Abstract
The objective of this study was to investigate Al3+-induced IAA transport, distribution, and the relation of these two processes to Al3+-inhibition of root growth in alfalfa. Alfalfa seedlings with or without apical buds were exposed to 0 or 100 μM AlCl3 and were foliar sprayed with water or 6 mg L−1 IAA. Aluminium stress resulted in disordered arrangement of cells, deformed cell shapes, altered cell structure, and a shorter length of the meristematic zone in root tips. Aluminium stress significantly decreased the IAA concentration in apical buds and root tips. The distribution of IAA fluorescence signals in root tips was disturbed, and the IAA transportation from shoot base to root tip was inhibited. The highest intensity of fluorescence signals was detected in the apical meristematic zone. Exogenous application of IAA markedly alleviated the Al3+-induced inhibition of root growth by increasing IAA accumulation and recovering the damaged cell structure in root tips. In addition, Al3+ stress up-regulated expression of AUX1 and PIN2 genes. These results indicate that Al3+-induced reduction of root growth could be associated with the inhibitions of IAA synthesis in apical buds and IAA transportation in roots, as well as the imbalance of IAA distribution in root tips.
Aluminium (Al) is the most abundant metal and is widely distributed in nature in the form of silicates or other deposits1. A high content of soluble Al3+ in soils with a pH below 5 is very phytotoxic and becomes a major limiting factor of plant productivity in acidic soils2. The most obvious symptom of Al3+ toxicity is the inhibition of root growth3. Excessive Al3+ inhibits both cell elongation and division in roots, leading to swollen root apices with poor or no root-hair development. Several studies suggested that this may be caused by the interaction of Al3+ with signal transduction pathways regulating cell growth. It has been shown that Al3+ may be bound to the plasma membrane, disturbing ion transport and homeostasis as well as blocking Ca2+-dependent signalling cascades4. The binding of Al3+ to the plasma membrane may also lead to a disruption of membrane function and the promotion of oxidative stress, which prevent the release of secondary signalling molecules and influence the organization of the cell cytoskeleton5,6. In addition, Al3+ stress induces callose production in plasmodesmata, which may block auxin or indole acetic acid (IAA) transport through the symplast and result in a Al3+-induced inhibition of root cell elongation7,8.
Plant hormones are involved in plant adaptation to environmental stresses, including Al3+ stress9,10,11. The involvement of auxin in a plant’s response to heavy metal toxicity has been investigated in recent years. For example, cadmium toxicity altered IAA distribution by increasing the activity of IAA oxidase in roots of Medicago truncatula seedlings12 and disturbed auxin homeostasis by affecting the distribution, metabolism, and transport of auxin in Arabidopsis seedlings13,14. Additionally, auxin transport via AUXIN RESISTANT 1 (AUX1) has been shown to play a positive role in plant tolerance to arsenite stress by influencing reactive oxygen species (ROS)-mediated signalling pathways15. Finally, copper (Cu2+) treatments modulated auxin redistribution via regulation of PINFORMED1 (PIN1) in Arabidopsis roots, and exogenous application of auxin reduced the toxic effect of Cu2+ on sunflower (Helianthus annuus), leading to an increase in root length and root hair formation16,17. However, limited information is available on the roles that auxin plays in plant responses to Al3+ stress.
Alfalfa (Medicago sativa L.) is an important legume and is used as a forage crop worldwide, but aluminium toxicity is a major factor limiting alfalfa production in soils with low pH18,19. Understanding the mechanisms underlying IAA regulation of alfalfa’s response to Al3+ is important for developing Al3+-tolerant germplasm through genetic modification or molecular breeding. Our previous study showed that IAA concentrations in apical buds and root tips of Al3+-stressed seedlings decreased in a short period (1–3 d) of Al3+ stress20, which may reduce the growth of alfalfa seedlings under Al3+ stress. In the present study, we aimed to further investigate the following questions: (1) What was the reason of IAA accumulation decrease in the Al3+-stressed root tips? (2) What was the effect of Al3+ stress on cell structure and IAA distribution in root tips? (3) Was there any effect of IAA on the cell structure formation of the Al3+-stressed root tips?
Results
IAA alleviated Al3+-induced plant growth
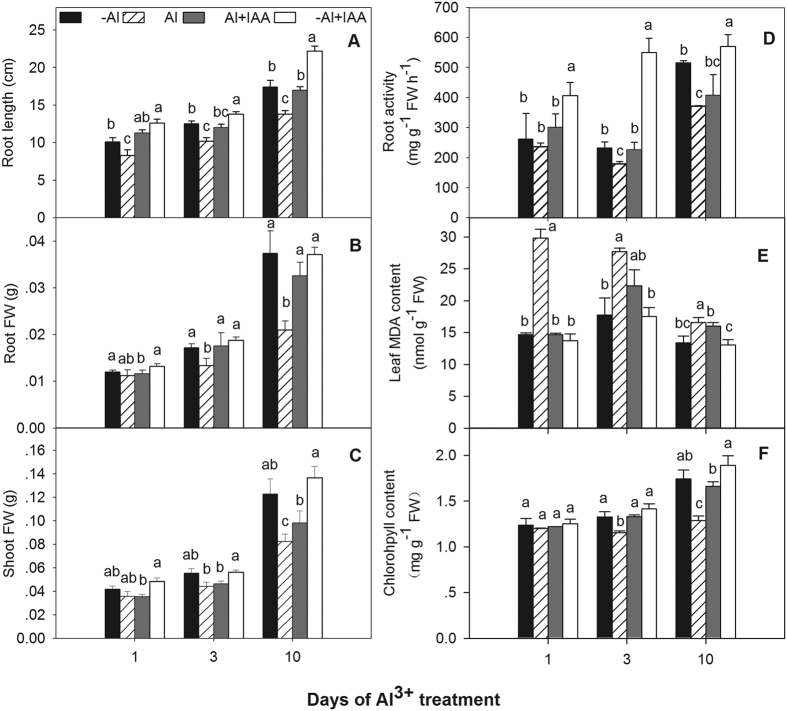
The primary Al3+ toxicity in plants is the inhibition of root growth. When alfalfa seedlings were exposed to varying concentrations of AlCl3 (0, 50, 100, 200 or 300 μM) for 48 h, the inhibition of roots elongation was positively dependent on AlCl3 concentrations, in which the higher decline rate of relative root growth was in the Al3+ concentration of 100 μM (Supplemental Fig. 1). Root length of alfalfa exposed to 100 μM AlCl3 was significantly reduced by 17–21% relative to plants without Al3+ treatments (-Al, control) from 1 to 10 d of treatment (Fig. 1A). Root fresh weight (Fig. 1B), shoot fresh weight (Fig. 1C), root activity (Fig. 1D) and total chlorophyll content (Fig. 1F) were significantly decreased after 3 d of Al3+ treatment and were 56.1, 32.9, 27.9 and 26.2% lower than those in control plants at 10 d of Al3+ treatment, respectively. The MDA content in leaves of Al3+-stressed plants (Fig. 1E) was significantly increased compared to control plants during the experimental periods.
