Abstract
The ability to rationally manipulate and augment the cytoplasmic membrane can be used to overcome many of the challenges faced by conventional cellular therapies and provide innovative opportunities when combined with new biotechnologies. The focus of this review is on emerging strategies used in cell functionalization, highlighting both pioneering approaches and recent developments. These will be discussed within the context of future directions in this rapidly evolving field.
Keywords: Functionalizing, cells, membrane, biomaterials
Introduction
The demand for cell functionalization
New biotechnologies, such as organ-on-a-chip1 and 3D bioprinting,2 are providing researchers with increasingly innovative approaches to studying disease, engineering tissue and promoting in vivo regeneration. These biotechnologies often demand unnatural functions from cells, for instance, in cell therapy, we would ideally like to target cells to a particular area of the body, often to promote an unnatural response within a hostile environment, while being able to visualize the entire process in vivo.3,4 Cells were never intended for use in allogeneic therapy,5 nor were tissues meant to be engineered in an incubator,6 or embryos in a test tube.7 Such applications are greatly removed from how a cell is programmed to function within an organism, which limits cells to pre-defined functions (e.g. cell-specific signaling pathways8 and surface markers9) and imposes tight constraints based on physiological conditions (e.g. temperature10 and oxygen tension11). Indeed, we are now at a stage where the cell itself could be considered the major restrictive factor, thus, effective methods to re-engineer cells are required to keep up with the rapid pace of biotechnological development.
An emerging strategy to overcome these limitations is cytoplasmic membrane modification, which can be used to either supplement the existing capabilities of a cell, or provide entirely new, non-native functionality. This cell functionalization approach has allowed us, for instance, to provide cells with additional binding sites12 and nutrients,13 protection in harsh environments,14 increased adhesion to scaffolds15 and magnetic contrast.16 Compared to genetic modification, these strategies are simpler, faster and can be used to deliver a greater variety of materials to a wider range of cells. The scope of this review will cover both the active and passive delivery of soft biomaterials (e.g. proteins, biopolymers and carbohydrates) with a specific focus on non-specific membrane binding and instances where cells have been augmented with added functionality. We will present a selection of both pioneering and recent approaches to cell functionalization, discuss their relative merits, and conclude by considering the challenges and future directions of this exciting new field.
The cell membrane as an addressable canvas
The cytoplasmic membrane was first identified by Wilhelm Pfeffer in 1877, who proposed a membrane theory that suggested cells were filled with an aqueous solution contained by a physical, semi-permeable barrier.17 In 1925, Gorter and Grendel famously used a Langmuir trough to demonstrate that the cytoplasmic membrane of erythrocytes was only two molecules thick.18 This led to the well-recognized phospholipid bilayer model, in which the cytoplasmic membrane was considered a lamellar bilayer stabilized by hydrophobic interactions between the fatty acid tails of the constituent phospholipids. It is now known that up to 1000 different lipids contribute to the bilayer structure, which has a thickness of approximately 75 Å.19 This lipid sandwich supports a wide array of proteins, which can be embedded within the bilayer (integral), loosely bound to the surface (peripheral) or attached via a lipid anchor (lipid-bound). This was elegantly portrayed in Singer and Nicolson’s fluid mosaic model in 1972, which depicted proteins laterally diffusing throughout a dynamic phospholipid bilayer, considered to be an isotropic fluid.20 An important advance on this model was the incorporation of lipid microdomains, which were proposed as non-equilibrium, two-dimensional aggregates of phospholipids and proteins, essential in membrane trafficking and turnover.21 Finally, an often overlooked component of the cytoplasmic membrane is the glycocalyx, a layer of glycans present on the outer membrane leaflet that is used by cells to interact with the extracellular environment.22 These components form the basis of the current understanding of the cytoplasmic membrane structure.
A cell biologist will (correctly) consider the phospholipid bilayer a protective structural barrier, with the proteins and carbohydrates as functional components regulating mass transport,23 adhesion24 and signaling.25 An alternative view of the cytoplasmic membrane is that of a canvas of addressable molecules and moieties, which can be exploited as targets for cell functionalization. Carolyn Bertozzi memorably described the cell membrane as a “sea of functionality,”26 and when viewed from this perspective, the phospholipid bilayer becomes a dynamic hydrophobic continuum into which lipid anchors may be inserted, while proteins, glycans and phospholipid head groups are simply a collection of chemically addressable functional groups. Having said this, a multitude of factors must be considered when designing a cell surface modification strategy. First and foremost is the maintenance of cell viability, which necessitates functionalization methodologies that employ aqueous conditions, physiological pH and ionic strength, as well as ambient temperature and pressure. Even under these cell-amenable conditions, the introduction of membrane-active chemical species can still lead to cytotoxicity through processes such membrane thinning or hole formation,27 while blocking or modifying specific glycans can also be detrimental to cell function. Second, the temporal persistence of the exogenous material must be considered. Unlike genetic modification, which can be used for long-term transgene expression, cell functionalization strategies are intrinsically transient due to membrane turnover and mitosis that continuously dilute membrane-bound species. Finally, the spatial location and orientation of the membrane guest molecule can be important for certain applications, for instance, a targeting antibody may require a linker to project it away from the membrane surface into extracellular space, while a receptor protein or glycan will need to transduce signals across the membrane bilayer. Fortunately, there exists an array of well-developed cell functionalization strategies that cater to different, individual requirements. The remainder of this review will discuss the relative merits and notable successes of three broad approaches; cell surface chemistry, non-covalent membrane labeling and extended cellular coating (Figure 1).
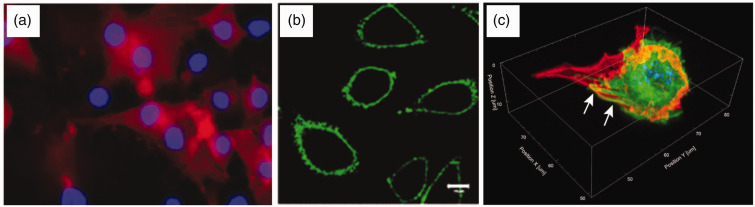
Figure 1.
