Abstract
Background
Inhaled corticosteroids are commonly prescribed for patients with severe COPD. They have been associated with increased risk of pneumonia but not with increased pneumonia-associated or overall mortality.
Methods
To further examine the effects of inhaled corticosteroids on pneumonia incidence, and mortality in COPD patients, we searched for potentially relevant articles in PubMed, Medline, CENTRAL, EMBASE, Scopus, Web of Science and manufacturers’ web clinical trial registries from 1994 to February 4, 2014. Additionally, we checked the included and excluded studies’ bibliographies. We subsequently performed systematic review and meta-analysis of included randomized controlled trials and observational studies on the topic.
Results
We identified 38 studies: 29 randomized controlled trials and nine observational studies. The estimated unadjusted risk of pneumonia was increased in randomized trials: RR 1.61; 95% CI 1.35-1.93, p<0.001; as well as in observational studies: OR 1.89; 95% CI 1.39-2.58, p<·001. Six randomized trials and seven observational studies were useful in estimating unadjusted risk of pneumonia case-fatality: RR 0.91; 95% CI 0.52-1.59, p=0.74; and OR 0.72; 95% CI 0.59-0.88, p=0.001, respectively. Twenty-nine randomized trials and six observational studies allowed estimation of unadjusted risk of overall mortality: RR 0.95; 95% CI 0.85-1.05, p=0.31; and OR 0.79; 95% CI 0.65-0.97, p=0.02, respectively.
Conclusions
Despite a substantial and significant increase in unadjusted risk of pneumonia associated with inhaled corticosteroid use, pneumonia fatality and overall mortality were found not to be increased in randomized controlled trials and were decreased in observational studies.
Keywords: Case-fatality, drop-out, bias, heterogeneity
Introduction
Inhaled corticosteroids (ICS) are among the most commonly prescribed anti-inflammatory medications. They reduce the incidence of disease exacerbations in COPD patients, which may translate into their improved overall health status. The Global Initiative for Chronic Obstructive Lung Disease recommends ICS for management of patients with severe COPD and those with frequent exacerbations.1 The TORCH trial2, published in 2007, was the first to report increased risk of pneumonia in patients using ICS. Since then, numerous prospective randomized trials have reported increased risk of pneumonia with ICS use.3-7 These trials reported pneumonia events as pre-specified adverse events; however they lacked rigorous diagnostic ascertainment or radiological confirmation of pneumonia. Moreover, the reported increased risk of pneumonia was not uniformly adjusted for the pertinent confounders. We have previously studied and reported that the increased risk of pneumonia is attenuated upon adjustment for demographics, comorbidities and concurrent medications.8 It is conceivable that the observed increased unadjusted risk of pneumonia can be at least partly explained by higher doses, potency and longer use of ICS, especially in older patients with more severe COPD.9
A previous meta-analysis of randomized controlled trials (RCTs) published before June 2008 reported no difference in pneumonia-associated and overall mortality despite significantly increased risk of pneumonia in ICS users, however, the authors noted that the included studies lacked power to detect a difference in pneumonia-associated or overall mortality.10 Although RCTs carry less risk of inherent bias compared to observational studies, the observed limitations with pneumonia ascertainment, relatively low prevalence rate, reporting bias and confounding limited the ability to interpret RCT’s regarding this issue.
Recent, large observational studies have demonstrated either similar or improved mortality in patients using ICS.8, 11-13 Other improved outcomes have been reported: decreased risk of development of parapneumonic effusion,14 lesser need for mechanical ventilation11 and fewer pulmonary complications at admission and during hospitalization.15 The observational studies did not rely solely on the clinical diagnosis of pneumonia; rather most included confirmatory radiographic assessments. Moreover, the recent observational studies evaluated patients with pneumonia events necessitating admission to the hospital, in comparison to the patients with mostly ambulatory pneumonia events enrolled in RCTs.
Given the discrepancies in estimates of risks of pneumonia, pneumonia-associated and overall mortality in COPD patients utilizing ICS and a number of recently published studies on the topic, we systematically reviewed all available RCTs and observational studies and pooled the results into two separate meta-analyses.
Materials and Methods
Search strategy
Three authors (E.F., V.B. and E.G.) independently and in duplicate searched PubMed, Medline, CENTRAL, EMBASE, Scopus, Web of Science and manufacturers’ web clinical trial registries (GlaxoSmithKline, AstraZeneca) with the clinical trial filters using multiple search terms with no language restrictions, from 1994 to February 4, 2014. To identify additional relevant articles, we checked the bibliographies from included and excluded studies, as well as related systematic reviews. All abstracts returned from the preliminary search were reviewed in three combinations of duplicates. Ten random manuscripts were reviewed for eligibility by all three reviewers together to help standardize the selection strategy. Then, the same reviewers each independently assessed approximately two-thirds of all full-text manuscripts for the eligibility. Disagreements regarding eligibility between two reviewers were resolved through consensus and with an input from the third reviewer.
Outcome measures
Among all studies of ICS use in COPD, those which measured pneumonia and reported study-period mortality were analyzed in detail. We considered all pneumonia events irrespective of severity. We also analyzed more reliable overall mortality outcomes, as well as pneumonia-associated mortality (number of pneumonia cases who died divided by number of patients in the ICS versus non-ICS group) and pneumonia case-fatality (number of pneumonia cases who died divided by all pneumonia cases in the ICS versus non-ICS group, respectively). At the outcome level, we assessed risk of bias by using GRADE profiler, version 3.6 (GRADE working group).
Quantitative data synthesis and sensitivity analysis
We used Review Manager Software, version 5.2 (Nordic Cochrane Center, Copenhagen, Denmark), to calculate pooled relative risk (RR) for RCTs and odds ratio (OR) for observational studies with respective 95% confidence intervals (CIs) using a random effects model. We reported the outcomes data according to an “intention to treat” (ITT) analysis. All reported p-values are two-sided, with significance set at less than 0.05. The statistical heterogeneity was assessed using the I2 statistic. Where substantial statistical heterogeneity was present, we explored potential sources of heterogeneity in the subgroup analyses. Sensitivity analyses were performed to explore the influence of statistical models (fixed vs random effects) on the effect size, the influence of individual trials, per protocol analysis and the inclusion of “double-zero” events (zero outcomes in both ICS and non-ICS groups).
Additional details on eligibility, search, data extraction, study characteristics and quality assessment are available in the Supplemental file and e-Figures 1-4.
