Abstract
Objectives: efficacy and tolerability of WS® 5570 for the treatment of acute mild-to-moderate depression, has been demonstrated in various studies. Here, we present a subgroup analysis of a double blind, randomised trial to compare the therapeutic efficacy of WS® 5570 with paroxetine in patients suffering from a major depressive episode with moderate symptom intensity.
Methods: moderate depression was defined by a baseline Hamilton Depression Rating Scale (HAM-D) total score between 22 and 25. Patients received, after a single blind placebo run-in phase of 3–7 d, either 3 × 300 mg/d WS® 5570 or 20 mg/d paroxetine for six weeks. The change of the HAM-D total score was used to describe the efficacy of WS® 5570 compared with paroxetine in the subgroup of patients with moderate depression.
Results: the reductions of the HAM-D total score were significantly more pronounced in patients treated with 3 × 300 mg/d WS® 5570 compared to 20 mg/d paroxetine.
Conclusions: patients treated with WS® 5570 not only showed a reduction in depression severity score but also yielded greater response and remission rates compared with patients treated with paroxetine.
Keypoints
Various studies showed the efficacy and tolerability of WS® 5570 for the treatment of acute mild-to-moderate depression.
Beneficial effects of WS® 5570 have been also shown in patients with moderate-to-severe depression.
In this study reductions of the HAM-D total score were significantly more pronounced in patients with moderate depression treated with WS® 5570 compared with paroxetine.
Patients treated with WS® 5570 not only showed a reduction in depression severity score but also yielded greater response and remission rates compared with patients treated with paroxetine.
Keywords: Hypericum extract, moderate major depressive episode, paroxetine, St. John’s wort, WS® 5570
Introduction
According to the World Health Organization (2012) more than 350 million people of all ages are affected by depression. Major depression is now the second cause of disability worldwide, as communicated by a recent report (Ferrari et al. 2013). These data reinforce the importance of treating depressive patients with potent but also cost-effective interventions to alleviate the burden of this disease. Over the last decades, numerous antidepressants have been introduced for the acute treatment of major depression. Amongst these synthetic antidepressants (e.g., monoamine oxidase [MAO] inhibitors, selective serotonin reuptake inhibitors [SSRIs], dual serotonin–noradrenaline reuptake inhibitors, tricyclic antidepressants [TCAs] as well as melatonergic drugs) and phytopharmaceuticals (e.g., St John’s Wort) are the most commonly used remedies.
Extracts of the St. John’s wort plant (Hypericum perforatum) have been used in clinical practice for decades for a wide range of mood disorders including depression, but mainly in German-speaking countries (Linde et al. 2008). Recent evidences have consistently demonstrated the efficacy and tolerability of H. perforatum in the treatment of patients with major depression and with mild-to-moderate symptom intensity in both, randomised clinical trials and in clinical practice (Lemmer et al. 1999; Gaster & Holroyd 2000; Kasper 2001; Rodriguez-Landa & Contreras 2003; Clement et al. 2006; Carpenter et al. 2008; Linde et al. 2008; Kasper et al. 2008a, 2010; Gastpar 2013). Thereby, broad evidence has been derived from Hypericum extract WS® 5570 (Hyperiplant® Rx, Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany). There is clear evidence from recent double-blind, randomised, controlled clinical trials that WS® 5570 is efficacious in the treatment of acute, mild-to-moderate major depression. In particular, WS® 5570 exerts effects on the core symptoms’ intensity in randomised trials and clinical practice (Lecrubier et al. 2002; Kasper et al. 2006, 2007, 2008b; Gastpar 2013). The extract is more efficacious than placebo and, as shown in non-inferiority trials, at least as potent as standard antidepressants (TCAs or SSRIs). Preliminary data also suggest that WS® 5570 is effective in moderate-to-severe depression. Szegedi et al. (2005) could demonstrate that Hypericum extract WS® 5570 was at least as effective as paroxetine in the acute treatment of moderate-to-severe depression (Szegedi et al. 2005). Moreover, significantly greater response rates were reported in the Hypericum group than in the paroxetine group and more patients from the Hypericum group were symptom-free after the acute treatment than in the paroxetine treatment group. WS® 5570 shows an excellent safety profile, which is similar to placebo and better tolerated than standard antidepressants except for mild, transient, gastrointestinal disturbance or skin irritation (Lemmer et al. 1999; Lecrubier et al. 2002; Kasper et al. 2010; Gastpar 2013). Although the overall safety profile is superior to conventional antidepressants, it is noteworthy that interactions with other drugs may occur (Izzo 2004; Borrelli & Izzo 2009; Caraci et al. 2011).