Fluorescence microscopy images of functionalized cells. (a) An example of cell surface chemistry, with human foetal osteoblasts (nuclei-labeled blue with DAPI) metabolically labeled with L-azidohomoalanine were conjugated to a biotinylated alkyne that was subsequently visualized using fluorescent streptavidin (labeled red).28 Reprinted (adapted) with permission from Borcard et al. Bioconjugate Chemistry 22, 1422-32 Copyright 2011 American Chemical Society. (b) An example of non-covalent membrane labeling, in which a polyethylene glycol/oleyl chain was used to anchor proteins such as GFP (labeled green) into NIH3T3 cells.29 Reproduced with kind permission from John Wiley and Sons: Kato et al.29 (c) An example of an extended cellular coating, whereby matrix proteins including fibronectin (labeled red) were used to “shrink wrap” C2C12 cells (nuclei-labeled blue with DAPI, actin fibres labeled in green and indicated with arrows).30 Reproduced with kind permission from Springer Science + Business Media: Palchesko et al.,30 Figure 4(e). (A color version of this figure is available in the online journal.)
Covalent cell surface chemistry and bio-orthogonal labeling
Designing chemical syntheses under cytocompatible conditions is challenging, as cell viability has to be prioritized over more common objectives, such as reaction yield and rate. With this in mind, an attractive strategy is to use bio-inspired enzymatic reactions that have evolved to work under physiological conditions. For example, the McEver and Wohlgemuth Groups used α-1,3-fucosyltransferase to enzymatically modify selectin ligands with guanosine diphosphate fucose, in order to present the glycoprotein sialyl Lewis X (sLeX) on cord blood cells31 and human mesenchymal stem cells (hMSCs).32 Here, promoting the surface expression of sLeX was shown to mediate a non-native cell rolling response to endothelial selectins. Cell rolling was also targeted by the Karp Group in 2008,101 who used biotinylation of cell-surface amines to streptavidin-link biotinylated sLeX (Figure 2(a)). This report was preceded by a very similar “ProtEx” technology, developed by the Shirwan Group in 2005,33 which showed that streptavidin fusion proteins could persistently label cells in vitro and in vivo. This approach has been used to enhance graft survival with CD95L,34,35 inhibit cancer cell growth with CD8036,37 and produce whole-cell vaccines bearing GM-CSF/TNFα co-stimulators.38 Despite being less abundant than amines, thiol groups present on cysteine-bearing proteins are an attractive target for cell surface chemistry, and their capacity for click chemistry reactivity has been used, for instance, in the binding of maleimide-functionalized, drug-loaded liposomes.39 Overall, these direct cell surface modifications represent an excellent approach, albeit one that is restricted by constrained reaction conditions and a limited number and range of addressable groups.
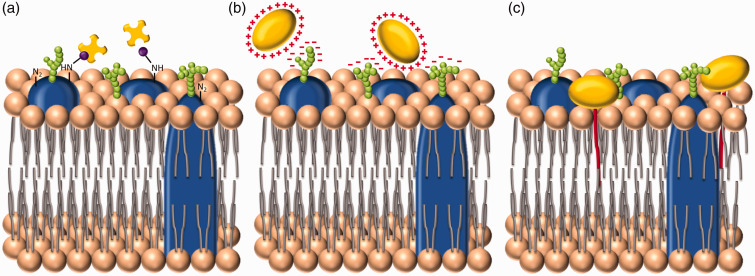
Figure 2.
Three broad approaches to cell membrane functionalization. (a) The first method is direct surface chemistry, performed on functional groups present on the cell membrane. Here, for instance, amine groups present on membrane proteins have been biotinylated (purple) to allow the addition of streptavidin (yellow). This approach is commonly used to deliver species labeled with streptavidin or biotin.87 (b) The second method is to increase the cationic surface charge of the exogenous species to facilitate attractive electrostatic interactions with negatively charged moieties present predominantly within the glycocalyx. (c) The third strategy uses hydrophobic interactions between a conjugated lipid tail and the phospholipid bilayer, to anchor the exogenous species to the cell membrane. (A color version of this figure is available in the online journal.)
A major breakthrough in this field was the development of “bio-orthogonal” chemistry, which was pioneered by the Bertozzi Group in 1997 (Figure 3).40 This built upon an established technique known as “metabolic labeling,” whereby culture medium supplemented with certain non-canonical amino acids or monosaccharides allowed the incorporation of new functional groups into the proteome or glycome.41,42 While metabolic labeling has been used in its own right as a functionalization tool to modulate virus–cell interactions,43 the Bertozzi Group extended the scope of this technology by introducing ketones as a reactive base for click chemistry reactions.40 This two-step, bio-orthogonal approach provided rapid kinetics with high specificity (i.e. no side reactivity) under physiological conditions, as well as great versatility. Indeed, azides, alkynes, thiols and methacryloyls have been successfully incorporated into cell surface glycans,44–47 amino acids15,28,48,49 and lipids,50 with applications that include the selective killing of cells,51 drug conjugation,46 cell-surface click gelation52 and artificial adhesion to 2D or 3D substrates.15,45 Metabolic labeling and bio-orthogonal strategies still suffer from limitations associated with tightly regulated biosynthetic pathways (more of an issue for amino acids than glycans), interference from specific metabolic pathways (a particular issue with ketone labeling), cytotoxicity arising from certain mediators (such as copper ions in certain azide–alkyne reactions) and the necessity to include a compatible, “clickable” functional group on the secondary reactive species.53 Most of these issues can be overcome with careful experimental design, and metabolic labeling coupled with bio-orthogonal bioconjugate chemistry remains an elegant approach to cell functionalization. Furthermore, metabolic labeling is the only approach discussed in this review, other than ProtEx, that has been effectively performed in vivo.54
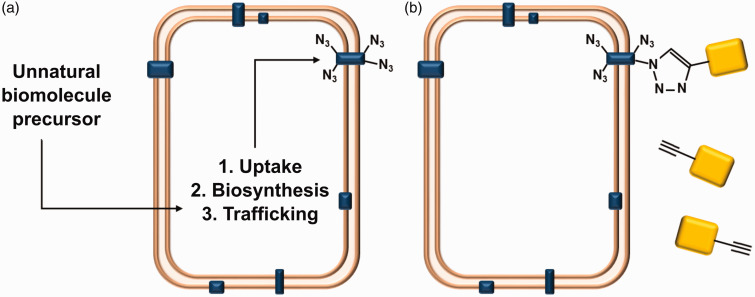
Figure 3.
Metabolic labeling and biorthogonal chemistry. (a) Unnatural biomolecular precursors, included as cell media additives, can be taken up by cells and become incorporated into lipids, carbohydrates or proteins (blue), including those at the cell membrane. (b) Metabolic labeling can be used to present reactive groups that can bind a secondary species (yellow). This is usually mediated by orthogonal click chemistry, in this example, an alkynated secondary species is bound to a cell metabolically labeled with azide groups. (A color version of this figure is available in the online journal.)
