Abstract
Human respiratory syncytial virus (RSV) is the leading cause of severe lower respiratory tract infection, such as bronchiolitis, bronchitis, or pneumonia, in both infants and the elderly. Despite the global burden of diseases attributable to RSV infection, no clinically approved vaccine is available, and a humanized monoclonal antibody for prophylaxis is not readily affordable in developing countries. There are several hurdles to the successful development of RSV vaccines: immune-vulnerable target populations such as premature infants, pregnant women, and immunocompromised people; safety concerns associated with vaccine-enhanced diseases; repeated infection; and waning memory. To develop successful strategies for the prevention of RSV infection, it is necessary to understand the protective and pathologic roles of host immune responses to RSV infection. In this review, we will summarize the positive and negative relationship between RSV infection and host immunity and discuss strategies for the development of the first successful RSV vaccine.
Keywords: Immunity, respiratory syncytial viruses, vaccine, vaccine-enhanced diseases
INTRODUCTION
Human respiratory syncytial virus (RSV), an enveloped, nonsegmented negative-sense RNA virus of the family Paramyxoviridae is the leading cause of severe lower respiratory tract infection in both infants and the elderly, despite limited viral variation and protective immunity.1 Most infants are infected during the first year of life, and re-infections occur throughout life. The immature immune system of infants is associated with pathologic symptoms and mortality as well as RSV vaccine-enhanced disease. Additionally, RSV infection is likely to be associated with specific side effects, such as asthma-like lesions following RSV re-infection. RSV infection is also a serious problem in elderly persons due to their weak immune systems. According to a retrospective cohort study, adult hospitalization due to RSV infection is associated with substantial rates of complications and mortality.2 These facts have increased the public health concern related to RSV worldwide; however, no approved vaccine for RSV is available. Developing an effective RSV vaccine is problematic, as the major target populations are infants and immunocompromised adults. The efficacy and safety of any vaccine are important aspects in its development.
In this review, we discuss the latest research on protective immunity against RSV infection and suggest what should be considered for the development of safe and effective vaccines against RSV infection.
RSV INFECTION AND INNATE IMMUNITY
Viruses are recognized mainly by Toll-like receptors (TLRs) and other pattern recognition receptors, which detect structural components including viral nucleic acids and surface glycoproteins as pathogen-associated molecular patterns. The recognition of viruses by these innate immune receptors commonly induces type I interferon (IFN) production, which mediates strong antiviral defenses. Similar to other viruses, RSV infection elicits host innate immune responses, in which innate receptors expressed on resident leukocytes and lung epithelial cells play key roles.3,4 TLRs are directly involved in activating innate immunity against RSV by recognizing certain conserved viral motifs.5,6 For instance, the fusion (F) protein of RSV has been observed to activate TLR4.7 Moreover, RSV induces production of inflammatory cytokines and chemokines through TLR2 and TLR6, which activate innate immunity by promoting TNF-α, interleukin (IL)-6, MCP-1, and RANTES production.8 The early inflammatory signals generated by RSV-TLR interactions during RSV infection are likely to recruit neutrophils and natural killer (NK) cells into the lung, which are important for clearing RSV-infected cells. Indeed, TLR4-deficient mice challenged with RSV, though not influenza virus, exhibited impaired NK cell and CD14+ cell pulmonary trafficking, deficient NK cell function, impaired IL-12 expression, and impaired virus clearance compared to control mice.9 However, Ehl, et al.10 reported that the absence of TLR4 had no impact on NK cell recruitment, NK cell activity, or recruitment of other pulmonary inflammatory cells, arguing against a significant role for TLR4 in primary murine RSV infection. In humans, Awomoyi, et al.11 suggested that a defect in TLR4 signaling is linked to RSV-induced pathology in preterm, high-risk infants. Supporting these findings, Tulic, et al.12 demonstrated that peripheral blood mononuclear cells isolated from children with variant forms of TLR4 exhibited reduced expression of the receptor on the surface and reduced response to RSV, suggesting that weakened immune responses contribute to enhanced susceptibility to RSV infection in these individuals. Thus, it is likely that TLR-dependent signaling is important for activating early inflammatory responses to RSV and that aberrant TLR signaling contributes to RSV-induced disease in humans. The RIG-I-like receptors (RLRs), including RIG-I and MDA5, detect viral dsRNA, 5'-triphosphorylated uncapped viral RNA, or ssRNA genome bearing 5'-triphosphates, and activate the downstream NF-κB and IRFs pathways through the common adaptor, mitochondrial anti-viral signaling protein (MAVS). Bhoj, et al.3 demonstrated in vitro and in vivo that MAVS is essential for the production of type I IFN and many inflammatory cytokines in response to RSV infection. However, Myd88-/-Mavs-/- mice mounted a normal cytotoxic T-lymphocyte response and exhibited delayed yet effective viral clearance, suggesting that comparable adaptive immune responses could be induced in the absence of innate immunity mediated by the MAVS and MyD88 pathways.3 Therefore, it is clear that innate immunity is involved in RSV recognition and the subsequent immune response, and further studies of anti-RSV innate immunity are required for the development of effective vaccines and therapeutics against RSV infection.
