Abstract
Neuronal calcium sensor-1 (NCS-1) mediates changes in cellular function by regulating various target proteins. Many potential targets have been identified but the physiological significance of only a few has been established. Upon temperature elevation, Caenorhabditis elegans exhibits reversible paralysis. In the absence of NCS-1, worms show delayed onset and a shorter duration of paralysis. This phenotype can be rescued by re-expression of ncs-1 in AIY neurons. Mutants with defects in four potential NCS-1 targets (arf-1.1, pifk-1, trp-1 and trp-2) showed qualitatively similar phenotypes to ncs-1 null worms, although the effect of pifk-1 mutation on time to paralysis was considerably delayed. Inhibition of pifk-1 also resulted in a locomotion phenotype. Analysis of double mutants showed no additive effects between mutations in ncs-1 and trp-1 or trp-2. In contrast, double mutants of arf-1.1 and ncs-1 had an intermediate phenotype, consistent with NCS-1 and ARF-1.1 acting in the same pathway. Over-expression of arf-1.1 in the AIY neurons was sufficient to rescue partially the phenotype of both the arf-1.1 and the ncs-1 null worms. These findings suggest that ARF-1.1 interacts with NCS-1 in AIY neurons and potentially pifk-1 in the Ca2+ signaling pathway that leads to inhibited locomotion at an elevated temperature.
Many aspects of neuronal function and subsequent changes in organism behaviour are regulated by changes in the concentration of intracellular free calcium ([Ca2+]i) in the neurons. Changes in [Ca2+]i are transduced through Ca2+ sensor proteins and neurons express a large number of these proteins1 including synaptotagmin I which is required for fast neurotransmission2, and many different EF-hand containing proteins1 related to the ubiquitous Ca2+ sensor protein calmodulin3. Calmodulin has many functions in neurons acting via targets such as Ca2+/calmodulin-dependent protein kinase II4. Other EF-hand containing Ca2+ sensors expressed in neurons include the calcium-binding protein (CaBP) family5,6 and the neuronal calcium sensor (NCS) protein family7,8,9.
Ca2+ sensor proteins undergo a conformational change on Ca2+ binding that results in a change in their interaction and regulation of one or more target effector proteins. The specificity of neuronal Ca2+ signaling is in large part determined by differential signal processing by these Ca2+ sensor proteins through their specific target proteins3,10,11. The rules underlying the sensing and specificity of Ca2+ signaling in neurons have begun to emerge in recent years but still remain to be fully understood.
The Neuronal Calcium Sensor (NCS) family of proteins consists of 5 sub-families including Class A (NCS-1/frequenin), Class B (visinin-like proteins (VILIPs), Class C (recoverin), Class D (guanylate cyclase activating proteins (GCAPs) and Class E (K+ channel interacting proteins (KChIPs)7. NCS-1 was first identified in Drosophila12 but orthologues of NCS-1 have been subsequently identified in all eukaryotes, from yeast to man. Genetic and functional studies have determined that NCS-1 has multiple physiological functions8,13,14,15. In Saccharomyces cerevisiae, NCS-1 (Frq1) is essential for survival due to its requirement for activation of the phosphatidylinositol-4-kinase enzyme Pik116 although its absence is not lethal in other organisms. Some of the roles of NCS-1 may also be specific to particular organisms such as its specific role in temperature-dependent behaviours in Caenorhabditis elegans as a consequence of its restricted neuronal expression17,18,19. In mammalian cells NCS-1 regulates Ca2+ -dependent exocytosis20, long-term depression21 axonal growth and neuronal regeneration22 and channel function23,24. Studies at an organism level have identified key NCS-1 functions8,9. In mice, knock-out of NCS-1 has relatively subtle effects but results in an increase in anxiety and depressive behaviour25 and over-expression of NCS-1 in adult mouse dentate gyrus neurons affected exploratory behaviour and the acquisition of spatial memory by regulating the surface expression of dopamine D2 receptors in the hippocampus26. In C. elegans NCS-1 is expressed in sensory neurons and interneurons and is involved in neuronal pathways that control long term memory for thermosensation17 and has also been implicated in chemotaxis27.
NCS-1 has many identified interacting partners28 some of which are unique for NCS-1 but others that are also regulated by other Ca2+ sensors particularly calmodulin29. There are some physiological effects of NCS-1 that can be attributed directly to one of its identified target proteins. The NCS-1 target proteins linked to function include phosphatidylinositol-4-kinase (PI4K) IIIβ30,31 and its orthologue Pik1 in yeast16, interleukin receptor accessory protein like-1 (IL1RAPL1)32, TRPC5 channels33, InsP(3) receptors34, Ric8a35, P/Q-type Ca2+ channels23,36,37 and dopamine D2 and D3 receptors38,39,40. Some of the protein interactions are known, however, only from in vitro binding assays and so their biological importance is unclear. It is possible, for example, that those binding partners that are also calmodulin targets in vitro are not regulated by NCS-1 under physiological conditions41.
One mammalian NCS-1 binding partner is ARF130,42, which was found to bind to NCS-1 in a Ca2+ -dependent manner, and interestingly both ARF1 and NCS-1 directly interact with and stimulate the activity of PI4KIIIβ. In non-neuronal cells over-expression of ARF1 reversed the inhibitory effect of NCS-1 on traffic from the Golgi complex, suggesting the possibility of a functionally relevant interaction between the two proteins30. Subsequently, both NCS-1 and ARF-1 were found to be required for inner ear development in zebrafish potentially via a common pathway43. To explore further whether an ARF1/NCS-1 interaction was required in a physiological context for an NCS-1-dependent effect, we took advantage of an assay for temperature-dependent changes in locomotion in C. elegans44, in which NCS-1 is required to be expressed in the pair of AIY neurons19. Analysis of worms with mutations in various potential NCS-1 target proteins established a potential role for pifk-1, the orthologue of PI4KIIIβ, trp-1, trp-2 and arf-1.1 in the temperature-dependent locomotion (TDL) assay. In particular, we observed a genetic interaction between arf-1.1 and ncs-1. ARF1.1 function in the TDL assay required its expression in AIY neurons. These findings suggest that both ARF1.1 and NCS-1 are components of the same temperature-dependent Ca2+ signaling pathway in AIY neurons.
Results
Characterisation of a modified temperature-dependent locomotion assay
Our previous work showed that acute elevation of temperature from 20 °C to 28 °C for 10 mins resulted in a significant slowing of movement of wild-type (N2) worms44. In contrast, ncs-1 null worms (qa401) showed not a slowing but instead a small but significantly faster rate of movement at the elevated temperature19. NCS-1 is normally expressed in the pair of AIY neurons17, which are involved in the known circuit that functions in thermotaxis in the worm45. The TDL phenotype in the ncs-1 null worms was completely reversed by the pan-neuronal expression of ncs-1, driven by a Prab-3 promoter, or the AIY-specific expression of ncs-1, driven by a Pttx-3 promoter19. In order to develop a more detailed and potentially more informative assay, we examined worm thrashing at an elevated temperature in real-time. We determined that in response to elevated temperature, worm thrashing rate decreased; however, this was a reflection of a proportion of worms undergoing temperature-dependent paralysis (Fig. 1A,B). In the assay of thrashing in N2 worms, it was observed that more than 80% of worms were paralysed within 10 mins of temperature elevation (Fig. 1A,B). Surprisingly, however, many of the worms showed a temporary recovery to normal thrashing behaviour before becoming paralysed again at later times (Supplementary video 1). It was observed at various times during the assay that those worms that were not paralysed moved at a similar rate, indicating that the paralysis was an all-or-none effect (Fig. 1A, inset). Based on these observations, we subsequently used a revised assay in which we determined the mean time to first paralysis and the duration of the first period of paralysis for individual worms within populations.
