Abstract
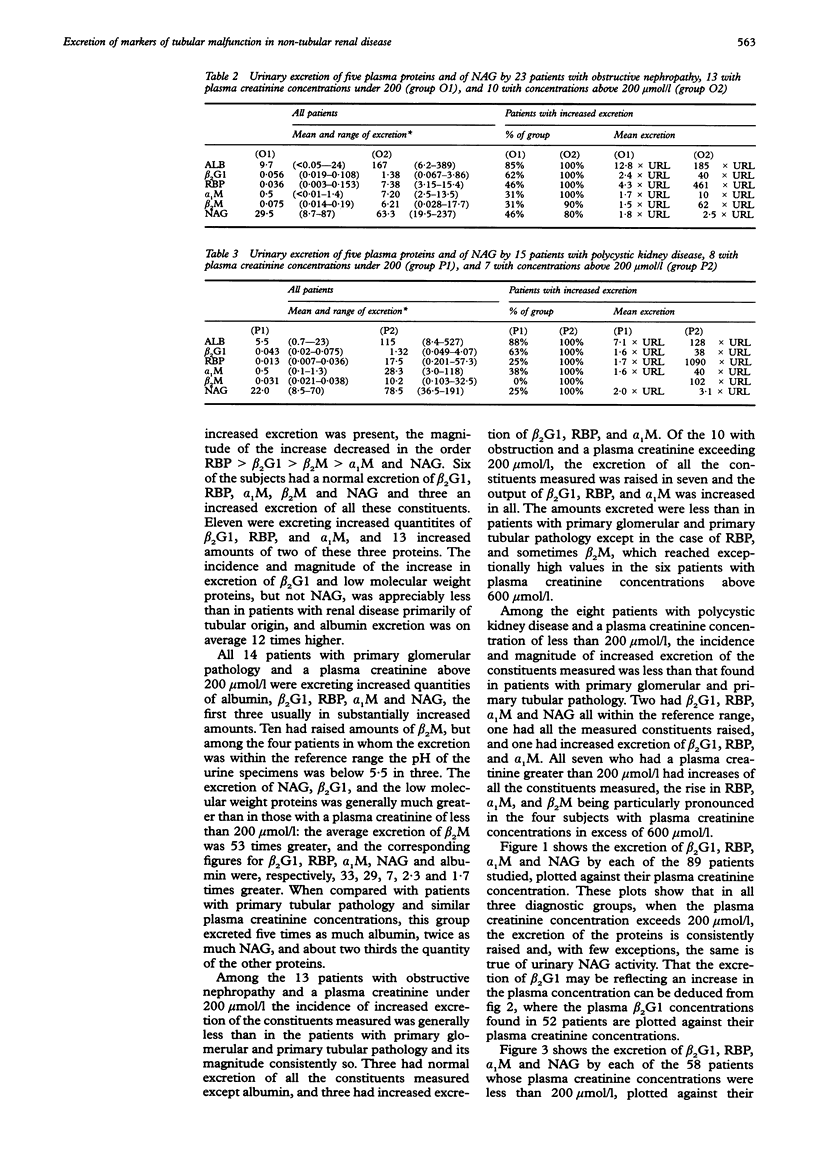
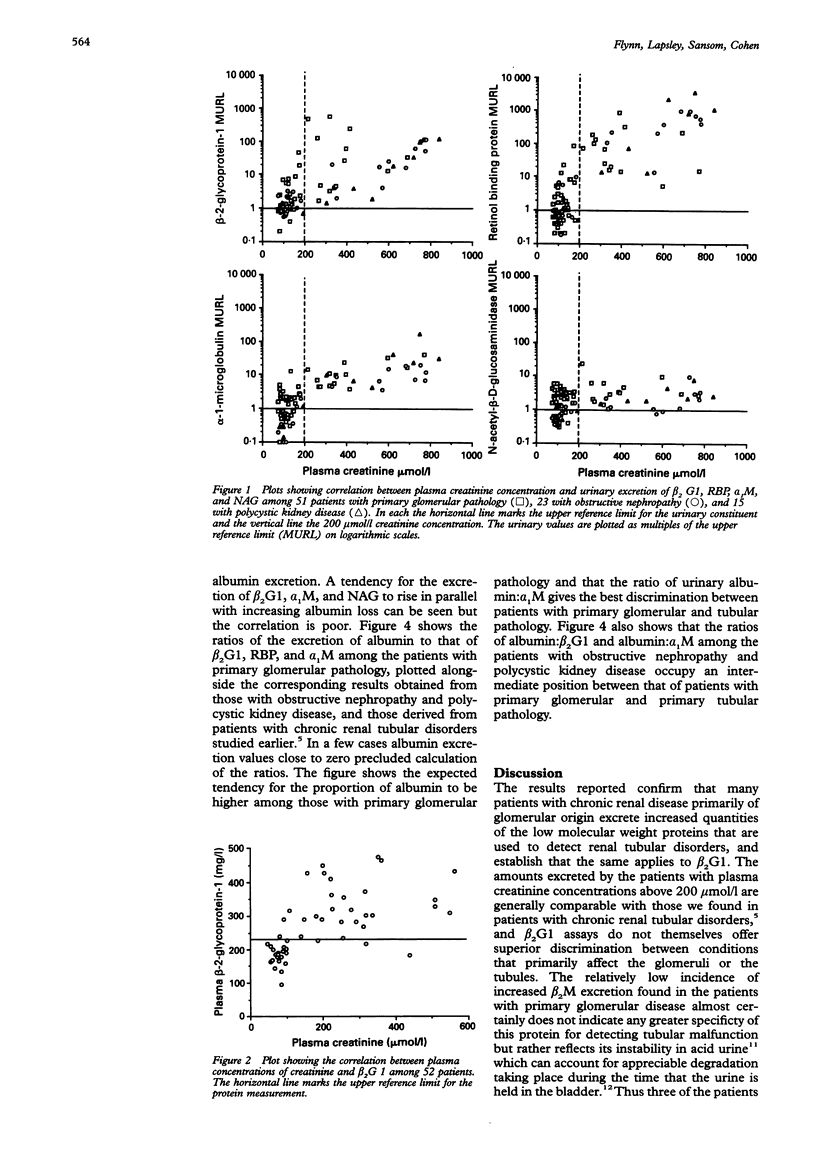
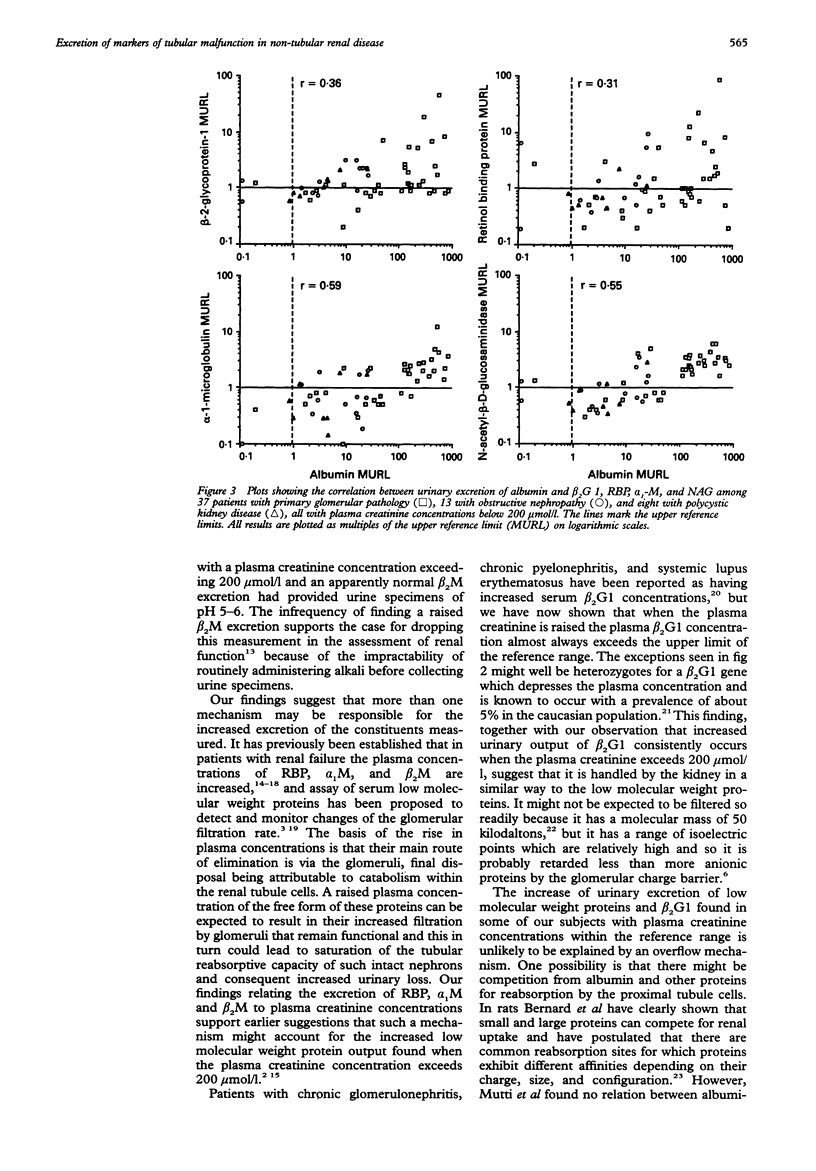
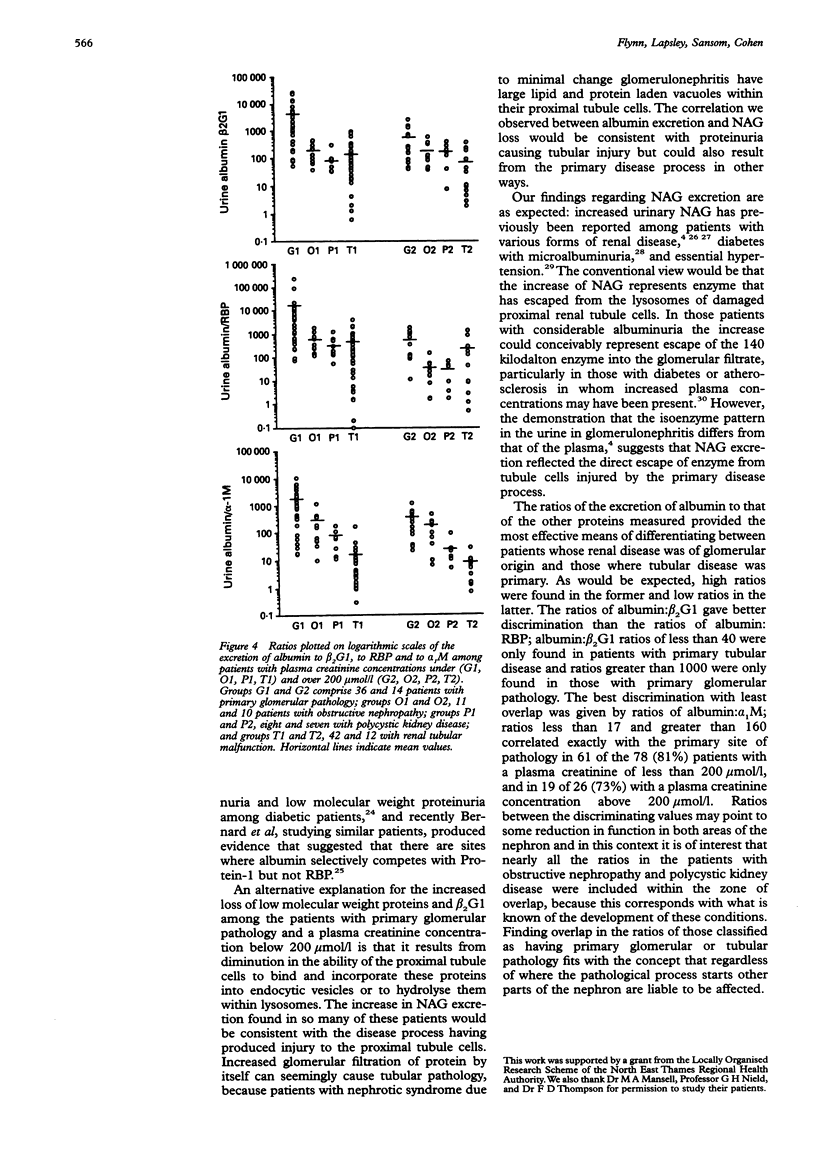
AIM: To determine whether urinary beta 2-glycoprotein-1 assays can provide improved discrimination between chronic renal diseases which are primarily of tubular or glomerular origin. METHODS: Urinary beta 2-glycoprotein-1, retinol-binding protein, alpha 1-microglobulin, beta 2-microglobulin, N-acetyl-beta-D-glucosa-minidase and albumin were measured in 51 patients with primary glomerular disease, 23 with obstructive nephropathy, and 15 with polycystic kidney disease, and expressed per mmol of creatinine. Plasma beta 2-glycoprotein-1 was assayed in 52 patients and plasma creatinine in all 89. The findings were compared between the diagnostic groups and with previously published data relating to primary tubular disorders. RESULTS: All 31 patients with plasma creatinine greater than 200 mumol/l excreted increased amounts of beta 2-glycoprotein-1, retinol-binding protein, and alpha 1-microglobulin, and 29 had increased N-acetyl-beta-D-glucosaminidase; the quantities were generally similar to those found in comparable patients with primary tubular pathology. Among 58 with plasma creatinine concentrations under 200 mumol/l, increases in beta 2-glycoprotein-1, retinol-binding protein, and alpha 1-microglobulin excretion were less common and much smaller, especially in those with obstructive nephropathy and polycystic disease. The ratios of the excretion of