B-CELL IMMUNITY TO RSV AND VACCINE DEVELOPMENT
Although natural infection with RSV induces neutralizing antibodies, the levels of which are correlated with protection against reinfection with RSV of the same strain, it does not provide long-lasting protective immunity.1,13 In humans, levels of RSV-specific antibodies decline more than 4-fold within 1 year following a natural RSV infection,14 and this decline was reported to correlate with susceptibility to recurrent symptomatic RSV infection.1 In particular, RSV-specific nasal IgA was correlated more strongly with protection from RSV infection, although the levels were less well maintained than those of serum neutralizing antibodies.15 As RSV antibodies are important for protection following RSV infection, strategies to modulate antibody persistence and confer protection against RSV are needed.
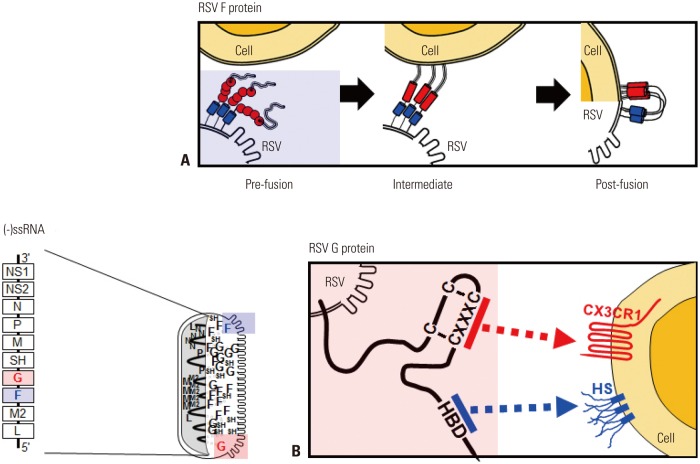
The major glycoproteins on the surface of RSV, the F and attachment (G) proteins, control the initial phases of viral infection and are the major protective antigens targeted by neutralizing antibodies.16 The F protein is highly conserved between RSV A and B subtypes (90% similarity) and elicits production of neutralizing antibodies and a T cell response against RSV challenge. F protein mediates virus entry via membrane fusion and exists on the RSV membrane in both prefusion (pre-F) and postfusion (post-F) conformations. Briefly, F-protein trimers in the envelope of resting RSV (pre-F form) are triggered, refolded, and attached to the target cell membrane (intermediate stage). The trimer then folds in half, and the viral and cellular membranes fuse (post-F form) (Fig. 1A). Although epitopes for neutralizing antibody production reside in both conformations, post-F exhibits less effective neutralization through immunoglobulins produced via natural infection with RSV, suggesting that pre-F has greater potency.17 The atomic-level structure of pre-F has increased our understanding of the targets of neutralizing antibodies produced via vaccination with RSV F protein. Identification of neutralization-sensitive epitopes in pre-F as a candidate for a subunit vaccine or for prophylactic monoclonal antibody development is a promising strategy for the development of an RSV vaccine.
Fig. 1. RSV genome, proteins, and their functions. In the schematic diagram of the RSV genome, 10 genes are indicated in the order NS1-NS2-N-P-M-SH-G-F-M2-L. Filled square, genes encoding proteins targeted by neutralizing antibodies. (A) Simplified diagram of RSV F protein-mediated membrane fusion. (B) Attachment of RSV G protein to the host cell membrane is mediated by HS and HBD and/or the CX3C-CX3CR1 interaction. Pre-fusion, pre-fusion form of the F protein; Intermediate, refolding of the F protein to initiate membrane attachment; Post-fusion, membrane fusion between RSV and the target cell; HS, heparan sulfate; HBD, heparin-binding domain; RSV, respiratory syncytial virus.