Figure 1. Changes in locomotion over time following temperature elevation.
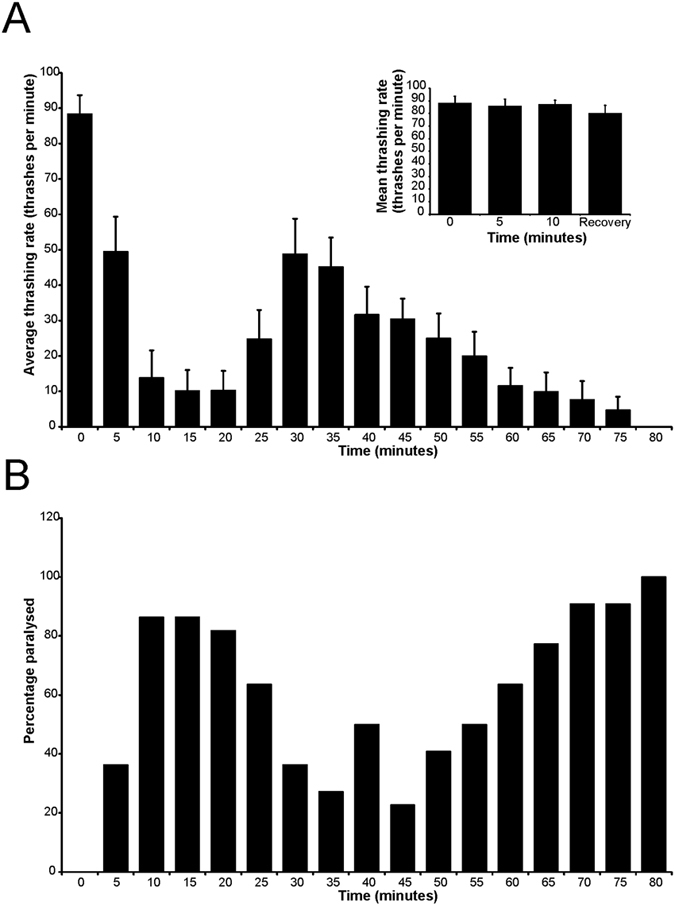
N2 worms were examined following elevation of temperature from 20 °C ± 0.5 to a final temperature of 28.0 °C ± 0.5. The rate of thrashing of individual worms was determined and the data shown as the mean rate of thrashing for the population ± SEM (A). The inset shows the rate of thrashing of those worms that were not paralysed, showing that they thrashed at the same rate before and during the time when other worms became paralysed and after the initial recovery period. The percentage of worm that were paralysed at each time point was also determined (B).
From initial experiments, an elevated temperature of 28.5 ± 0.5 °C was selected as optimal in showing consistency in worm responses. A temperature shift to 26.5 oC resulted in an observable difference in time to paralysis of ncs-1 null mutants versus N2 worms; but this represented paralysis of only 33% of the ncs-1 null worms. In contrast, a shift to 30 °C showed little difference in time to paralysis between ncs-1 null mutants and N2 worms (Supplementary Fig. 1).
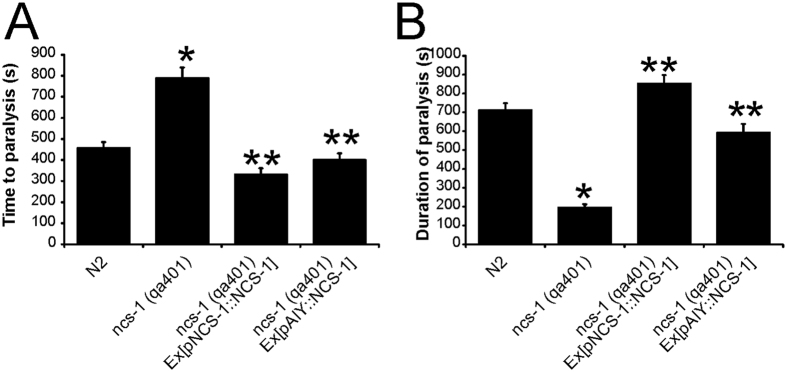
Use of the revised assay in which multiple individual worms were observed revealed that on average N2 worms became paralysed within 500s, but subsequently recovered after another 700s. The ncs-1 null worms showed both an increase in the mean time to paralysis compared to N2 worms (73% increase) and a shorter (73% decrease) duration of paralysis (Fig. 2). Both aspects of this phenotype were rescued following expression of NCS-1 under its endogenous promoter. AIY-specific expression of NCS-1 also fully rescued the time to paralysis, while the duration of paralysis was only partially rescued with AIY-specific expression.
Figure 2. Analysis of the temperature-dependent locomotion of null ncs-1 and NCS-1 rescue strains.
Various C. elegans strains, as indicated, were analysed using the TDL assay as described in the Methods section. Locomotion of worms was initially assessed at 20 °C and then following the temperature shift to 28.5 °C. Multiple animals were tested for each strain and mean values for time to paralysis (A) and the duration of paralysis (B) were determined and expressed as mean ± SEM. The numbers of animals used for each strain were N ≥ 30. All data sets were compared with N2 control worms and statistical differences for the onset and duration of paralysis for each strain was determined using one-way ANOVA and Dunnett’s correction for multiple comparisons (*p < 0.001 versus N2; **p < 0.001 versus qa401).
An important aspect for the subsequent analyses was that all worm strains assayed in this study showed similar basal thrashing rate before temperature elevation (Supplementary Table 1) and also all showed similar stereotypical thrashing behaviour.
Assessment of the temperature-dependent locomotion of C. elegans strains with mutations in potential NCS-1 target proteins
As a first approach to identifying potential orthologues of Arf1 or other identified NCS-1 targets involved in this NCS-1-dependent pathway, we examined the phenotypes of various worm mutants in the revised TDL assay. We would argue that mutants of any downstream targets for NCS-1 should show a similar phenotype in the assay as ncs-1 null worms, i.e., a longer time to paralysis and shorter duration of paralysis. A large number of potential NCS-1 interacting proteins are known particularly from the study of mammalian proteins11, some of which have orthologues in C. elegans (Supplementary Table 2). We excluded those candidates that do not have a clearly recognizable orthologue in C. elegans (e.g. IL1RAPL1). From the list of the existing worm orthologues, we then excluded any candidates that were known not to be expressed in AIY neurons (e.g., the dopamine D2 receptor orthologues dop-2 and dop-3) or those where available mutants have very severe locomotion defects that would preclude their use in the TDL assay (e.g., the voltage-gated Ca2+ channel subunit unc-2). Following from this analysis we obtained mutant strains for the following target candidates: pifk-1 (orthologue of mammalian PI4KIIIβ), trp-1 and trp-2 (orthologues of TRPC5), arf-1.1 and arf-1.2 (orthologues of ARF1) and grk-2 (orthologue of GRK2).