More than 150 active and mutually influencing ingredients have been identified within Hypericum extracts, whereby naphthodianthrones (e.g., hypericin), flavonoids, bi-flavonoids, xanthons and phloroglucinol (e.g., hyperforin) may contribute to the pharmacological effect of Hypericum perforatum (Linde et al. 2008). Because of the large amount of ingredients, the mechanism of action has not been fully elucidated yet. It is known that Hypericum extracts, which contain hypericin and hyperforin, have a broad spectrum of actions modulating all neurotransmitter systems with approximately the same affinity. Therefore, it is suggested that Hypericum extracts work in a similar manner as tricyclic antidepressants and SSRIs in reducing monoamine reuptake and enhancing synaptic availability of serotonin, dopamine and norepinephrine (Muller 2003). For a long time hypericin was assumed to be the main active principle in Hypericum extracts. However, studies found that the phloroglucinol derivate hyperforin mostly accounts for the antidepressant effect rather than hypericin (Chatterjee et al. 1998; Laakmann et al. 1998; Butterweck & Schmidt 2007; Linde et al. 2008). Hyperforin and hypericin have different pharmacokinetic effects and are represented with different concentrations within the plant (higher hyperforin than hypericin concentrations) (Chatterjee et al. 1998; Russo et al. 2014). Hyperforin is suggested to be a potent reuptake inhibitor of serotonin, dopamine, noradrenaline, gamma-aminobutyric acid (GABA) and L-glutamate (Chatterjee et al. 1998; Muller 2003). The antidepressant effect may be elicited by affecting the sodium gradient leading to an inhibition of transmitter reuptake rather than inhibiting the transmitter binding sites of the transporter proteins (Muller 2003). Hypericin shows only activity as a MAO inhibitor in vitro but has neither been associated with MAO inhibition nor with synaptosomal uptake of serotonin, noradrenaline, dopamine and GABA in vivo (Muller et al. 1997; Wonnemann et al. 2001; Russo et al. 2014). In order to reach the full pharmacologic effect of Hypericum extracts, researchers suggest that the interplay of hyperforin, hypericin and some yet undefined flavonoids mostly account for the observed clinical efficacy of Hypericum extracts (Chatterjee et al. 1998; Laakmann et al. 1998; Wurglics et al. 2001a, 2001b; Butterweck & Schmidt 2007).
Although the ingredients of Hypericum extracts are roughly the same across products, Hypericum products should not be interchangeably used for the treatment of mild-to-moderate depression as large differences in hypericin and hyperforin concentrations may occur (Wurglics et al. 2001a). The indication, composition (raw plant material used, extraction process and solvents) and quality of Hypericum extracts can greatly vary across products – even sometimes within the same batch (Wurglics et al. 2001a; Linde et al. 2008). This can have an effect on the antidepressant effect of the hypericum extract. High hyperforin concentrations may be crucial for the antidepressant effect, as demonstrated by Laakmann et al. (1998) who compared two Hypericum extracts with different hyperforin contents (5% vs. 0.5%) in patients with mild-to-moderate depression. At the end of treatment, patients treated with the 5% hyperforin extract revealed greater reductions in Hamilton Depression Rating Scale (HAM-D) total scores than patients having received the 0.5% hyperforin extract. Therefore, standardisation of ingredient extraction should be aimed at enabling acceptable hyperforin concentrations of Hypericum products to offer an efficacious remedy for the treatment of major depression with various symptom intensity.
Efficacy and tolerability of Hypericum extract WS® 5570 for the treatment of mild-to-moderate major depression has been proven through various randomised clinical trials and systematic reviews. Evidence that Hypericum extract WS® 5570 is also efficacious in patients with moderate-to-severe depression is still sparse. So far, data from two randomised clinical trials support the hypothesis that WS® 5570 is beneficial in patients with moderate-to-severe depression (Lecrubier et al. 2002; Szegedi et al. 2005). This subgroup analysis on moderately depressed patients treated with WS® 5570 tries to support the hypothesis that WS® 5570 is an effective remedy in patients with major depression and moderate symptom intensity.
Methods
Study design
This analysis refers to a subgroup of a double-blind, double dummy, randomised phase III trial (published by Szegedi et al. 2005) to compare the therapeutic efficacy of 3 × 300 mg/d Hypericum extract WS® 5570 with 20 mg/d paroxetine in patients suffering from moderate depression. After a single-blind placebo run-in phase of 3–7 days, patients either received WS® 5570 or the SSRI paroxetine for six weeks of double-blind treatment. Efficacy was evaluated after 7, 14, 28 and 42 d of acute treatment. All patients gave their written informed consent.
Participants
Male and female outpatients with an age range of 18–70 years were recruited from 21 psychiatric primary care centres in Germany. To be included into the main study patients had to meet criteria for unipolar depression without psychotic features (Diagnostic and Statistical Manual of Mental Disorders, fourth edition [DSM-IV] 296.22, 296.23, 296.32, 296.33) persisting for at least two weeks up to a year. In addition, patients had to have a total score of ≥22 points on the 17-item HAM-D and ≥2 points for the item ‘depressive mood’ at screening and baseline. Patients were considered suffering from moderate depression if the HAM-D total score was <25 points at baseline. No concomitant psychotropic medication was allowed during the study phase, and patients with other psychiatric disorders were excluded from the study. Table 1 shows the reasons for premature study termination in both patients treated with the Hypericum extract WS® 5570 and paroxetine. Further details on the recruitment procedure, exclusion criteria, interventions and blinding of this study can be found elsewhere (Szegedi et al. 2005).
Table 1.