The RSV G protein is involved in attachment.16 The heparin-binding domain and the third and fourth cysteines separated by three amino acids (CX3C) in the cysteine noose of the membrane-anchored G protein bind their respective targets, heparan sulfate18 and CX3CR1, on the surface of most cell types (Fig. 1B).18,19 The RSV A- and B-type G proteins share only 53% identity,20 and the antigenic variability of RSV is a barrier to vaccine development. However, recent research suggests that the G protein has a conserved region from amino acids 148 to 198, which contains four conserved cysteine residues forming a noose (amino acid positions 173, 176, 182, and 186), and the central conserved region is targeted by antibodies protective against RSV challenge.17 The cysteine noose has a CX3C chemokine motif within amino acids 182 to 186 that shares structural similarity with the chemokine fractalkine (CX3CL1) and can bind to CX3CR1-expressing leukocytes competitive with CX3CL1.21 Although a monoclonal antibody against the CX3C region of the G protein was found to have no neutralizing activity in RSV-infected Hep2 cells,22 it directly blocked the binding between CX3C and CX3CRl and reduced viral replication in both prophylaxis and treatment mouse models of RSV challenge.23 A non-neutralizing monoclonal antibody against a G protein epitope (amino acid positions 174–187) was reported to provide passive protection via both ADCC and complement-mediated cytolysis.24 Previous works suggested that a recombinant G protein core fragment of amino acids 131 to 230 (Gcf) induced strong immunoglobulin responses (RSV-spe-cific IgG and IgA) in mice as well as potent chemotactic activity in a cell migration assay.25 An excellent vector-based mucosal vaccine comprising a recombinant replication-deficient adenovirus expressing the tandem repeats of Gcf also induced strong RSV-specific B-cell responses and showed protective efficacy against human RSV A and B.26,27
As prophylactic vaccine candidates or therapeutics, conserved RSV antigens and neutralizing and/or non-neutralizing antibodies to these target antigens could play a role in providing protection against RSV infection.
T-CELL IMMUNITY AGAINST RSV INFECTION
T cells are believed to be essential for the clearance of RSV from the lungs and host defense. In mice, adoptive transfer of RSV-specific memory T cells cleared primary RSV infection,28 and depletion of CD4 or CD8 T cells markedly prolonged the duration of RSV shedding,29 suggesting that T cells contribute to the elimination of infected cells during acute RSV infection. In humans, the numbers and functional capacity of RSV-specific T cells might be implicated in viral clearance, as aging and/or immunodeficiency affecting T-cell function resulted in more severe disease and higher levels of virus shedding.30,31 A recent human study has shown that a CD8 resident memory T cell subset was maintained at an increased level during the recovery period, and their abundance was highly correlated with virus clearance and protection against RSV infection, despite lower cytotoxicity compared with peripheral blood CD8 T cells.32 In addition, a number of RSV gene products are implicated in the modulation of the immune system, including T-cell immunity (reviewed in Openshaw and Chiu33). For example, RSV infection of DCs impairs their activation of protective T cells yet promotes their proliferation and activation of pathologic Th2 cells.34 RSV infection might modulate the assembly of immunological synapses35 or affect the expression of co-stimulatory molecules and cytokine production.36 Thus, it is clear that a greater understanding of the role of T-cell immunity in RSV infection and viral counter-regulation is required for the development of a vaccine that provides long-term protective immunity.