Mutant strains were tested in the TDL assay in comparison with the behaviour of N2 and ncs-1 null worms (Fig. 3). Mutant strains of trp-1 (two separate alleles), trp-2, and arf-1.1 showed changes in time of onset and duration of paralysis similar to that seen in the ncs-1 null worms with an increase in time to paralysis and a decrease in duration. For pifk-1 mutants, the phenotype was qualitatively similar to ncs-1 null worms. The quantitative effect on time to paralysis was, however, much larger for the pifk-1 mutant (increased by 296%) than what was seen with ncs-1 null worms (increased by 74%). In the case of the two arf-1.2 mutant lines tested, these did not give a consistently similar phenotype compared to that for the ncs-1 null worms. For example, the arf-1.2 (ok1322) strain had a time to paralysis that was not significantly different from N2 worms, while the other mutant strain (ok796) had a significantly reduced time to paralysis. The two grk-2 mutant strains did not replicate the ncs-1 null worm phenotype, but showed a decreased time to paralysis and no effect on its duration. From this analysis, we concluded that of those tested there are four potential target proteins for NCS-1 (arf-1.1, pifk-1, trp-1 and trp-2) that could function in the pathway controlling the temperature-dependent behaviour based on similar mutant phenotypes in the onset and duration of paralysis in the TDL assay. Of these mutant strains, three (arf-1.1, trp-1 and trp-2) had the most similar phenotype to the ncs-1 null worms in the assay.
Figure 3. Assay of the temperature dependent locomotion of various mutant C. elegans strains.
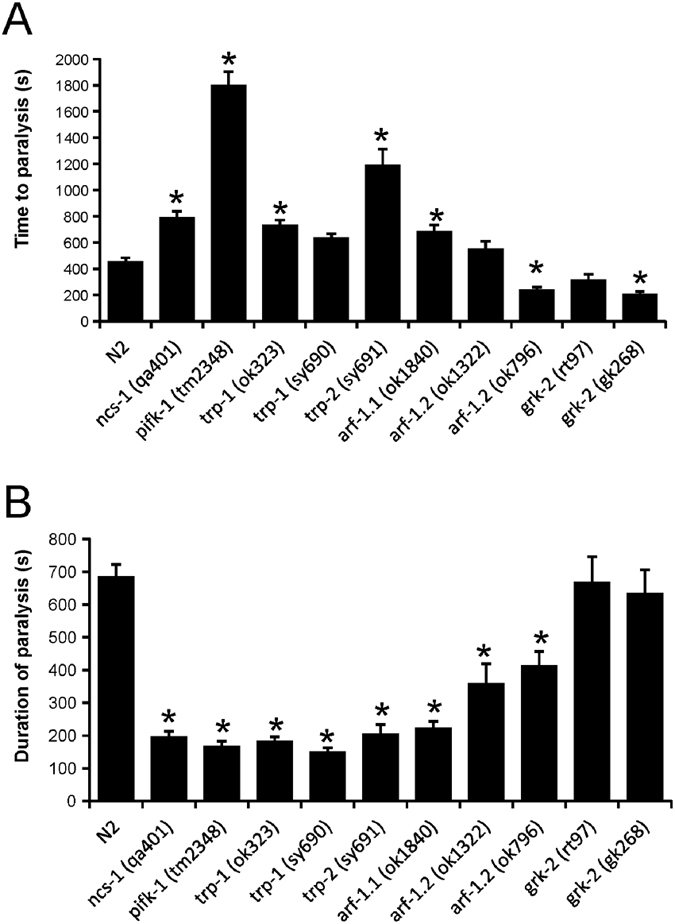
Temperature-dependent locomotion assays were carried out on various strains of C. elegans, as indicated, to provide mean values for the time to the start of paralysis after the shift to 28.5 °C and the duration of paralysis. Multiple animals were tested for each strain (N = ≥20) and mean values for time to paralysis (A) and the duration of paralysis (B) were determined. All data are expressed as mean ± S.E.M. Statistical differences were identified by comparing averaged data to that of N2 wild-type worms, using one-way ANOVA with Dunnett’s correction for multiple comparisons (*p < 0.05 versus N2).
Effect of double mutations on temperature-dependent locomotion
In order to investigate further the potential downstream targets in the TDL behaviour and to follow up the findings from single mutant strains, we generated double mutants to assess any genetic interaction. Genetic crosses were confirmed and validated through use of Polymerase Chain Reaction (PCR) analysis of genomic DNA. After confirming the presence of the ncs-1 mutation in the trp-1, trp-2 or arf-1.1 mutant background, we analysed the behavioural phenotypes of the resulting double mutant lines in the revised TDL assay. Where multiple independent lines were generated, the data from their analysis were pooled together.
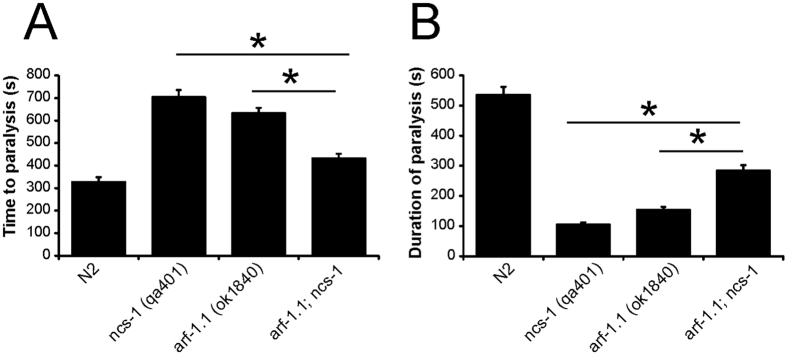
The trp-1; ncs-1 and trp-2; ncs-1 double mutant strains showed defects comparable to N2 worms in their temperature-dependent locomotion phenotypes for both time to paralysis and duration of paralysis. Both phenotypic aspects were of essentially the same magnitude as that observed with single ncs-1, trp-1 or trp-2 single mutant strains (Fig. 4). The lack of any additive effect in the double mutants would be consistent with the proteins acting together or at least within a common pathway. The arf-1.1; ncs-1 double mutant strains gave a different outcome in the revised TDL assay. The double mutants showed a significant increase in the time to paralysis (32% increase) and a decrease in the duration of paralysis (47% decrease) compared to N2 worms, but they also consistently (seen with four independently derived lines) showed an intermediate phenotype between those of the single mutants or N2 worms in the time of onset and duration of paralysis (Fig. 5). This intermediate phenotype seen with the double mutant is indicative of reciprocal sign epistasis, supporting the notion that NCS-1 and ARF-1.1 function together in the regulation of the revised TDL behaviour.
Figure 4. Assay of the temperature-dependent locomotion of the ncs-1 mutation in the presence or absence of trp-1 and trp-2.
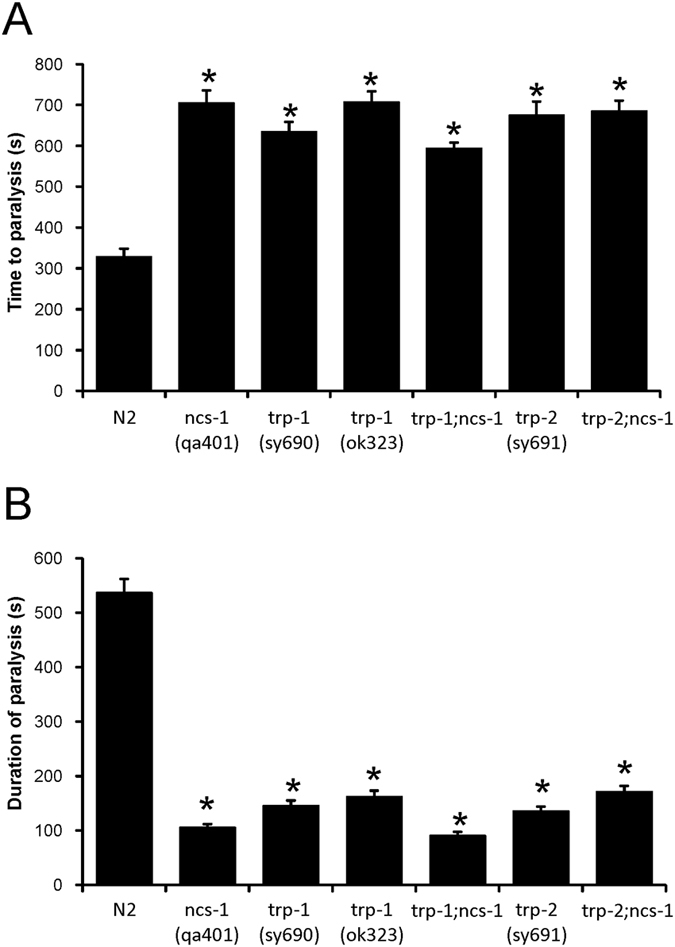
Temperature-dependent locomotion assays were carried out on various indicated strains of C. elegans to determine the onset and duration of paralysis after the shift to 28.5 °C. The double mutants were derived from genetic crosses of qa401 with either sy691 or ok323. Multiple animals (N ≥ 40) were tested for each strain and mean values for time to paralysis (A) and the duration of paralysis (B) were determined. All data are expressed as mean ± S.E.M. Statistical differences were identified by comparing averaged data to that of N2 wild-type worms, using one-way ANOVA with Dunnett’s correction for multiple comparisons (*p < 0.001 versus N2).
