Abstract
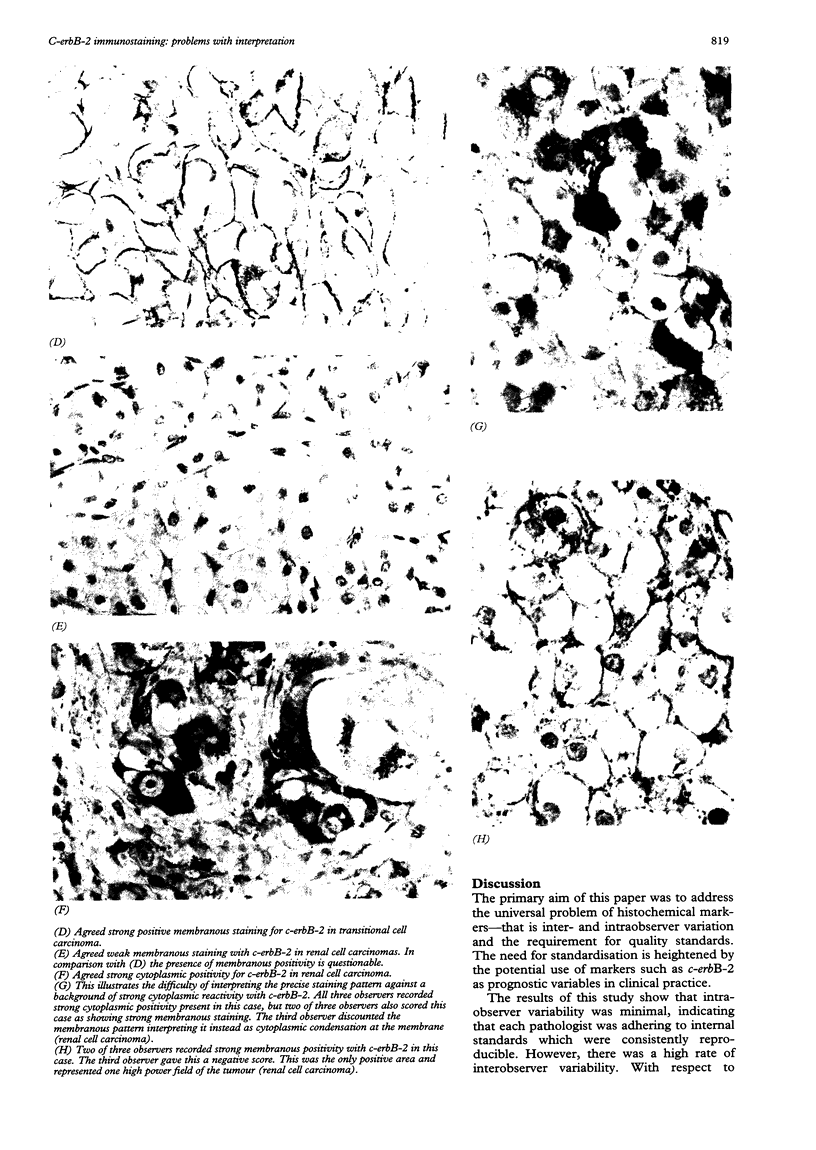
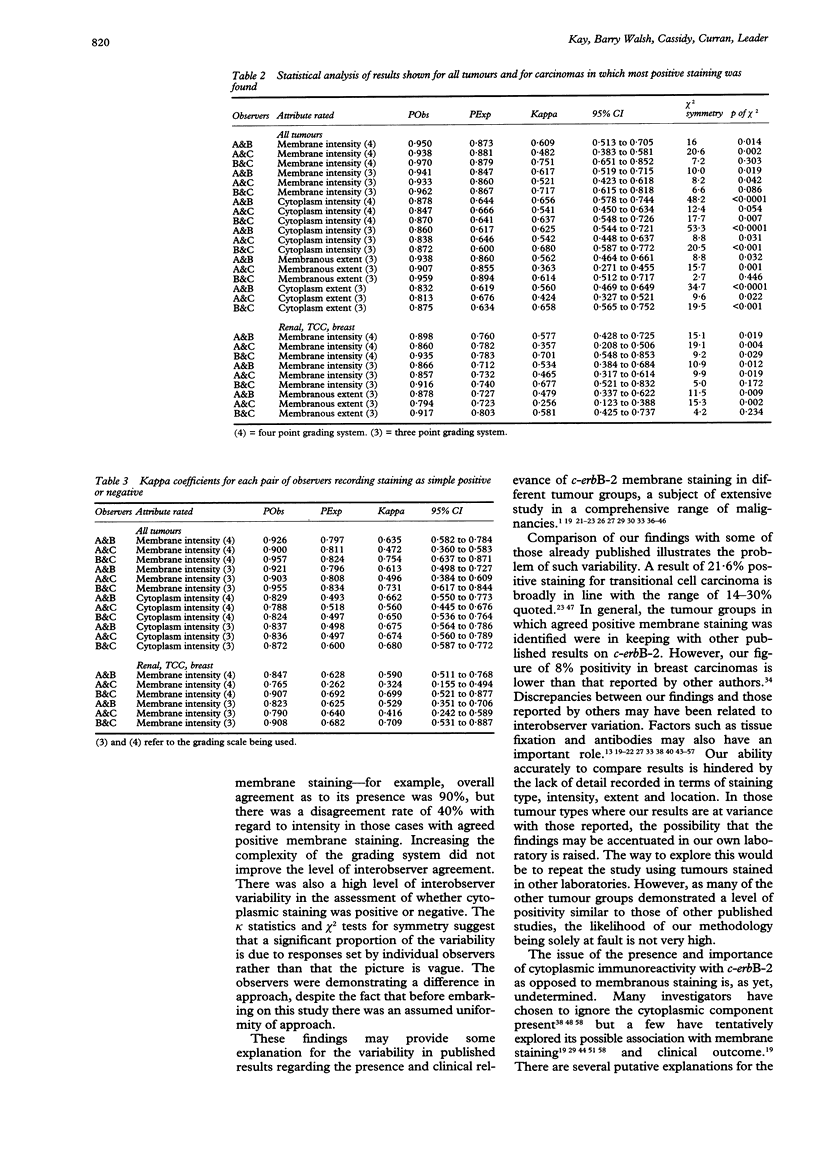
AIMS--To assess the consistency and reproducibility of assessment of c-erbB-2 immunostaining, and to examine some of the problems relating to inter- and intraobserver variability in the documentation of positive staining; to profile the spectrum of cytoplasmic and membranous staining in a wide range of tumour types. METHODS--A total of 283 neoplasms were examined for immunohistochemical expression of the c-erbB-2 oncoprotein. Three independent observers were required to assess intensity both of membrane and cytoplasmic staining on a three point and then a four point scale. Extent of positive staining was also assessed on a two point scale. A minimum of two weeks elapsed between assessments using the differing scales. RESULTS--Positive membrane staining was documented by one or more observers in 16.6% of tumours examined. This positivity was largely restricted to bladder, renal, and breast tumours. The overall level of disagreement as to the presence or absence of membranous staining was 11.3%. Cytoplasmic staining was identified in 55.5% of tumours studied. The level of disagreement as to the presence or absence of cytoplasmic staining was 26.5%. CONCLUSIONS--Intraobserver variability was minimal, indicating that each pathologist was adhering to internal reproducible standards. Interobserver variability was greater, indicating that the interpretation of c-erbB-2 immunostaining may require set guidelines. It is suggested that assessment should be referenced to a standard positive control, that a three tier system for grading of intensity and a two tier system for grading of extent should be adopted, and that the evaluation should be agreed by at least two pathologists. The presence of cytoplasmic staining should continue to be routinely recorded until its biological role and clinical implications are fully understood.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Sudo C., Ogawara H., Toyoshima K., Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986 Jun 27;232(4758):1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- Arnaout A. H., Dawson P. M., Soomro S., Taylor P., Theodorou N. A., Feldmann M., Fendly B. M., Shepard H. M., Shousha S. HER2 (c-erbB-2) oncoprotein expression in colorectal adenocarcinoma: an immunohistological study using three different antibodies. J Clin Pathol. 1992 Aug;45(8):726–727. doi: 10.1136/jcp.45.8.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Barnes D. M. Breast cancer and a proto-oncogene. BMJ. 1989 Oct 28;299(6707):1061–1062. doi: 10.1136/bmj.299.6707.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R. D. Cellular and molecular aspects of neoplastic progression in the mammary gland. Eur J Cancer Clin Oncol. 1988 Jan;24(1):15–20. doi: 10.1016/0277-5379(88)90171-x. [DOI] [PubMed] [Google Scholar]