Figure 1.
Root length (A), shoot (B), root (C) fresh weight (FW), root activity (D), leaf MDA content (E), and total chlorophyll content (F) in alfalfa seedlings grown for 1, 3 and 10 days on ½-strength Hoagland’s medium (pH 4.5) with treatments of no aluminium (-Al), aluminium stress (Al) (100 μM AlCl3), aluminium with additional IAA (Al + IAA) (100 μM AlCl3 + IAA) and -Al with additional IAA (-Al + IAA). Data are means ± SE of three replicates. Bars with different letters indicate a significant difference at P < 0.05 (least significant difference test).
Compared with Al3+ treatment alone, the addition of IAA (Al + IAA) markedly increased root length (Fig. 1A), root fresh weight (Fig. 1B), shoot fresh weight (Fig. 1C), and total chlorophyll content (Fig. 1F) after 10 d of the experiment. The MDA content in leaves treated with Al + IAA decreased to levels near those of the control plants (-Al) (Fig. 1E).
IAA alleviated Al3+-induced damage of root tip structure
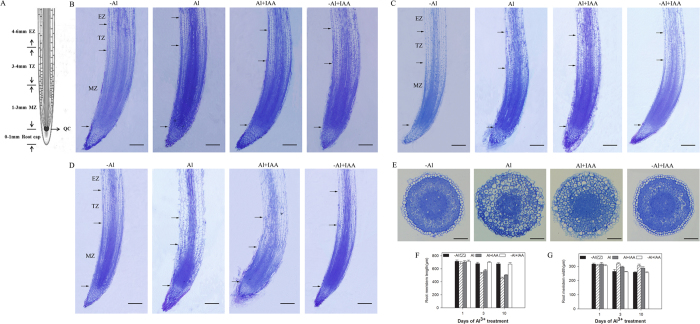
Root tips mainly contain four regions: the root cap (0–1 mm from the tip), meristematic zone (MZ 1–3 mm), transition zone (TZ 3–4 mm) and elongation zone (EZ 4–6 mm) (Fig. 2A). Compared with the control, Al3+ treatments decreased the root meristem length at 1, 3 and 10 d (Fig. 2B–D,F) and enhanced the root meristem width at 3 and 10 d (Fig. 2G). The tissue structure in the whole root tip was damaged by the addition of Al3+. The cell arrangement was also disordered, and the cell shape was distorted from a rectangle into an irregular shape in the epidermis and cortex and even in the stele of the TZ and EZ (Fig. 2C,D). The cell length enlarged in the cortex in all root tip zones, the epidermal cells in the root cap detached, and the root tips became obtuse (Fig. 2D). Compared with the control (-Al) and based on the cross-section in the middle of the MZ (Fig. 2E), the epidermal cell was seriously damaged, the cell and vacuole enlarged, the intercellular space diminished, the thickness of the cortex increased, and the diameter of the stele decreased. However, the addition of IAA (Al + IAA) protected the tissue structure of the Al3+-stressed root tip in which the cell arrangement, cell shape and cell length as well as the thickness of the cortex were close to those of the control plant (no Al3+ addition), and the meristem length increased compared with Al3+ treatment alone (Fig. 2A–F).
Figure 2. Images of root tips (0–6 mm) in the middle longitudinal section of alfalfa seedlings grown for 1, 3 and 10 days on ½-strength Hoagland’s medium (pH 4.5) with treatments of no aluminium (-Al), aluminium stress (Al) (100 μM AlCl3), aluminium with additional IAA (Al + IAA) (100 μM AlCl3 + IAA) and -Al with additional IAA (-Al + IAA).
Root tip (A) was divided into the root cap, the transition zone (TZ), the meristematic zone (MZ) and the elongation zone (EZ) and is indicated by arrowheads. The quiescent centre was marked as QC. A cross-section was cut in the middle of the meristematic zone (E) on day 10. Scale bars in longitudinal sections indicate 100 μm; in cross-sections, they indicate 50 μm. The length (F) and width (G) of the root meristems were measured. Data are means ± SE of three replicates. Bars with different letters indicate a significant difference at P < 0.05 (least significant difference test).