Non-covalent interactions with the cytoplasmic membrane
An extremely facile approach to cell functionalization is to generate a membrane-active biomaterial in isolation, rather than trying to perform in situ chemical reactions at the cell surface. Perhaps, the simplest approach is to generate a cationic molecule that will interact with anionic proteoglycans present within the cell glycocalyx (Figure 2(b)). One of the first examples of this approach was reported in 1972, when Danon et al.55 showed that chemically cationized ferritin could effectively contrast label cell membranes for electron microscopy. This approach used a relatively simple chemical reaction, whereby acidic amino acids on the protein shell were converted into non-native cationic residues via carbodiimide-mediated nucleophilic addition of reactive diamines. This approach was very recently applied to the superparamagnetic protein magnetoferritin, where hMSCs were contrast labeled for magnetic resonance imaging, using incubation periods as short as 1 min.16 The magnetization of hMSCs was reduced when the biosynthesis of sulfated proteoglycans was significantly inhibited, which was evidence that these anionic glycocalyx species play a major role in mediating the electrostatic binding of chemically cationized magnetoferritin.
A complementary approach, developed by the Liu Group at Harvard University, used aggressive site-directed mutagenesis to produce “supercharged proteins” possessing an unnaturally large number of charged residues.56 Here, a thermodynamically stable variant of the green fluorescent protein (GFP) bearing a theoretical net charge of +36 was shown to efficiently interact with membrane proteoglycans and was used to deliver proteins and DNA to a range of different cells.57–59 Both chemical cationization and supercharging, however, involve making widespread modifications to the surface of a protein, which can lead to conformational changes in secondary and tertiary structure and subsequent loss of biological activity. In general, however, cationization represents a sound approach for delivering robust proteins (e.g. ferritin, GFP) to the cell membrane, however, this approach is likely to be challenging for more structurally sensitive proteins, while certain cationic species have also been shown to induce cytotoxicity via membrane thinning and hole formation.27
An alternative strategy to induce artificial membrane binding is to use a hydrophobic moiety to anchor a species to the phospholipid bilayer (Figure 2(c)). A pioneering example was introduced by Kim and Peacock,60 who decorated hybridoma cells with anti-mouse antibodies using palmitate protein A. This advance was achieved by exploiting the ability of the palmitate lipid to intercalate with the cytoplasmic membrane, and the affinity of protein A for the Fc region of antibodies. Using palmitated proteins as a scaffold ensures that any bound antibodies are oriented away from the cell surface, which effectively presents the binding paratope. A decade later, this technology was adopted by the Dennis Group, who used palmitate protein G and tissue-specific antibodies to target chondrogenic progenitor cells to cartilage,61 as well as hMSCs to endothelial cells,12 the colon62 and infarcted heart tissue.63 In 2000, the Tykocinski Group broadened this approach beyond antibodies by expressing fusion proteins containing the immunoglobulin Fc region, which could specifically bind membrane-anchored palmitate protein A.64 Variations on this “protein transfer” technique have been used to induce cell-rolling by functionalizing MSCs with CD162,65 as well as eliciting anti-tumor responses using an array of co-stimulators and cytokines, including CD80,66,67 CD254,68,69 CCL21,68,69 CD95L68 and CD137L.68,69
In 1995, the Selvaraj and Tykocinski Groups introduced a biomimetic method known as “cell surface painting” using proteins recombinantly-tagged with glycosylphosphatidylinositol (GPI).70,71 The GPI tail, present in many native membrane-bound proteins, inserts into lipid raft domains in the cytoplasmic membrane and anchors the fusion protein to the cell, without the need for any intermediary species. Importantly, the original function of the anchored protein is retained, which allowed cells to be painted with a wide range of proteins, including CD80,70–75 CD86,71,72 CD1,76 IL-12,77 TIMP-1,78 TCR,79 CCL580 and the I-domain of CD11a.81 A major drawback to both protein transfer and cell surface painting is the reliance upon fusion proteins, which limits versatility, and can be time-consuming to prepare at sufficient quantities. With this in mind, a number of groups have used liposome-based delivery vectors to present antigens82 and synthetic membrane receptor mimics,83 increase the cellular association of GPI-anchored proteins84 and provide binding sites for secondary species, such as biotinylated SLeX.85 Liposome-based approaches, however, are often limited by poor encapsulation efficiency, particularly with large biomolecular species.86
The limitations surrounding fusion proteins and liposomes can be circumvented in several ways, for instance, by using synthetic glycoprotein analogues87 or metal-chelating lipids bearing nickel nitriloacetic acid (NTA) bound to polyhistidine-tagged proteins.88 Another, bioconjugation approach involves the direct covalent coupling of lipids to proteins (lipidation) to display hydrophobic tails such as myristyl,89,90 palmityl,90,91 stearyl90,92 or oleyl.93–95 Here, the membrane affinity of lipidated proteins can be tuned to some extent by increasing the molecular weight of the lipid,96 while membrane persistence can be increased by using branched lipid tails.29 A limitation of protein lipidation, however, is the requirement of organic co-solvents or detergents to prevent protein aggregation. A new technology that circumvents these issues is “cell priming,” which uses chemically cationized proteins electrostatically conjugated to a responsive poly(ethylene glycol) (PEG)-based surfactant corona. Conformational rearrangement of the amphiphilic polymer surfactant promotes protein stability and aqueous solubility (due to the hydrophilic PEG segment),97,98 and mediates membrane tethering for around one week in culture (via the hydrophobic tail).13 Myoglobin conjugates retained their oxygen-binding capacity99 and were delivered to hMSCs to provide an in situ oxygen reservoir to enhance the production of matrix fibres at the centre of engineered cartilage constructs.13 Importantly, both cell priming and protein lipidation necessitate careful modification of the protein surface, as aggressive bioconjugation strategies can lead to denaturation and subsequent loss of biological function.100 With this in mind, orthogonal or site-specific modifications are an attractive option, however, these approaches are not feasible for all proteins.
Extended cellular coatings
An entirely different approach to cell functionalization is to wrap or patch cells with thin polymeric microsheets. For instance, agarose, carrageenan or low-methoxy pectin biopolymers have been used to generate a 50-µm thick gel veneer around newly fertilized toad eggs.102,103 This process is an excellent example of a single-cell coating (rather than gel encapsulation) and was shown to be effective at preventing microbial infection and improving post-hatching survival rates. This study, however, was demonstrated using relatively large cells (diameter = 1–1.5 mm) and has not been applied to smaller cells (diameter <100 µm). Palchesko et al.30 reported a more advanced, microscale technology that used extracellular matrix protein sheets to “shrink wrap” endothelial cells, myoblasts and cardiomyocytes. This global coating of functional biomolecules was shown to be effective at modulating the structure, adhesion and behavior of the coated cells and was used in the study of cell–matrix interactions.