Figure 5. Assay of the temperature dependent locomotion of arf-1.1; ncs-1 mutants.
Temperature-dependent locomotion assays were carried out on various indicated strains of C. elegans to determine the onset and duration of paralysis after the shift to 28.5 °C. Multiple animals (N ≥ 60) were tested for each strain and mean values for time to paralysis (A) and the duration of paralysis (B) determined. All data are expressed as mean ± S.E.M. Statistical differences were identified by comparing averaged data to that of N2 wild-type worms, using one-way ANOVA with Dunnett’s correction for multiple comparisons (*p < 0.001).
Effect of inhibition of PIFK-1 on temperature-dependent locomotion
Since ncs-1 and pifk-1 reside close to each other on the X chromosome, generation of ncs-1 pifk-1 double mutants would be problematical. We were able, however, to take advantage of an inhibitor of mammalian (PI4K) IIIβ inhibitor PIK-9346. Pre-treatment of N2 worms for one hour with 19 μM PIK-93 resulted in a longer time to paralysis and shorter duration of paralysis as seen with the ncs-1 null worms (Fig. 6). To check the specificity of the action of the drug, we examined whether it would have any effect on pifk-1 mutant worms. No difference in the behaviour of the pifk-1 mutant worms was observed in drug-treated versus untreated worms (Fig. 6).
Figure 6. Effect of PIK-93 on temperature-dependent locomotion in N2, pifk-1 and ncs-1 null strains.
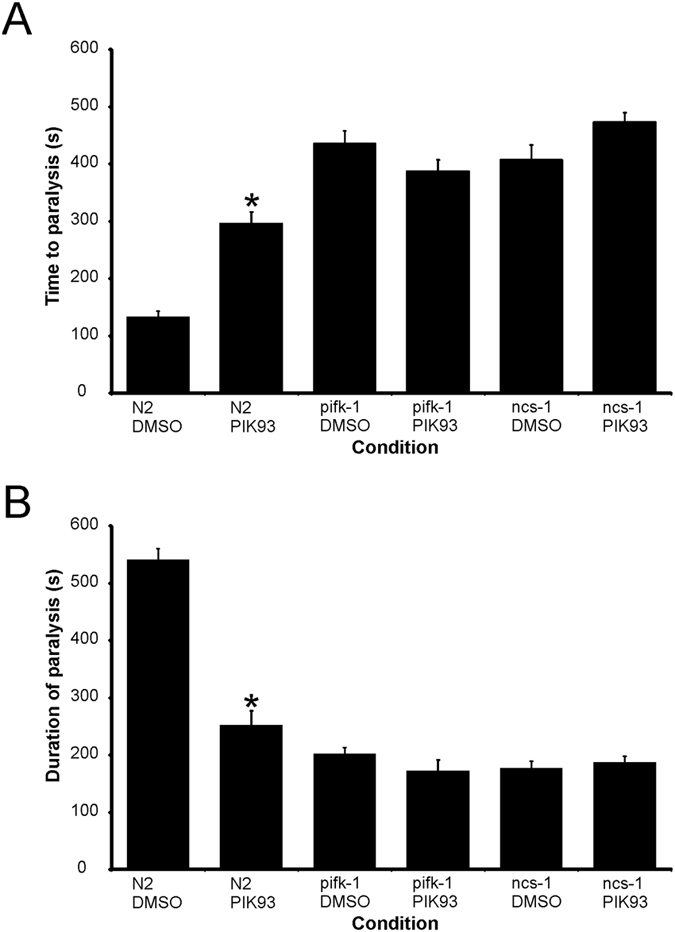
Various worm stains as indicated were pre-treated for one hour with vehicle (1% DMSO) or 19 μM PIK-93. Temperature-dependent locomotion assays were carried out to determine the onset and duration of paralysis after the shift to 28.5 °C. Multiple animals (N = 30) were tested for each strain and mean values for time to paralysis (A) and the duration of paralysis (B) were determined. All data are expressed as mean ± S.E.M. Statistical differences were identified by comparing averaged data to that of N2 wild-type worms, using one-way ANOVA with Dunnett’s correction for multiple comparisons (*p < 0.001 versus vehicle control).
NCS-1 is able to activate mammalian (PI4K)IIIβ. Interestingly, PIK-93 also had no additional effect on the time to paralysis and shorter duration of paralysis in ncs-1 null worms, supporting the involvement of pifk-1 in the action of ncs-1.