Al3+ altered IAA distribution in plants
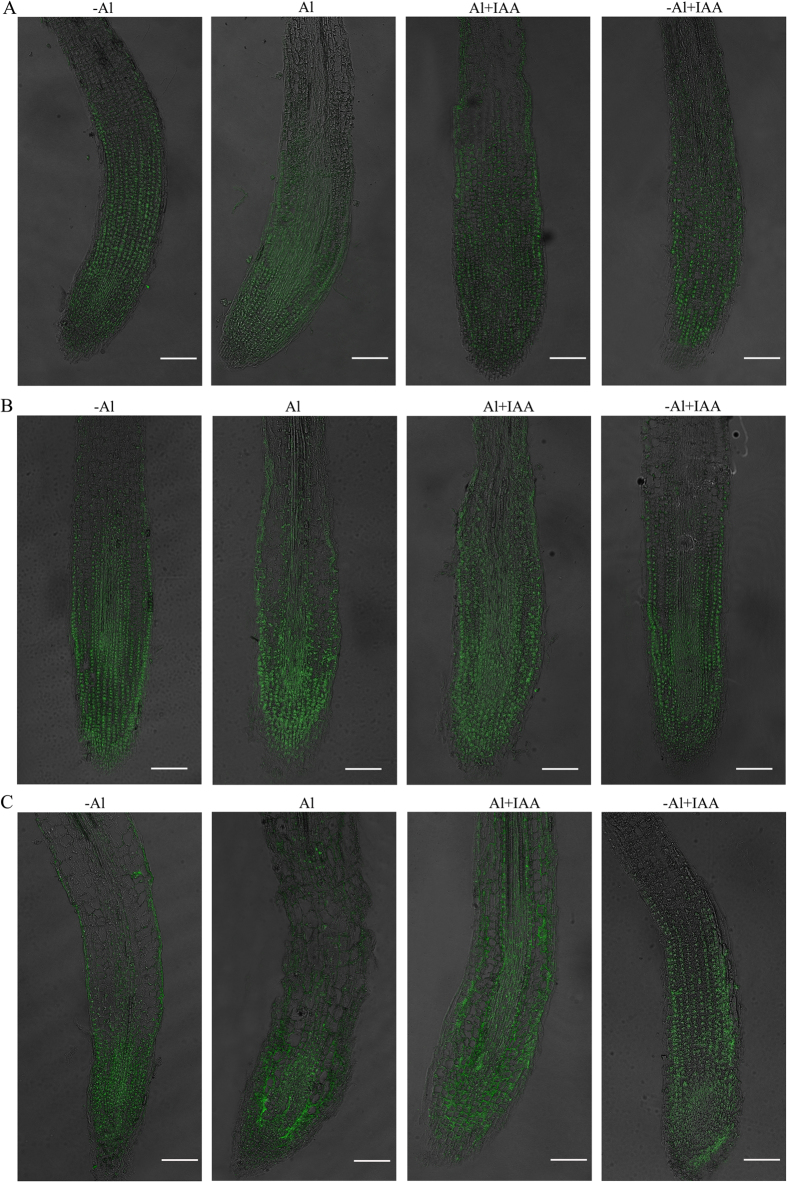
To explore the IAA distribution in alfalfa root tips, the immunohistochemical method of staining with antibodies was used in this study. In the control (-Al) and -Al + IAA treatments, a high intensity of homogeneous fluorescence signals was detected as a continuous dotted line in the epidermis, cortex (close to the endodermis), endodermis and stele of the TZ and distal MZ (Fig. 3A,B), whereas weak signals was detected in the EZ during the 10-d experimental period (Fig. 3C). However, in the Al3+ treatment alone, the distribution of fluorescence signals in root tips was disturbed; the dotted line characterizing fluorescence signals disappeared (Fig. 3B,C). Discontinuous fluorescence signals were detected in the epidermis, cortex (close to the endodermis), endodermis, and stele of the TZ and distal MZ. On the other hand, a higher intensity of fluorescence signals was detected in all tissues of the apical MZ compared with the control (-Al) treatment after 10 d of Al3+ treatment (Fig. 3B,C), which means that the distribution of IAA was altered in root tips. Higher IAA accumulation in the apical MZ and lower IAA accumulation in the TZ compared with the control (-Al) treatment indicated that IAA transportation in root tips was inhibited.
Figure 3. Immunolocalization of IAA in the middle longitudinal section of alfalfa seedlings grown for 1, 3 and 10 days on ½-strength Hoagland’s medium (pH 4.5) with treatments of no aluminium (-Al), aluminium stress (Al) (100 μM AlCl3), aluminium with additional IAA (Al + IAA) (100 μM AlCl3 + IAA) and -Al with additional IAA (-Al + IAA).
Scale bars in each image indicate 100 μm.
In the combined treatment of Al3+ with IAA (Al + IAA), the IAA signals were less disturbed by Al3+ stress (Fig. 3A–C); the dotted lines of fluorescence signals were clearer and more continuous than in Al3+ treatment alone after 3 d of Al3+ treatment (Fig. 3A,B). The distributional intensity of fluorescence signals in the whole root tip was also close to that of the control plant. The IAA signals in the epidermis, cortex and stele of the TZ were increased compared with the Al3+ (Al) treatment, indicating that exogenous application of IAA could compensate for the lack of IAA in the TZ under Al3+ (Al) treatment. Either the primary antibody or the secondary antibody was omitted during the complete procedure, and no signals were observed in the two antibody breeding procedures (Supplementary Fig. 2).