albumin to the other proteins provided the clearest discrimination between the patients with glomerular or tubular malfunction, but an area of overlap was present which embraced those with obstructive nephropathy and polycystic disease. CONCLUSIONS: Increased excretion of beta 2-glycoprotein-1 due to a raised plasma concentration or diminution of tubular reabsorption, or both, is common in all the forms of renal disease investigated, and both plasma creatinine and urinary albumin must be taken into account when interpreting results. Ratios of urinary albumin: beta 2-glycoprotein-1 greater than 1000 are highly suggestive of primary glomerular disease and those less than 40 of primary tubular disease. Used in this way, beta 2-glycoprotein-1 assays provide superior discrimination between glomerular and tubular malfunction when compared with retinol binding protein but the best discrimination is provided by albumin: alpha 1-microglobulin ratios.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderman M. H., Melcher L., Drayer D. E., Reidenberg M. M. Increased excretion of urinary N-acetyl-beta-glucosaminidase in essential hypertension and its decline with antihypertensive therapy. N Engl J Med. 1983 Nov 17;309(20):1213–1217. doi: 10.1056/NEJM198311173092004. [DOI] [PubMed] [Google Scholar]
- Belfiore F., Napoli E., Vecchio L. L., Rabuazzo A. M. Increased serum activity of beta-n-acetyl-glucoseaminidase in atherosclerosis. Am J Med Sci. 1974 Oct;268(4):235–239. doi: 10.1097/00000441-197410000-00004. [DOI] [PubMed] [Google Scholar]
