A double-blind, placebo-controlled, dose-ranging study to investigate the safety and efficacy of CY 208-243 in patients with Parkinson’s disease. Neurology. 1989;39:856–858. doi: 10.1212/wnl.39.6.856. [DOI] [PubMed] [Google Scholar]
- 49.Hutcheson JD, Setola V, Roth BL, Merryman WD. Serotonin receptors and heart valve disease--it was meant 2B. Pharmacol Ther. 2011;132:146–157. doi: 10.1016/j.pharmthera.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pendleton RG, Samler L, Kaiser C, Ridley PT. Studies on renal dopamine receptors with a new agonist. European Journal of Pharmacology. 1978;51:19–28. doi: 10.1016/0014-2999(78)90057-2. [DOI] [PubMed] [Google Scholar]
- 51.Setler PE, Sarau HM, Zirkle CL, Saunders HL. The central effects of a novel dopamine agonist. European Journal of Pharmacology. 1978;50:419–430. doi: 10.1016/0014-2999(78)90148-6. [DOI] [PubMed] [Google Scholar]
- 52.Kaiser C, Dandridge PA, Garvey E, Hahn RA, Sarau HM, Setler PE, et al. Absolute stereochemistry and dopaminergic activity of enantiomers of 2,3,4,5-tetrahydro-7,8-dihydroxy-1-phenyl-1H-3-benzazepine. J Med Chem. 1982;25:697–703. doi: 10.1021/jm00348a017. [DOI] [PubMed] [Google Scholar]
- 53.Kaiser C, Jain T. Dopamine receptors: functions, subtypes and emerging concepts. Medicinal Research Reviews-1. 1985;5:145–229. doi: 10.1002/med.2610050202. [DOI] [PubMed] [Google Scholar]
- 54.Neumeyer JL, Kula NS, Bergman J, Baldessarini RJ. Receptor affinities of dopamine D1 receptor-selective novel phenylbenzazepines. European Journal of Pharmacology. 2003;474:137–140. doi: 10.1016/s0014-2999(03)02008-9. [DOI] [PubMed] [Google Scholar]
- 55.Watts VJ, Lawler CP, Gilmore JH, Southerland SB, Nichols DE, Mailman RB. Dopamine D1 receptors: efficacy of full (dihydrexidine) vs. partial (SKF38393) agonists in primates vs. rodents. European Journal of Pharmacology. 1993;242:165–172. doi: 10.1016/0014-2999(93)90076-t. [DOI] [PubMed] [Google Scholar]
- 56.Gilmore JH, Watts VJ, Lawler CP, Noll EP, Nichols DE, Mailman RB. “Full” dopamine D1 agonists in human caudate: biochemical properties and therapeutic implications. Neuropharmacology. 1995;34:481–488. doi: 10.1016/0028-3908(95)00014-w. [DOI] [PubMed] [Google Scholar]
- 57.Watts VJ, Lawler CP, Gonzales AJ, Zhou QY, Civelli O, Nichols DE, et al. Spare receptors and intrinsic activity: studies with D1 dopamine receptor agonists. Synapse. 1995;21:177–187. doi: 10.1002/syn.890210211. [DOI] [PubMed] [Google Scholar]
- 58.George MS, Molnar CE, Grenesko EL, Anderson B, Mu Q, Johnson K, et al. A single 20 mg dose of dihydrexidine (DAR-0100), a full dopamine D(1) agonist, is safe and tolerated in patients with schizophrenia. Schizophrenia Research. 2007;93:42–50. doi: 10.1016/j.schres.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 59.Downes RP, Waddington JL. Grooming and vacuous chewing induced by SK&F 83959, an agonist of dopamine ‘D1-like’ receptors that inhibits dopamine-sensitive adenylyl cyclase. European Journal of Pharmacology. 1993;234:135–136. doi: 10.1016/0014-2999(93)90718-w. [DOI] [PubMed] [Google Scholar]
- 60.Gnanalingham KK, Erol DD, Hunter AJ, Smith LA, Jenner P, Marsden CD. Differential anti-parkinsonian effects of benzazepine D 1 dopamine agonists with varying efficacies in the MPTP-treated common marmoset. Psychopharmacology (Berlin) 1995;117:275–286. doi: 10.1007/BF02246102. [DOI] [PubMed] [Google Scholar]
- 61.Gnanalingham KK, Hunter AJ, Jenner P, Marsden CD. The differential behavioural effects of benzazepine D1 dopamine agonists with varying efficacies, co-administered with quinpirole in primate and rodent models of Parkinson’s disease. Psychopharmacology (Berlin) 1995;117:287–297. doi: 10.1007/BF02246103. [DOI] [PubMed] [Google Scholar]