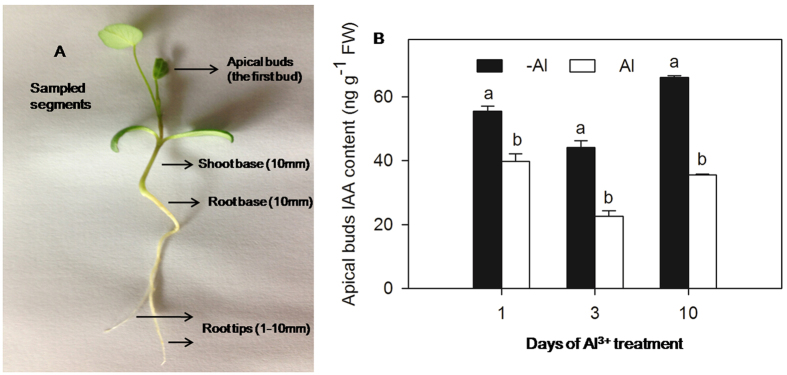
To evaluate the mechanism by which exogenous application of IAA alleviates Al3+ toxicity, IAA concentration in different parts of the plant was measured. Sampled segments are shown in Fig. 4A. When alfalfa was exposed to 100 μM AlCl3, IAA concentrations in apical buds (Fig. 4B) and root tips at 1, 3, and 10 d as well as in the shoot base at 3 and 10 d were significantly reduced compared to control plants (Table 1). However, the degree of reduction in apical buds (25–50%) was lower than in root tips (75–90%) (Table 1). IAA concentrations in the root base were significantly higher in both the Al3+ treatment and Al3+ with IAA treatment (Al + IAA) compared to both the treatment without Al3+ (-Al) and -Al3+ with IAA treatment (-Al + IAA), respectively, after 3 d (Table 1).
Figure 4.
Sampled segments are shown in (A); IAA concentrations in apical buds of alfalfa seedlings grown for 1, 3 and 10 days on ½-strength Hoagland’s medium (pH 4.5) in the treatments of no aluminium (-Al) and aluminium stress (Al) (100 μM AlCl3) (B). Data are means ± SE of three replicates. Bars with different letters indicate a significant difference at P < 0.05 (least significant difference test).
Table 1. IAA concentrations in root tips, root base and shoot base, and IAA[root tips]/IAA[shoot base] radio in alfalfa seedlings grown for 1, 3 and 10 days on ½-strength Hoagland’s medium (pH 4.5) with treatments of no Aluminum(-Al), Aluminum stress (Al) (100 μM AlCl3), Aluminum with additional IAA (Al + IAA) (100 μM AlCl3 + IAA) and -Al with additional IAA(-Al + IAA).
| Treatments | 1d | 3d | 10d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root tips | Root base | Shoot base | Root tips/shoot base* | Root tips | Root base | Shoot base | Root tips/shoot base* | Root tips | Root base | Shoot base | Root tips/shoot base* | |
| -Al | 1.276 ± 0.215b | / | 12.81 ± 1.784b | 9.961 | 2.397 ± 0.407a | 3.672 ± 0.481b | 34.49 ± 1.230b | 6.950 | 1.193 ± 0.131b | 3.435 ± 0.420b | 23.43 ± 0.504b | 5.092 |
| Al | 0.185 ± 0.044c | / | 11.93 ± 1.279b | 1.551 | 0.219 ± 0.058b | 6.079 ± 0.687a | 17.79 ± 2.557c | 1.231 | 0.315 ± 0.038c | 5.117 ± 0.507a | 13.53 ± 1.890d | 2.328 |
| Al + IAA | 0.772 ± 0.189bc | / | 22.75 ± 0.787a | 3.393 | 0.348 ± 0.069b | 5.491 ± 0.001a | 42.83 ± 3.435a | 0.813 | 0.562 ± 0.199c | 6.084 ± 0.615a | 19.10 ± 0.748c | 2.942 |
| -Al + IAA | 2.506 ± 0.405a | / | 24.13 ± 2.052a | 10.385 | 2.996 ± 0.119a | 2.941 ± 0.318b | 43.82 ± 3.155a | 6.837 | 1.721 ± 0.184a | 4.905 ± 0.009ab | 27.91 ± 0.491a | 6.166 |
Data are means ± SE of three replicates. Columns with different letters indicate a significant difference at P < 0.05 (least significant difference test).
*Root tips/shoot base is the radio of 100·IAA[root tips]/IAA[shoot base].
Compared to Al3+ treatment alone, Al + IAA significantly increased (by 40–140%) IAA concentrations in the shoot base throughout the experimental period; however, the increased IAA concentration in root tips reached a significant level only at 1 d of Al + IAA treatment (Table 1). In contrast to the treatment without Al3+ (-Al), -Al + IAA induced an increase in IAA concentrations in both the shoot base and root tips, by 20–88% and 25–96%, respectively, throughout the experimental period (Table 1). After exogenous application of IAA, the degree of increase in IAA in root tips was lower in the plants treated with Al3+ than in those without Al3+. In addition, regardless of IAA treatment, the ratio of IAA concentrations between root tips and shoot base was lower in the presence of Al3+ than without Al3+ throughout the experimental period (Table 1).
To further explore the effect of Al3+ on IAA distribution in the plant, apical buds that were a primary site for IAA synthesis were removed 24 h before Al3+ treatment; this was done to eliminate the effect of endogenous IAA synthesis. Compared with plants with intact apical buds, the IAA concentration in root tips was significantly decreased in plants without apical buds in both the -Al and Al treatment, by 89.8% and 87.6%, respectively (Fig. 5). In bud-removed plants, exogenous application of IAA significantly increased IAA concentrations in root tips in both -Al + IAA and Al + IAA treatments, 25-fold and 10-fold higher than those in -Al3+ and Al3+ treatments, respectively (Fig. 5). The degree of increase was lower in the Al3+ treatment than in the treatment without Al3+.