Reasons for premature study termination for patients with moderate depression treated with WS® 5570 3 × 300 mg/d or paroxetine.
| Reasons for premature discontinuation | WS® 5570 (n = 31) | Paroxetine (n = 33) |
|---|---|---|
| Withdrawal of consent without giving the reason | 3 (9.1) | |
| Lack of efficacy | 1 (3.0) | |
| Adverse event(s) | 1 (3.2) | 2 (6.1) |
| Lost to follow-up | 1 (3.0) | |
| Total | 1 (3.2) | 7 (21.2) |
All values are expressed as n (%).
Interventions
Hypericum extract WS® 5570 is a stabilised dry extract from the St. John’s wort plant (drug-to-extract ratio 3–7:1), extraction solvent methanol 80% (v/v). Main constituents include 3–6% hyperforin, 0.1–0.3% hypericin, not less than 6% flavonoids, and not less than 1.5% rutin. Patients received three coated tablets with 300 mg of the extract per day. Paroxetine was provided in 20 mg capsules. During the treatment phase patients took either 3 × 300 mg WS® 5570 plus a paroxetine placebo (Hypericum group) or 20 mg paroxetine and three WS® 5570 placebos (paroxetine group). The coated tablets and capsules were indistinguishable from placebo in terms of their outward appearance.
Efficacy measures
In order to compare the efficacy of 3 × 300 mg/d WS® 5570 and paroxetine, the change of the total score of the HAM-D between baseline and the end of the acute treatment period was used as primary outcome measure (Hamilton 1960). Interviews were performed by trained psychiatrists and psychologists. In the subgroup of patients with moderate depression the changes of the HAM-D total score during the acute treatment period is used to describe the efficacy of 3 × 300 mg/d WS® 5570 compared with 20 mg/d paroxetine. Furthermore the responder rates (patients with at least 50% reduction of the HAM-D total score between baseline and 42 d of treatment) and rates of patients with remission (patients with HAM-D total scores of at least 7 points after 42 d of treatment) were compared between the treatment groups.
Statistical analysis
The trial was conducted with a planned adaptive interim analysis to enable early termination if the presence or absence of treatment differences were clear. According to the results of the interim analysis the study was continued with a second study part. The subgroup analysis was performed using pooled data of the first (data collected before the interim analysis) and the second (data collected after the interim analysis) study part. In order to compare the efficacy of the treatments the changes of the HAM-D total score during the acute treatment period (between baseline and weeks 7, 14, 28 and 42) were compared between the treatment groups using two-sided t-tests. Rates of responders and remitters were compared between the treatment groups using two-sided χ 2 tests.
Results
Participants
The subgroup of patients suffering from moderate depression consisted of 64 patients treated with either 3 × 300 mg/d WS® 5570 (n = 31) or 20 mg/d paroxetine (n = 33). Baseline characteristics of these patients are shown in Table 2. Mean age was slightly higher in the Hypericum group than in the paroxetine group, 67.7% of patients treated with 3 × 300 mg/d WS® 5570 and 54.6% of patients having received 20 mg/d paroxetine were women. Patients from the Hypericum and paroxetine group had a HAM-D baseline score of 23.1 and 22.9, respectively, and 41.9% of patients from the Hypericum group and 57.6% from the paroxetine group were presented with recurrent depression.
Table 2.
Baseline characteristics of patients with moderate depression treated with WS® 5570 3 × 300 mg/d or paroxetine [mean (SD) or absolute frequency (percent) of patients and two-sided p value; pooled data; FAS, LOCF].
| WS® 5570 3 × 300 mg/d (n = 31) | Paroxetine 20 mg/d (n = 33) | p Value (test) | |
|---|---|---|---|
| HAM-D Score mean (SD) | 23.1 (0.9) | 22.9 (0.8) | 0.248 (t-test) |
| Age (years) mean (SD) | 48.6 (11.9) | 42.9 (11.6) | 0.056 (t-test) |
| Duration of current episodes (days) mean (SD) | 165 (153) | 121 (88) | 0.178 (t-test) |
| No. of women n (%) | 21 (67.7) | 18 (54.6) | 0.280 (χ2-test) |
| No. of patients with recurrent depression n (%) | 13 (41.9) | 19 (57.6) | 0.211 (χ2-test) |
Interventional therapy
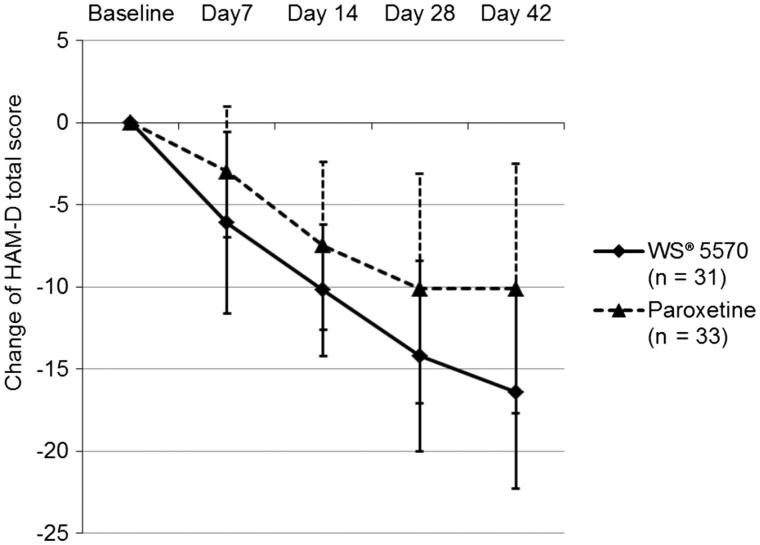
The changes of the HAM-D total score during the course of the trial are summarised in Table 3. In both groups substantial improvements of the depression score were observed during six weeks of acute treatment depicted by continued marked reductions in HAM-D total scores. Beginning after 7 d of treatment, the mean reduction of the HAM-D total score was statistically significantly more pronounced in patients treated with 3 × 300 mg/d WS® 5570 compared with 20 mg/d paroxetine (Figure 1). These findings clearly suggest that WS® 5570 is superior to paroxetine in reducing depressive symptom intensity.