- 62.Perreault ML, Shen MY, Fan T, George SR. Regulation of c-fos expression by the dopamine D1-D2 receptor heteromer. Neuroscience. 2015;285:194–203. doi: 10.1016/j.neuroscience.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee SM, Kant A, Blake D, Murthy V, Boyd K, Wyrick SJ, et al. SKF-83959 is not a highly-biased functionally selective D1 dopamine receptor ligand with activity at phospholipase C. Neuropharmacology. 2014;86:145–154. doi: 10.1016/j.neuropharm.2014.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SM, Yang Y, Mailman RB. Dopamine D1 Receptor Signaling: Does GalphaQ-Phospholipase C Actually Play a Role? The Journal of pharmacology and experimental therapeutics. 2014;351:9–17. doi: 10.1124/jpet.114.214411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Meszaros J, et al. Evidence against dopamine D1/D2 receptor heteromers. Mol Psychiatry. 2015 doi: 10.1038/mp.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mailman R, Huang X, Nichols DE. Parkinson’s disease and D1 dopamine receptors. Current Opinion in Investigational Drugs. 2001;2:1582–1591. [PubMed] [Google Scholar]
- 67.Starr MS, Starr BS. Seizure promotion by D1 agonists does not correlate with other dopaminergic properties. J Neural Transm Park Dis Dement Sect. 1993;6:27–34. doi: 10.1007/BF02252620. [DOI] [PubMed] [Google Scholar]
- 68.Truex LL, Foreman MM, Riggs RM, Nichols DE. Effects of modifications of the 4-(3,4-dihydroxyphenyl)-1,2,3,4-tetrahydroisoquinoline structure on dopamine sensitive rat retinal adenylate cyclase activity. Society for Neuroscience Abstracts. 1985;11:315. [Google Scholar]
- 69.Andersen PH, Nielsen EB, Scheel-Kruger J, Jansen JA, Hohlweg R. Thienopyridine derivatives identified as the first selective, full efficacy, dopamine D1 receptor agonists. European Journal of Pharmacology. 1987;137:291–292. doi: 10.1016/0014-2999(87)90240-8. [DOI] [PubMed] [Google Scholar]
- 70.Lovenberg TW, Brewster WK, Mottola DM, Lee RC, Riggs RM, Nichols DE, et al. Dihydrexidine, a novel selective high potency full dopamine D-1 receptor agonist. Eur J Pharmacol. 1989;166:111–113. doi: 10.1016/0014-2999(89)90690-0. [DOI] [PubMed] [Google Scholar]
- 71.Lovenberg TW, Roth RH, Nichols DE, Mailman RB. D1 dopamine receptors of NS20Y neuroblastoma cells are functionally similar to rat striatal D1 receptors. Journal of Neurochemistry. 1991;57:1563–1569. doi: 10.1111/j.1471-4159.1991.tb06352.x. [DOI] [PubMed] [Google Scholar]
- 72.Mottola DM, Brewster WK, Cook LL, Nichols DE, Mailman RB. Dihydrexidine, a novel full efficacy D 1 dopamine receptor agonist. Journal of Pharmacology and Experimental Therapeutics. 1992;262:383–393. [PubMed] [Google Scholar]
- 73.Brewster WK, Nichols DE, Riggs RM, Mottola DM, Lovenberg TW, Lewis MH, et al. trans-10,11-Dihydroxy-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine: A highly potent selective dopamine D1 full agonist. J Med Chem. 1990;33:1756. doi: 10.1021/jm00168a034. [DOI] [PubMed] [Google Scholar]
- 74.Taylor JR, Lawrence MS, Redmond DE, Jr, Elsworth JD, Roth RH, Nichols DE, et al. Dihydrexidine, a full dopamine D1 agonist, reduces MPTP-induced parkinsonism in monkeys. European Journal of Pharmacology. 1991;199:389–391. doi: 10.1016/0014-2999(91)90508-n. [DOI] [PubMed] [Google Scholar]
- 75.Arnsten AFT, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 76.Darney KJ, Jr, Lewis MH, Brewster WK, Nichols DE, Mailman RB. Behavioral effects in the rat of dihydrexidine, a high-potency, full-efficacy D1 dopamine receptor agonist. Neuropsychopharmacology. 1991;5:187–195. [PubMed] [Google Scholar]
- 77.Kebabian JW, Briggs C, Britton DR, Asin K, DeNinno M, MacKenzie RG, et al. A68930: a potent and specific agonist for the D-1 dopamine receptor. American Journal of Hypertension. 1990;3:40S–42S. doi: 10.1093/ajh/3.6.40s. [DOI] [PubMed] [Google Scholar]