Despite their importance in RSV clearance, T cells are also associated with RSV disease severity. For example, transfer of CTLs augmented lung pathology,28 and in vivo depletion of CD8 T cells29 resulted in reduced illness in mice. In the 1960s, formalin-inactivated RSV (FI-RSV) underwent a clinical trial as a vaccine candidate yet failed to induce protective immunity to RSV challenge.37 Instead, the vaccinated subjects developed exacerbated symptoms that were associated with strong Th2 responses such as severe lung eosinophilia.38,39 This aberrant response can be mimicked in murine models by priming with inactivated RSV40 or recombinant vaccinia virus expressing RSV G protein,29,41 which is associated with Th2 cytokines and a specific oligoclonal CD4 T-cell response.42 In a mouse model, a strong RSV M2-specific CD8 T-cell response suppressed Th2-type cytokine production and subsequent pulmonary eo-sinophilia, suggesting that CD8 T cells play a regulatory role in the differentiation and activation of Th cells.43 However, it is unlikely that Th2-biased responses and vaccine-enhanced diseases are an intrinsic property of G antigen, as other vaccine platforms targeting G protein did not exhibit these properties.44 Nonetheless, the data from animal models and humans concerning vaccine-associated pathology can be used to form a consensus that Th2-biased CD4 T-cell responses and subsequent vaccine-enhanced diseases should be avoided in future RSV vaccine trials. However, other studies failed to find an association between clinical severity and Th2-like T-cell responses.45,46 Thus, it remains to be determined whether the balance between Th1- and Th2-type responses induced by vaccination has an effect on subsequent RSV infection and RSV-induced disease severity.
Recently, emerging evidence has suggested that IL-17 and possibly Th17-type responses are involved in the pathophysiology of RSV infection and airway inflammation.47,48,49 In mice, neutralization of IL-17 during RSV infection resulted in reduced inflammation, inhibition of mucus production, decreased viral load, and increased RSV-specific CD8 T cells.49 The level of IL-17 in tracheal aspirates from hospitalized infants also increased significantly compared to the control, suggesting a pathogenic role for IL-17. Tregs might also play an important role in the regulation of cellular infiltration in the lungs and disease severity following acute RSV infection. Depletion of Tregs following RSV infection resulted in increased cellular trafficking and enhanced disease severity without affecting viral clearance.50,51 However, the significance of Th17-type and Treg responses to RSV infection requires further studies in both humans and animal models.
T cells are essential for the generation of protective immunity against RSV. A successful RSV vaccine should elicit a balanced immune response without vaccine-enhanced disease as well as protective memory. Our understanding of T-cell–mediated immunity is a work in progress, and further studies on the protective and pathologic roles of T cells are required.
RSV VACCINES IN CLINICAL TRIALS
Vaccination might be the most effective and economic strategy for obtaining protective immunity against RSV. Since the failure of the FI-RSV vaccine, many groups have investigated RSV immunity and vaccine-enhanced disease. Several RSV vaccine candidates are undergoing clinical trials (Table 1).
Table 1. Clinical Trials of Vaccines for RSV Infection.
| Vaccine | Age (yrs) | Developer | Phase | Reference | |
|---|---|---|---|---|---|
| Type | Antigen | ||||
| Live-attenuated | RSV-PIV3 chimeric virus (MEDI-534) | 0.2-9 | MedImmune | 1, 2 | Gomez, et al.57; Bernstein, et al.58; Yang, et al.59 |
| rA2cp248/404/1030ΔSH (MEDI-559) | 0.4-2 | MedImmune | 1, 2 | Schickli, et al.60; Malkin, et al.61 | |
| RSV MEDI ΔM2-2 | 0.5-49 | NIAID | 1 | Bermingham and Collins62; Karron, et al.63 | |
| RSV ΔNS2 Δ1313 I1314L | 0.5-5 | NIAID | 1 | Luongo, et al.64 | |
| rA2cp248/404/1030ΔSH containing five independent attenuating elements | 0.5-2 | NIAID | 1 | Luongo, et al.65 | |
| RSV LID ΔM2-2 | 0.5-2 | NIAID | 1 | ||
| M2-2 deleted RSV (D46cpΔM2-2) | 0.5-5 | NIAID | 1 | Karron, et al.63 | |
| Subunit | RSV-F | 2-6, 18-49, >60 | Novavax | 1, 2, 3 | Calder, et al.66; Collins and Mottet67; López, et al.68; Glenn, et al.69 |
| RSV-sF/GLA (MEDI-7510) | >60 | MedImmune | 1, 2 | ||
| RSV F | 18-45 | Novartis | 1 | ||
| RSV-SHe/DepoVaxTM and alum | 50-64 | Dalhousie University, ImmunoVaccine Technologies | 1 | https://www.imvaccine.com/infectvac.php | |
| Vectored | Chimpanzee Adenovirus and Modified Vaccinia Ankara encoding the F, N, and M2-1 proteins of RSV (PanAd3-RSV, MVA-RSV) | 18-75 | ReiThera Srl | 1 | Pierantoni, et al.70 |
| Adenovirus encoding full length RSV F protein (Ad26.RSV.FA2 & Ad35.RSV.FA2) | 18-50 | Crucell Holland BV | 1 | Widjojoatmodjo, et al.71 | |
| Chimpanzee Adenovirus encoding RSV protein (ChAd155-RSV; GSK3389245A) | 18-45 | GSK | 1 | http://www.gsk-clinicalstudyregister.com/study/201974#ps | |
| Modified Vaccinia Ankara encoding two surface proteins of RSV (MVA-mBN294B) | 18-65 | Bavarian Nordic | 1 | http://www.bavarian-nordic.com/ | |
| Immuno-stimulant | GSK3003891A | 18-45 | GSK | 1, 2 | http://www.gsk-clinicalstudyregister.com/compounds/gsk3003891a#ps |
RSV, respiratory syncytial virus; PIV3, parainfluenza virus type 3; cp, cold-passaged; NIAID, National Institute of Allergy and Infectious Diseases; GSK, GlaxoSmithKline.