Figure 5. IAA concentrations in root tips (0–10 mm) of alfalfa seedlings (normal or apical bud removed) grown for 24 h on ½-strength Hoagland’s medium (pH 4.5) with treatments of no aluminium (-Al), aluminium stress (Al) (100 μM AlCl3), aluminium with additional IAA (Al+IAA) (100 μM AlCl3 + IAA) and -Al with additional IAA (-Al + IAA).
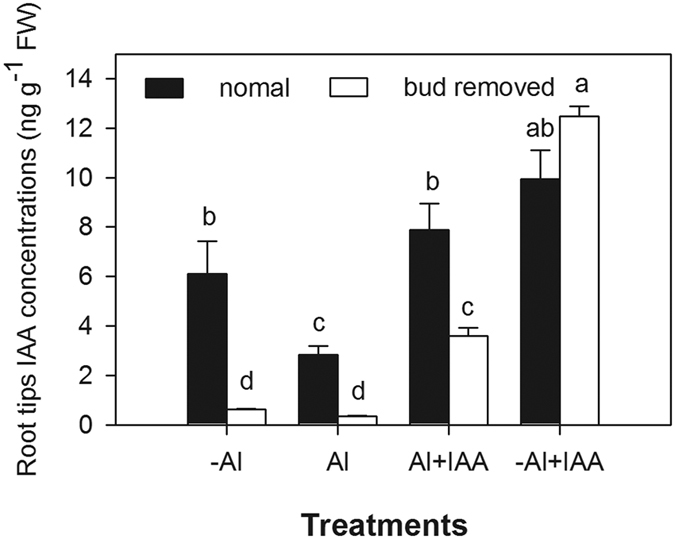
Data are means ± SE of three replicates. Bars with different letters indicate a significant difference at P < 0.05 (least significant difference test).
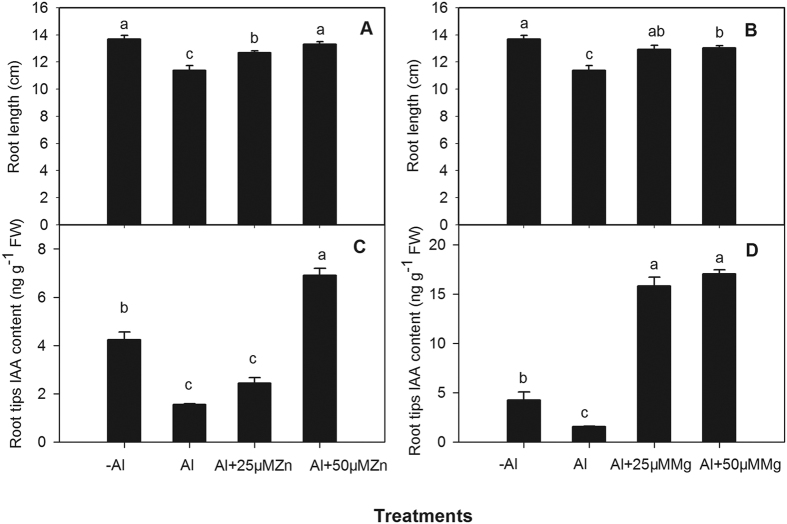
Magnesium (Mg2+) and zinc (Zn2+) alleviated Al3+-induced inhibition of root elongation by increased IAA accumulation in root tips
Compared with Al3+ treatment alone, the addition of Mg2+ (Fig. 6A) and Zn2+ (Fig. 6B) to the Al3+ treatment significantly increased root length, which reached the same level as the control (-Al) after 1 d of treatment. The addition of 25 μM and 50 μM Mg2+ with excess Al3+ for 1 d also significantly increased IAA concentrations in the root tips 9.2-fold and 9.5-fold, respectively, in comparison with Al3+ treatment (Fig. 6C). The addition of 25 μM or 50 μM Zn2+ significantly increased IAA concentrations in the root tips by 56.73 and 340%, respectively (Fig. 6D).
Figure 6.
Root length and IAA concentrations in root tips (0–10 mm) of alfalfa seedlings grown for 24 h on ½-strength Hoagland’s medium (pH 4.5) with treatments of no aluminium (-Al), aluminium stress (Al) (100 μM AlCl3), aluminium together with 25 μM Zn2+ (Al + 25 μM Zn), aluminium together with 50 μM Zn2+ (Al + 50 μM Zn) (A,C), aluminium together with 25 μM Mg2+ (Al + 25 μM Mg) and aluminium together with 50 μM Mg2+ (Al + 50 μM Mg)(B,D). Data are means ± SE of three replicates. Bars with different letters indicate a significant difference at P < 0.05 (least significant difference test).
IAA decreased Al3+-induced callose production in plants
Compared to the treatment without Al3+ (-Al), callose content in root tips increased by 10.9, 20.4 and 27.0% at 1, 3 and 10 d of Al3+ treatment, respectively (Fig. 7A). Callose content in shoots under Al3+ treatment also increased by 45.9% at 10 d but showed no significant difference from the content under -Al treatment at 3 d (Fig. 7B). This finding suggests that shoot callose formation was slower than root callose formation under Al3+ stress. Compared to Al3+ treatment, callose content reduction was only 9.1% in root tips and 17.2% in shoots in the Al + IAA treatment throughout the experimental period (Fig. 7).
Figure 7.