- 78.Kebabian JW, Britton DR, DeNinno MP, Perner R, Smith L, Jenner P, et al. A-77636: a potent and selective dopamine D1 receptor agonist with antiparkinsonian activity in marmosets. Eur J Pharmacol. 1992;229:203–209. doi: 10.1016/0014-2999(92)90556-j. [DOI] [PubMed] [Google Scholar]
- 79.DeNinno MP, Schoenleber R, MacKenzie R, Britton DR, Asin KE, Briggs C, et al. A68930: a potent agonist selective for the dopamine D1 receptor. European Journal of Pharmacology. 1991;199:209–219. doi: 10.1016/0014-2999(91)90459-4. [DOI] [PubMed] [Google Scholar]
- 80.Blanchet P, Bedard PJ, Britton DR, Kebabian JW. Differential effect of selective D-1 and D-2 dopamine receptor agonists on levodopa-induced dyskinesia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine- exposed monkeys. Journal of Pharmacology and Experimental Therapeutics. 1993;267:275–279. [PubMed] [Google Scholar]
- 81.Pearce RK, Jackson M, Smith L, Jenner P, Marsden CD. Chronic L-DOPA administration induces dyskinesias in the 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine-treated common marmoset (Callithrix Jacchus) Mov Disord. 1995;10:731–740. doi: 10.1002/mds.870100606. [DOI] [PubMed] [Google Scholar]
- 82.Asin KE, Wirtshafter D. Effects of repeated dopamine D 1 receptor stimulation on rotation and c-fos expression. European Journal of Pharmacology. 1993;235:167–168. doi: 10.1016/0014-2999(93)90840-e. [DOI] [PubMed] [Google Scholar]
- 83.Britton DR, Kebabian JW, Curzon P. Rapid reversal of denervation supersensitivity of dopamine D1 receptors by l-dopa or a novel dopamine D1 receptor agonist, A68930. European Journal of Pharmacology. 1991;200:89–93. doi: 10.1016/0014-2999(91)90670-l. [DOI] [PubMed] [Google Scholar]
- 84.Blanchet PJ, Grondin R, Bedard PJ, Shiosaki K, Britton DR. Dopamine D 1 receptor desensitization profile in MPTP-lesioned primates. European Journal of Pharmacology. 1996;309:13–20. doi: 10.1016/0014-2999(96)00309-3. [DOI] [PubMed] [Google Scholar]
- 85.Michaelides MR, Hong Y, DiDomenico S, Jr, Asin KE, Britton DR, Lin CW, et al. (5aR,11bS)-4,5,5a,6,7,11b-hexahydro-2-propyl-3-thia-5-azacyclopent-1-ena[c]-phenanthrene-9,10-diol (A-86929) a potent and selective dopamine D1 agonist that maintains behavioral efficacy following repeated administration and characterization of its diacetyl prodrug (ABT-431) Journal of Medicinal Chemistry. 1995;38:3445–3447. doi: 10.1021/jm00018a002. [DOI] [PubMed] [Google Scholar]
- 86.Shiosaki K, Jenner P, Asin KE, Britton DR, Lin CW, Michaelides M, et al. ABT-431: the diacetyl prodrug of A-86929, a potent and selective dopamine D 1 receptor agonist: in vitro characterization and effects in animal models of Parkinson’s disease. Journal of Pharmacology and Experimental Therapeutics. 1996;276:150–160. [PubMed] [Google Scholar]
- 87.Rascol O, Blin O, Thalamas C, Descombes S, Soubrouillard C, Azulay P, et al. ABT-431, a D1 receptor agonist prodrug, has efficacy in Parkinson’s disease. Annals of Neurology. 1999;45:736–741. doi: 10.1002/1531-8249(199906)45:6<736::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 88.Ghosh D, Snyder SE, Watts VJ, Mailman RB, Nichols DE. 9-Dihydroxy-2,3,7,11b-tetrahydro-1H-naph[1,2,3-de]isoquinoline: a potent full dopamine D1 agonist containing a rigid-beta-phenyldopamine pharmacophore. Journal of Medicinal Chemistry. 1996;39:549–555. doi: 10.1021/jm950707+. [DOI] [PubMed] [Google Scholar]
- 89.Gulwadi AG, Korpinen CD, Mailman RB, Nichols DE, Sit SY, Taber MT. Dinapsoline: characterization of a D 1 dopamine receptor agonist in a rat model of Parkinson’s disease. Journal of Pharmacology and Experimental Therapeutics. 2001;296:338–344. [PubMed] [Google Scholar]