Table 3.
Change of HAM-D total score during the course of the acute treatment phase [mean (SD) and p value of the two-sided t-test; pooled data; FAS, LOCF].
| WS® 5570 3 × 300 mg/d (n = 31) | Paroxetine 20 mg/d (n = 33) | p Value | |
|---|---|---|---|
| Day 7 – baseline | −6.1 (5.5) | −3.0 (4.0) | 0.012 |
| Day 14 – baseline | −10.2 (4.0) | −7.5 (5.1) | 0.020 |
| Day 28 – baseline | −14.2 (5.8) | −10.1 (7.0) | 0.014 |
| Day 42 – baseline | −16.4 (5.9) | −10.1 (7.6) | <0.001 |
Figure 1.
Change of Hamilton Depression Rating Scale (HAM-D) total score during the course of the acute treatment phase [mean (SD) and p value of the two-sided t-test; pooled data; FAS, LOCF].
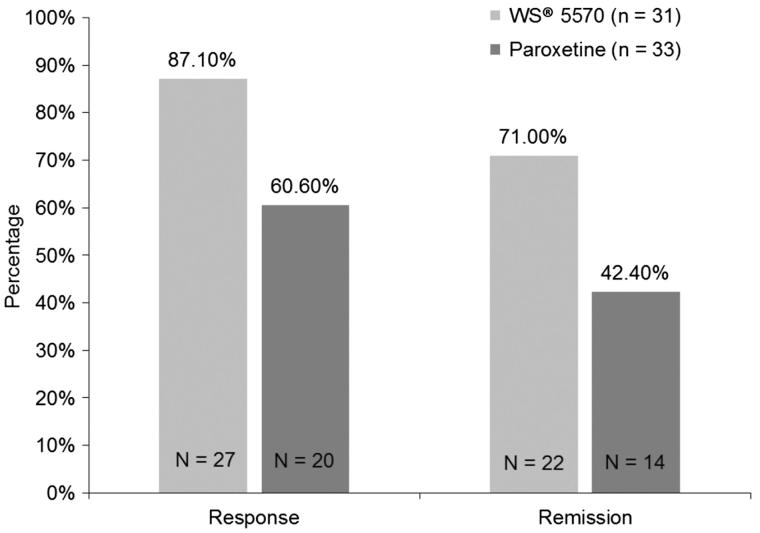
Twenty-seven (87.1%) patients treated with 3 × 300 mg/d WS® 5570 were responders (reduction of HAM-D total score of at least 50%) compared with 20 patients (60.6%) in the paroxetine 20 mg/d group. This difference between the treatment groups was statistically significant (p = 0.017, χ 2 test, two-sided). Twenty-two patients (71.0%) that had received 3 × 300 mg/d WS® 5570 were remitters (HAM-D total score of at least 7 points after six weeks of acute treatment) compared with 14 patients (42.4%) in the paroxetine 20 mg/d group (p = 0.001, χ 2 test, two-sided, Table 4; Figure 2).
Table 4.
Response and remission after six weeks of acute treatment [absolute (relative frequency) and p value of the two-sided χ 2-test; pooled data; FAS, LOCF].
| WS® 5570 3 × 300 mg/day (n = 31) | paroxetine 20 mg/d (n = 33) | p Value | |
|---|---|---|---|
| Response HAM-D total score reduction ≥50% n (%) |
27 (87.1) | 20 (60.6) | 0.017 |
| Remission HAM-D total score reduction ≤7 n (%) |
22 (71.0) | 14 (42.4) | 0.001 |
Figure 2.
Response and remission after six weeks of acute treatment [absolute (relative frequency) and p value of the two-sided χ2 test; pooled data; FAS, LOCF].
Safety and tolerability
During the treatment period six of 31 patients randomised to Hypericum (19%) reported 15 potentially suspected adverse drug reactions and 20 of the 33 treated with paroxetine (61%) reported 61 suspected adverse drug reactions. Only few suspected adverse drug reactions were reported in the Hypericum group, whereby gastrointestinal disorders (three events in three patients), general disorders and administration site conditions (three events in three patients), and nervous system disorders (three events in three patients) were the most commonly reported cases. In the paroxetine group the highest incidence of suspected cases of adverse drug reactions was found for gastrointestinal disorders (22 events in 21 patients), followed by nervous system disorders (16 events in 16 patients). Table 5 summarises all suspected adverse drug reactions that occurred during the acute treatment period in both patient groups.
Table 5.