- Cohen J. A., Weiner D. B., More K. F., Kokai Y., Williams W. V., Maguire H. C., Jr, LiVolsi V. A., Greene M. I. Expression pattern of the neu (NGL) gene-encoded growth factor receptor protein (p185neu) in normal and transformed epithelial tissues of the digestive tract. Oncogene. 1989 Jan;4(1):81–88. [PubMed] [Google Scholar]
- Coombs L. M., Pigott D. A., Sweeney E., Proctor A. J., Eydmann M. E., Parkinson C., Knowles M. A. Amplification and over-expression of c-erbB-2 in transitional cell carcinoma of the urinary bladder. Br J Cancer. 1991 Apr;63(4):601–608. doi: 10.1038/bjc.1991.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett I. P., Henry J. A., Angus B., Watchorn C. J., Wilkinson L., Hennessy C., Gullick W. J., Tuzi N. L., May F. E., Westley B. R. NCL-CB11, a new monoclonal antibody recognizing the internal domain of the c-erbB-2 oncogene protein effective for use on formalin-fixed, paraffin-embedded tissue. J Pathol. 1990 May;161(1):15–25. doi: 10.1002/path.1711610105. [DOI] [PubMed] [Google Scholar]
- Coussens L., Yang-Feng T. L., Liao Y. C., Chen E., Gray A., McGrath J., Seeburg P. H., Libermann T. A., Schlessinger J., Francke U. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985 Dec 6;230(4730):1132–1139. doi: 10.1126/science.2999974. [DOI] [PubMed] [Google Scholar]
- D'Emilia J., Bulovas K., D'Ercole K., Wolf B., Steele G., Jr, Summerhayes I. C. Expression of the c-erbB-2 gene product (p185) at different stages of neoplastic progression in the colon. Oncogene. 1989 Oct;4(10):1233–1239. [PubMed] [Google Scholar]
- De Potter C. R., Van Daele S., Van de Vijver M. J., Pauwels C., Maertens G., De Boever J., Vandekerckhove D., Roels H. The expression of the neu oncogene product in breast lesions and in normal fetal and adult human tissues. Histopathology. 1989 Oct;15(4):351–362. doi: 10.1111/j.1365-2559.1989.tb01587.x. [DOI] [PubMed] [Google Scholar]
- Fendly B. M., Winget M., Hudziak R. M., Lipari M. T., Napier M. A., Ullrich A. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 1990 Mar 1;50(5):1550–1558. [PubMed] [Google Scholar]
- Fox S. B., Day C. A., Rogers S. Lack of c-erbB-2 oncoprotein expression in male breast carcinoma. J Clin Pathol. 1991 Nov;44(11):960–961. doi: 10.1136/jcp.44.11.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman M., Ravia Y., Assaf D., Yamamoto T., Rozin R., Shiloh Y. Amplification of c-myc and c-erbB-2 proto-oncogenes in human solid tumors: frequency and clinical significance. Int J Cancer. 1989 Nov 15;44(5):802–805. doi: 10.1002/ijc.2910440509. [DOI] [PubMed] [Google Scholar]