Live-attenuated RSV vaccines that lack vaccine-associated enhanced RSV disease might be suitable for RSV-naïve infants and young children.52 These vaccines offer several advantages: 1) needle-free administration via the nasal cavity, 2) upper respiratory tract infection of the live-attenuated RSV and local mucosal immunity even in the presence of maternal neutralizing antibody, and 3) broad stimulation of innate, humoral and cellular immunity.53 Despite the fact that balancing attenuation and immunogenicity is difficult54 and that the vaccine itself is extremely unstable,53 several candidate live-attenuated vaccines that use temperature sensitivity and/or reverse genetics to derive attenuation are currently undergoing clinical trials (Table 1).
Infants less than 0.5 years old, children from 0.5 to 2 years of age, pregnant women, and the elderly are the target populations for RSV vaccines. The immature and/or pre-existing immunity in certain groups necessitates effective vaccine platforms. Although subunit vaccines are expected to elicit a strong neutralizing antibody response,21 animal and human studies indicate that some subunit vaccine candidates weaken cellular immunity and elicit a Th2-skewed immune response accompanied by severe vaccine-enhanced disease.55 Currently, subunit vaccine candidates including F and SHe proteins are being evaluated in clinical trials (Table 1).
Since the advent of recombinant vector technology, many vectored vaccines have been used as gene carrier systems, and viral vectors such as adenovirus and vaccinia virus have undergone clinical trials for RSV vaccine development. According to the delivery methods, the vectored vaccines might direct the production of mRNA and protein antigens inside host cells and induce both cellular and humoral immune responses.56 As shown in Table 1, various RSV genes of interest–such as F, G, N, and M2-1–are delivered to human subjects by means of these vectored vaccine platforms.
CONCLUSION
For many decades, RSV vaccine development has been a high priority in global healthcare, and much effort has been devoted to the study of host immunity and the development of safe and effective vaccine strategies to prevent and/or treat RSV infection. Presently, several vaccine candidates are being evaluated in clinical trials targeting children, pregnant women, and the elderly, and this will continue until an effective vaccine is developed. The greater our understanding of the host immunity, viral immune evasion, and immunopathology of RSV infection, the closer we move towards the first licensed RSV vaccine.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant number: HI13C0826).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 2.Volling C, Hassan K, Mazzulli T, Green K, Al-Den A, Hunter P, et al. Respiratory syncytial virus infection-associated hospitalization in adults: a retrospective cohort study. BMC Infect Dis. 2014;14:665. doi: 10.1186/s12879-014-0665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres JP, et al. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc Natl Acad Sci U S A. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Rudd BD, Smit JJ, Flavell RA, Alexopoulou L, Schaller MA, Gruber A, et al. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 6.Schlender J, Hornung V, Finke S, Günthner-Biller M, Marozin S, Brzózka K, et al. Inhibition of toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J Virol. 2005;79:5507–5515. doi: 10.1128/JVI.79.9.5507-5515.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurt-Jones EA, Popova L, Kwinn L, Haynes LM, Jones LP, Tripp RA, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 8.Murawski MR, Bowen GN, Cerny AM, Anderson LJ, Haynes LM, Tripp RA, et al. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J Virol. 2009;83:1492–1500. doi: 10.1128/JVI.00671-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes LM, Moore DD, Kurt-Jones EA, Finberg RW, Anderson LJ, Tripp RA. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehl S, Bischoff R, Ostler T, Vallbracht S, Schulte-Mönting J, Poltorak A, et al. The role of Toll-like receptor 4 versus interleukin-12 in immunity to respiratory syncytial virus. Eur J Immunol. 2004;34:1146–1153. doi: 10.1002/eji.200324449. [DOI] [PubMed] [Google Scholar]