Results
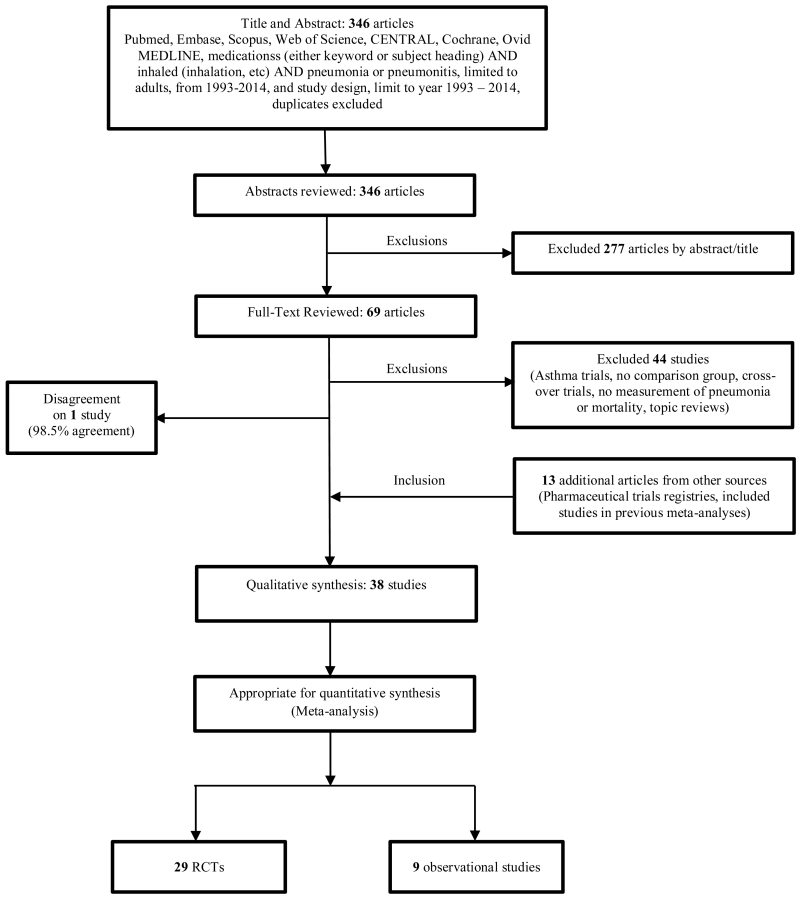
We identified 38 studies, 29 RCTs and nine observational. The flowchart is shown in Figure 1. Twenty-five studies initially fulfilled our inclusion criteria. Thirteen additional studies were identified; four through the reviews of web-based pharmaceutical clinical trial registries and nine through the reviews of bibliographies of the previous meta-analyses.10, 16 We then investigated why the nine latter studies were not included in our initial search results and discovered that their published versions did not contain either of the specific inclusive terms: “pneumonia” or “pneumoni*”. Study characteristics are shown in Tables 1 (29 RCTs)3-7, 17-40 and 2 (9 observational studies).8, 11-14, 41-44
Figure 1. Study flow-chart.
Table 1. Characteristics of randomized controlled trials.
| Source | COPD Patients |
Setting | Duration (weeks) |
Interventions | Enrolled/ Analyzed |
Outcomes | Risk of bias | Funding |
|---|---|---|---|---|---|---|---|---|
| Aaron 200717 | Moderate to severe COPD |
Outpatient | 52 | Tiotropium 18 μg +placebo Tiotropium 18 μg +salmeterol 25 μg Tiotropium 18 μg +Fluticasone/salmeterol 250/25 μg |
156/82 148/84 145/108 |
Pneumonia Overall mortality |
High Low |
The Canadian Institutes of Health Research and the Ontario Thoracic Society |
| Anzueto 20094 | Moderate to severe COPD |
Outpatient | 52 | Fluticasone propionate/ salmeterol 250 / 50 μg Salmeterol 50 μg |
394/269 403/247 |
Pneumonia Overall mortality |
Low Low |
GlaxoSmithKline. |
| Burge 200030 | Moderate to severe COPD |
Outpatient | 156 | Fluticasone propionate 500 μg Placebo |
376/212 375/175 |
Pneumonia Overall mortality |
High Low |
Glaxo Wellcome Research and Development |
| Calverley 200318 - Maintenance therapy with budesonide and formoterol in COPD |
Moderate to severe COPD |
Outpatient | 52 | Budesonide/formoterol 320/9 μg Budesonide 400 μg Formoterol 9 μg Placebo |
254/180 257/155 255/144 256/150 |
Pneumonia Overall mortality |
High Low |
AstraZeneca, Lund, Sweden |
| Calverley 200319- Combined salmeterol and fluticasone in the treatment of COPD |
Moderate to severe COPD |
Outpatient | 52 | Fluticasone/salmeterol 500/50 μg Fluticasone propionate 500 μg Salmeterol 50 μg Placebo |
358/269 374/266 372/253 361/221 |
Pneumonia Overall mortality |
High Low |
GlaxoSmithKline |
| Calverley 201020 |
Moderate to severe COPD |
Outpatient | 48 | Beclomethasone/ formoterol MDI 100/6μg Budesonide/formoterol DPI 200/6 μg Formoterol DPI 12 μg |
237/205 242/212 239/204 |
Pneumonia Overall mortality |
High Low |
Chiesi Farmaceutici S.p.A |
| Calverley 20115 |
Moderate to severe COPD |
Outpatient | 104 | Salmeterol/fluticasone propionate 50/500 μg Tiotropium bromide 18 μg |
658/426 665/386 |
Pneumonia Pneumonia mortality Overall mortality |
Low Low low |
GlaxoSmithKline and Nycomed |
| Crim 200921 (TORCH) |
Moderate to severe COPD |
Outpatient | 156 | Fluticasone 500 μg Salmeterol 50 μg Salmeterol/fluticasone 50/500 μg Placebo |
1534/947 1521/960 1533/1011 1524/851 |
Pneumonia Pneumonia mortality Overall mortality |
High High Low |
GlaxoSmithKline |
| Doherty22 2012 |
Moderate to severe COPD |
Outpatient | 26 | Mometasone /formoterol 400/10 μg Mometasone /formoterol 200/10 μg, Mometasone 400 μg, Formoterol 10 μg, or Placebo |
225/190 239/202 253/202 243/193 236/169 |
Pneumonia Overall mortality |
High Low |
Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc. |
| Dransfield 20137 |
Moderate to severe COPD |
Outpatient | Pooled 2 studies; 104 and 102 |
Vilanterol 25 μg Fluticasone furoate 50 μg + vilanterol 25 μg Fluticasone furoate 100 + vilanterol 25 μg Fluticasone 200 μg + vilanterol 25 μg |
409/294 408/315 403/312 402/301 |
Pneumonia Pneumonia mortality Overall mortality |
Low Low Low |
GlaxoSmithKline |
| Ferguson 20083 |
Moderate to severe COPD |
Outpatient | 52 | Fluticasone propionate/ salmeterol 250/50 μg Salmeterol 50 μg |
394/277 388/239 |
Pneumonia Overall mortality |
High Low |
GlaxoSmithKline |
| FLTA 302534 | Moderate to severe COPD |
Outpatient | 24 | Fluticasone propionate 250 μg Fluticasone propionate 500 μg Placebo |
216/140 218/147 206/127 |
Pneumonia Overall mortality |
High Low |
Glaxo Wellcome Research and Development |
| Kardos 200723 | Moderate to severe COPD |
Outpatient | 48 | Fluticasone propionate/salmeterol 500/50 μg Salmeterol 50 μg |
507/408 487/384 |
Pneumonia Pneumonia mortality Overall mortality |
High High Low |
Mainly funded by GlaxoSmithKline but also received funds from Altana, AstraZeneca and Novartis |
| Kerwin 201340 | Moderate to severe COPD |
Outpatient | 24 | Fluticasone/vilanterol 100/25 μg Fluticasone/vilanterol 50/25 μg Fluticasone 100 μg Vilanterol 25 μg Placebo |
206/151 206/147 206/145 205/142 207/138 |
Pneumonia Overall mortality |
Low low |
GlaxoSmithKline |
| Mahler 200239 | Moderate to severe COPD |
Outpatient | 24 | Fluticasone 500 μg Salmeterol 50 μg Fluticasone/salmeterol 500/50 μg Placebo |
168/100 160/115 165/113 181/112 |
Pneumonia Pneumonia mortality Overall mortality |
High High High **People who were hospitalized were withdrawn from the study |
GlaxoSmithKline |
| Martinez 201324 |
Moderate to severe COPD |
Outpatient | 24 | Fluticasone furoate 100 μg Fluticasone furoate 200 μg Vilanterol 25 μg Fluticasone furoate / vilanterol 100/25 μg Fluticasone furoate / vilanterol 200/25 μg Placebo |
204/155 203/160 203/161 204/144 205/158 205/146 |
Pneumonia Overall mortality |
Low Low |
GlaxoSmithKline |
| Paggiaro 199833 |
Moderate to severe COPD |
Outpatient | 24 | Fluiticasone propionate 500 μg Placebo |
142/123 139/112 |
Pneumonia Overall mortality (not specified in article, pooled data from previous meta-analysis) |
High High |
Glaxo Wellcome Research and Development, Greenford, Middlesex UB6 0HE, UK |
| Pauwels 199932 |
Moderate to severe COPD |
Outpatient | 156 | Budesonide 400 μg Placebo |
634/458 643/454 |
Pneumonia Overall mortality |
High Low |
Astra Draco, Lund, Sweden |
| Rennard 200925 | Moderate to severe COPD |
Outpatient | 52 | Budesonide/formoterol pMDI 320/9 μg Budesonide/formoterol pMDI 160/9 μg Formoterol DPI 9 μg Placebo |
494/360 494/351 495/338 481/306 |
Pneumonia Overall mortality |
High Low |
AstraZeneca LP, Wilmington, DE, USA |
| SCO10047035 | Moderate to severe COPD |
Outpatient | 24 | Fluticasone propionate/ Salmeterol 250/50 μg Salmeterol 50 μg |