- 90.Lewis MM, Watts VJ, Lawler CP, Nichols DE, Mailman RB. Homologous desensitization of the D1A dopamine receptor: efficacy in causing desensitization dissociates from both receptor occupancy and functional potency. Journal of Pharmacology and Experimental Therapeutics. 1998;286:345–353. [PubMed] [Google Scholar]
- 91.Ryman-Rasmussen JP, Griffith A, Oloff S, Vaidehi N, Brown JT, Goddard WA, III, et al. Functional selectivity of dopamine D(1) receptor agonists in regulating the fate of internalized receptors. Neuropharmacology. 2007;52:562–575. doi: 10.1016/j.neuropharm.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Blanchet PJ, Fang J, Gillespie M, Sabounjian L, Locke KW, Gammans R, et al. Effects of the full dopamine D1 receptor agonist dihydrexidine in Parkinson’s disease. Clinical Neuropharmacology. 1998;21:339–343. [PubMed] [Google Scholar]
- 93.Amenta F, Ferrante F, Ricci A. Pharmacological characterisation and autoradiographic localisation of dopamine receptor subtypes in the cardiovascular system and in the kidney. Hypertens Res. 1995;18(Suppl 1):S23–S27. doi: 10.1291/hypres.18.supplementi_s23. [DOI] [PubMed] [Google Scholar]
- 94.Mu Q, Johnson K, Morgan PS, Grenesko EL, Molnar CE, Anderson B, et al. A single 20 mg dose of the full D(1) dopamine agonist dihydrexidine (DAR-0100) increases prefrontal perfusion in schizophrenia. Schizophrenia Research. 2007;94:332–341. doi: 10.1016/j.schres.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 95.Rosell DR, Zaluda LC, McClure MM, Perez-Rodriguez MM, Strike KS, Barch DM, et al. Effects of the D1 dopamine receptor agonist dihydrexidine (DAR-0100A) on working memory in schizotypal personality disorder. Neuropsychopharmacology. 2015;40:446–453. doi: 10.1038/npp.2014.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin CW, Bianchi BR, Miller TR, Stashko MA, Wang SS, Curzon P, et al. Persistent activation of the dopamine D 1 receptor contributes to prolonged receptor desensitization: studies with A-77636. Journal of Pharmacology and Experimental Therapeutics. 1996;276:1022–1029. [PubMed] [Google Scholar]
- 97.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 98.Lehmann HE, Ban TA. The history of the psychopharmacology of schizophrenia. Can J Psychiatry. 1997;42:152–162. doi: 10.1177/070674379704200205. [DOI] [PubMed] [Google Scholar]
- 99.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 100.Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl) 1987;91:415–433. doi: 10.1007/BF00216006. [DOI] [PubMed] [Google Scholar]
- 101.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D’Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc Natl Acad Sci U S A. 1996;93:9235–9240. doi: 10.1073/pnas.93.17.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 103.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hietala J, Syvalahti E, Vuorio K, Rakkolainen V, Bergman J, Haaparanta M, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346:1130–1131. doi: 10.1016/s0140-6736(95)91801-9. [DOI] [PubMed] [Google Scholar]
- 105.McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia - A positron emission tomographic [F-18]fluorodopa study. Arch Gen Psychiat. 2004;61:134–142. doi: 10.1001/archpsyc.61.2.134. [DOI] [PubMed] [Google Scholar]
- 106.Reith J, Benkelfat C, Sherwin A, Yasuhara Y, Kuwabara H, Andermann F, et al. Elevated Dopa Decarboxylase Activity in Living Brain of Patients with Psychosis. P Natl Acad Sci USA. 1994;91:11651–11654. doi: 10.1073/pnas.91.24.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry. 2005;20:15–27. doi: 10.1016/j.eurpsy.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 109.Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: Combined results of the North American trials. J Clin Psychiat. 1997;58:538–546. doi: 10.4088/jcp.v58n1205. [DOI] [PubMed] [Google Scholar]