- 11.Awomoyi AA, Rallabhandi P, Pollin TI, Lorenz E, Sztein MB, Boukhvalova MS, et al. Association of TLR4 polymorphisms with symptomatic respiratory syncytial virus infection in high-risk infants and young children. J Immunol. 2007;179:3171–3177. doi: 10.4049/jimmunol.179.5.3171. [DOI] [PubMed] [Google Scholar]
- 12.Tulic MK, Hurrelbrink RJ, Prêle CM, Laing IA, Upham JW, Le Souef P, et al. TLR4 polymorphisms mediate impaired responses to respiratory syncytial virus and lipopolysaccharide. J Immunol. 2007;179:132–140. doi: 10.4049/jimmunol.179.1.132. [DOI] [PubMed] [Google Scholar]
- 13.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 14.Falsey AR, Singh HK, Walsh EE. Serum antibody decay in adults following natural respiratory syncytial virus infection. J Med Virol. 2006;78:1493–1497. doi: 10.1002/jmv.20724. [DOI] [PubMed] [Google Scholar]
- 15.Habibi MS, Jozwik A, Makris S, Dunning J, Paras A, DeVincenzo JP, et al. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am J Respir Crit Care Med. 2015;191:1040–1049. doi: 10.1164/rccm.201412-2256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLellan JS, Ray WC, Peeples ME. Structure and function of respiratory syncytial virus surface glycoproteins. Curr Top Microbiol Immunol. 2013;372:83–104. doi: 10.1007/978-3-642-38919-1_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham BS, Modjarrad K, McLellan JS. Novel antigens for RSV vaccines. Curr Opin Immunol. 2015;35:30–38. doi: 10.1016/j.coi.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman SA, Hendry RM, Beeler JA. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J Virol. 1999;73:6610–6617. doi: 10.1128/jvi.73.8.6610-6617.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol. 2001;2:732–738. doi: 10.1038/90675. [DOI] [PubMed] [Google Scholar]
- 20.Oshansky CM, Zhang W, Moore E, Tripp RA. The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiol. 2009;4:279–297. doi: 10.2217/fmb.09.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorquera PA, Oakley KE, Tripp RA. Advances in and the potential of vaccines for respiratory syncytial virus. Expert Rev Respir Med. 2013;7:411–427. doi: 10.1586/17476348.2013.814409. [DOI] [PubMed] [Google Scholar]
- 22.Anderson LJ, Bingham P, Hierholzer JC. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J Virol. 1988;62:4232–4238. doi: 10.1128/jvi.62.11.4232-4238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collarini EJ, Lee FE, Foord O, Park M, Sperinde G, Wu H, et al. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J Immunol. 2009;183:6338–6345. doi: 10.4049/jimmunol.0901373. [DOI] [PubMed] [Google Scholar]
- 24.Corbeil S, Seguin C, Trudel M. Involvement of the complement system in the protection of mice from challenge with respiratory syncytial virus long strain following passive immunization with monoclonal antibody 18A2B2. Vaccine. 1996;14:521–525. doi: 10.1016/0264-410X(95)00222-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Joo DH, Lee JB, Shim BS, Cheon IS, Jang JE, et al. Dual role of respiratory syncytial virus glycoprotein fragment as a mucosal immunogen and chemotactic adjuvant. PLoS One. 2012;7:e32226. doi: 10.1371/journal.pone.0032226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu JR, Kim S, Lee JB, Chang J. Single intranasal immunization with recombinant adenovirus-based vaccine induces protective immunity against respiratory syncytial virus infection. J Virol. 2008;82:2350–2357. doi: 10.1128/JVI.02372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jang JE, Lee JB, Kim KH, Park SM, Shim BS, Cheon IS, et al. Evaluation of protective efficacy of respiratory syncytial virus vaccine against A and B subgroup human isolates in Korea. PLoS One. 2011;6:e23797. doi: 10.1371/journal.pone.0023797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cherukuri A, Patton K, Gasser RA, Jr, Zuo F, Woo J, Esser MT, et al. Adults 65 years old and older have reduced numbers of functional memory T cells to respiratory syncytial virus fusion protein. Clin Vaccine Immunol. 2013;20:239–247. doi: 10.1128/CVI.00580-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall CB, Powell KR, MacDonald NE, Gala CL, Menegus ME, Suffin SC, et al. Respiratory syncytial viral infection in children with compromised immune function. N Engl J Med. 1986;315:77–81. doi: 10.1056/NEJM198607103150201. [DOI] [PubMed] [Google Scholar]