518/459 532/458 |
Pneumonia Overall mortality |
High Low |
GlaxoSmithKline |
| SCO4004136 | Moderate to severe COPD |
Outpatient | 156 | Fluticasone propionate/ Salmeterol 250/50 μg Salmeterol 50 μg |
92/56 94/55 |
Pneumonia Pneumonia mortality Overall mortality |
High High Low |
GlaxoSmithKline |
| Sharafkhaneh 20126 |
Moderate to severe COPD |
Outpatient | 52 | Budesonide/formoterol pMDI 320/9 μg Budesonide/formoterol pMDI 160/9 μg Formoterol DPI 9 μg |
407/290 408/290 404/271 |
Pneumonia Pneumonia mortality Overall mortality |
High High Low |
AstraZeneca LP, Wilmington, DE, USA |
| Szafranski 200331 |
Moderate to severe COPD |
Outpatient | 52 | Budesonide/formoterol 320/9 μg Budesonide 200 μg Formoterol 4.5 μg Placebo |
208/149 198/136 201/137 205/115 |
Pneumonia Overall mortality |
High Low |
AstraZeneca |
| Tashkin 200827 |
Moderate to severe COPD |
Outpatient | 26 | Budesonide/formoterol pMDI 320/9 μg Budesonide/formoterol pMDI 160/9 μg Budesonide pMDI 320 μg + formoterol DPI 9 μg Budesonide pMDI 320 μg Formoterol DPI 9μg Placebo |
277/238 281/243 287/239 275/212 284/223 300/223 |
Pneumonia Overall mortality |
High Low |
AstraZeneca LP, |
| Tashkin 201226 |
Moderate to severe COPD |
Outpatient | 52; 26- week treatment *, 26- week safety extension |
Mometasone/ formoterol 400/10 μg, Mometasone/ formoterol 200/10 μg, Mometasone 400 μg, Formoterol 10 μg, Placebo |
217/176 207/169 210/164 209/172 212/159 |
Pneumonia Overall mortality |
Low High |
Supported by Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc. |
| Van der valk 200237 |
Moderate to severe COPD |
Outpatient | 26 | Fluticasone 500 μg Placebo |
123/122 121/120 |
Pneumonia Overall Mortality |
High Low |
Supported by the Netherlands Asthma Foundation, Amicon Health Insurance Co., Boehringer Ingelheim, and GlaxoSmithKline BV |
| Vestbo 1999 | Moderate to severe COPD |
Outpatient | 156 | Budesonide Placebo |
145/109 145/94 |
Pneumonia Overall Mortality |
High Low |
ASTRA Denmark A/S, ASTRA Pharmaceutical Production AB Sweden |
| Vogelmeier 201329 |
Moderate to severe COPD |
Outpatient | 26 | QVA 149 (LABA indacaterol and the LAMA glycopyrronium in fixed combination) 110/50 μg Salmeterol/fluticasone 50μg/500μg |
259/215 264/217 |
Pmeumonia Overall mortality |
High Low |
Main funding from Novartis Pharma AG. Authors on advisory board of multiple pharmaceuticals |
| Wouters 200538 |
Moderate to severe COPD |
Outpatient | 52 | Salmeterol/fluticasone 50μg/500μg Salmeterol 50μg |
189/155 184/138 |
Pneumonia Overall mortality |
High Low |
GlaxoSmithKline |
Only the 26-week treatment phase was included in the meta-analysis. The extension phase did not have placebo group and only some patients from the treatment group was randomized to continue in the extension phase.
Table 2. Characteristics of observational studies.
| Study | Patients Characteristics | Setting | Duration | Exposure | Number of patients |
Outcomes | Risk of bias |
|---|---|---|---|---|---|---|---|
| CASE – CONTROL STUDIES | |||||||
| Ernst 2007 (Retrospective) |
Population-based cohort design with a nested case control analysis for COPD patients 66 years of age or older |
Inpatient | 14 years (1988- 2001) |
ICS Non-ICS |
40366 79344 |
Pneumonia (Crude & Adjusted) Overall mortality (Crude) |
High* Low |
| Joo 2010 (Retrospective) |
Nested case control study in a cohort of Veterans Affairs (VA) patients with newly diagnosed COPD patients 65 years of age or older |
Inpatient | 4 years (1998- 2002) |
ICS Non-ICS |
24091 121495 |
Pneumonia (Crude & Adjusted) Pneumonia (30 day) mortality (Crude) |
High* Low |
| COHORT STUDIES | |||||||
| Chen 2011 (Retrospective) |
Cohort of COPD patients, 65 years of age or older who had a discharge diagnosis of pneumonia |
Inpatient | 5 years (2002- 2007) |
ICS Non-ICS |
8271 7497 |
Pneumonia (Crude) Pneumonia (30 day) mortality (Crude & Adjusted) Overall (90 day) mortality (Crude & Adjusted) |
High* Low Low |
| Cheng 2011 (Prospective) |
Cohort of moderate to severe COPD patients |
Inpatient | 2 years (2007- 2008) |
ICS Non-ICS |
125 149 |
Pneumonia (Crude & Adjusted) Zero pneumonia deaths |
Low Low |
| Festic 2014 (Retrospective) |
Cohort of adult patients with pneumonia or other risk factors for ARDS |
Inpatient | 26 weeks (March 2009– August 2009) |
ICS Non ICS |
226 363 |
Pneumonia (Crude & Adjusted) Pneumonia mortality (Crude) Overall (90 day) mortality (Crude) |
Low Low Low |
| Ko 2008 (Prospective) |
Cohort of patients with acute exacerbation of COPD with concomitant pneumonia |
Inpatient | 1 year (2004- 2005) |
ICS Non ICS |
42 36 |
Pneumonia (Crude) Pneumonia (Deaths during the same hospitalization) mortality (Crude) Overall (Deaths in the following 12 months) mortality (Crude) |
Low Low Low |
| Malo de Molina 2010 (Retrospective) |
Cohort of hospitalized patients with diagnosis of pneumonia who had a pre- existing diagnosis of COPD, age 65 or older |
Inpatient | 1 year (1999- 2000) |
ICS Non-ICS |
2420 3933 |
Pneumonia (Crude) Pneumonia (30 day) mortality (Crude & Adjusted) Overall (90 day) mortality (Crude & Adjusted) |
High* Low Low |
| Sellares 2013 (Prospective) |
Community acquired pneumonia patients admitted through emergency room, age 16 or older |
Inpatient | 12 years (January 1997 – July 2008) |
ICS Non-ICS |
340 394 |
Pneumonia (Crude) Pneumonia mortality (Crude) |
Low Low |
| Singanayagam 2011 (Prospective) |
Spirometry-confirmed COPD patients presenting with a primary diagnosis of community acquired pneumonia |
Inpatient | 4 years (2005- 2009) |
ICS Non-ICS |
376 114 |
Complicated pneumonia (Crude) Pneumonia (30 day) mortality (Crude & Adjusted) Overall (6 month) mortality (Crude & Adjusted) |
Low Low Low |
The 29 RCTs included 33,472 patients, of whom 18,715 received ICS and 14,757 received control treatment. The duration of trials ranged from 24 weeks to three years, with median duration of 12 months. The most studied ICS was fluticasone with 22,216 participants in 19 trials, followed by budesonide with 8,768 participants in eight trials, and 2 mometasone trials with 2,488 participants. We considered all RCTs as high quality studies based on the sequence generation and double-blinding. At the outcome-level RCTs were judged to be at ‘high’ rather than ‘very high’ risk of bias, as this bias would be expected to be non-differential.