- Bernard A. M., Lauwerys R. R., Noël A., Vandeleene B., Lambert A. Urine protein 1: a sex-dependent marker of tubular or glomerular dysfunction. Clin Chem. 1989 Oct;35(10):2141–2142. [PubMed] [Google Scholar]
- Bernard A., Viau C., Ouled A., Lauwerys R. Competition between low- and high-molecular-weight proteins for renal tubular uptake. Nephron. 1987;45(2):115–118. doi: 10.1159/000184090. [DOI] [PubMed] [Google Scholar]
- Bernard A., Vyskocyl A., Mahieu P., Lauwerys R. Effect of renal insufficiency on the concentration of free retinol-binding protein in urine and serum. Clin Chim Acta. 1988 Jan 15;171(1):85–93. doi: 10.1016/0009-8981(88)90293-8. [DOI] [PubMed] [Google Scholar]
- Cohnen G. Immunochemical quantitation of beta2-glycoprotein I in various diseases. J Lab Clin Med. 1970 Feb;75(2):212–216. [PubMed] [Google Scholar]
- Cooper E. H., Forbes M. A., Crockson R. A., MacLennan I. C. Proximal renal tubular function in myelomatosis: observations in the fourth Medical Research Council trial. J Clin Pathol. 1984 Aug;37(8):852–858. doi: 10.1136/jcp.37.8.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey P. G., Gosling P. beta 2-Microglobulin instability in pathological urine. Clin Chem. 1982 Jun;28(6):1330–1333. [PubMed] [Google Scholar]
- Evrin P. E., Wibell L. The serum levels and urinary excretion of 2 -microglobulin in apparently healthy subjects. Scand J Clin Lab Invest. 1972 Feb;29(1):69–74. doi: 10.3109/00365517209081057. [DOI] [PubMed] [Google Scholar]