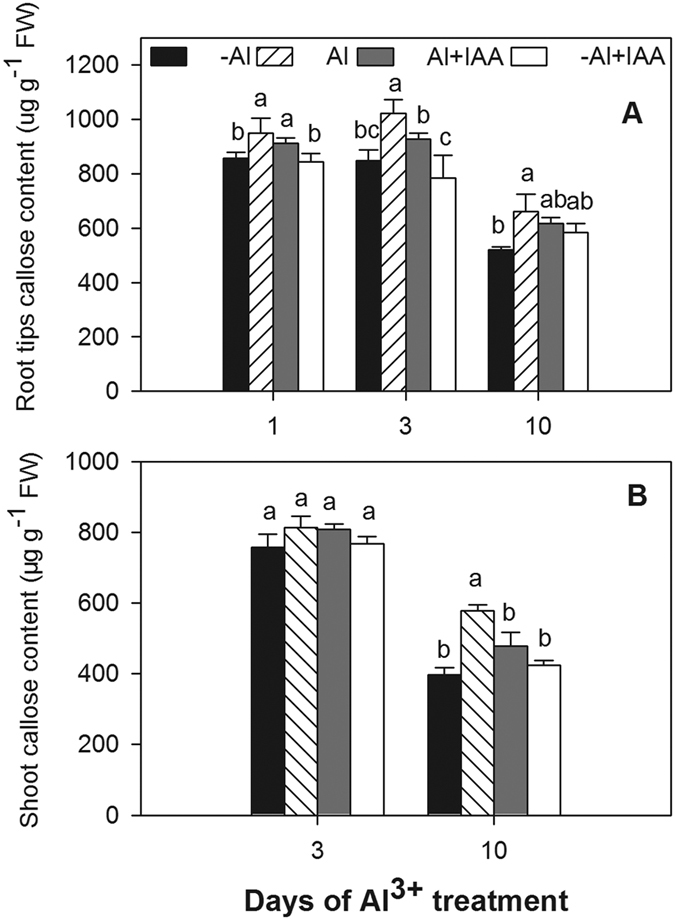
Callose content in root tips (0–10 mm) (A) and shoot (B) of alfalfa seedlings grown for 1, 3 and 10 days on ½-strength Hoagland’s medium (pH 4.5) with treatments of no aluminium (-Al), aluminium stress (Al) (100 μM AlCl3), aluminium with additional IAA (Al + IAA) (100 μM AlCl3 + IAA) and -Al with additional IAA (-Al + IAA). Data are means ± SE of three replicates. Bars with different letters indicate a significant difference at P < 0.05 (least significant difference test).
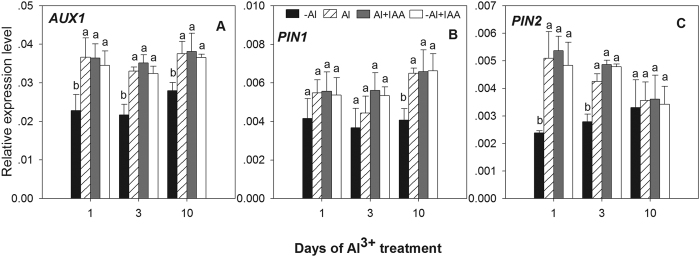
Al3+ up-regulated expression levels of IAA transport related genes
The relative transcription levels of AUX1 in root tips were significantly up-regulated by Al3+ treatment by 60.5, 52.1 and 30.1% at 1, 3, and 10 d of treatment, respectively (Fig. 8A). The relative expression levels of PIN2 in Al3+ treatment were also significantly up-regulated by 114.1, 52.3 and 7.7% at 1, 3, and 10 d, respectively (Fig. 8C), whereas the expression levels of PIN1 did not show a significant increase within the first 3 d but exhibited a significant increase at 10 d (Fig. 8B). The relative transcription levels of the three genes were all up-regulated after exogenous application of IAA in both -Al + IAA and Al + IAA treatments compared with -Al treatment, but there was no significant difference in the expression level of the three genes among the treatments of -Al + IAA, Al + IAA and Al (Fig. 8A–C).
Figure 8.
Expression of AUX1, PIN1 and PIN2 in root tips (0–10 mm) (A–C) of alfalfa seedlings grown for 1, 3 and 10 days on ½-strength Hoagland’s medium (pH 4.5) in the treatments of no aluminium (-Al) and aluminium stress (Al) (100 μM AlCl3) aluminium with additional IAA (Al + IAA) (100 μM AlCl3 + IAA) and -Al with additional IAA (-Al + IAA). Data are means ± SE of three replicates. Bars with different letters indicate a significant difference at P < 0.05 (least significant difference test).
Discussion
Al3+ inhibition of IAA synthesis in apical buds
Various environmental and endogenous signals can be integrated into changes of auxin distribution through their effects on local auxin biosynthesis10,21. When alfalfa was exposed to Al3+-stressed conditions, IAA concentrations in apical buds and root tips were significantly decreased; the IAA concentrations in root tips in both -Al and Al treatments decreased to a very low level after the apical buds were removed. These results indicate that the synthesis of IAA is mainly in apical buds and strongly decreased by Al3+ stress, which leads to the reduction of IAA concentration in root tips.