Effect of ARF1.1 rescue on temperature-dependent locomotion
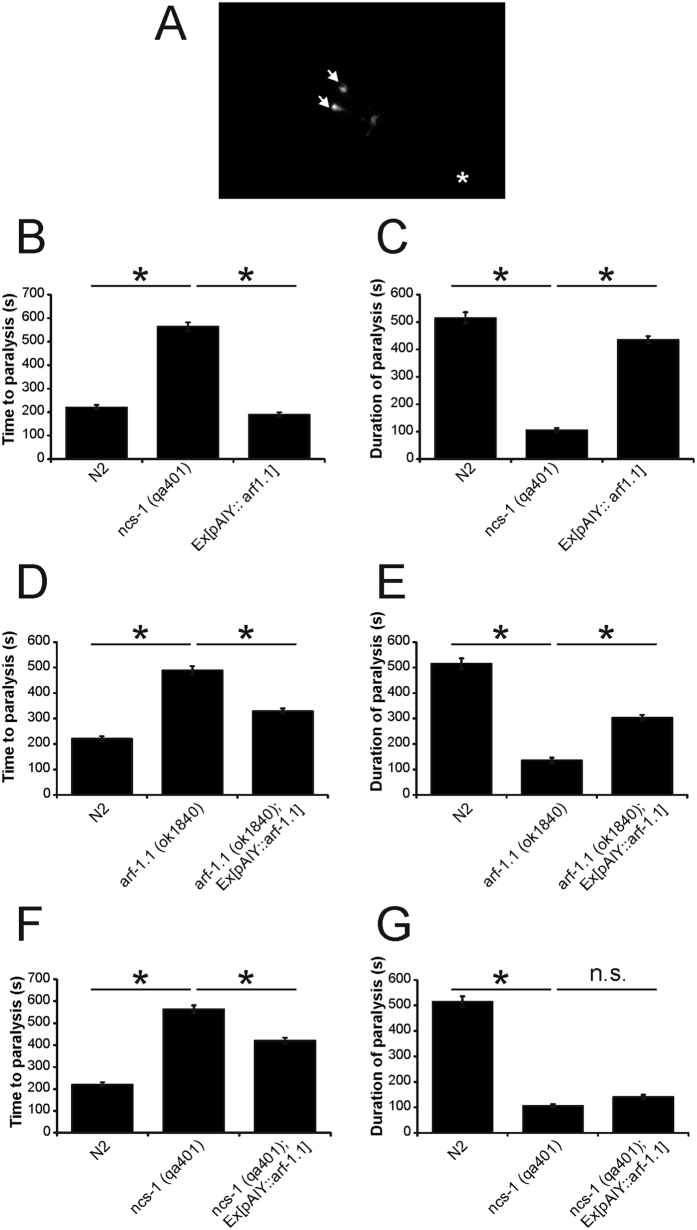
The results from analysis of single and double mutants suggest that ARF-1.1 has a regulatory role in the control of the temperature-dependent behaviour that we have shown involves NCS-1 and that the two proteins may interact in the same signaling pathway. NCS-1 expression in the AIY neurons can rescue the phenotype in ncs-1 null worms. In order to substantiate further the possible role of ARF-1.1, we aimed to examine whether expression of ARF-1.1 specifically in AIY neurons (Fig. 7A) was sufficient to rescue the arf-1.1 mutant phenotype in the TDL assay. We first examined whether overexpression of ARF-1.1 in AIY neurons would have any effect by itself. Such over-expression had no major effect in the TDL assay (Fig. 7B,C): there was no effect on the time to paralysis, although there was a significant albeit small reduction in the duration of paralysis (15% reduction compared to a 79% reduction in ncs-1 null worms). Expression of ARF-1.1 in AIY neurons of the arf-1.1 mutant (Fig. 7D,E) resulted in a partial rescue of both the increase in time to paralysis and its duration. A similar extent of rescue was observed with pan-neuronal expression of ARF-1.1 driven by the rab-3 promoter (Supplementary Fig. 2). These finding are consistent with the neuronal-dependent part of the phenotype of the arf-1.1 mutant worms being attributed wholly to ARF-1.1 function in AIY neurons.
Figure 7. Assay of the temperature-dependent locomotion after ARF-1.1 overexpression or rescue.
GFP expression is driven by pAIY specifically in the two AIY neurons indicated by arrows. No expression was observed elsewhere (A). The asterisk shows the position of the posterior tip of the imaged worm. Transgenic worms were generated to express ARF-1.1 within AIY neurons in a wild-type background (N ≥ 56) (B,C), in arf-1.1 mutant worms (N ≥ 40) (D,E) or in ncs-1 null mutant worms (N ≥ 56) (F,G). Temperature-dependent locomotion assays were carried out on various indicated strains of C. elegans to determine the time to the start of paralysis after the shift to 28.5 °C and the duration of paralysis. Multiple animals were tested for each strain and mean values for time to paralysis (B,D,F) and the duration of paralysis (C,E,G) were determined. Each transgenic data set presented used a conglomerate of at least 3 separate transgenic lines, which were pooled together. All data are expressed as mean ± S.E.M. Statistical differences were identified by comparing averaged data to those of N2 wild-type worms using one-way ANOVA with Dunnett’s correction for multiple comparisons (*p < 0.001).
As we had noted a potential genetic interaction between ncs-1 and arf-1.1 from the analysis of the arf-1.1; ncs-1 double mutant, we also examined the effect of overexpression of arf-1.1 in AIY neurons in ncs-1 null worms. ARF-1.1 overexpression in AIY (Fig. 7F,G) had little effect on the duration of paralysis, but significantly suppressed the delayed onset of paralysis in ncs-1 null worms (90% increase versus the 151% increase in ncs-1 null single mutants). This is again consistent with a functional interaction of the two proteins in the AIY neurons.
Discussion
A key aspect to fully understanding how Ca2+ signaling is related to changes in neuronal function that lead to alterations in behaviour is the need to identify the components of the Ca2+ signaling pathways, including the essential proteins involved. Previous work has shown a requirement for the Ca2+ sensor protein NCS-1 in thermotaxis responses17 and in changes in locomotion rate following temperature elevation19. The ability to manipulate genetically protein expression in the worm in a specific manner, along with knowledge of the precise neurons involved in particular behavioural responses, provides an opportunity to investigate components of Ca2+ signaling pathways that function at the level of the organism.
C. elegans has long been used as a model organism to study cellular/genetic mechanisms that underlie neurosensory perception and neuroethology in general. The temperature-dependent locomotion (TDL) behaviour examined in this paper is particularly novel, in that it potentially reflects a cellular/genetic mechanism that allows the animal to adapt to harsh temperatures. The TDL phenotype is not only a thermosensory-driven behaviour, but it demonstrates adaptive responses within the context of a prolonged lethal environmental situation (high temperature). The nematode first continues to behave as normal during the initial exposure to the high temperature. In response to continued high temperatures, the animal stops moving – possibly to conserve energy or outlast the high temperature. As the high temperature continues, the animal then begins to move again, presumably with the revised goal of escaping the environment.
We have now developed our original temperature-dependent locomotion (TDL) assay from analysis of locomotion at a single time point at an elevated temperature44 to a quantitative assay that allows assessment of two key parameters by following the behaviour of individual worms. This gives information on the mean time to paralysis and the mean duration of the reversible paralysis occurring at the elevated temperature. The aim behind this was to provide the ability for a more discriminating comparison of temperature-dependent phenotypes between worm strains. The behaviour that we assayed uses known neural elements of the well-characterised thermotaxis response of worms47,48, including the involvement of the AFD and AIY classes of neurons19,44. The TDL behaviour differs, however, in its genetic requirement compared to those for both thermotaxis and temperature avoidance behaviour. The latter, for example, does not require NCS-1 function17,49 and thermotaxis is affected by genes not required for the TDL response44,50.
Multiple potential targets for NCS-1 regulation in response to Ca2+ signaling have been identified. The best characterised target in mammals is the dopamine D2 receptor, whose regulation by NCS-138 has clear physiological roles in affecting behaviour in mice26,51. In most cases, however, the physiological significance of the potential identified targets is unclear. To date, no NCS-1 target protein interactions have been directly examined using biochemical or cellular assays for the worm proteins. The majority of the available information comes from study of mammalian proteins. The advantage of using C. elegans in the present study comes from the ability to use genetic approaches to study the involvement of the proposed target proteins in a behavioural phenotype at an organismal level, thus allowing physiological validation of the nature of the NCS-1 signaling pathway. Importantly, NCS-1 is highly conserved and the worm protein is 76% identical to the human protein. Clearly identifiable orthologues of potential target proteins are identifiable in worms with varying levels of sequence identity to the human orthologues of the proteins (ARF1.1 61% identity; ARF-1.2, 94% identity; GRK-2, 67% identity; PIFK-1, 31% identity; TRP-1 40% identity; TRP-2 47% identity), suggesting that these interactions would be likely conserved from worms to man, as known for many other examples of conserved protein-protein interactions. Note that the functional interaction of NCS-1 and PI4KIIIβ orthologues is conserved from yeast to mammalian proteins16,30. A three-way interaction between NCS-1, ARF-1 and PI4KIIIβ has been characterised using biochemical approaches and shown to affect secretory function in non-neuronal mammalian cells30. The significance of the NCS-1 interaction with ARF-1 for neuronal function in vivo has not been established. We set out therefore to test its relevance, along with the involvement of other potential NCS-1 targets in a defined behavioural assay, where NCS-1 function was known to be required in the pair of AIY neurons in the worm.