There were nine observational studies, which included 292,430 patients, of whom 76,521 were on ICS and 215,909 were not on ICS. Seven studies were cohort and two were case-control studies. Eight studies assessed risk of pneumonia requiring admission to the hospital and one included patients admitted to either emergency room or hospital. While quality of pneumonia ascertainment was more systematic compared to RCTs, all observational studies were also judged to be at ’very high’ risk of bias due to high heterogeneity and conferred overall lower confidence in pneumonia and mortality outcomes (Table 3).
Table 3. Outcome-level GRADE assessment tables.
| Outcome: Pneumonia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quality assessment | Summary of Findings | ||||||||||
| Participants (studies) Follow up |
Risk of bias |
Inconsistency | Indirectness | Imprecision | Publication bias |
Overall quality of evidence |
Study event rates (%) | Relative effect (95% CI) |
Anticipated absolute effects |
||
| Non-ICS | ICS | Risk with Non-ICS |
Risk difference with ICS (95% CI) |
||||||||
| RCTs | |||||||||||
| 33472 (29 studies) 12 months |
serious1 | no serious inconsistency2 |
no serious indirectness |
no serious imprecision3 |
undetected | ⊕⊕⊕⊖ MODERATE1,2,3 due to risk of bias |
563/14757 (3.8%) |
1062/18715 (5.7%) |
RR 1.61 (1.35 to 1.93) |
Study population | |
|
38 per
1000 |
23 more per 1000 (from 13 more to 35 more) |
||||||||||
| Moderate | |||||||||||
|
16 per
1000 |
10 more per 1000 (from 6 more to 15 more) |
||||||||||
| Observational | |||||||||||
| 292430 (9 studies) |
serious4 | very serious5 | no serious indirectness |
no serious imprecision |
undetected | ⊕⊖⊖⊖ VERY LOW4,5 due to risk of bias, inconsistency |
38063/215909 (17.6%) |
26458/76521 (34.6%) |
OR 1.89 (1.39 to 2.58) |
Study population | |
|
176 per
1000 |
112 more per 1000 (from 53 more to 180 more) |
||||||||||
| Moderate | |||||||||||
|
1000
per 1000 |
- | ||||||||||
| Outcome: Pneumonia fatality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quality assessment | Summary of Findings | ||||||||||
| Participants (studies) |
Risk of bias |
Inconsistency | Indirectness | Imprecision | Publication bias |
Overall quality of evidence |
Study event rates (%) | Relative effect (95% CI) |
Anticipated absolute effects |
||
| Non-ICS | ICS | Risk with Non-ICS |
Risk difference with ICS (95% CI) |
||||||||
| RCTs | |||||||||||
| 1159 (6 studies) |
serious1 | no serious inconsistency |
no serious indirectness |
serious2 | undetected | ⊕⊕⊖⊖ LOW1,2 due to risk of bias, imprecision |
18/376 (4.8%) |
36/783 (4.6%) |
RR 0.91 (0.52 to 1.59) |
Study population | |
|
48 per
1000 |
4 fewer per 1000 (from 23 fewer to 28 more) |
||||||||||
| Moderate | |||||||||||
|
27 per
1000 |
2 fewer per 1000 (from 13 fewer to 16 more) |
||||||||||
| Observational | |||||||||||
| 37672 (7 studies) |
serious1 | serious3 | no serious indirectness |
no serious imprecision |
undetected | ⊕⊖⊖⊖ VERY LOW1,3 due to risk of bias, inconsistency |
3395/23067 (14.7%) |
1467/14605 (10%) |
OR 0.72 (0.59 to 0.88) |
Study population | |
|
147 per
1000 |
37 fewer per 1000 (from 15 fewer to 55 fewer) |
||||||||||
| Moderate | |||||||||||
|
110 per
1000 |
28 fewer per 1000 (from 12 fewer to 42 fewer) |
||||||||||
| Outcome: Overall mortality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quality assessment | Summary of Findings | ||||||||||
| Participants (studies) |
Risk of bias |
Inconsistency | Indirectness | Imprecision | Publication bias |
Overall quality of evidence |
Study event rates (%) | Relative effect (95% CI) |
Anticipated absolute effects |
||
| Non-ICS | ICS | Risk with Non-ICS |
Risk difference with ICS (95% CI) |
||||||||
| RCTs | |||||||||||
| 33472 (29 studies) |
no serious risk of bias |
no serious inconsistency |
no serious indirectness |
no serious imprecision |
undetected |
⊕
⊕
⊕
⊕
HIGH |
657/14757 (4.5%) |
660/18715 (3.5%) |
RR 0.95 (0.85 to 1.05) |
Study population | |
|
45 per
1000 |
2 fewer per 1000 (from 7 fewer to 2 more) |
||||||||||
| Moderate | |||||||||||
|
14 per
1000 |
1 fewer per 1000 (from 2 fewer to 1 more) |
||||||||||
| Observational | |||||||||||
| 47220 (6 studies) |
serious1 | serious2 | no serious indirectness |
no serious imprecision |
undetected | ⊕⊖⊖⊖ VERY LOW1,2 due to risk of bias, inconsistency |
3974/29948 (13.3%) |
2253/17272 (13%) |
OR 0.79 (0.65 to 0.97) |
Study population | |
|
133 per
1000 |
25 fewer per 1000 (from 3 fewer to 42 fewer) |
||||||||||
| Moderate | |||||||||||
|
168 per
1000 |
30 fewer per 1000 (from 4 fewer to 52 fewer) |
||||||||||
No systematic ascertainment of pneumonia outcome
Heterogeneity present but not substantial
Several studies showing non-significant risk and wide confidence intervals represent only 4% of overall weight in RCTs
Unclear length of duration of respiratory symptoms/unresolving exacerbations and step up therapy with ICS prior to admission with pneumonia
Maximum heterogeneity noted
Difficulty with ascertainment of pneumonia-relatedness
Wide confidence intervals in majority of the estimable studies
Substantial heterogeneity
Non-randomized, retrospective, observational design
Substantial heterogeneity
We attempted to obtain additional unpublished information on 11 trials by contacting corresponding authors by email. Five investigators replied to the first email sent and three were able to provide usable information on three trials. We received no replies to the second email attempts.