- 110.Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, et al. Effects of Clozapine on Positive and Negative Symptoms in Outpatients with Schizophrenia. Am J Psychiat. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- 111.Kasper S, Lerman MN, McQuade RD, Saha A, Carson WH, Ali M, et al. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol. 2003;6:325–337. doi: 10.1017/S1461145703003651. [DOI] [PubMed] [Google Scholar]
- 112.Meltzer HY, Casey DE, Garver DL, Marder SR, Masand PS, Miller D, et al. Assessing the effects of atypical antipsychotics on negative symptoms. J Clin Psychiat. 1998;59:28–34. [PubMed] [Google Scholar]
- 113.Green MF, Marshall BD, Wirshing WC, Ames D, Marder SR, McGurk S, et al. Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am J Psychiat. 1997;154:799–804. doi: 10.1176/ajp.154.6.799. [DOI] [PubMed] [Google Scholar]
- 114.Kern RS, Green MF, Marshall BD, Wirshing WC, Wirshing D, McGurk S, et al. Risperidone vs. haloperidol on reaction time, manual dexterity, and motor learning in treatment-resistant schizophrenia patients. Biol Psychiat. 1998;44:726–732. doi: 10.1016/s0006-3223(98)00088-2. [DOI] [PubMed] [Google Scholar]
- 115.Green MF, Marder SR, Glynn SM, McGurk SR, Wirshing WC, Wirshing DA, et al. The neurocognitive effects of low-dose haloperidol: A two-year comparison with risperidone. Biol Psychiat. 2002;51:972–978. doi: 10.1016/s0006-3223(02)01370-7. [DOI] [PubMed] [Google Scholar]
- 116.Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;161:985–995. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- 117.Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, et al. Long-term neurocognitive effects of olanzapine or low-dose haloperidol in first-episode psychosis. Biol Psychiatry. 2006;59:97–105. doi: 10.1016/j.biopsych.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 118.Keefe RS, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 119.Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 120.Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 121.Nuechterlein KH, Dawson ME, Gitlin M, Ventura J, Goldstein MJ, Snyder KS, et al. Developmental Processes in Schizophrenic Disorders: longitudinal studies of vulnerability and stress. Schizophr Bull. 1992;18:387–425. doi: 10.1093/schbul/18.3.387. [DOI] [PubMed] [Google Scholar]
- 122.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 123.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 124.Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980;286:74–76. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- 125.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 126.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 127.Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: Involvement in working memory. Science. 1991;251:947–950. doi: 10.1126/science.1825731. [DOI] [PubMed] [Google Scholar]
- 128.Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 129.Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of dopamine D1 receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Arnsten AF. Catecholamine regulation of the prefrontal cortex. J Psychopharmacol. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- 132.Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9:404–416. doi: 10.1177/1073858403252674. [DOI] [PubMed] [Google Scholar]
- 133.Vijayraghavan S, Wang M, Birnbaum SG, Bruce CJ, Williams GV, Arnsten AFT. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neuroscience. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 134.Weinberger DR, Berman KF, Chase TN. Mesocortical dopaminergic function and human cognition. Ann N Y Acad Sci. 1988;537:330–338. doi: 10.1111/j.1749-6632.1988.tb42117.x. [DOI] [PubMed] [Google Scholar]