Analysis of single worm mutant strains showed that worms bearing mutation in four of the potential NCS-1 targets (arf-1.1, pifk-1, trp-1 and trp-2) showed similar temperature-dependent behaviours to those of ncs-1 null worms. One caveat, however, is that only single alleles were tested for pifk-1, and trp-2. We were able to show, however, that PIK-93, a specific inhibitor of PI4K IIIβ, resulted in a longer time to paralysis and shorter duration of paralysis, as seen with the ncs-1 null worms and that this appeared to be a specific action on pifk-1. The lack of effect of PIK-93 on ncs-1 null worms suggested an involvement of pifk-1 in the action of ncs-1 on temperature-dependent locomotion.
Based on the similar effects of mutations on time to paralysis and duration in the TDL assay, three genes (arf-1.1, trp-1 and trp-2) had the most similar phenotype to the ncs-1 null worms. This connection was further investigated by analysis of double mutant strains. The lack of additivity of the phenotypes of the trp-1; ncs-1 and trp-2; ncs-1 double mutant strains would be consistent with the proteins functioning in the same pathway. It was striking, however, that the arf-1.1; ncs-1 double mutant showed indications of an intermediate phenotype compared to the single mutants. This type of genetic interaction is known as reciprocal sign epistasis, which is when two deleterious mutations or genes are more beneficial together than when they are alone, because the mutations impinge on the same process. These results, therefore, support the notion that the NCS-1 and ARF-1.1 function together in the regulation of the TDL behaviour.
It was important to establish that ARF-1.1 does indeed act in the same AIY neurons in which NCS-1 expression is required for normal TDL behaviour. ARF-1.1 is expressed in many cell types in the worm, but expression of ARF-1.1 in only the AIY neurons was sufficient to rescue the TDL phenotype, as effectively as pan-neuronal ARF-1.1 expression. This suggests that the neuronal-dependent part of the arf-1.1 phenotype is entirely due to the ARF-1.1 function in the AIY neurons. Interestingly, overexpression of ARF-1.1 in AIY neurons in the ncs-1 null worms was able to rescue the time to paralysis, but not the duration of paralysis. This latter result parallels the finding that AIY-specific expression of ncs-1 is more effective in rescuing the “onset of paralysis” phenotype versus the “duration of paralysis” phenotype of the ncs-1 mutants. Thus, this suggests that AIY neurons are more important in ARF-1.1- and NCS-1-dependent regulation of the onset of paralysis than in the regulation of its duration in the TDL assay.
This is the first description of this TDL assay (of onset and duration of paralysis) and the genes/cells that are involved. Both parameters of onset and duration of paralysis will presumably involve many steps, including temperature sensation, sensory information processing, motor output, as well as muscle contraction. Therefore, it is not surprising that each parameter could involve multiple genes and cells, some common and some distinct. The two parameters obviously have some common features, as NCS-1 expression in the AIY neurons alone is required for both phenotypes (Fig. 2). Some of our other data, however, point to other cells and genes being involved. Indeed, particular aspects of the locomotor circuitry could be important. Additionally, the arf-1.1 rescue experiments indicate that driving expression in AIY alone only partially rescues the arf-1.1 phenotype, but is as good at rescue as driving arf-1.1 expression throughout the nervous system. This could point to an involvement of non-neuronal arf-1.1 in the phenotype. Considerably more work would be required to investigate all aspects of the cells and genes involved.
Overall, the results presented here suggest an involvement of TRP-1, TRP-2, ARF-1.1 and PIFK-1 in the effects of increased temperature on locomotion. In addition, our findings support the notion that ARF-1.1 interacts with NCS-1 in AIY neurons within the Ca2+ signaling pathway that determines the effect of elevated temperature on C. elegans locomotion. This establishes that ARF-1.1 is a physiologically relevant partner in NCS-1 function.
Methods
C. elegans strains and culture
The existing C. elegans strains used in this project were Bristol N2 (wild-type) or mutant strains obtained, unless otherwise indicated, from the Caenorhabditis Genetics Centre (CGC) (University of Minnesota, USA). The mutant strains were: arf-1.1(ok1840), arf-1.2(ok1233), arf-1.2(ok796), grk-2(rt97), grk-2(gk268), ncs-1(qa401), pifk-1(tm2348 from the National Bioresource Project for the “Experimental Animal Nematode C. elegans”, Tokyo, Japan), trp-1(ok323), trp-1(sy690), trp-2(sy691). The ncs-1 rescue strains were described previously18. C. elegans strains were grown and maintained on standard nematode growth media52,53 supplemented with the relevant salts (NGM). Strains were maintained following standard protocol as described previously54 and maintained at 20 °C using Escherichia coli (E. coli) OP50 strain as the food source.
Double mutant generation
Double mutant strains were generated by mating two existing strains and isolating the homozygous double mutant progeny by PCR-based genotyping.
Validation of double mutants was confirmed over multiple generations by PCR using the following primers:
ncs-1 forward, CAGTTGAGCATCGTTATTCTG
ncs-1 wild type reverse, CAGTTGAGCATCGTTATTCTG
ncs-1 mutant reverse, CCGTATTTGAACGTTGCTAC
trp-1 forward, CAACAGTTGCTCACCTCTATC
trp-1 wild type reverse, CCTCCGCTACCAACATTGGTTC
trp-1 mutant reverse, CGAATTTGTTGTGGAGGCAG
trp-2 forward, CACTGATGACGTGGATCGCAAGG
trp-2 reverse, CTAAGGGTGAAATATGACGAG
arf-1.1 forward, CATCGCCAACCAAGGAAAG
arf-1.1 wild type reverse, CCACATCAGACCTTCGTAGAG
arf-1.1 mutant reverse, CGACAGAGATCACCAAACATTG
The double mutant strains generated in this study were: trp-1; ncs-1 (AMG 145–148), trp-2; ncs-1 (AMG 128), arf-1.1; ncs-1 (AMG 149–152).
Plasmids used for C. elegans injections
The GFP marker plasmid for pan-neuronal expression of GFP (pRAB100 [Prab3::GFP]) was obtained from the Nonet Laboratory (Washington, University of St. Louis, USA). The AIY neuron-specific expression plasmid (PAIY::MCS) was obtained from the Hobert Lab (Columbia University Medical Center, N.Y.). All plasmids for injection were generated as Gateway DEST vectors (Life Technologies) to produce tissue-specific expression vectors as described previously55.
Microinjection
Larval stage 4 to Day 1 adult C. elegans, including N2, qa401 (ncs-1 null), and RB1535 (arf-1.1 null) were injected using a micropipette needle into their germline cells within the dorsal gonad. Injection mixtures contained a final concentration of 100 ng/μl DNA. Of this 100 ng, 10 ng/μl was the plasmid of interest (ncs-1 or arf-1.1 expression plasmid), 40 ng/μl of GFP marker plasmid and 50 ng/μl of empty pBluescript plasmid, the final volume was made up to 100 μl using dH2O. Multiple, independently-derived separate lines for each plasmid injection were generated and their phenotypes assessed. The transgenic strains generated in this study were: Ex[PAIY::arf-1.1] injected into a wild-type background, arf-1.1 (ok1840); Ex[PAIY::arf-1.1], arf-1.1 (ok1840); Ex[Prab3::arf-1.1], ncs-1 (qa401); Ex[PAIY::arf-1.1].