Number of subjects with suspected cases of ADRs and number of suspected cases by SOC and PT (beginning of event between first and last intake of investigational product).
| Treatment |
||||
|---|---|---|---|---|
| WS® 5570 3 × 300 mg/d |
Paroxetine 20 mg/d |
|||
| System organ class and preferred term | n (%) | Total ADR | n (%) | Total ADR |
| All | ||||
| Total patients with suspected cases of ADRs | 6 (19.35) | 15 | 20 (60.61) | 61 |
| Total patients | 31 (100) | 33 (100) | ||
| Gastrointestinal disorders | ||||
| Total | 2 (6.45) | 3 | 16 (48.48) | 22 |
| Abdominal pain | 2 (6.45) | 2 | 1 (3.03) | 1 |
| Constipation | 1 (3.23) | 1 | 1 (3.03) | 1 |
| Diarrhoea NOS | 4 (12.12) | 5 | ||
| Dry mouth | 4 (12.12) | 4 | ||
| Nausea | 11 (33.33) | 11 | ||
| General disorders and administration site conditions | ||||
| Total | 3 (9.68) | 3 | 6 (18.18) | 7 |
| Fatigue | 2 (6.45) | 2 | 4 (12.12) | 4 |
| Weakness | 1 (3.23) | 1 | 3 (9.09) | 3 |
| Immune system disorders | ||||
| Total | 1 (3.23) | 1 | ||
| Hypersensitivity NOS | 1 (3.23) | 1 | ||
| Nervous system disorders | ||||
| Total | 3 (9.68) | 3 | 11 (33.33) | 16 |
| Depressed level of consciousness | 1 (3.23) | 1 | 1 (3.03) | 1 |
| Dizziness (Exc. Vertigo) | 2 (6.45) | 2 | 6 (18.18) | 6 |
| Headache NOS | 4 (12.12) | 4 | ||
| Insomnia NEC | 1 (3.03) | 1 | ||
| Somnolence | 2 (6.06) | 2 | ||
| Tremor NEC | 2 (6.06) | 2 | ||
| Psychiatric disorders | ||||
| Total | 2 (6.45) | 3 | 5 (15.15) | 8 |
| Agitation | 1 (3.23) | 1 | 1 (3.03) | 1 |
| Flat affect | 1 (3.23) | 2 | 1 (3.03) | 1 |
| Loss of libido | 1 (3.03) | 1 | ||
| Restlessness | 1 (3.03) | 1 | ||
| Sleep disorder NOS | 4 (12.12) | 4 | ||
| Reproductive system and breast disorders | ||||
| Total | 1 | 1 (3.03) | 1 | |
| Ejaculation disorder NOS | 1 | 1(3.03) | 1 | |
| Skin and subcutaneous tissue disorders | ||||
| Total | 2 (6.45) | 2 | 6 (18.18) | 7 |
| Photosensitivity reaction | 1 (3.23) | 1 | 6 (18.18) | 7 |
| NOS | 1 (3.23) | 1 | ||
| Sweating increased | ||||
ADRs: adverse drug reactions; SOC: system organ class; PT: preferred term; NOS: not otherwise specified; NEC: not elsewhere classified.
Conclusion
In patients suffering from moderate depression, a daily dose of 3 × 300 mg/d Hypericum extract WS® 5570 was significantly superior to the SSRI paroxetine 20 mg with respect to the reduction of the HAM-D total score during the acute treatment period. After six weeks of treatment significantly more patients treated with WS® 5570 responded to treatment and more patients showed remission compared with the reference group. These results confirm and strengthen the results of the whole sample presented in the Szegedi et al. (2005) study. Moreover, the present analysis reinforces the currently available clinical data on non-inferiority of WS® 5570 compared with synthetic antidepressants in patients with an acute moderate depressive episode. Some clinical trials treating moderately depressed patients with other Hypericum products could mainly show non-inferiority but no superiority to synthetic antidepressants (Vorbach et al. 1997; Philipp et al. 1999; Gastpar et al. 2005, 2006).
Moreover, the greater response and remission rate under WS® 5570 is an important finding and, in line with two other clinical trials, where a trend to superiority regarding response and remission rate was found in mild-to-moderate depressed patients (Schrader 2000; Fava et al. 2005). The high remission rate is of importance as ∼50% of patients with unipolar depression will eventually relapse, a predictor of which is incomplete remission, and therefore there is a strong need for continued antidepressant therapy to maintain the remission period (Kasper et al. 2010). Evidence elaborated so far suggests that WS® 5570 can be successfully applied for prophylactic continuation treatment in patients prone to relapse after recovery from an acute episode of depression without an increase in the number of adverse events (Szegedi et al. 2005; Anghelescu et al. 2006; Kasper et al. 2007, 2008b, 2010).
To comply with long-term therapy patients require a remedy that is not only efficacious but also well tolerated. The findings of this subgroup analysis show a favourable safety profile of Hypericum compared with paroxetine. This is in line with previous studies and corroborate the hypothesis that the safety profile of Hypericum extract is more favourable than in synthetic antidepressants (Lemmer et al. 1999; Lecrubier et al. 2002; Kasper et al. 2010; Gastpar 2013). Weight gain or sexual dysfunction, two common side effects of synthetic antidepressants, can negatively affect a patient’s quality of life and drug compliance, but has not been linked to WS® 5570 in this subgroup analysis and in previous studies (Kasper et al. 2010). A good safety and tolerability profile is therefore crucial in maintenance therapy. Moreover, less patients treated with Hypericum dropped out of clinical trials due to adverse effects compared with patients treated with first or second generation antidepressants (Linde et al. 2008). This may suggest that compliance rates are generally higher in patients treated with Hypericum than with synthetic antidepressants.