In 2008, the Rubner Group reported that photolithography and layer-by-layer assembly could be used to generate cell-binding patches of polymer104 or mucin/lectin.105 These so-called “cell backpacks” were persistently attached to one side of T-cells and monocytes, respectively, and have been used to magnetize cells,104 promote non-native cellular assembly,106 provide resistance to phagocytosis107 and deliver therapeutics by “hitchhiking” on the surface of monocytes.108 The major limitation of this approach is the requirement for time-consuming, layer-by-layer deposition of polymeric material, however, this was recently addressed by the Guan Group, who used microcontact printing as a simpler, cheaper and higher throughput method for generating cellular backpacks.109,110
Discussion and new developments
The strategies discussed above could be broadly considered as passive labeling technologies, whereby cells exposed to bulk media, reagents or biomaterials are functionalized in an indiscriminate fashion. Recently, there has been a focus on more controlled approaches that allow targeted delivery of discrete biomaterial payloads to specific areas of individual cells. In 2011, for example, the Cojoc Group reported on a new approach whereby liposomes were maneuvered toward the surface of individual neurons using optical tweezers.111,112 The optically trapped liposomes were then ruptured, using an external pulse of ultraviolet radiation, which released proteins and chemical stimuli that directionally stimulated adjacent neurons. A similar approach was developed by the Perriman Group to optically deliver membrane-free coacervate microdroplets to the cytoplasmic membrane of MSCs (Figure 4). Here, the coacervate microdroplets were optimized to undergo spontaneous fusion with the cell membrane, without the need for external stimulation.113 Moreover, the coacervate microdroplets could be pre-loaded with biomolecules (e.g. proteins, nucleic acids or small molecular dyes), allowing cells to be “paintballed” with discrete patches of functional payload. While these approaches are intrinsically low throughput, with respect to total cell number, they represent extremely powerful diagnostic and experimental tools for site-specific or single-cell functionalization.
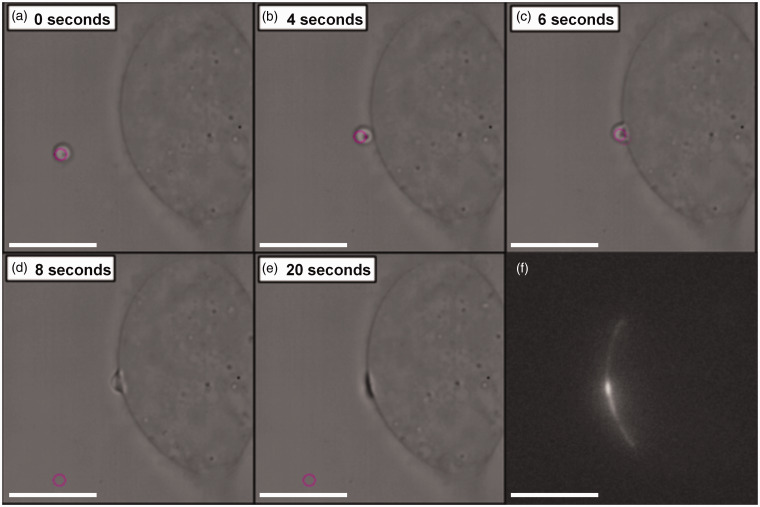
Figure 4.
Cell paintballing using coacervate microdroplets. Armstrong et al. recently demonstrated that membrane-free coacervate microdroplets can be actively loaded with biomaterial payloads of protein or nucleotides, and then delivered to the cell membrane using optical tweezers.113 (a)–(e) Time-lapse bright field microscope images showing an optical trap (pink circle) maneuvering a GFP-loaded coacervate microdroplet toward a human mesenchymal stem cell to initiate a targeted fusion event. (f) Fluorescence microscopy revealed fluorescence emission from the GFP payload present at the site of delivery
In summary, it is clear that rational reconfiguration of the cytoplasmic membrane is a highly effective pathway to endow cells with new functionality to enhance cell-based biotechnologies. Indeed, the rapid pace of biotechnological advance makes this an opportune moment to add an extra dimension to the host of cell-based therapies at our fingertips, whether this is targeting cells to diseased tissues or tumors, engineering whole cell vaccines, interfacing cells with materials for bioelectronics and biosensing, regenerative medicine or disease modeling. What is surprising, is that cell functionalization remains an under-exploited tool, particularly when contrasted with the success of transfection “toolkits” that have made genetic manipulation a routine undertaking. This disparity may be rationalized, in part, by the inherently interdisciplinary nature of cell functionalization, which draws on expertize from disparate fields of synthetic chemistry, materials science, biochemistry and cell biology, and generally necessitates a more considered, bespoke approach. To this end, we hope that this review will serve as an aid to a greater understanding of the subtle differences between strategies, encourage the adoption of current techniques, and inspire the development of new cell functionalization methodologies.
Acknowledgements
We thank the Elizabeth Blackwell Institute for funding JPKA, and EPSRC (Early Career Fellowship EP/K026720/1) for support of AWP.