- Haldane J. S., Hird V., Hughes C. M., Gullick W. J. c-erbB-2 oncogene expression in ovarian cancer. J Pathol. 1990 Nov;162(3):231–237. doi: 10.1002/path.1711620309. [DOI] [PubMed] [Google Scholar]
- Hale R. J., Buckley C. H., Fox H., Williams J. Prognostic value of c-erbB-2 expression in uterine cervical carcinoma. J Clin Pathol. 1992 Jul;45(7):594–596. doi: 10.1136/jcp.45.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Hughes C. M., Staddon S. L., Richman P. I., Gullick W. J., Lemoine N. R. The c-erb B-2 proto-oncogene in human pancreatic cancer. J Pathol. 1990 Jul;161(3):195–200. doi: 10.1002/path.1711610305. [DOI] [PubMed] [Google Scholar]
- Hanna W., Kahn H. J., Andrulis I., Pawson T. Distribution and patterns of staining of Neu oncogene product in benign and malignant breast diseases. Mod Pathol. 1990 Jul;3(4):455–461. [PubMed] [Google Scholar]
- Haugen D. R., Akslen L. A., Varhaug J. E., Lillehaug J. R. Expression of c-erbB-2 protein in papillary thyroid carcinomas. Br J Cancer. 1992 Jun;65(6):832–837. doi: 10.1038/bjc.1992.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung M. C., Schechter A. L., Chevray P. Y., Stern D. F., Weinberg R. A. Molecular cloning of the neu gene: absence of gross structural alteration in oncogenic alleles. Proc Natl Acad Sci U S A. 1986 Jan;83(2):261–264. doi: 10.1073/pnas.83.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Filipe M. I., Gullick W. J., Linehan J., Morris R. W. c-erbB-2 proto-oncogene expression and its relationship to survival in gastric carcinoma: an immunohistochemical study on archival material. Int J Cancer. 1991 Jul 9;48(5):668–671. doi: 10.1002/ijc.2910480506. [DOI] [PubMed] [Google Scholar]
- Kahn H. J., Hanna W., Auger M., Andrulis I. Expression and amplification of neu oncogene in pleomorphic adenomas of salivary gland. Arch Pathol Lab Med. 1992 Jan;116(1):80–83. [PubMed] [Google Scholar]
- Keatings L., Sinclair J., Wright C., Corbett I. P., Watchorn C., Hennessy C., Angus B., Lennard T., Horne C. H. c-erbB-2 oncoprotein expression in mammary and extramammary Paget's disease: an immunohistochemical study. Histopathology. 1990 Sep;17(3):243–247. doi: 10.1111/j.1365-2559.1990.tb00714.x. [DOI] [PubMed] [Google Scholar]