Thrashing assay
Basal locomotion of all C. elegans strains used in this study was assessed. Day 1 adult hermaphrodite animals were transferred into a 50 μl droplet of Dent’s solution (140 mM NaCl, 6 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, pH 7.4 supplemented with bovine serum albumin at 0.1 mg/ml). Individual worms were allowed to acclimatise to the Dent’s solution for 10 minutes before thrashing rate was quantified. Thrashing was defined as one sinusoidal movement of the animal. Thrashing was measured over a 1-minute period for each individual worm. All experiments were completed at room temperature (~22 °C), using worms cultivated at 20 °C. Animals were first placed onto an unseeded NGM plate to remove any excess OP50 E. coli prior to locomotion being tested. None of the strains showed any differences in basal locomotion (Supplementary Table 1) indicating that differences in locomotion following temperature elevation could not be attributed to a general locomotion defect.
Temperature-Dependent Locomotion Assay
A 35 mm petri dish lid was placed onto a Peltier effect thermoelectric plate (TEtech) to which a 150 μl droplet of Dent’s solution was added. Temperature throughout the assay was monitored manually in real time with a thermocoupler placed directly in the solution. Approximately five 1-day to 2-day old worms were placed into the Dent’s solution at room temperature (~20 ± 0.5 °C) and left to acclimatise for 5 minutes. Then current to the Peltier device was increased by heating the Dent’s solution to 27 °C in 1–2 minutes, at which point the timer was started manually. The temperature was allowed to rise over 1 min to a final temperature of 28.5 ± 0.5 °C. Two different parameters were measured for this assay: firstly, the time to paralysis, defined as a complete cessation of motility, using thrashing as a measure of locomotion; secondly, the onset of resumption of thrashing, defined as the time of the first forward thrashing movement. This was assessed while the worms were still at the elevated temperature and defined as the first appearance of two complete thrashes in a row. Multiple worms were observed in the same microscope field. Times for onset and end of paralysis were noted for each individual worm within each assay. Duration of paralysis was calculated by subtracting the onset of paralysis time from the end of paralysis time.
Additional Information
How to cite this article: Todd, P. A. C. et al. Interaction of ARF-1.1 and neuronal calcium sensor-1 in the control of the temperature-dependency of locomotion in Caenorhabditis elegans. Sci. Rep. 6, 30023; doi: 10.1038/srep30023 (2016).
Supplementary Material
Acknowledgments
Strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR). The worm strain tm2348 was obtained from the National Bioresource Project for the “Experimental Animal Nematode C. elegans”, Tokyo, Japan. The AIY neuron-specific expression plasmid (PAIY::MCS) was obtained from the Hobert Lab (Columbia University Medical Center, N.Y.). This work was supported by a Wellcome Trust Prize Studentship grant to PACT (099796/Z/12/Z).
Footnotes
Author Contributions P.A.C.T., L.P.H., J.W.B. and R.D.B. conceived and designed the experiments. P.A.C.T. performed all experimental work reported and analysed the data. H.V.M. carried out the generation of transgenic worms by germ line injection. R.D.B. and P.A.C.T. wrote the paper and all of the other authors read and discussed the manuscript.
References
- McCue H. V., Haynes L. P. & Burgoyne R. D. The Diversity of Calcium Sensor Proteins in the Regulation of Neuronal Function. Cold Spring Harb Perspect Biol 2, a004085, 10.1101/cshperspect.a004085 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R. et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410, 41–49 (2001). [DOI] [PubMed] [Google Scholar]
- Ikura M. & Ames J. B. Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: two ways to promote multifunctionality. Proc. Natl. Acad. Sci. USA 103, 1159–1164 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T. Neuronal Ca2+/calmodulin-dependent protein kinase II–discovery, progress in a quarter of a century, and perspective: implication for learning and memory. Biological & pharmaceutical bulletin 28, 1342–1354 (2005). [DOI] [PubMed] [Google Scholar]
- Haeseleer F. et al. Five members of a novel Ca2+ binding protein (CABP) subfamily with similarity to calmodulin. J. Biol. Chem. 275, 1247–1260 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. P., McCue H. V. & Burgoyne R. D. Evolution and functional diversity of the Calcium Binding Proteins (CaBPs). Front Mol Neurosci 5, 9, 10.3389/fnmol.2012.00009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. & Weiss J. L. The neuronal calcium sensor family of Ca2+ -binding proteins. Biochem. J 353, 1–12 (2001). [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci 8, 182–193 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. & Haynes L. P. Understanding the physiological roles of the neuronal calcium sensor proteins. Mol Brain 5, 2, 10.1186/1756-6606-5-2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames J. B. & Lim S. Molecular structure and target recognition of neuronal calcium sensor proteins. Biochim Biophys Acta 1820, 1205–1213, 10.1016/j.bbagen.2011.10.003S0304-4165(11)00246-7 [pii] (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne R. D. & Haynes L. P. Sense and specificity in neuronal calcium signalling. Biochim Biophys Acta 1853, 1921–1932, 10.1016/j.bbamcr.2014.10.029 ( 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O. et al. Frequenin - A novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron 11, 15–28 (1993). [DOI] [PubMed] [Google Scholar]
- Dason J. S., Romero-Pozuelo J., Atwood H. L. & Ferrus A. Multiple roles for frequenin/NCS-1 in synaptic function and development. Mol Neurobiol 45, 388–402, 10.1007/s12035-012-8250-4 (2012). [DOI] [PubMed] [Google Scholar]
- Mikhaylova M., Hradsky J. & Kreutz M. R. Between promiscuity and specificity: novel roles of EF-hand calcium sensors in neuronal Ca2+ signalling. J Neurochem 118, 695–713, 10.1111/j.1471-4159.2011.07372.x (2011). [DOI] [PubMed] [Google Scholar]
- Kerrigan T. L., Daniel J. W., Regan P. L. & Cho K. The role of neuronal calcium sensors in balancing synaptic plasticity and synaptic dysfunction. Front Mol Neurosci 5, 57, 10.3389/fnmol.2012.00057 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks K. B., Wang B. Q., Schnieders E. A. & Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nature Cell Biology 1, 234–241 (1999). [DOI] [PubMed] [Google Scholar]
- Gomez M. et al. Ca2+ signalling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron 30, 241–248 (2001). [DOI] [PubMed] [Google Scholar]
- Wang D., O’Halloran D. & Goodman M. B. GCY-8, PDE-2, and NCS-1 are critical elements of the cGMP-dependent thermotransduction cascade in the AFD neurons responsible for C. elegans thermotaxis. The Journal of general physiology 142, 437–449, 10.1085/jgp.201310959 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin V. M., Johnson J. R., Haynes L. P., Barclay J. W. & Burgoyne R. D. Identification of key structural elements for neuronal calcium sensor-1 function in the regulation of the temperature-dependency of locomotion in C. elegans. Mol Brain 6, 39, 10.1186/1756-6606-6-39 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFerran B. W., Graham M. E. & Burgoyne R. D. NCS-1, the mammalian homologue of frequenin is expressed in chromaffin and PC12 cells and regulates neurosecretion from dense-core granules. J. Biol. Chem. 273, 22768–22772 (1998). [DOI] [PubMed] [Google Scholar]