Author contributions
JPKA and AWP contributed equally to this paper. The manuscript was written by JPKA and AWP.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science 2009; 328: 1662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derby B. Printing and prototyping of tissues and scaffolds. Science 2012; 338: 921–7. [DOI] [PubMed] [Google Scholar]
- 3.George JC. Stem cell therapy in acute myocardial infarction: a review of clinical trials. Transl Res 2010; 155: 10–19. [DOI] [PubMed] [Google Scholar]
- 4.Strauer BE, Kornowski R. Stem cell therapy in perspective. Circulation 2003; 107: 929–34. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol 1991; 49: 281–355. [DOI] [PubMed] [Google Scholar]
- 6.Cima LG, Vacanti JP, Vacanti C, Ingber D, Mooney D, Langer R. Tissue engineering by cell transplantation using degradable polymer substrates. J Biomech Eng 1991; 113: 143–51. [DOI] [PubMed] [Google Scholar]
- 7.Edwards RG, Bavister BD, Steptoe PC. Early stages of fertilization in vitro of human oocytes matured in vitro. Nature 1969; 221: 632–5. [DOI] [PubMed] [Google Scholar]
- 8.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1 associated protein TRADD signals cell death and NF kappa B activation. Cell 1995; 81: 495–504. [DOI] [PubMed] [Google Scholar]
- 9.Dzionek A, Inagaki Y, Okawa K, Nagafune J, Rock J, Sohma Y, Winkels G, Zysk M, Yamaguchi Y, Schmitz J. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum Immunol 2002; 63: 1133–48. [DOI] [PubMed] [Google Scholar]
- 10.Bloemkolk J-W, Gray MR, Merchant F, Mosmann TR. Effect of temperature on hybridoma cell cycle and MAb production. Biotechnol Bioeng 1992; 40: 427–31. [DOI] [PubMed] [Google Scholar]
- 11.Malda J, Klein TJ, Upton Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng 2007; 13: 2153–62. [DOI] [PubMed] [Google Scholar]
- 12.Ko IK, Kean TJ, Dennis JE. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials 2009; 30: 3702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong JPK, Shakur R, Horne JP, Dickinson SC, Armstrong CT, Lau K, Kadiwala J, Lowe A, Mann S, Anderson JLR, Perriman AW, Hollander AP. Artificial membrane binding proteins stimulate oxygenation of stem cells during tissue engineering of large cartilage constructs. Nat Comm 2015; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kampf N. The use of polymers for coating of cells. Polym Advan Technol 2003; 905: 896–905. [Google Scholar]
- 15.Juillerat FK, Borcard F, Staedler D, Scaletta C, Applegate LA, Comas H, Gauckler LJ, Gerber-Lemaire S, Juillerat-Jeanneret L, Gonzenbach UT. Functionalization of microstructured open-porous bioceramic scaffolds with human fetal bone cells. Bioconjugate Chem 2012; 23: 2278–90. [DOI] [PubMed] [Google Scholar]
- 16.Carreira SC, Armstrong JPK, Seddon AM, Perriman AW, Hartley-Davies R, Schwarzacher W. Ultra-fast stem cell labelling using cationised magnetoferritin. Nanoscale 2016; 8: 7474–83. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer W. Osmotic investigations: studies on cell mechanics, Leipzig: Englemann, 1877. [Google Scholar]
- 18.Gorter E, Grendel F. On bimolecular layers of lipoids on the chromocytes of the blood. J Exp Med 1925; 41: 439–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edidin M. Lipids on the frontier: a century of cell-membrane bilayers. Nat Rev Mol Cell Biol 2003; 4: 414–8. [DOI] [PubMed] [Google Scholar]
- 20.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science 1972; 175: 720–31. [DOI] [PubMed] [Google Scholar]
- 21.Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci 2005; 118: 1099–102. [DOI] [PubMed] [Google Scholar]
- 22.Lanctot PM, Gage FH, Varki AP. The glycans of stem cells. Curr Opin Chem Biol 2007; 11: 373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kyte J. Molecular considerations relevant to the mechanism of active transport. Nature 1981; 292: 201–4. [DOI] [PubMed] [Google Scholar]
- 24.Mager MD, LaPointe V, Stevens MM. Exploring and exploiting chemistry at the cell surface. Nat Chem 2011; 3: 582–9. [DOI] [PubMed] [Google Scholar]
- 25.Kholodenko BN. Cell-signalling dynamics in time and space. Nat Rev Mol Cell Biol 2006; 7: 165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sletten EM, Bertozzi CR. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew Chem Int Ed 2009; 48: 6974–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nel AE, Mädler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 2009; 8: 543–57. [DOI] [PubMed] [Google Scholar]
- 28.Borcard F, Godinat A, Staedler D, Blanco HC, Dumont A-L, Chapuis-Bernasconi C, Scaletta C, Applegate LA, Juillerat FK, Gonzenbach UT, Gerber-Lemaire S, Juillerat-Jeanneret L. Covalent cell surface functionalization of human fetal osteoblasts for tissue engineering. Bioconjugate Chem 2011; 22: 1422–32. [DOI] [PubMed] [Google Scholar]
- 29.Kato K, Itoh C, Yasukouchi T, Nagamune T. Rapid protein anchoring into the membranes of mammalian cells using oleyl chain and poly(ethylene glycol) derivatives. Biotechnol Prog 2004; 20: 897–904. [DOI] [PubMed] [Google Scholar]
- 30.Palchesko RN, Szymanski JM, Sahu AM, Feinberg AW. Shrink wrapping cells in a defined extracellular matrix to modulate the chemo-mechanical microenvironment. Cell Mol Bioeng 2014; 7: 355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia L, McDaniel JM, Yago T, Doeden A, Mcever RP. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood 2004; 104: 3091–6. [DOI] [PubMed] [Google Scholar]
- 32.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med 2008; 14: 181–7. [DOI] [PubMed] [Google Scholar]
- 33.Singh NP, Yolcu ES, Askenasy N, Shirwan H. ProtEx: a novel technology to display exogenous proteins on the cell surface for immunomodulation. Ann N Y Acad Sci 2005; 1056: 344–58. [DOI] [PubMed] [Google Scholar]
- 34.Yolcu ES, Askenasy N, Singh NP, Cherradi S-EL, Shirwan H. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity 2002; 17: 795–808. [DOI] [PubMed] [Google Scholar]