Mg2+ is an important cofactor of phenylpyruvate decarboxylase, which is involved in the biosynthesis of IAA22. Zn2+ is necessary for the formation of IAA from tryptophan, one of the essential ingredients for IAA synthesis, and its concentrations indirectly affect the IAA content in plants23. The two nutrients are also extremely important for maintaining membrane function, cell extension and antioxidant enzyme activities24. Al3+ stress interfered with uptake or transport of nutrients and caused a decrease of Mg content in plant roots of maize (Zea mays), rice (Oryza.sativa), wheat (Triticum aestivum) and alfalfa6,24,25. Exogenous application of Mg2+ in culture medium could alleviate Al3+ toxicity in many plant species26; meanwhile, up-regulation of Mg2+ transporter genes, particularly those for high-affinity Mg2+ transporters, could contribute to amelioration of Al3+ toxicity by enhancing Mg2+ uptake in Arabidopsis27. The proposed mechanisms for Al3+ toxicity alleviation by Mg2+ ions include (i) increase of organic acid synthesis and exudation; (ii) reduction in Al3+ saturation at the apoplastic exchange sites; and (iii) decrease of Al3+ activity at the root cell plasma membrane surface, and restored plasma membrane H+-ATPase activity26,28. In present study, the absence of Mg2+ and Zn2+ resulted in a significant decrease in IAA concentration in root tips of Al3+-stressed alfalfa. On the other hand, application of Mg2+ and Zn2+ significantly increased IAA concentrations in root tips and also enhanced root growth, indicating that Al3+-induced deficiency of Mg2+ and Zn2+ in roots could be associated with the reduction of IAA synthesis in apical buds. The effect of Mg2+ on alleviating Al3+-induced IAA reduction in root tip was higher than Zn2+, The IAA concentrations in root tips under Al3+ stress was 6.5-fold and 2.5-fold higher in treatments of 25 μM and 50 μM Mg2+ in comparison with treatments of 25 μM and 50 μM Zn2+, respectively. The function of the Mg2+ in increasing the IAA concentration in Al3+-stressed root tip may associate with increase22 of IAA biosynthesis and decrease of Al3+ activity on the root cell plasma membrane surface reflected by a normal cell structure and a convenient array of IAA fluorescence signals in Al3+-stressed root tips after exogenous application IAA.
Al3+ inhibition of IAA transport and disruption of IAA distribution in root tip
IAA is transported from auxin-synthesized young shoot tissues via phloem to the root tip and is then redirected to the epidermis and outer cortex10,29. Inhibition of basipetal auxin flow disturbs auxin distribution in plants and causes adverse effects on root growth and morphology10. In the present study, the ratio of IAA concentration between root tip and shoot base was 2–6 times lower in the presence Al3+ than in the absence of Al3+; meanwhile, after exogenous application of IAA to plants with or without apical buds, the increased degrees of IAA accumulation in root tips were lower in the plants treated with Al3+ than in those with no Al3+. In addition, the distribution of fluorescence signals in root tips of Al3+-stressed alfalfa was disturbed; the dotted lines of fluorescence signals were not clear and were discontinuous in the endodermis, the cortex (close to the endodermis) and the stele of the TZ and distal MZ. These two results and the phenomenon overall strongly demonstrated that Al3+ inhibited IAA transport from shoots to root tips. As a result, a higher amount of IAA accumulated in the root base, but a lower amount accumulated in root tips.
Although Al3+ stress caused a reduction of IAA concentration in the root tip, stronger IAA fluorescence signals were presented in the apical MZ, including the quiescent centre (QC), a highly efficient operation centre of IAA polar transport30,31. As well as fewer IAA signals were observed in the TZ and EZ under Al3+ treatment. Using the isotopic tracer method, Kollmeier et al.7 found that Al3+ treatment increased auxin accumulation in the MZ and DTZ (the distal transition zone) but reduced auxin levels in the EZ of maize root tips. These results indicated that Al3+ stress decreased IAA accumulation in root tips, but the IAA distribution was unbalanced. The meristematic, transition and elongation zones determined primary root development and root growth. Appropriate concentrations of auxin in the distinct zones could promote cell division and root elongation; for example, an external supply of auxin to the EZ was able to partially overcome the inhibition of root growth in maize caused by Al3+ stress7, but excessive auxin will reduce cell division and root elongation. Over-accumulation of IAA in the apical meristem might have a negative effect on cell division and root elongation. The reduction and imbalance of IAA accumulation in root tips of Al3+-stressed alfalfa could together influence cell division, elongation and differentiation in the distinct zones of roots; as a result, restricted root development and growth.
Al3+-induced cellular response in root tips inhibited IAA transport and root growth
High concentrations of Al3+ ions inhibited root development at the organ, tissue, and cellular levels32. The Al3+ ions were located in the epidermis and stele of Al3+-stressed roots20, which could inhibit cell division and elongation33,34, changed cell patterns35 in the epidermis and stele, and even damaged the epidermis and stele36. An obvious characteristic of root development under Al3+-stressed conditions was swollen and short root tips, including fibrous roots. Changes in both cell division and cell elongation could alter root tip morphology. In the present study, the diameter of the stele was decreased, while the cortex width was enlarged due to cell enlargements under Al3+ stress, which directly caused swelling of the root tip. The cell enlargement was attributed to the Al3+-induced vacuole enlargements. Vacuole enlargement might be necessary for plants to store excessive Al3+ and adapt to conditions of Al3+ stress37,38, but this change might disturb root functions such as nutrient absorption, and reduced root growth; thus, vacuole enlargement might be an important characteristic of plant Al3+ toxicity.
In addition to the change in cross-section structure of cells, the longitudinal cell arrangement was disordered, the cell shapes were distorted from a rectangle into an irregular shape, and the cell size was enlarged in the cortex and even in the stele of the TZ and EZ. The cell structure changes in the longitudinal direction coincided with the distribution of IAA fluorescence signal intensities in the longitudinal direction of the cortex and stele; in which, the dotted lines of IAA fluorescence signals were discontinuous and disordered. These longitudinal changes in cell structure might inhibit IAA down-transport from the upper root to root tips through the stele, as well as backflow through the epidermis and outer cortex cells and lead to an imbalance in IAA accumulation in root tips. Meanwhile, Al3+ stress promoted differentiation of the root meristematic zone and shorted the length of the MZ, which led to an early appearance of the TZ and EZ, as well as the cells in the TZ and EZ were seriously enlarged. Doncheva et al.39 reported that Al3+ ions could inhibit cell division in the proximal meristem (250–800 μm from the tip), but stimulated cell division in the elongation zone of the root tip. Thus, the meristematic zone was very sensitive to Al3+, and the cell division inhibition in MZ directly reduced the amount of new cell supplement in roots, as a consequence, fundamentally restricted root elongation.