Pneumonia
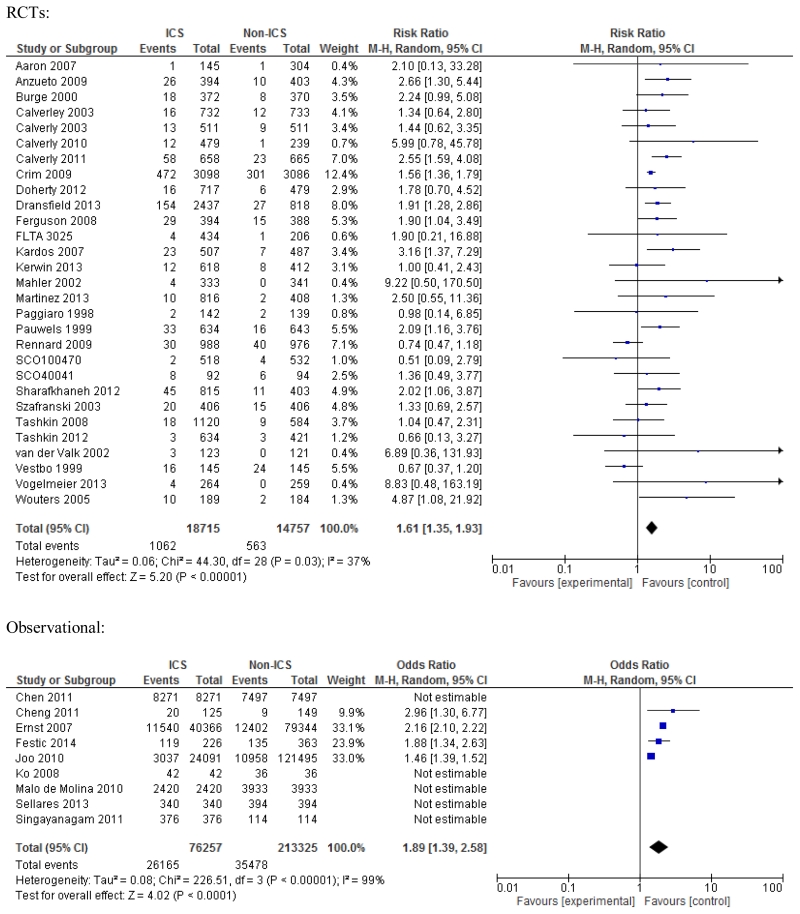
All 29 RCTs were usable for estimating unadjusted increased risk of pneumonia with the use of ICS; RR 1.61; 95% CI 1.35-1.93, p<0.001 (Figure 2). The heterogeneity was deemed acceptable (I2=37%). Nineteen trials with fluticasone showed significantly increased risk of pneumonia, RR 1.76; 95% CI 1.53-2.02, p<0.001; while eight trials of budesonide and two trials of mometasone showed a non-significant risk, RR 1.27; 95% CI 0.86-1.87, p=0.23; and RR 1.36; 95% CI 0.57-3.22, p=0.49; respectively (e-Figure 5, Online Supplement). However, the difference among these three ICS subgroups was not significant (p=0.27, I2=23%). Eight trials that allowed use of ICS in the run-in period showed slightly higher risk of pneumonia compared to 13 trials without ICS in run-in period, RR 1.70; 95% CI 1.13-2.55, p=0.01; versus RR 1.59; 95% CI 1.40-1.80, p<0.001; respectively. Two trials with oral corticosteroids used in the run-in period showed even higher risk of pneumonia, RR 2.14; 95% CI 1.29-3.58, p=0.003.
Figure 2. Meta-analysis of RCTs and observational studies for pneumonia. Risk estimates shown are relative risk (RR) for RCTs and odds ratio (OR) for observational studies.
Four observational studies (2 cohort and 2 case-control) allowed estimation of unadjusted risk of pneumonia, which was increased, OR 1.89; 95% CI 1.39-2.58, p<0.001; with the near-maximum heterogeneity noted (Figure 2). Excluding two case-control studies improved heterogeneity somewhat (I2=62%) and the estimated OR for pneumonia remained similar, OR 1.74; 95% CI 0.97-3.13, p=0.06.
Notably, there was no statistically significant difference (none is singular) between subgroups within any of the subgroup-level analyses.
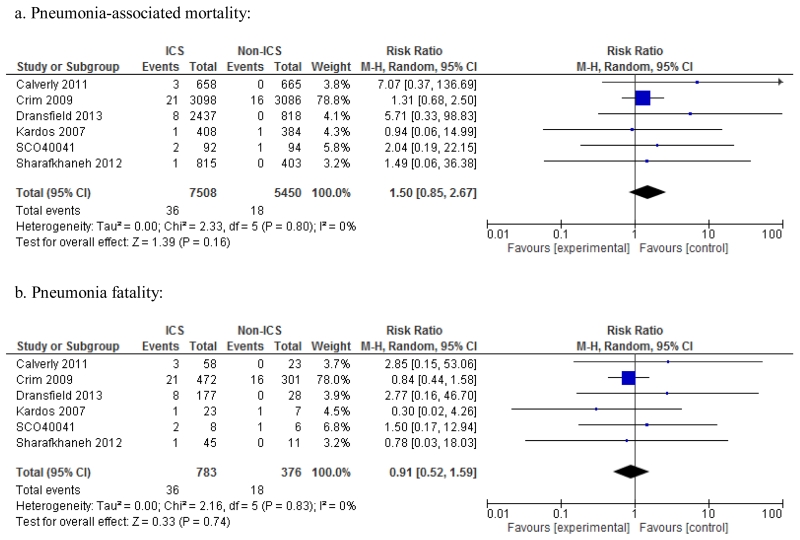
Pneumonia-associated mortality and case-fatality
Six of 29 RCTs reported mortality experience among patients with pneumonia, hence they were useful in estimating risk of pneumonia-related mortality and fatality. There was no significant differences between ICS and non-ICS arms in either pneumonia-associated mortality; RR 1.50; 95% CI 0.85-2.67, p=0.16, or pneumonia fatality; RR 0.91; 95% CI 0.52-1.59, p=0.74 (Figure 3), with no heterogeneity among these trials, I2=0%. This suggested that significantly increased risk of pneumonia in ICS group was not proportionately followed by higher risks of pneumonia-associated mortality or pneumonia fatality, which were not significantly different between the ICS and non-ICS groups.
Figure 3. Meta-analysis of RCTs for pneumonia-associated mortality and pneumonia fatality. Risk estimates shown are relative risks (RR).
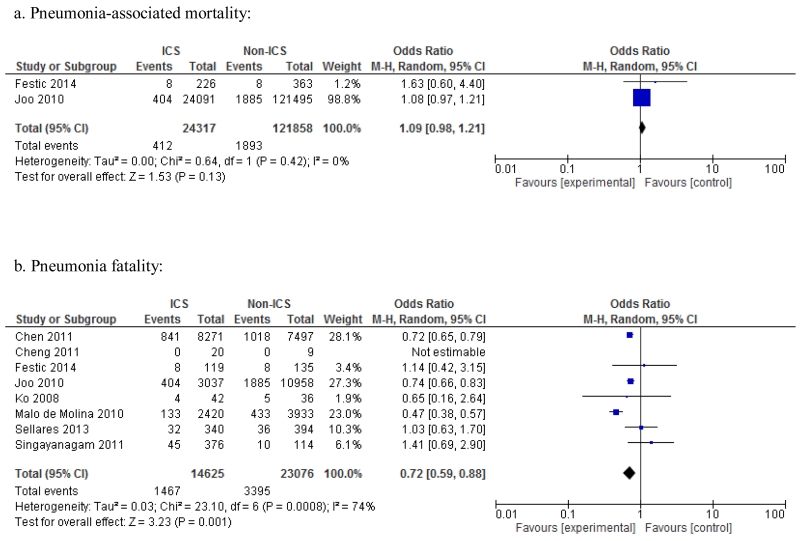
Only two observational studies allowed estimation of pneumonia-associated mortality (number of pneumonia cases who died divided by number of all patients, with or without pneumonia, in the ICS versus non-ICS groups). There was no difference in pneumonia-associated mortality between the ICS versus non-ICS groups; OR 1.09; 95% CI 0.98-1.21, p=0.13, I2=0% (Figure 4). Seven observational studies allowed estimation of unadjusted risk of pneumonia fatality (number of pneumonia cases who died divided by all pneumonia cases in the ICS versus non-ICS groups). There was a significant difference in pneumonia fatality between the ICS and non-ICS groups; OR 0.72; 95% CI 0.59-0.88, p=0.001, however, substantial heterogeneity was observed (I2=74%., p<0.001).