- 135.Kahn RS, Harvey PD, Davidson M, Keefe RS, Apter S, Neale JM, et al. Neuropsychological correlates of central monoamine function in chronic schizophrenia: relationship between CSF metabolites and cognitive function. Schizophr Res. 1994;11:217–224. doi: 10.1016/0920-9964(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 136.Daniel DG, Weinberger DR, Jones DW, Zigun JR, Coppola R, Handel S, et al. The effect of amphetamine on regional cerebral blood flow during cognitive activation in schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1991;11:1907–1917. doi: 10.1523/JNEUROSCI.11-07-01907.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 139.Knable MB, Hyde TM, Murray AM, Herman MM, Kleinman JE. A postmortem study of frontal cortical dopamine D1 receptors in schizophrenics, psychiatric controls, and normal controls. Biol Psychiatry. 1996;40:1191–1199. doi: 10.1016/S0006-3223(96)00116-3. [DOI] [PubMed] [Google Scholar]
- 140.Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry. 1997;54:1089–1095. doi: 10.1001/archpsyc.1997.01830240045007. [DOI] [PubMed] [Google Scholar]
- 141.Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban NB, et al. Increased prefrontal cortical D1 receptors in drug naive patients with schizophrenia: a PET study with [11C]NNC112. J Psychopharmacol. 2011 doi: 10.1177/0269881111409265. [DOI] [PubMed] [Google Scholar]
- 142.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- 143.Karlsson P, Farde L, Halldin C, Sedvall G. PET study of D(1) dopamine receptor binding in neuroleptic-naive patients with schizophrenia. Am J Psychiatry. 2002;159:761–767. doi: 10.1176/appi.ajp.159.5.761. [DOI] [PubMed] [Google Scholar]
- 144.Slifstein M, van de Giessen E, Van Snellenberg JX, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extra-striatal dopamine release in schizophrenia: a PET fMRI study. JAMA Psychiatry. 2014 doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McClure MM, Harvey PD, Goodman M, Triebwasser J, New A, Koenigsberg HW, et al. Pergolide treatment of cognitive deficits associated with schizotypal personality disorder: continued evidence of the importance of the dopamine system in the schizophrenia spectrum. Neuropsychopharmacology. 2010;35:1356–1362. doi: 10.1038/npp.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Knoerzer TA, Nichols DE, Brewster WK, Watts VJ, Mottola D, Mailman RB. Dopaminergic benzo[a]phenanthridines: resolution and pharmacological evaluation of the enantiomers of dihydrexidine, the full efficacy D1 dopamine receptor agonist. J Med Chem. 1994;37:2453–2460. doi: 10.1021/jm00041a025. [DOI] [PubMed] [Google Scholar]
- 147.Slifstein M, Suckow RF, Javitch JA, Cooper T, Lieberman J, Abi-Dargham A. Characterization of in vivo pharmacokinetic properties of the dopamine D1 receptor agonist DAR-0100A in nonhuman primates using PET with [11C] NNC112 and [11C] raclopride. J Cereb Blood Flow Metab. 2011;31:293–304. doi: 10.1038/jcbfm.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lewis MA, Hunihan L, Watson J, Gentles RG, Hu S, Huang Y, et al. Discovery of D1 Dopamine Receptor Positive Allosteric Modulators (PAM) Characterization of Pharmacology and Identification of Residues Which Regulate Species Selectivity. Journal of Pharmacology and Experimental Therapeutics. 2015 doi: 10.1124/jpet.115.224071. [DOI] [PubMed] [Google Scholar]
- 149.Beadle DBCDA, Hao JL, Jr, Krushinski JH, Reinhard MR, Schaus JM, Wolfangel CD. WPO, editor. 3,4-dihydroisoquinolin-2(1h)-yl compounds. Lilly Inc; 2014. https://wwwgooglecom/patents/WO2014193781A1?cl=en. [Google Scholar]
- 150.Heidenreich BA, Mailman RB, Nichols DE, Napier TC. Partial and full dopamine D1 agonists produce comparable increases in ventral pallidal neuronal activity: contribution of endogenous dopamine. The Journal of pharmacology and experimental therapeutics. 1995;273:516–525. [PubMed] [Google Scholar]