Although the tolerability profile of Hypericum extracts is comparable with placebo and superior to synthetic antidepressants the most relevant safety concern pertains to drug–herb interaction. In interaction studies and case reports Hypericum extracts have been found to induce the activity of the cytochrome P450 (CYP) metabolising enzymes (notably CYP3A4) and P-glycoprotein (P-GP) with an effect on reduced drug plasma levels of cyclosporine, tacrolimus, antiretroviral drugs (indinavir and other protease inhibitors in anti-HIV treatment), anticancer drugs (irinotecan, imatinib), benzodiazepines and their derivates (e.g., midazolam), theophylline, digoxin, anticoagulants of the coumarin type (e.g., phenprocoumon and warfarin), amitriptyline and nortriptyline, fexofenadine, methadone, simvastatin, finasteride, serotonin syndrome during co-administration with SSRIs, and to reduce the efficacy of contraception (Borrelli & Izzo 2009; Izzo & Ernst 2009; Nahrstedt & Butterweck 2010; Schellander & Donnerer 2010; Caraci et al. 2011; Russo et al. 2014). The hyperforin concentration seems to be the main driver for increased expression of CYP enzymes (CYP3A4 and CYPC19) and P-GP (Russo et al. 2014). CYPs were found to play a crucial role in the metabolism of many drug classes and P-GP is responsible for increased excretion of drugs (Russo et al. 2014). However, it is noteworthy that the risk for interaction with other drugs is very low (Kasper et al. 2010).
Optimal dosage of WS® 5570 has not been conclusively established (Kasper et al. 2006). Antidepressant effects are usually reached with a dosage between 600 and 1200 mg. A recent study by Kasper et al. (2010) revealed that doses of 600 and 1200 mg led to the same antidepressant effect in patients with mild-to-moderate major depression. Patients treated with 1200 mg had a slightly greater remission rate compared with patients treated with 600 mg. There were no additional or more severe adverse events in the higher dose group reported (Kasper et al. 2006). This suggests that a higher dose of WS® 5570 does not lead to a stronger antidepressant effect than a lower dose of 600 mg. A recent meta-analysis could confirm these results and demonstrated that even with higher doses of WS® 5570 no monotonic relationship between drug dose and adverse events could be found (Kasper et al. 2010; Gastpar 2013).
Limitations
It is, of course, noteworthy that this subgroup analysis focuses on a part of the original study sample by the study of Szegedi et al. (2005), where the results of the whole study sample can be found. Furthermore, authors from a recent Cochrane systematic review criticised that studies from German-speaking countries were more favourable to their antidepressant efficacy than studies from other countries (Linde et al. 2008). This may be partially accounted for the long tradition of Hypericum products and high prescription rate by physicians in German-speaking countries. Moreover, results cannot be easily transferred to other Hypericum extracts or clinical trials as the indication, composition and quality of Hypericum extracts can greatly vary across products (Wurglics et al. 2001a; Linde et al. 2008). In addition, only a minority of Hypericum extracts have been tested in randomised, double-blind, controlled clinical trials. As the quality and therapeutic suitability of each Hypericum product may depend on the chosen manufacturing process, each of these products needs its own clinical proof of efficacy (Lemmer et al. 1999). In terms of WS® 5570, broad scientific evidence has been derived from clinical studies.
More and more patients and physicians request a remedy that is efficacious, safe and can be used for long-term application with an extremely good tolerability. There is evidence that the antidepressant effect of Hypericum WS® 5570 is comparable with synthetic antidepressants not only in mild-to-moderate, but also in moderate-to-severe major depressive episode. This subgroup analysis of patients with moderate major depression supports these findings and shows that patients treated with 3 × 300 mg/d WS® 5570 not only have a reduction in their depression severity score but also have a greater response and remission rate and a better tolerability than patients treated with paroxetine. Due to these findings Hypericum may be considered as first-line treatment for patients with mild–to-moderate depressive episode.
Acknowledgements
We thank the participating patients, research coordinators, psychiatrists and psychologists; and Mirja Gross (SAN GmbH) for providing editorial assistance of the manuscript.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Funding information
This work was supported by Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany.