- 35.Askenasy N, Yolcu E, Wang Z, Shirwan H. Display of Fas ligand protein on cardiac vasculature as a novel means of regulating allograft rejection. Circulation 2003; 107: 1525–31. [DOI] [PubMed] [Google Scholar]
- 36.Singh NP, Yolcu ES, Taylor DD, Singh NP, Yolcu ES, Taylor DD, Gercel-Taylor C, Metzinger DS, Dreisbach SK, Shirwan H. A novel approach to cancer immunotherapy: tumor cells decorated with CD80 generate effective antitumor immunity. Cancer Res 2003; 63: 4067–73. [PubMed] [Google Scholar]
- 37.Singh NP, Miller RW, Yolcu ES, Kilinc MO, Oechsli M, Huseby R, Taylor DD, Perry MT, Larocca RV, Shirwan H. Primary tumor cells resected from cancer patients and decorated with a novel form of CD80 protein serve as effective antigen-presenting cells for the induction of autologous T cell immune responses ex vivo. Hum Gene Ther 2006; 17: 334–46. [DOI] [PubMed] [Google Scholar]
- 38.Yin W, He Q, Hu Z, Chen Z, Qifeng M, Zhichun S, Zhihui Q, Xiaoxia N, Li J, Gao J. A novel therapeutic vaccine of GM-CSF/TNFalpha surface-modified RM-1 cells against the orthotopic prostatic cancer. Vaccine 2010; 28: 4937–44. [DOI] [PubMed] [Google Scholar]
- 39.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med 2010; 16: 1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahal LK, Bertozzi CR. Engineered cell surfaces: fertile ground for molecular landscaping. Chem Biol 1997; 4: 415–22. [DOI] [PubMed] [Google Scholar]
- 41.Wilson MJ, Hatfield DL. Incorporation of modified amino acids into proteins in vivo. Biochim Biophys Acta 1984; 781: 205–15. [DOI] [PubMed] [Google Scholar]
- 42.Kayser H, Zeitler R, Kannicht C, Grunow D, Nuck R, Reutter W. Biosynthesis of a nonphysiological sialic acid in different rat organs, using N-propanoyl-D-hexosamines as precursors. J Biol Chem 1992; 267: 16934–8. [PubMed] [Google Scholar]
- 43.Keppler OT, Stehling P, Herrmann M, Kayser H, Grunow D, Reutter W, Pawlita M. Biosynthetic modulation of sialic acid-dependent virus-receptor interactions of two primate polyoma viruses. J Biol Chem 1995; 270: 1308–14. [DOI] [PubMed] [Google Scholar]
- 44.Saxon E, Bertozzi CR. Cell surface engineering by a modified staudinger reaction. Science 2000; 287: 2007–11. [DOI] [PubMed] [Google Scholar]
- 45.Du J, Che P, Wang Z, Aich U, Yarema KJ. Designing a binding interface for control of cancer cell adhesion via 3D topography and metabolic oligosaccharide engineering. Biomaterials 2011; 32: 5427–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okeley NM, Toki BE, Zhang X, Jeffrey SC, Burke PJ, Alley SC, Senter PD. Metabolic engineering of monoclonal antibody carbohydrates for antibody – drug conjugation. Bioconjugate Chem 2013; 24: 1650–5. [DOI] [PubMed] [Google Scholar]
- 47.Sprung R, Nandi A, Chen Y, Kim SC, Barma D, Falck JR, Zhao Y. Tagging-via-substrate strategy for probing O-GlcNAc modified proteins. J Proteome Res 2005; 4: 950–7. [DOI] [PubMed] [Google Scholar]
- 48.Kho Y, Kim SC, Jiang C, Barma D, Kwon SW, Cheng J, Janis J, Weinbaum C, Tamanoi F, Falck J, Zhao Y. A tagging-via-substrate technology for detection and proteomics of farnesylated proteins. Proc Natl Acad Sci U S A 2004; 101: 12479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiick KL, Saxon E, Tirrell DA, Bertozzi CR. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc Natl Acad Sci U S A 2001; 99: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin DDO, Vilas GL, Prescher JA, Rajaiah G, Falck JR, Bertozzi CR, Berthiaume LG. Rapid detection, discovery, and identification of post- translationally myristoylated proteins during apoptosis using a bio-orthogonal azidomyristate analog. FASEB J 2008; 22: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mahal LK, Yarema KJ, Bertozzi CR. Engineering chemical reactivity on cell surfaces through -oligosaccharide biosynthesis. Science 1997; 276: 1125–8. [DOI] [PubMed] [Google Scholar]
- 52.Iwasaki Y, Matsunaga A, Fujii S. Preparation of biointeractive glycoprotein-conjugated hydrogels through metabolic oligosacchalide engineering. Bioconjugate Chem 2014; 25: 1626–31. [DOI] [PubMed] [Google Scholar]
- 53.Patterson DM, Nazarova LA, Prescher JA. Finding the right (bioorthogonal) chemistry. ACS Chem Biol 2014; 9: 592–605. [DOI] [PubMed] [Google Scholar]
- 54.Prescher JA, Bertozzi CR. Chemistry in living systems. Nat Chem Biol 2005; 1: 13–21. [DOI] [PubMed] [Google Scholar]
- 55.Danon D, Goldstein L, Marikovsky Y, Skutelsky E. Use of cationized ferritin as a label of negative charges on cell surfaces. J Ultra Mol Struct Res 1972; 38: 500–10. [DOI] [PubMed] [Google Scholar]
- 56.Lawrence MS, Phillips KJ, Liu DR. Supercharging proteins can impart unusual resilience. J Am Chem Soc 2007; 129: 10110–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNaughton BR, Cronican JJ, Thompson DB, Liu DR. Mammalian cell penetration, siRNA transfection, and DNA transfection by supercharged proteins. Proc Natl Acad Sci U S A 2009; 106: 6111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cronican JJ, Thompson DB, Beier KT, McNaughton BR, Cepko CL, Liu DR. Potent delivery of functional proteins into mammalian cells. ACS Chem Biol 2010; 5: 747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson DB, Villaseñor R, Dorr BM, Zerial M, Liu DR. Cellular uptake mechanisms and endosomal trafficking of supercharged proteins. Chem Biol 2012; 19: 831–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SA, Peacock JS. The use of palmitate-conjugated protein A for coating cells with artificial receptors which facilitate intercellular interactions. J Immunol Methods 1993; 158: 57–65. [DOI] [PubMed] [Google Scholar]
- 61.Dennis JE, Cohen N, Goldberg VM, Caplan AI. Targeted delivery of progenitor cells for cartilage repair. J Orthop Res 2004; 22: 735–41. [DOI] [PubMed] [Google Scholar]