- King C. R., Kraus M. H., Aaronson S. A. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science. 1985 Sep 6;229(4717):974–976. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- Kokai Y., Dobashi K., Weiner D. B., Myers J. N., Nowell P. C., Greene M. I. Phosphorylation process induced by epidermal growth factor alters the oncogenic and cellular neu (NGL) gene products. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5389–5393. doi: 10.1073/pnas.85.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokai Y., Myers J. N., Wada T., Brown V. I., LeVea C. M., Davis J. G., Dobashi K., Greene M. I. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989 Jul 28;58(2):287–292. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- Lee J., Koh D., Ong C. N. Statistical evaluation of agreement between two methods for measuring a quantitative variable. Comput Biol Med. 1989;19(1):61–70. doi: 10.1016/0010-4825(89)90036-x. [DOI] [PubMed] [Google Scholar]
- Lemoine N. R., Wyllie F. S., Lillehaug J. R., Staddon S. L., Hughes C. M., Aasland R., Shaw J., Varhaug J. E., Brown C. L., Gullick W. J. Absence of abnormalities of the c-erbB-1 and c-erbB-2 proto-oncogenes in human thyroid neoplasia. Eur J Cancer. 1990;26(7):777–779. doi: 10.1016/0277-5379(90)90149-n. [DOI] [PubMed] [Google Scholar]
- McCann A., Dervan P. A., Johnston P. A., Gullick W. J., Carney D. N. c-erbB-2 oncoprotein expression in primary human tumors. Cancer. 1990 Jan 1;65(1):88–92. doi: 10.1002/1097-0142(19900101)65:1<88::aid-cncr2820650119>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- McKenzie S. J., Marks P. J., Lam T., Morgan J., Panicali D. L., Trimpe K. L., Carney W. P. Generation and characterization of monoclonal antibodies specific for the human neu oncogene product, p185. Oncogene. 1989 May;4(5):543–548. [PubMed] [Google Scholar]
- Mellon K., Thompson S., Charlton R. G., Marsh C., Robinson M., Lane D. P., Harris A. L., Horne C. H., Neal D. E. p53, c-erbB-2 and the epidermal growth factor receptor in the benign and malignant prostate. J Urol. 1992 Feb;147(2):496–499. doi: 10.1016/s0022-5347(17)37287-7. [DOI] [PubMed] [Google Scholar]
- Moriyama M., Morishita Y., Mori S., Akiyama T., Kato T. Ultrastructural localization of c-erbB-2 gene product in transitional cell carcinoma of the urinary tract. Ultrastruct Pathol. 1990 Sep-Oct;14(5):399–405. doi: 10.3109/01913129009007219. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Nicotra M. R., Bigotti A., Venturo I., Slamon D. J., Fendly B. M., Ullrich A. Expression of the p185 encoded by HER2 oncogene in normal and transformed human tissues. Int J Cancer. 1990 Mar 15;45(3):457–461. doi: 10.1002/ijc.2910450314. [DOI] [PubMed] [Google Scholar]