- Flynn F. V. Assessment of renal function: selected developments. Clin Biochem. 1990 Feb;23(1):49–54. doi: 10.1016/0009-9120(90)90435-w. [DOI] [PubMed] [Google Scholar]
- Gibb D. M., Tomlinson P. A., Dalton N. R., Turner C., Shah V., Barratt T. M. Renal tubular proteinuria and microalbuminuria in diabetic patients. Arch Dis Child. 1989 Jan;64(1):129–134. doi: 10.1136/adc.64.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultberg B., Ravnskov U. The excretion of N-acetyl-beta-glucosaminidase in glomerulonephritis. Clin Nephrol. 1981 Jan;15(1):33–38. [PubMed] [Google Scholar]
- Itoh Y., Kawai T. Human alpha 1-microglobulin: its measurement and clinical significance. J Clin Lab Anal. 1990;4(5):376–384. doi: 10.1002/jcla.1860040511. [DOI] [PubMed] [Google Scholar]
- Jung K., Schulze B. D., Sydow K., Pergande M., Precht K., Schreiber G. Diagnostic value of low-molecular mass proteins in serum for the detection of reduced glomerular filtration rate. J Clin Chem Clin Biochem. 1987 Aug;25(8):499–503. doi: 10.1515/cclm.1987.25.8.499. [DOI] [PubMed] [Google Scholar]
- Maack T., Johnson V., Kau S. T., Figueiredo J., Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979 Sep;16(3):251–270. doi: 10.1038/ki.1979.128. [DOI] [PubMed] [Google Scholar]
- Mutti A., Alinovi R., Ghiggeri G. M., Bergamaschi E., Candiano G., Rasi A., Gusmano R., Franchini I., Borghetti A. Urinary excretion of brush-border antigen and plasma proteins in early stages of diabetic nephropathy. Clin Chim Acta. 1990 Apr 30;188(2):93–100. doi: 10.1016/0009-8981(90)90153-j. [DOI] [PubMed] [Google Scholar]