- Jo J. et al. Metabotropic glutamate receptor-mediated LTD involves two interacting Ca2+ sensors, NCS-1 and PICK1. Neuron 60, 1095–1111, S0896-6273(08)00950-1 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip P. K., Wong L. F., Sears T. A., Yanez-Munoz R. J. & McMahon S. B. Cortical overexpression of neuronal calcium sensor-1 induces functional plasticity in spinal cord following unilateral pyramidal tract injury in rat. PLoS Biol 8, e1000399, 10.1371/journal.pbio.1000399 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. L., Archer D. A. & Burgoyne R. D. NCS-1/frequenin functions in an autocrine pathway regulating Ca2+ channels in bovine adrenal chromaffin cells. J. Biol. Chem. 275, 40082–40087 (2000). [DOI] [PubMed] [Google Scholar]
- Weiss J. L., Hui H. & Burgoyne R. D. Neuronal calcium sensor-1 regulation of calcium channels, secretion, and neuronal outgrowth. Cell Mol Neurobiol 30, 1283–1292, 10.1007/s10571-010-9588-7 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rezende V. B. et al. NCS-1 deficiency causes anxiety and depressive-like behavior with impaired non-aversive memory in mice. Physiology & behavior 130, 91–98, 10.1016/j.physbeh.2014.03.005 (2014). [DOI] [PubMed] [Google Scholar]
- Saab B. J. et al. NCS-1 in the dentate gyrus promotes exploration, synaptic plasticity, and rapid acquisition of spatial memory. Neuron 63, 643–656, S0896-6273(09)00626-6.10.1016/j.neuron.2009.08.014 (2009). [DOI] [PubMed] [Google Scholar]
- Hukema R. K., Rademakers S., Dekkers M. P., Burghoorn J. & Jansen G. Antagonistic sensory cues generate gustatory plasticity in Caenorhabditis elegans. EMBO J 25, 312–322, 760094010.1038/sj.emboj.7600940 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. P. et al. Analysis of the interacting partners of the neuronal calcium-binding proteins L-CaBP1, hippocalcin, NCS-1 and neurocalcin. Proteomics 6, 1822–1832 (2006). [DOI] [PubMed] [Google Scholar]
- Schaad N. C. et al. Direct modulation of calmodulin targets by the neuronal calcium sensor NCS-1. Proc. Natl. Acad. Sci. USA 93, 9253–9258 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. P., Thomas G. M. H. & Burgoyne R. D. Interaction of neuronal calcium sensor-1 and ARF1 allows bidirectional control of phosphatidylinositol 4-kinase beta and TGN-plasma membrane traffic. J. Biol. Chem. 280, 6047–6054 (2005). [DOI] [PubMed] [Google Scholar]
- de Barry J. et al. Functional implication of neuronal calcium sensor-1 and PI4 kinase-b interaction in regulated exocytosis of PC12 cells. J Biol Chem 281, 18098–18111 (2006). [DOI] [PubMed] [Google Scholar]
- Bahi N. et al. IL1 receptor accessory protein like, a protein involved in X-linked mental retardation, interacts with Neuronal Calcium Sensor-1 and regulates exocytosis. Human Mol. Genetics 12, 1415–1425 (2003). [DOI] [PubMed] [Google Scholar]
- Hui H. et al. Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J Physiol 572, 165–172 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecker C. et al. Neuronal calcium sensor-1 enhancement of InsP(3) receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest 116, 1668–1674 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Pozuelo J. et al. The guanine-exchange factor Ric8a binds the calcium sensor NCS-1 to regulate synapse number and probability of release. J Cell Sci 127, 4246–4259, 10.1242/jcs.152603 (2014). [DOI] [PubMed] [Google Scholar]
- Lian L. Y. et al. Demonstration of Neuronal Calcium Sensor-1 binding to the Cav2.1 P/Q-type calcium channel. Biochemistry 53, 6052–6062, 10.1021/bi500568v (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J. et al. Modulation of CaV2.1 channels by neuronal calcium sensor-1 induces short-term synaptic facilitation. Molecular and cellular neurosciences 63, 124–131, 10.1016/j.mcn.2014.11.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbani N., Negyessy L., Lin R., Goldman-Rakic P. & Levenson R. Interaction with the neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. J. Neurosci. 22, 8476–8486 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian L. Y. et al. Characterisation of the Interaction of the C-Terminus of the Dopamine D2 Receptor with Neuronal Calcium Sensor-1. PLoS ONE 6, e27779, 10.1371/journal.pone.0027779PONE-D-11-17757 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandalaneni S. et al. Neuronal Calcium Sensor-1 binds the D2 dopamine receptor and G-protein coupled receptor kinase 1 (GRK1) peptides using different modes of interactions. J Biol Chem, 10.1074/jbc.M114.627059 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D. J., Burgoyne R. D. & Haynes L. P. Neuronal calcium sensor proteins are unable to modulate NFAT activation in mammalian cells. Biochim Biophys Acta 1780, 240–248, S0304-4165(07)00256-510.1016/j.bbagen.2007.10.011 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L. P., Sherwood M. W., Dolman N. J. & Burgoyne R. D. Specificity, promiscuity and localization of ARF protein interactions with NCS-1 and phosphatidylinositol-4 kinase-IIIb. Traffic 8, 1080–1092 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petko J. A. et al. Proteomic and functional analysis of NCS-1 binding proteins reveals novel signaling pathways required for inner ear development in zebrafish. BMC neuroscience 10, 27, 1471-2202-10-2710.1186/1471-2202-10-27 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. R. et al. PKC-2 Phosphorylation of UNC-18 Ser322 in AFD Neurons Regulates Temperature Dependency of Locomotion. J Neurosci 32, 7042–7051, 32/20/7042.10.1523/JNEUROSCI.4029-11.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I., Sasakura H. & Kuhara A. Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol 17, 712–719, 10.1016/j.conb.2007.11.010S0959-4388(07)00137-7 (2007). [DOI] [PubMed] [Google Scholar]
- Knight Z. A. et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell 125, 733–747, 10.1016/j.cell.2006.03.035 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida Y., Sugi T., Nonomura M. & Mori I. Identification of the AFD neuron as the site of action of the CREB protein in Caenorhabditis elegans thermotaxis. EMBO reports 12, 855–862, 10.1038/embor.2011.120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura H. & Mori I. Behavioral plasticity, learning, and memory in C. elegans. Curr Opin Neurobiol 23, 93–99, S0959-4388(12)00147-X.10.1016/j.conb.2012.09.005 (2012). [DOI] [PubMed] [Google Scholar]
- Ghosh R., Mohammadi A., Kruglyak L. & Ryu W. S. Multiparameter behavioral profiling reveals distinct thermal response regimes in Caenorhabditis elegans. BMC biology 10, 85, 10.1186/1741-7007-10-85 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi Y., Kimura K. D., Ohta A. & Mori I. Diverse regulation of sensory signaling by C. elegans nPKC-epsilon/eta TTX-4. EMBO J 24, 2127–2137, 10.1038/sj.emboj.7600697 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng E. et al. Neuronal calcium sensor-1 deletion in the mouse decreases motivation and dopamine release in the nucleus accumbens. Behavioural brain research, 10.1016/j.bbr.2015.12.037 (2015). [DOI] [PubMed] [Google Scholar]
- Graham M. E. et al. UNC-18 Modulates Ethanol Sensitivity in Caenorhabditis elegans. Mol Biol Cell 20, 43–55, E08-07-0689 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R. et al. Binding of UNC-18 to the N-terminus of syntaxin is essential for neurotransmission in Caenorhabditis elegans. Biochem J 418, 73–80, 10.1042/BJ20081956 (2009). [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Rajamanoharan D., McCue H. V., Rankin K. & Barclay J. W. Small Heat Shock Proteins are Novel Common Determinants of Alcohol and Nicotine Sensitivity in Caenorhabditis elegans. Genetics, 10.1534/genetics.115.185025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.