- Ong G., Gullick W., Sikora K. Oncoprotein stability after tumour resection. Br J Cancer. 1990 Apr;61(4):538–542. doi: 10.1038/bjc.1990.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivière A., Becker J., Löning T. Comparative investigation of c-erbB2/neu expression in head and neck tumors and mammary cancer. Cancer. 1991 Apr 15;67(8):2142–2149. doi: 10.1002/1097-0142(19910415)67:8<2142::aid-cncr2820670823>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Rotter M., Block T., Busch R., Thanner S., Höfler H. Expression of HER-2/neu in renal-cell carcinoma. Correlation with histologic subtypes and differentiation. Int J Cancer. 1992 Sep 9;52(2):213–217. doi: 10.1002/ijc.2910520210. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Hung M. C., Vaidyanathan L., Weinberg R. A., Yang-Feng T. L., Francke U., Ullrich A., Coussens L. The neu gene: an erbB-homologous gene distinct from and unlinked to the gene encoding the EGF receptor. Science. 1985 Sep 6;229(4717):976–978. doi: 10.1126/science.2992090. [DOI] [PubMed] [Google Scholar]
- Semba K., Kamata N., Toyoshima K., Yamamoto T. A v-erbB-related protooncogene, c-erbB-2, is distinct from the c-erbB-1/epidermal growth factor-receptor gene and is amplified in a human salivary gland adenocarcinoma. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6497–6501. doi: 10.1073/pnas.82.19.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton T. P., Niehans G. A., Gu F., Litz C. E., Hagen K., Qiu Q., Kiang D. T., Strickler J. G. Detection of c-erbB-2 activation in paraffin-embedded tissue by immunohistochemistry. Hum Pathol. 1992 Oct;23(10):1141–1150. doi: 10.1016/0046-8177(92)90032-x. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Kamps M. P. EGF-stimulated tyrosine phosphorylation of p185neu: a potential model for receptor interactions. EMBO J. 1988 Apr;7(4):995–1001. doi: 10.1002/j.1460-2075.1988.tb02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M., Wetzler M., Josefberg Z., Deutch A., Gutman M., Assaf D., Kris R., Shiloh Y., Givol D., Schlessinger J. Sporadic amplification of the HER2/neu protooncogene in adenocarcinomas of various tissues. Cancer Res. 1988 Mar 15;48(6):1517–1520. [PubMed] [Google Scholar]
- Tandon A. K., Clark G. M., Chamness G. C., Ullrich A., McGuire W. L. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989 Aug;7(8):1120–1128. doi: 10.1200/JCO.1989.7.8.1120. [DOI] [PubMed] [Google Scholar]
- Tauchi K., Hori S., Itoh H., Osamura R. Y., Tokuda Y., Tajima T. Immunohistochemical studies on oncogene products (c-erbB-2, EGFR, c-myc) and estrogen receptor in benign and malignant breast lesions. With special reference to their prognostic significance in carcinoma. Virchows Arch A Pathol Anat Histopathol. 1989;416(1):65–73. doi: 10.1007/BF01606471. [DOI] [PubMed] [Google Scholar]