- Norden A. G., Fulcher L. M., Lapsley M., Flynn F. V. Excretion of beta 2-glycoprotein I (apolipoprotein H) in renal tubular disease. Clin Chem. 1991 Jan;37(1):74–77. [PubMed] [Google Scholar]
- Sansom P. A., Marlow C. T., Lapsley M., Flynn F. V. A sandwich enzyme-linked immunosorbent assay for beta 2-glycoprotein I. Ann Clin Biochem. 1991 May;28(Pt 3):283–289. doi: 10.1177/000456329102800315. [DOI] [PubMed] [Google Scholar]
- Scarpioni L., Dall'aglio P. P., Poisetti P. G., Buzio C. Retinol binding protein in serum and in urine of glomerular and tubular nephropathies. Clin Chim Acta. 1976 Apr 15;68(2):107–113. doi: 10.1016/0009-8981(76)90409-5. [DOI] [PubMed] [Google Scholar]
- Schardijn G. H., Statius van Eps L. W. Beta 2-microglobulin: its significance in the evaluation of renal function. Kidney Int. 1987 Nov;32(5):635–641. doi: 10.1038/ki.1987.255. [DOI] [PubMed] [Google Scholar]
- Schousboe I. Purification, characterization and identification of an agglutinin in human serum. Biochim Biophys Acta. 1979 Aug 28;579(2):396–408. doi: 10.1016/0005-2795(79)90067-9. [DOI] [PubMed] [Google Scholar]
- Sepehrnia B., Kamboh M. I., Adams-Campbell L. L., Bunker C. H., Nwankwo M., Majumder P. P., Ferrell R. E. Genetic studies of human apolipoproteins. VIII. Role of the apolipoprotein H polymorphism in relation to serum lipoprotein concentrations. Hum Genet. 1989 May;82(2):118–122. doi: 10.1007/BF00284041. [DOI] [PubMed] [Google Scholar]