- 62.Ko IK, Kim B-G, Awadallah A, Mikulan J, Lin P, Letterio JJ, Dennis JE. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther 2010; 18: 1365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kean TJ, Duesler L, Young RG, Dadabayev A, Olenyik A, Penn M, Wagner J, Fink DJ, Caplan AI, Dennis JE. Development of a peptide-targeted, myocardial ischemia-homing, mesenchymal stem cell. J Drug Target 2012; 20: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen A, Zheng G, Tykocinski ML. Hierarchical costimulator thresholds for distinct immune responses: application of a novel two-step Fc fusion protein transfer method. J Immunol 2000; 164: 705–11. [DOI] [PubMed] [Google Scholar]
- 65.Lo CY, Antonopoulos A, Dell A, Haslam SM, Lee T, Neelamegham S. The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials 2013; 34: 8213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng G, Chen A, Sterner RE, Zhang PJ, Pan T, Kiyatkin N, Tykocinski ML. Induction of antitumor immunity via intratumoral tetra-costimulator protein transfer. Cancer Res 2001; 61: 8127–34. [PubMed] [Google Scholar]
- 67.Zheng G, Liu S, Wang P, Xu Y, Chen A. Arming tumor-reactive T cells with costimulator B7-1 enhances therapeutic efficacy of the T cells. Cancer Res 2006; 66: 6793–9. [DOI] [PubMed] [Google Scholar]
- 68.Liu S, Breiter DR, Zheng G, Chen A. Enhanced antitumor responses elicited by combinatorial protein transfer of chemotactic and costimulatory molecules. J Immunol 2007; 178: 3301–6. [DOI] [PubMed] [Google Scholar]
- 69.Liu S, Foster BA, Chen T, Zheng G, Chen A. Modifying dendritic cells via protein transfer for antitumor therapeutics. Clin Cancer Res 2007; 13: 283–91. [DOI] [PubMed] [Google Scholar]
- 70.McHugh RS, Ahmed SN, Wang Y-C, Sell KW, Selvaraj P. Construction, purification, and functional incorporation on tumor cells of glycolipid-anchored human B7-1 (CD80). Proc Natl Acad Sci U S A 1995; 92: 8059–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brunschwig EB, Levine E, Trefzer U, Tykocinski M. Glycosylphosphatidylinositol-modified murine B7-1 and B7-2 retain costimulator function. J Immunol 1995; 155: 5498–505. [PubMed] [Google Scholar]
- 72.Brunschwig EB, Fayen JD, Medof ME, Tykocinski ML. Protein transfer of glycosyl-phosphatidylinositol (GPI)-modified murine B7-1 and B7-2 costimulators. J Immunother 1999; 22: 390–400. [DOI] [PubMed] [Google Scholar]
- 73.McHugh RS, Nagarajan S, Wang Y-C, Sell KW, Selvaraj P. Protein transfer of glycosyl-phosphatidylinositol-B7-1 into tumor cell membranes: a novel approach to tumor immunotherapy. Cancer 1999; 59: 2433–7. [PubMed] [Google Scholar]
- 74.Poloso N, Nagarajan S, Bumgarner GW, Zampell JC, Selvaraj P. Designer cancer vaccines made easy: protein transfer of immunostimulatory molecules for use in therapeutic tumor vaccines. J Immunol 2001; 6: 760–75. [DOI] [PubMed] [Google Scholar]
- 75.Poloso NJ, Nagarajan S, Bumgarner GW, Selvaraj P. Development of therapeutic vaccines by direct modification of cell membranes from surgically removed human tumor tissue with immunostimulatory molecules. Vaccine 2001; 19: 2029–38. [DOI] [PubMed] [Google Scholar]
- 76.Geho DH, Fayen JD, Jackman RM, Branch D, Moody DB, Porcelli SA, Tykocinski ML. Glycosyl-phosphatidylinositol reanchoring unmasks distinct antigen-presenting pathways for CD1b and CD1c. J Immunol 2000; 165: 1272–7. [DOI] [PubMed] [Google Scholar]
- 77.Nagarajan S, Selvaraj P. Glycolipid-anchored IL-12 expressed on tumor cell surface induces antitumor immune response. Cancer Res 2002; 62: 2869–74. [PubMed] [Google Scholar]
- 78.Djafarzadeh R, Noessner E, Engelmann H, Schendel DJ, Notohamiprodjo M, von Luettichau I, Nelson PJ. GPI-anchored TIMP-1 treatment renders renal cell carcinoma sensitive to FAS-meditated killing. Oncogene 2006; 25: 1496–508. [DOI] [PubMed] [Google Scholar]
- 79.Lin AY, Devaux B, Green A, Sagerström C, Elliott JF, Davis MM. Expression of T cell antigen receptor heterodimers in a lipid-linked form. Science 1987; 249: 23–5. [DOI] [PubMed] [Google Scholar]
- 80.Notohamiprodjo M, Djafarzadeh R, Mojaat A, von Lüttichau I, Gröne H-J, Nelson PJ. Generation of GPI-linked CCL5 based chemokine receptor antagonists for the suppression of acute vascular damage during allograft transplantation. Protein Eng Des Sel 2006; 19: 27–35. [DOI] [PubMed] [Google Scholar]
- 81.Knorr BR, Dustin ML. The lymphocyte function – associated antigen 1 I domain is a transient binding module for intercellular adhesion molecule (ICAM)-1 and ICAM-3 in hydrodynamic flow. J Exp Med 1997; 186: 719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Coeshott C, Grey HM. Transfer of antigen-presenting capacity to Ia-negative cells upon fusion with Ia-bearing liposomes. J Immunol 1985; 134: 1343–8. [PubMed] [Google Scholar]
- 83.Peterson BR. Synthetic mimics of mammalian cell surface receptors: prosthetic molecules that augment living cells. Org Biomol Chem 2005; 3: 3607–12. [DOI] [PubMed] [Google Scholar]
- 84.Legler DF, Doucey M-A, Schneider P, Chapatte L, Bender FC, Bron C. Differential insertion of GPI-anchored GFPs into lipid rafts of live cells. FASEB J 2004; 19: 73–5. [DOI] [PubMed] [Google Scholar]
- 85.Sarkar D, Vemula PK, Weian Z, Gupta A, Karnik R, Karp JM. Engineered mesenchymal stem cells with self-assembled vesicles for systemic cell targeting. Biomaterials 2010; 31: 5266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barenholz Y. Lipsome application: problems and prospects. Curr Opin Colloid In 2001; 6: 66–77. [Google Scholar]
- 87.Rabuka D, Forstner MB, Groves JT, Bertozzi CR. Noncovalent cell surface engineering: incorporation of bioactive synthetic glycopolymers into cellular membranes. J Am Chem Soc 2008; 130: 5947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Broekhoven CL, Parish CR, Vassiliou G, Altin JG. Engrafting costimulator molecules onto tumor cell surfaces with chelator lipids: a potentially convenient approach in cancer vaccine development. J Immunol 2015; 164: 2433–43. [DOI] [PubMed] [Google Scholar]