References
- Anghelescu IG, Kohnen R, Szegedi A, Klement S, Kieser M. Comparison of Hypericum extract WS 5570 and paroxetine in ongoing treatment after recovery from an episode of moderate to severe depression: results from a randomized multicenter study. Pharmacopsychiatry. 2006;39(6):213–219. doi: 10.1055/s-2006-951388. [DOI] [PubMed] [Google Scholar]
- Borrelli F, Izzo AA. Herb-drug interactions with St John's wort (Hypericum perforatum): an update on clinical observations. AAPS J. 2009;11(4):710–727. doi: 10.1208/s12248-009-9146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterweck V, Schmidt M. St. John's wort: role of active compounds for its mechanism of action and efficacy. Wien Med Wochenschr. 2007;157(13–14):356–361. doi: 10.1007/s10354-007-0440-8. [DOI] [PubMed] [Google Scholar]
- Caraci F, Crupi R, Drago F, Spina E. Metabolic drug interactions between antidepressants and anticancer drugs: focus on selective serotonin reuptake inhibitors and Hypericum extract. Curr Drug Metab. 2011;12(6):570–577. doi: 10.2174/138920011795713706. [DOI] [PubMed] [Google Scholar]
- Carpenter C, Crigger N, Kugler R, Loya A. Hypericum and nurses: a comprehensive literature review on the efficacy of St. John's Wort in the treatment of depression. J Holist Nurs. 2008;26(3):200–207. doi: 10.1177/0898010107313243. [DOI] [PubMed] [Google Scholar]
- Chatterjee SS, Bhattacharya SK, Wonnemann M, Singer A, Muller WE. Hyperforin as a possible antidepressant component of Hypericum extracts. Life Sci. 1998;63(6):499–510. doi: 10.1016/s0024-3205(98)00299-9. [DOI] [PubMed] [Google Scholar]
- Clement K, Covertson CR, Johnson MJ, Dearing K. St. John's wort and the treatment of mild to moderate depression: a systematic review. Holist Nurs Pract. 2006;20(4):197–203. doi: 10.1097/00004650-200607000-00008. [DOI] [PubMed] [Google Scholar]
- Fava M, Alpert J, Nierenberg AA, Mischoulon D, Otto MW, Zajecka J, Murck H, Rosenbaum J. A Double-blind, randomized trial of St John's wort, fluoxetine, and placebo in major depressive disorder. J Clin Psychopharmacol. 2005;25(5):441–447. doi: 10.1097/01.jcp.0000178416.60426.29. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaster B, Holroyd J. St John's wort for depression: a systematic review. Arch Intern Med. 2000;160(2):152–156. doi: 10.1001/archinte.160.2.152. [DOI] [PubMed] [Google Scholar]
- Gastpar M. Hypericum extract WS (R) 5570 for depression–an overview. Int J Psychiatry Clin Pract. 2013;17(Suppl. 1):1–7. doi: 10.3109/13651501.2013.813554. [DOI] [PubMed] [Google Scholar]
- Gastpar M, Singer A, Zeller K. Efficacy and tolerability of Hypericum extract STW3 in long-term treatment with a once-daily dosage in comparison with sertraline. Pharmacopsychiatry. 2005;38(2):78–86. doi: 10.1055/s-2005-837807. [DOI] [PubMed] [Google Scholar]
- Gastpar M, Singer A, Zeller K. Comparative efficacy and safety of a once-daily dosage of Hypericum extract STW3-VI and citalopram in patients with moderate depression: a double-blind, randomised, multicentre, placebo-controlled study. Pharma-copsychiatry. 2006;39(2):66–75. doi: 10.1055/s-2006-931544. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA. Drug interactions with St. John's Wort (Hypericum perforatum): a review of the clinical evidence. Int J Clin Pharmacol Ther. 2004;42(3):139–148. doi: 10.5414/cpp42139. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs. Drugs. 2009;69:1777–1798. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kasper S. Hypericum perforatum–a review of clinical studies. Pharmacopsychiatry. 2001;34(Suppl. 1):S51–S55. doi: 10.1055/s-2001-15467. [DOI] [PubMed] [Google Scholar]
- Kasper S, Anghelescu IG, Szegedi A, Dienel A, Kieser M. Superior efficacy of St John's wort extract WS 5570 compared to placebo in patients with major depression: a randomized, double-blind, placebo-controlled, multi-center trial. BMC Med. 2006;4:14. doi: 10.1186/1741-7015-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Anghelescu IG, Szegedi A, Dienel A, Kieser M. Placebo controlled continuation treatment with Hypericum extract WS 5570 after recovery from a mild or moderate depressive episode. Wien Med Wochenschr. 2007;157(13–14):362–366. doi: 10.1007/s10354-007-0441-7. [DOI] [PubMed] [Google Scholar]
- Kasper S, Gastpar M, Moller HJ, Muller WE, Volz HP, Dienel A, Kieser M. Better tolerability of St. John's wort extract WS 5570 compared to treatment with SSRIs: a reanalysis of data from controlled clinical trials in acute major depression. Int Clin Psychopharmacol. 2010;25(4):204–213. doi: 10.1097/yic.0b013e328335dc1a. [DOI] [PubMed] [Google Scholar]
- Kasper S, Gastpar M, Muller WE, Volz HP, Dienel A, Kieser M, Möller HJ. Efficacy of St. John's wort extract WS 5570 in acute treatment of mild depression: a reanalysis of data from controlled clinical trials. Eur Arch Psychiatry Clin Neurosci. 2008a;258(1):59–63. doi: 10.1007/s00406-007-0779-2. [DOI] [PubMed] [Google Scholar]