- Sherman R. L., Drayer D. E., Leyland-Jones B. R., Reidenberg M. M. N-acetyl-beta-glucosaminidase and beta 2-microglobulin. Their urinary excretion in patients with renal parenchymal disease. Arch Intern Med. 1983 Jun;143(6):1183–1185. doi: 10.1001/archinte.143.6.1183. [DOI] [PubMed] [Google Scholar]
- Swedenborg P., Hultberg B., Thysell H. Urinary beta-hexosaminidase excretion in polycystic kidney disease. Acta Med Scand. 1981;210(6):471–473. doi: 10.1111/j.0954-6820.1981.tb09852.x. [DOI] [PubMed] [Google Scholar]
- Topping M. D., Forster H. W., Dolman C., Luczynska C. M., Bernard A. M. Measurement of urinary retinol-binding protein by enzyme-linked immunosorbent assay, and its application to detection of tubular proteinuria. Clin Chem. 1986 Oct;32(10):1863–1866. [PubMed] [Google Scholar]
- Wibell L., Evrin P. E., Berggård I. Serum 2 -microglobulin in renal disease. Nephron. 1973;10(5):320–331. doi: 10.1159/000180203. [DOI] [PubMed] [Google Scholar]
- Yu H., Yanagisawa Y., Forbes M. A., Cooper E. H., Crockson R. A., MacLennan I. C. Alpha-1-microglobulin: an indicator protein for renal tubular function. J Clin Pathol. 1983 Mar;36(3):253–259. doi: 10.1136/jcp.36.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen C. T., Kind P. R., Price R. G., Praill P. F., Richardson A. C. Colorimetric assay for N-acetyl-beta-D-glucosaminidase (NAG) in pathological urine using the omega-nitrostyryl substrate: the development of a kit and the comparison of manual procedure with the automated fluorimetric method. Ann Clin Biochem. 1984 Jul;21(Pt 4):295–300. doi: 10.1177/000456328402100411. [DOI] [PubMed] [Google Scholar]