- 89.Breydo L, Sun Y, Makarava N, Lee C-I, Novitskaia V, Bocharova O, Kao JPY, Baskakov IV. Nonpolar substitution at the C-terminus of the prion protein, a mimic of the glycosylphosphatidylinositol anchor, partially impairs amyloid fibril formation. Biochemistry 2007; 46: 852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abe H, Goto M, Kamiya N. Protein lipidation catalyzed by microbial transglutaminase. Chem Eur J 2011; 17: 14004–8. [DOI] [PubMed] [Google Scholar]
- 91.Chou SH, Shetty AV, Geng Y, Xu L, Munirathinam G, Pipathsouk A, Tan I, Morris T, Wang B, Chen A, Zheng G. Palmitate-derivatized human IL-2: a potential anticancer immunotherapeutic of low systemic toxicity. Cancer Immunol Immunother 2013; 62: 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi G, Mukthavaram R, Kesari S, Simberg D. Distearoyl anchor-painted erythrocytes with prolonged ligand retention and circulation properties in vivo. Adv Healthc Mater 2014; 3: 142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He X, Bonaparte N, Kim S, Acharya B, Lee J-Y, Chi L, Lee H-J, Paik Y-K, Moon P-G, Baek M-C, Lee E-K, Kim J-H, Kim I, -S, Lee B-H. Enhanced delivery of T cells to tumor after chemotherapy using membrane-anchored, apoptosis-targeted peptide. J Control Release 2012; 162: 521–8. [DOI] [PubMed] [Google Scholar]
- 94.Tomita U, Yamaguchi S, Sugimoto Y, Takamori S, Nagamune T. Poly(ethylene glycol)-lipid-conjugated antibodies enhance dendritic cell phagocytosis of apoptotic cancer cells. Pharmaceuticals 2012; 5: 405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tomita U, Yamaguchi S, Maeda Y, Chujo K, Minamihata K, Nagamune T. Protein cell-surface display through in situ enzymatic modification of proteins with a poly(ethylene glycol)-lipid. Biotechnol Bioeng 2013; 110: 2785–9. [DOI] [PubMed] [Google Scholar]
- 96.Antos JM, Miller GM, Grotenbreg GM, Ploegh HL. Lipid modification of proteins through sortase-catalyzed transpeptidation. J Am Chem Soc 2008; 130: 16338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gallat F-X, Brogan APS, Fichou Y, McGrath N, Moulin M, Härtlein M, Combet J, Wuttke J, Mann S, Zaccai G, Jackson CJ, Perriman AW, Weik M. A polymer surfactant corona dynamically replaces water in solvent-free protein liquids and ensures macromolecular flexibility and activity. J Am Chem Soc 2012; 134: 13168–71. [DOI] [PubMed] [Google Scholar]
- 98.Brogan APS, Sessions RB, Perriman AW, Mann S. Molecular dynamics simulations reveal a dielectric-responsive coronal structure in protein – polymer surfactant hybrid nanoconstructs. J Am Chem Soc 2014; 136: 16824–31. [DOI] [PubMed] [Google Scholar]
- 99.Perriman AW, Brogan APS, Cölfen H, Tsoureas N, Owen GR, Mann S. Reversible dioxygen binding in solvent-free liquid myoglobin. Nat Chem 2010; 2: 622–6. [DOI] [PubMed] [Google Scholar]
- 100.Stephanopoulos N, Francis MB. Choosing an effective protein bioconjugation strategy. Nat Chem Biol 2011; 7: 876–84. [DOI] [PubMed] [Google Scholar]
- 101.Sarkar D, Vemula PK, Teo GSL, Spelke D, Karnik R, Wee LY, Karp JM. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjugate Chem 2008; 19: 2105–9. [DOI] [PubMed] [Google Scholar]
- 102.Kampf N, Zohar C, Nussinovitch A. Hydrocolloid coating of Xenopus laevis embryos. Biotechnol Prog 2000; 16: 480–7. [DOI] [PubMed] [Google Scholar]
- 103.Kampf N, Zohar C, Nussinovitch A. Alginate coating of xenopus laevis embryos. Biotechnol Prog 2000; 16: 497–505. [DOI] [PubMed] [Google Scholar]
- 104.Swiston AJ, Cheng C, Um SH, Irvine DJ, Cohen RE, Rubner MF. Surface functionalization of living cells with multilayer patches. Nano Lett 2008; 8: 4446–53. [DOI] [PubMed] [Google Scholar]
- 105.Polak R, Crouzier T, Lim RM, Ribbeck K, Beppu MM, Pitombo RNM, Cohen RE, Rubner MF. Sugar-mediated disassembly of mucin/lectin multi layers and their use as pH-tolerant, on- demand sacrificial layers. Biomacromolecules 2014; 15: 3093–8. [DOI] [PubMed] [Google Scholar]
- 106.Swiston AJ, Gilbert JB, Irvine DJ, Cohen RE, Rubner MF. Freely suspended cellular “backpacks” lead to cell aggregate self-assembly. Biomacromolecules 2010; 11: 1826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doshi N, Swiston AJ, Gilbert JB, Alcaraz ML, Cohen RE, Rubner MF, Mitragotri S. Cell-based drug delivery devices using phagocytosis-resistant backpacks. Adv Mater 2011; 23: H105–9. [DOI] [PubMed] [Google Scholar]
- 108.Anselmo AC, Gilbert JB, Kumar S, Gupta V, Cohen RE, Rubner MF, Mitragotri S. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: a generalized strategy to deliver drugs to treat inflammation. J Control Release 2015; 199: 29–36. [DOI] [PubMed] [Google Scholar]
- 109.Xia J, Wang Z, Huang D, Yan Y, Li Y, Guan J. Asymmetric biodegradable microdevices for cell-borne drug delivery. ACS Appl Mater Interfaces 2015; 7: 6293–9. [DOI] [PubMed] [Google Scholar]
- 110.Wang Z, Xia J, Yan Y, Tsai A-C, Li Y, Ma T, Guan J. Facile functionalization and assembly of live cells with microcontact-printed polymeric biomaterials. Acta Biomater 2015; 11: 80–7. [DOI] [PubMed] [Google Scholar]
- 111.Pinato G, Raffaelli T, D’Este E, Tavano F, Cojoc D. Optical delivery of liposome encapsulated chemical stimuli to neuronal cells. J Biomed Opt 2011; 16: 095001–095001. [DOI] [PubMed] [Google Scholar]
- 112.D’Este E, Baj G, Beuzer P, Ferrari E, Pinato G, Tongiorgi E, Cojoc D. Use of optical tweezers technology for long-term, focal stimulation of specific subcellular neuronal compartments. Integr Biol 2011; 3: 568–77. [DOI] [PubMed] [Google Scholar]
- 113.Armstrong JPK, Olof SN, Jakimowicz MD, Hollander AP, Mann S, Davis SA, Miles MJ, Patil AJ, Perriman AW. Cell paintballing using optically targeted coacervate microdroplets. Chem Sci 2015; 6: 6106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]