- Thor A. D., Schwartz L. H., Koerner F. C., Edgerton S. M., Skates S. J., Yin S., McKenzie S. J., Panicali D. L., Marks P. J., Fingert H. J. Analysis of c-erbB-2 expression in breast carcinomas with clinical follow-up. Cancer Res. 1989 Dec 15;49(24 Pt 1):7147–7152. [PubMed] [Google Scholar]
- Tsutsumi Y., Naber S. P., DeLellis R. A., Wolfe H. J., Marks P. J., McKenzie S. J., Yin S. neu oncogene protein and epidermal growth factor receptor are independently expressed in benign and malignant breast tissues. Hum Pathol. 1990 Jul;21(7):750–758. doi: 10.1016/0046-8177(90)90035-4. [DOI] [PubMed] [Google Scholar]
- Tuzi N. L., Venter D. J., Reidlinger J. A., Dean C. J., Gullick W. J. Production and characterization of monoclonal antibodies to the c-erbB-2 proto-oncogene protein using a synthetic peptide immunogen. Biochem Soc Trans. 1988 Oct;16(5):675–677. doi: 10.1042/bst0160675. [DOI] [PubMed] [Google Scholar]
- Venter D. J., Tuzi N. L., Kumar S., Gullick W. J. Overexpression of the c-erbB-2 oncoprotein in human breast carcinomas: immunohistological assessment correlates with gene amplification. Lancet. 1987 Jul 11;2(8550):69–72. doi: 10.1016/s0140-6736(87)92736-x. [DOI] [PubMed] [Google Scholar]
- Wood D. P., Jr, Wartinger D. D., Reuter V., Cordon-Cardo C., Fair W. R., Chaganti R. S. DNA, RNA and immunohistochemical characterization of the HER-2/neu oncogene in transitional cell carcinoma of the bladder. J Urol. 1991 Nov;146(5):1398–1401. doi: 10.1016/s0022-5347(17)38123-5. [DOI] [PubMed] [Google Scholar]
- Wrba F., Gullick W. J., Fertl H., Amann G., Salzer-Kuntschik M. Immunohistochemical detection of the c-erbB-2 proto-oncogene product in normal, benign and malignant cartilage tissues. Histopathology. 1989 Jul;15(1):71–76. doi: 10.1111/j.1365-2559.1989.tb03042.x. [DOI] [PubMed] [Google Scholar]
- Wright C., Mellon K., Neal D. E., Johnston P., Corbett I. P., Horne C. H. Expression of c-erbB-2 protein product in bladder cancer. Br J Cancer. 1990 Nov;62(5):764–765. doi: 10.1038/bjc.1990.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Ikawa S., Akiyama T., Semba K., Nomura N., Miyajima N., Saito T., Toyoshima K. Similarity of protein encoded by the human c-erb-B-2 gene to epidermal growth factor receptor. Nature. 1986 Jan 16;319(6050):230–234. doi: 10.1038/319230a0. [DOI] [PubMed] [Google Scholar]
- van de Vijver M. J., Peterse J. L., Mooi W. J., Wisman P., Lomans J., Dalesio O., Nusse R. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988 Nov 10;319(19):1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]