Figure 4. Meta-analysis of observational studies for pneumonia-associated mortality and case fatality. Risk estimates shown are odds ratios (OR).
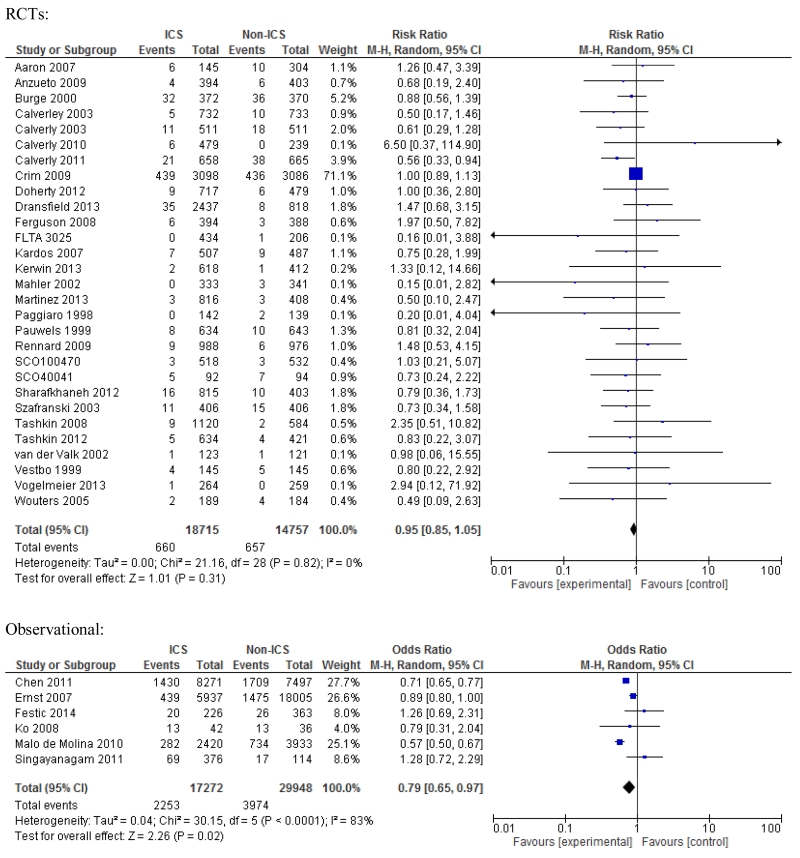
Overall mortality
All RCTs allowed estimation of unadjusted risk of overall mortality with ICS use and the risk was not different from the comparison arm, RR 0.95; 95% CI 0.85-1.05, p=0.31; I2=0% (Figure 5). The risk estimate was similar for all three studied ICS medications. Six estimable observational studies demonstrated decreased unadjusted risk of overall mortality with ICS use but with high heterogeneity, OR 0.79; 95% CI 0.65-0.97, p=0.02, I2=83% (Figure 4). Exclusion of a single case-control study improved heterogeneity somewhat (I2=72%) with no change in estimated risk, OR 0.75; 95% CI 0.60-0.94, p=0.01.
Figure 5. Meta-analysis of RCTs and observational studies for overall mortality. Risk estimates shown are relative risk (RR) for RCTs and odds ratio (OR) for observational studies.
Study drop-out rates
All 29 RCTs were assessed for the study drop-out rates. Compared with placebo, use of ICS was associated with a lower relative risk of trial drop-out, RR 0.84; 95% CI 0.81-0.88, p<0.001; I2=29% (e-Figure 6, Online Supplement).
Sensitivity analyses
Sensitivity analysis on effect size for statistical models showed no appreciable differences for any of the outcomes for fixed vs random effects. Excluding case-control studies decreased the heterogeneity without a change in overall results. Sensitivity analysis of all RCTs by “per protocol” (PP) strategy (excluding patients that did not complete the trial) rather than per ITT, decreased the estimated risks for pneumonia and overall mortality outcomes. This is as expected because the study completion was previously shown to be favored by ICS versus non-ICS arms.21 For the outcome of overall mortality (e-Figure 7, Online Supplement), a change to PP strategy resulted in a significant decrease in unadjusted risk of overall mortality for patients in ICS arms, RR 0.89; 95% CI 0.81-0.99, p=0.03; I2=0%). Sensitivity analysis with inclusion of “double-zero” events, and change in risk estimates from RR to OR or vice-versa did not significantly affect any results.
Discussion
Our findings confirm significantly increased unadjusted risk of pneumonia among ICS users in published RCTs as well as observational studies on the topic. Despite this observed risk, pneumonia-associated mortality, pneumonia fatality and overall mortality were not significantly different between the ICS and non-ICS group in RCTs. Surprisingly, observational studies showed significantly decreased risk of pneumonia fatality and overall mortality in the ICS group but with the substantial heterogeneity among these studies. The estimable risk of pneumonia-associated mortality in two observational studies was not different between ICS and non-ICS groups, but it was proportionately lower than the observed higher risk of incident pneumonia in those studies.
In terms of risk of pneumonia associated with ICS use, our results are remarkably similar to the results of a previous meta-analysis on the topic10, although the total number of patients in the current meta-analysis is twice as large. Therefore, the evidence for increased unadjusted risk of pneumonia with ICS use remains robust. Also, similar to previous reports,45 in our meta-analysis this risk was more prominent for fluticasone, which has higher corticosteroid receptor affinity compared to budesonide.46 Although the subgroup analysis showed that the risk of pneumonia was higher in trials where corticosteroids were used in the run-in period, none of the differences among subgroups were statistically significant. A proposed rationale for the observed increased risk of pneumonia with ICS use is combination of immunosuppressive effect of corticosteroids superimposed on an exposed COPD patient with chronically obstructed airways frequently colonized with bacteria.46 Of note, this risk is not uniform for all patients and is likely dependent on the host characteristics (age, COPD severity, functional status etc.) and medication effects (dose, duration and potency of ICS compounds).
The risk of incident pneumonia was estimated to be higher in observational studies than in RCTs. This could be likely explained by the retrospective inclusion of patients with pneumonia diagnosis without proper adjustment for the duration of preceding respiratory symptoms. A recent major trial showed that a half of all pneumonia events in the fluticasone arm were associated with an ongoing or unresolved COPD exacerbation.5 The data from this trial’s daily record cards showed more unresolved exacerbations preceding pneumonia events in the ICS-treated patients.5 While this effect is possible to study and analyze prospectively, the retrospective observational design would not allow this.