- 32.Jozwik A, Habibi MS, Paras A, Zhu J, Guvenel A, Dhariwal J, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Openshaw PJ, Chiu C. Protective and dysregulated T cell immunity in RSV infection. Curr Opin Virol. 2013;3:468–474. doi: 10.1016/j.coviro.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munir S, Hillyer P, Le Nouën C, Buchholz UJ, Rabin RL, Collins PL, et al. Respiratory syncytial virus interferon antagonist NS1 protein suppresses and skews the human T lymphocyte response. PLoS Pathog. 2011;7:e1001336. doi: 10.1371/journal.ppat.1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González PA, Prado CE, Leiva ED, Carreño LJ, Bueno SM, Riedel CA, et al. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci U S A. 2008;105:14999–15004. doi: 10.1073/pnas.0802555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wythe SE, Dodd JS, Openshaw PJ, Schwarze J. OX40 ligand and programmed cell death 1 ligand 2 expression on inflammatory dendritic cells regulates CD4 T cell cytokine production in the lung during viral disease. J Immunol. 2012;188:1647–1655. doi: 10.4049/jimmunol.1103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 38.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 39.Cohn L, Herrick C, Niu N, Homer R, Bottomly K. IL-4 promotes airway eosinophilia by suppressing IFN-gamma production: defining a novel role for IFN-gamma in the regulation of allergic airway inflammation. J Immunol. 2001;166:2760–2767. doi: 10.4049/jimmunol.166.4.2760. [DOI] [PubMed] [Google Scholar]
- 40.Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–2860. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 42.Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4(+) T cells. Immunity. 2001;15:637–646. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 43.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–432. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang J. Current progress on development of respiratory syncytial virus vaccine. BMB Rep. 2011;44:232–237. doi: 10.5483/BMBRep.2011.44.4.232. [DOI] [PubMed] [Google Scholar]
- 45.Brandenburg AH, Kleinjan A, van Het Land B, Moll HA, Timmerman HH, de Swart RL, et al. Type 1-like immune response is found in children with respiratory syncytial virus infection regardless of clinical severity. J Med Virol. 2000;62:267–277. [PubMed] [Google Scholar]
- 46.Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis. 2001;184:393–399. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]
- 47.Kallal LE, Hartigan AJ, Hogaboam CM, Schaller MA, Lukacs NW. Inefficient lymph node sensitization during respiratory viral infection promotes IL-17-mediated lung pathology. J Immunol. 2010;185:4137–4147. doi: 10.4049/jimmunol.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lukacs NW, Smit JJ, Mukherjee S, Morris SB, Nunez G, Lindell DM. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010;185:2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, et al. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol. 2011;179:248–258. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fulton RB, Meyerholz DK, Varga SM. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J Immunol. 2010;185:2382–2392. doi: 10.4049/jimmunol.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee DC, Harker JA, Tregoning JS, Atabani SF, Johansson C, Schwarze J, et al. CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J Virol. 2010;84:8790–8798. doi: 10.1128/JVI.00796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright PF, Karron RA, Belshe RB, Shi JR, Randolph VB, Collins PL, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25:7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:259–284. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins PL, Murphy BR. New generation live vaccines against human respiratory syncytial virus designed by reverse genetics. Proc Am Thorac Soc. 2005;2:166–173. doi: 10.1513/pats.200501-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malloy AM, Falsey AR, Ruckwardt TJ. Consequences of immature and senescent immune responses for infection with respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:211–231. doi: 10.1007/978-3-642-38919-1_11. [DOI] [PubMed] [Google Scholar]
- 56.Loomis RJ, Johnson PR. Gene-based vaccine approaches for respiratory syncytial virus. Curr Top Microbiol Immunol. 2013;372:307–324. doi: 10.1007/978-3-642-38919-1_15. [DOI] [PubMed] [Google Scholar]