- Kasper S, Volz HP, Moller HJ, Dienel A, Kieser M. Continuation and long-term maintenance treatment with Hypericum extract WS 5570 after recovery from an acute episode of moderate depression–a double-blind, randomized, placebo controlled long-term trial. Eur Neuropsychopharmacol. 2008b;18(11):803–813. doi: 10.1016/j.euroneuro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Laakmann G, Schule C, Baghai T, Kieser M. St. John's wort in mild to moderate depression: the relevance of hyperforin for the clinical efficacy. Pharmacopsychiatry. 1998;31(Suppl. 1):54–59. doi: 10.1055/s-2007-979346. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, Clerc G, Didi R, Kieser M. Efficacy of St. John's wort extract WS 5570 in major depression: a double-blind, placebo-controlled trial. Am J Psychiatry. 2002;159(8):1361–1366. doi: 10.1176/appi.ajp.159.8.1361. [DOI] [PubMed] [Google Scholar]
- Lemmer W, von den Driesch V, Klieser E. Efficacy and tolerability of Neuroplant(R) 300 film-coated tablets (St. John's Wort Special Extract WS 5572) Fortschritte der Medizin. 1999;117(3):143–154. [Google Scholar]
- Linde K, Berner MM, Kriston L. St John's wort for major depression. Cochrane Database Syst Rev. 2008;(4):CD000448. doi: 10.1002/14651858.CD000448.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WE. Current St John's wort research from mode of action to clinical efficacy. Pharmacol Res. 2003;47(2):101–109. doi: 10.1016/s1043-6618(02)00266-9. [DOI] [PubMed] [Google Scholar]
- Muller WE, Rolli M, Schafer C, Hafner U. Effects of Hypericum extract (LI 160) in biochemical models of antidepressant activity. Pharmacopsychiatry. 1997;30(Suppl. 2):102–107. doi: 10.1055/s-2007-979528. [DOI] [PubMed] [Google Scholar]
- Nahrstedt A, Butterweck V. Lessons learned from herbal medicinal products: the example of St. John's Wort (perpendicular). J Nat Prod. 2010;73(5):1015–1021. doi: 10.1021/np1000329. [DOI] [PubMed] [Google Scholar]
- Philipp M, Kohnen R, Hiller KO. Hypericum extract versus imipramine or placebo in patients with moderate depression: randomised multicentre study of treatment for eight weeks. BMJ. 1999;319(7224):1534–1538. doi: 10.1136/bmj.319.7224.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Landa JF, Contreras CM. A review of clinical and experimental observations about antidepressant actions and side effects produced by Hypericum perforatum extracts. Phytomedicine. 2003;10(8):688–699. doi: 10.1078/0944-7113-00340. [DOI] [PubMed] [Google Scholar]
- Russo E, Scicchitano F, Whalley BJ, Mazzitello C, Ciriaco M, Esposito S, Patane M, Upton R, Pugliese M, Chimirri S, et al. Hypericum perforatum: pharmacokinetic, mechanism of action, tolerability, and clinical drug-drug interactions. Phytother Res. 2013;28(5):643–655. doi: 10.1002/ptr.5050. [DOI] [PubMed] [Google Scholar]
- Schellander R, Donnerer J. Antidepressants: clinically relevant drug interactions to be considered. Pharmacology. 2010;86(4):203–215. doi: 10.1159/000319744. [DOI] [PubMed] [Google Scholar]
- Schrader E. Equivalence of St John's wort extract (Ze 117) and fluoxetine: a randomized, controlled study in mild-moderate depression. Int Clin Psychopharmacol. 2000;15(2):61–68. doi: 10.1097/00004850-200015020-00001. [DOI] [PubMed] [Google Scholar]
- Szegedi A, Kohnen R, Dienel A, Kieser M. Acute treatment of moderate to severe depression with hypericum extract WS 5570 (St John's wort): randomised controlled double blind non-inferiority trial versus paroxetine. BMJ. 2005;330(7490):503. doi: 10.1136/bmj.38356.655266.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorbach EU, Arnoldt KH, Hubner WD. Efficacy and tolerability of St. John's wort extract LI 160 versus imipramine in patients with severe depressive episodes according to ICD-10. Pharmacopsychiatry. 1997;30(Suppl. 2):81–85. doi: 10.1055/s-2007-979524. [DOI] [PubMed] [Google Scholar]
- Wonnemann M, Singer A, Siebert B, Muller WE. Evaluation of synaptosomal uptake inhibition of most relevant constituents of St. John's wort. Pharmacopsychiatry. 2001;34(Suppl. 1):S148–S151. doi: 10.1055/s-2001-15465. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2012. http://www.who.int/mental_health/management/depression/who_paper_depression_wfmh_2012.pdf [Google Scholar]
- Wurglics M, Westerhoff K, Kaunzinger A, Wilke A, Baumeister A, Baumeister A, Dressman J, Schubert-Zsilovecz M. Batch-to-batch reproducibility of St. John's wort preparations. Pharmacopsychiatry. 2001a;34(Suppl. 1):S152–S156. doi: 10.1055/s-2001-15453. [DOI] [PubMed] [Google Scholar]
- Wurglics M, Westerhoff K, Kaunzinger A, Wilke A, Baumeister A, Dressman J, Schubert-Zsilovecz M. Comparison of German St. John's wort products according to hyperforin and total hypericin content. J Am Pharm Assoc. 2001b;41(4):560–566. doi: 10.1016/s1086-5802(16)31280-3. [DOI] [PubMed] [Google Scholar]