Despite an increased unadjusted risk of pneumonia in RCTs, meta-analysis on pneumonia-associated and overall mortality outcomes showed no significant difference between ICS and non-ICS groups. This could be expected if pneumonia events were not severe enough to affect the mortality outcomes. However, both COPD and pneumonia are among the most frequent causes of death, and as such the overall mortality would be expected to be higher in a group of COPD patients with higher rates of serious pneumonia. Moreover, pooling of the observational studies with >75,000 pneumonia events showed similar results. All pneumonia events in observational studies were diagnosed upon the emergency room or hospital admission making these more severe than ambulatory pneumonia events not requiring hospitalization. Therefore, we propose that although ICS might predispose COPD patients to the increased risk of pneumonia, their anti-inflammatory or other mitigating effect might counterbalance the pneumonia risk and result in similar or improved mortality. A recently published RCT on the role of systemic corticosteroids among patients hospitalized for community-acquired pneumonia with high inflammatory response has indicated a protective effect of corticosteroids.47 This paradoxical beneficial effect of corticosteroids that has been earlier suggested15, 46 might be further supported by the higher adherence among ICS users in RCTs. A sensitivity analysis by PP strategy, which accounted for the imbalance in drop-out rates in RCTs, showed that patients in ICS arms had significantly decreased unadjusted overall mortality compared to patients in non-ICS arms. Of note, the mortality was assessed completely, regardless of the compliance or drop out from the study protocol.
In view of a recently suggested “double-effect” of ICS,46, 48 we also analyzed the risk of pneumonia case-fatality between ICS and non-ICS groups. In meta-analyses of both RCTs and observational studies, the estimated risks of pneumonia fatality were in the opposite direction from the observed increased risk for pneumonia, although patients who use ICS tend to be older 9 with more severe disease.
In a recent, population-based study Gershon et al. reported no difference in incidence of pneumonia requiring hospitalization in patients with newly prescribed combination of ICS and long acting beta agonists (LABA) compared to LABA alone.49 However, patients with new prescriptions for ICS/LABA had decreased risk of overall mortality compared to those using LABA alone. Post hoc addition of this study (with more than 12,000 patients) to our meta-analysis did not change results.
This meta-analysis has several limitations, mostly rooted in the trials and studies we included. As mentioned earlier, many of the trials did not systematically define pneumonia events or require radiographic confirmation for cases of suspected pneumonia. While this specific limitation was largely averted in observational studies, pooling of observational studies resulted in large heterogeneity due to other inherent biases. Although included studies used varying and subjective criteria for the ascertainment of pneumonia diagnosis, this bias was non-differential relative to ICS and non-ICS groups and the expected effect of this bias on the mortality outcomes would not be expected to be different. Indeed, the studies differed widely in their methodologies and only a few of the studies were estimable for the specific outcomes (e.g. pneumonia mortality), which might have affected the power of the meta-analysis to identify significant differences. Despite the above limitations, the overall similarity in the risk estimates for “pneumonia“ and changes in their direction for the mortality outcomes are considered as important findings. Our meta-analysis was prone to reporting bias as we depended solely on the investigator’s reporting of outcomes. Only about half of contributing authors replied to our enquiry and only a third was able to provide us the required information, hence the possibility for bias remains. Although all but one RCT17 were sponsored by the pharmaceutical industry, we did not observe evidence for publication bias. We reviewed the clinical trials registry and included both published and unpublished studies. Also, solid evidence for increased unadjusted risk of pneumonia has been the finding in both the non-industry and industry-sponsored studies.
We investigated only the crude outcome risks, both in RCTs and in observational studies. A random enrollment in RCTs and a large number of patients in observational studies likely eased this concern. Although a few observational studies reported adjusted analyses, their selected predictor and outcome variables varied drastically precluding meaningful pooling. Moreover, the available adjusted risk estimates in the individual studies were all very similar to the unadjusted ones. Without individual-patient data, we could not study any differences in pneumonia-associated and overall mortality based on COPD severity or presence of comorbidities. We grouped all patients utilizing ICS into ICS-user group, regardless of ICS being used alone or in combination with other agents. Similarly, those in non-ICS user group were not using ICS regardless of potential use of other pertinent medications. More portioned investigation of ICS alone or in combination with other medications were not done at this stage.
Finally, the grade of confidence in risk estimates from the included studies ranged from low (pneumonia-associated mortality) to high (overall mortality) in RCTs and was lower in observational studies. Previously noted major limitation with ascertainment of pneumonia and associated mortality,10 still remains. Only future prospective trials of ICS, which would systematically assess and monitor pneumonia as a prespecified outcome using objective pneumonia definitions could clarify this and other above-mentioned concerns.
Conclusion
Despite the fact that most RCTs and observational studies demonstrate increased risk of pneumonia associated with ICS use, the risks of pneumonia fatality and pneumonia-associated as well as overall mortality are, surprisingly, not increased in RCT’s and are reduced in observational studies. This paradox suggests an unexplained “double effect” of ICS in COPD patients and merits further study.
Supplementary Material
Acknowledgment
We acknowledge Drs. Victor Montori, Murad Hasan and Colin West, from Mayo Clinic, Rochester, MN for their methodological expertise and inputs.
We acknowledge Patricia Erwin, the head reference librarian at Mayo Clinic, Rochester, MN for her help with the library search.
We acknowledge Drs. Antoni Torres, Jacobo Sellares Torres, Pierre Ernst, Peter Calverley, Peter Cardos, and Aaron Shawn for the correspondence and contribution.
Funding: Supported in part by grants from the National Center for Advancing Translational Sciences (grant no. 5KL2TR000136-08 and grant no. CTSA UL1 TR000135), a component of the National Institutes of Health (NIH) and Mayo Foundation.
Footnotes
Declaration of interests: EF, VB, EG and PDS declare no existing conflicts of interest as it may relate to the manuscript. PDS declares the following financial activities outside the submitted work: Grants - GlaxoSmithKline, Forest, Boehringer Ingelheim, Novartis AG, Pfizer, Pearl Therapeutics; Consulting with fees paid to the institution – GlaxoSmithKline, Merck, University of Minnesota; Book royalties – Lippincott Williams & Wilkins
Contributions of all authors (EF, VB, EG, PDS) include:
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
Drafting the work or revising it critically for important intellectual content; AND
Final approval of the version to be published; AND
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- 1.From the Global Strategy for the Diagnosis, Management and Prevention of COPD Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011 cited; Available from: Available at: http://www.goldcopd.org/
- 2.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson GT, Anzueto A, Fei R, Emmett A, Knobil K, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 mug) or salmeterol (50 mug) on COPD exacerbations. Respir Med. 2008;102(8):1099–108. doi: 10.1016/j.rmed.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6(5):320–9. doi: 10.1080/15412550903140881. [DOI] [PubMed] [Google Scholar]
- 5.Calverley PM, Stockley RA, Seemungal TA, et al. Reported pneumonia in patients with COPD: findings from the INSPIRE study. Chest. 2011;139(3):505–12. doi: 10.1378/chest.09-2992. [DOI] [PubMed] [Google Scholar]
- 6.Sharafkhaneh A, Southard JG, Goldman M, Uryniak T, Martin UJ. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med. 2012;106(2):257–68. doi: 10.1016/j.rmed.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–23. doi: 10.1016/S2213-2600(13)70040-7. [DOI] [PubMed] [Google Scholar]