- 57.Gomez M, Mufson MA, Dubovsky F, Knightly C, Zeng W, Losonsky G. Phase-I study MEDI-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr Infect Dis J. 2009;28:655–658. doi: 10.1097/INF.0b013e318199c3b1. [DOI] [PubMed] [Google Scholar]
- 58.Bernstein DI, Malkin E, Abughali N, Falloon J, Yi T, Dubovsky F MI-CP149 Investigators. Phase 1 study of the safety and immunogenicity of a live, attenuated respiratory syncytial virus and parainfluenza virus type 3 vaccine in seronegative children. Pediatr Infect Dis J. 2012;31:109–114. doi: 10.1097/INF.0b013e31823386f1. [DOI] [PubMed] [Google Scholar]
- 59.Yang CF, Wang CK, Malkin E, Schickli JH, Shambaugh C, Zuo F, et al. Implication of respiratory syncytial virus (RSV) F transgene sequence heterogeneity observed in Phase 1 evaluation of MEDI-534, a live attenuated parainfluenza type 3 vectored RSV vaccine. Vaccine. 2013;31:2822–2827. doi: 10.1016/j.vaccine.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Schickli JH, Kaur J, Tang RS. Nonclinical phenotypic and genotypic analyses of a Phase 1 pediatric respiratory syncytial virus vaccine candidate MEDI-559 (rA2cp248/404/1030ΔSH) at permissive and non-permissive temperatures. Virus Res. 2012;169:38–47. doi: 10.1016/j.virusres.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 61.Malkin E, Yogev R, Abughali N, Sliman J, Wang CK, Zuo F, et al. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS One. 2013;8:e77104. doi: 10.1371/journal.pone.0077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bermingham A, Collins PL. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci U S A. 1999;96:11259–11264. doi: 10.1073/pnas.96.20.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karron RA, Luongo C, Thumar B, Loehr KM, Englund JA, Collins PL, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Sci Transl Med. 2015;7:312ra175. doi: 10.1126/scitranslmed.aac8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luongo C, Winter CC, Collins PL, Buchholz UJ. Respiratory syncytial virus modified by deletions of the NS2 gene and amino acid S1313 of the L polymerase protein is a temperature-sensitive, liveattenuated vaccine candidate that is phenotypically stable at physiological temperature. J Virol. 2013;87:1985–1996. doi: 10.1128/JVI.02769-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luongo C, Winter CC, Collins PL, Buchholz UJ. Increased genetic and phenotypic stability of a promising live-attenuated respiratory syncytial virus vaccine candidate by reverse genetics. J Virol. 2012;86:10792–10804. doi: 10.1128/JVI.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calder LJ, González-Reyes L, García-Barreno B, Wharton SA, Skehel JJ, Wiley DC, et al. Electron microscopy of the human respira tory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology. 2000;271:122–131. doi: 10.1006/viro.2000.0279. [DOI] [PubMed] [Google Scholar]
- 67.Collins PL, Mottet G. Post-translational processing and oligomerization of the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1991;72(Pt 12):3095–3101. doi: 10.1099/0022-1317-72-12-3095. [DOI] [PubMed] [Google Scholar]
- 68.López JA, Bustos R, Orvell C, Berois M, Arbiza J, García-Barreno B, et al. Antigenic structure of human respiratory syncytial virus fusion glycoprotein. J Virol. 1998;72:6922–6928. doi: 10.1128/jvi.72.8.6922-6928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glenn GM, Smith G, Fries L, Raghunandan R, Lu H, Zhou B, et al. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine. 2013;31:524–532. doi: 10.1016/j.vaccine.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Pierantoni A, Esposito ML, Ammendola V, Napolitano F, Grazioli F, Abbate A, et al. Mucosal delivery of a vectored RSV vaccine is safe and elicits protective immunity in rodents and nonhuman primates. Mol Ther Methods Clin Dev. 2015;2:15018. doi: 10.1038/mtm.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Widjojoatmodjo MN, Bogaert L, Meek B, Zahn R, Vellinga J, Custers J, et al. Recombinant low-seroprevalent adenoviral vectors Ad26 and Ad35 expressing the respiratory syncytial virus (RSV) fusion protein induce protective immunity against RSV infection in cotton rats. Vaccine. 2015;33:5406–5414. doi: 10.1016/j.vaccine.2015.08.056. [DOI] [PubMed] [Google Scholar]