- 8.Festic E, Bansal V, Gajic O, Lee AS, United States Critical Illness and Injury Trials Group: Lung Injury Prevention Study Prehospital use of inhaled corticosteroids and point prevalence of pneumonia at the time of hospital admission: secondary analysis of a multicenter cohort study. Mayo Clin Proc. 2014;89(2):154–62. doi: 10.1016/j.mayocp.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176(2):162–6. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease. Arch Intern Med. 2009;169(3):219–29. doi: 10.1001/archinternmed.2008.550. [DOI] [PubMed] [Google Scholar]
- 11.Chen D, Restrepo MI, Fine MJ, et al. Observational Study of Inhaled Corticosteroids on Outcomes for COPD Patients with Pneumonia. Am J Respir Crit Care Med. 2011;184(3):312–6. doi: 10.1164/rccm.201012-2070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Molina RM, Mortensen EM, Restrepo MI, Copeland LA, Pugh MJV, Anzueto A. Inhaled corticosteroid use is associated with lower mortality for subjects with COPD and hospitalised with pneumonia. Eur Respir J. 2010;36(4):751–7. doi: 10.1183/09031936.00077509. [DOI] [PubMed] [Google Scholar]
- 13.Singanayagam A, Chalmers JD, Akram AR, Hill AT. Impact of inhaled corticosteroid use on outcome in COPD patients admitted with pneumonia. Eur Respir J. 2011;38(1):36–41. doi: 10.1183/09031936.00077010. [DOI] [PubMed] [Google Scholar]
- 14.Sellares J, Lopez-Giraldo A, Lucena C, et al. Influence of previous use of inhaled corticoids on the development of pleural effusion in community-acquired pneumonia. Am J Respir Crit Care Med. 2013;187(11):1241–8. doi: 10.1164/rccm.201209-1732OC. [DOI] [PubMed] [Google Scholar]
- 15.Liapikou A, Polverino E, Ewig S, et al. Severity and outcomes of hospitalised community-acquired pneumonia in COPD patients. Eur Respir J. 2012;39(4):855–61. doi: 10.1183/09031936.00067111. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Loke YK. Risk of pneumonia associated with long-term use of inhaled corticosteroids in chronic obstructive pulmonary disease: a critical review and update. Curr Opin Pulm Med. 2010;16(2):118–22. doi: 10.1097/MCP.0b013e328334c085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaron SD, Vandemheen KL, Fergusson D, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: A randomized trial. Ann Intern Med. 2007;146(8):545–55. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 18.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(6):912–9. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 19.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 20.Calverley PMA, Kuna P, Monso E, et al. Beclomethasone/formoterol in the management of COPD: A randomised controlled trial. Respir Med. 2010;104(12):1858–68. doi: 10.1016/j.rmed.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–7. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 22.Doherty DE, Tashkin DP, Kerwin E, et al. Effects of mometasone furoate/formoterol fumarate fixed-dose combination formulation on chronic obstructive pulmonary disease (COPD): Results from a 52-week phase III trial in subjects with moderate-to-very severe COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:57–71. doi: 10.2147/COPD.S27320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of Salmeterol/Fluticasone Propionate versus Salmeterol on Exacerbations in Severe Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2007;175:144–9. doi: 10.1164/rccm.200602-244OC. [DOI] [PubMed] [Google Scholar]
- 24.Martinez FJ, Boscia J, Feldman G, et al. Fluticasone furoate/vilanterol (100/25; 200/25 mug) improves lung function in COPD: A randomised trial. Respir Med. 2013;107(4):550–9. doi: 10.1016/j.rmed.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Rennard SI, Tashkin DP, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5):549–65. doi: 10.2165/00003495-200969050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tashkin DP, Doherty DE, Kerwin E, et al. Efficacy and safety characteristics of mometasone furoate/formoterol fumarate fixed-dose combination in subjects with moderate to very severe COPD: findings from pooled analysis of two randomized, 52-week placebo-controlled trials. Int J Chron Obstruct Pulmon Dis. 2012;7:73–86. doi: 10.2147/COPD.S29444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tashkin DP, Rennard SI, Martin P, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs. 2008;68(14):1975–2000. doi: 10.2165/00003495-200868140-00004. [DOI] [PubMed] [Google Scholar]
- 28.Vestbo J, Sorensen T, Lange P, Brix A, Tone P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 1999;353(9167):1819–23. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 29.Vogelmeier CF, Bateman ED, Pallante J, Alagappan VKT, D’Andrea P, Chen H, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): A randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1(1):51–60. doi: 10.1016/S2213-2600(12)70052-8. [DOI] [PubMed] [Google Scholar]
- 30.Burge PS, Calverley PMA, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE Trial. BMJ. 2000;320(7245):1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(5):74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 32.Pauwels RA, Lofdahl CG, Laitinen LA, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med. 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 33.Paggiaro PL, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicenter randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. Lancet. 1998;351(9105):773–780. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 34.GlaxoSmithKline Clinical study register. Study No. FLTA3025. http://www.gskclinicalstudyregister.com/files/pdf/1231.pdf.
- 35.GlaxoSmithKline Clinical study register. Study No.SCO100470 http://www.gsk-clinicalstudyregister.com/files/pdf/1079.pdf.
- 36.GlaxoSmithKline Clinical study register. Study No.SCO40041. http://www.gsk-clinicalstudyregister.com/files/pdf/21086.pdf.
- 37.van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE Study. Am J Respir Crit Care Med. 2002;166(10):1358–1363. doi: 10.1164/rccm.200206-512OC. [DOI] [PubMed] [Google Scholar]
- 38.Wouters EF, Postma DS, Fokkens B, et al. for the COSMIC (COPD and Seretide; a Multicenter Intervention and Characterization) Study Group Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax. 2005;60(6):480–487. doi: 10.1136/thx.2004.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahler DA, Wire P, Horstman D, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(8):1084–1091. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- 40.Kerwin E, Scott-Wilson C, Sanford L, et al. A randomised trial of fluticasone furoate/vilanterol (50/25μg; 1--/25 μg) on lung function in COPD. Respir Med. 2013;(107):560–569. doi: 10.1016/j.rmed.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Cheng SL, Wang HC, Cheng SJ, Yu CJ. Elevated placenta growth factor predicts pneumonia in patients with chronic obstructive pulmonary disease under inhaled corticosteroids therapy. BMC Pulm. 2011;11:46. doi: 10.1186/1471-2466-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ernst P, Suissa S. Pneumonia in elderly patients with chronic obstructive pulmonary disease. Curr Infect Dis Rep. 2008;10(3):223–8. doi: 10.1007/s11908-008-0037-4. [DOI] [PubMed] [Google Scholar]
- 43.Joo MJ, Au DH, Fitzgibbon ML, Lee TA. Inhaled corticosteroids and risk of pneumonia in newly diagnosed COPD. Respir Med. 2010;104(2):246–52. doi: 10.1016/j.rmed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Ko FWS, Ip M, Chan PKS, Ng SS, Chau SS, Hui DSC. A one -year prospective study of infectious etiology in patients hospitalized with acute exacerbations of COPD and concomittant pneumonia. Respir Med. 2008;(102):1109–1116. doi: 10.1016/j.rmed.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halpin DMG, Gray J, Edwards SJ, Morais J, Singh D. Budesonide/formoterol vs. salmeterol/fluticasone in COPD: a systematic review and adjusted indirect comparison of pneumonia in randomised controlled trials. Int J Clin Pract. 2011;65(7):764–74. doi: 10.1111/j.1742-1241.2011.02685.x. [DOI] [PubMed] [Google Scholar]
- 46.Sibila O, Anzueto A, Restrepo MI. The paradoxical effect on pneumonia of chronic inhaled corticosteroids. Clin Pulm Med. 2013;20(1):6–10. doi: 10.1097/CPM.0b013e31827a2a60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres A, Sibila O, Ferrer M, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response. JAMA. 2015;313(7):677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 48.Festic E, Scanlon P. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease. A double effect of inhaled corticosteroids? Am J Respir Crit Care Med. 2015;191(2):141–8. doi: 10.1164/rccm.201409-1654PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gershon AS, Campitelli MA, Croxford R, et al. Combination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary disease. JAMA. 2014;312(11):1114–1121. doi: 10.1001/jama.2014.11432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.