Callose (β-1,3 binding glucan), a sensitive marker of Al3+ sensitivity, was quickly induced in the epidermis and cortex in Al3+-stressed plants. It was deposited along the plasmodesmata sleeve and blocked symplastic transport and communication5,36,40. The Al-induced callose accumulation in shoots and root tips blocked auxin movement down the stele to the root tips as well as the backward flow to the upper root in the epidermis and outer cortex cell, leading to a disturbed IAA distribution in root tips.
The accumulation of auxin in the root tip is mediated by its biosynthesis and transport10,29. Polar auxin transport is the central rate-limiting component of auxin transport29,41. Polar auxin transport in the root epidermis requires PIN2 and AUX141,42. PIN2 plays a pivotal role in mediating the backward (towards the root base) auxin flow in the epidermis and outer cortex of cells43. The AUX1 gene encodes a transmembrane protein and is believed to be associated with the influx of auxin across the plasma membrane42. Transcriptional analysis demonstrated that PIN2 and AUX1 were up-regulated in root tips exposed to Al3+, positively mediating polar auxin transport from the QC of root tips to the upper root, leading to a higher IAA accumulation in the epidermis and cortex of the meristematic zone; this was reflected by higher intensity of IAA fluorescence signals. Because the cell structure and arrangement in the cortex of the TZ and EZ were changed, and aluminum-induced cell change would alter cell cytoskeleton8, the auxin transport within both cells and inter-cellularly might be affected35,44. Therefore, the IAA fluorescence signals discontinued in the cortex in the TZ and the backward IAA transport was blocked, leading to a higher auxin accumulation in the meristematic zone.
PIN1 is another gene functioning in polar auxin transport and helps auxin flow from apical buds to the root tips through the stele10. Transcription of this gene was up-regulated in Al3+-stressed roots in the present study. This finding suggests that the PIN1 regulation of polar auxin transport through the stele was not affected by Al3+ stress in alfalfa and that the reduction of IAA transport through the stele to root tips may be regulated by other mechanisms in which the Al3+-induced cellular changes in arrangement, shape, size, maturity, inner structure and components would be important mechanisms for inhibiting IAA transport in root tips.
Exogenous application of IAA alleviated Al3+-induced inhibition of plant growth by protecting cell structure in root tips
Growth reduction due to excess Al3+ is a common feature in most plant species2. In the present study, Al3+ caused a reduction in root length, root weight, root activity, and total chlorophyll content in leaves. This was in agreement with several previous studies45. The Al3+-induced inhibition of root growth and damage in physiology, however, was significantly alleviated by exogenous application of IAA. Accumulation of IAA in root tips is essential for root growth in conditions of excessive metal stress46,47. Exogenous application of auxin alleviated cadmium toxicity in Arabidopsis due to its compensation for the lack of auxin in the root tips48. Exogenous application of IAA protected the cell structures of Al3+-stressed root tips (cell arrangement, cell shape and cell size); consequently, it restored the convenient array of IAA fluorescence signals in the form of a dotted line and balanced the distribution of IAA fluorescence signals in different zones of the root tip. Those results suggested that the Al3+-induced inhibition of alfalfa growth could be alleviated by auxin through reducing the cell structure damage in root tips.
In conclusion, our results demonstrated that IAA played a key role in alfalfa responses to Al3+ stress. IAA synthesis and transport in alfalfa was sensitive to Al3+ stress, as exhibited by the quick decrease in IAA concentrations in apical buds, shoot base and root tips of alfalfa seedlings in response to Al3+ stress. The significant reduction of IAA accumulation in apical buds and root tips, as well as the imbalance of IAA distribution among the meristematic zone, transition zone and elongation zone of Al3+-stressed root tips may be the fundamental factors attributed to the restriction of plant growth by Al3+ stress. Al3+ stress caused serious damage to the cell structure in terms of cell arrangement, shape, size, maturity, inner structure and components, which strongly disturbed IAA transport in root tips. Moreover, exogenous application of IAA could partly alleviate this disturbance, restore the convenient array of IAA fluorescence signals, enhance IAA accumulation in root tips, and increase root growth.
Material and Methods
Plant materials and growth conditions
An Al3+-tolerant alfalfa genotype, WL-525HQ49, was obtained from the Chinese National Seed Group Corporation, Ltd. and was used in this study. Seeds were soaked in distilled water for 1 h and then germinated on a filter paper moistened regularly with ½-strength Hoagland’s nutrient solution in a growth chamber at a temperature of 25 °C. After six days, uniform seedlings were transplanted to a foam board (12 holes/plate; 6 seedlings/hole) floating on an aerated ½-strength Hoagland’s nutrient solution (pH 5.8) in plastic containers. The composition of Hoagland’s nutrient solution is 2.5 mM KNO3, 0.5 mM NH4NO3, 1 mM MgSO4, 0.5 mM KH2PO4, 2 mM Ca(NO3)2, 25 μM FeSO4, 25 μM Na2-EDTA, 2.5 μM KI, 50 μM MnSO4, 50 μM H3BO3, 0.05 μM CuSO4, 15 μM ZnSO4, 0.5 μM NaMoO4 and 0.05 μM CoCl2. Solutions were renewed every 2 d, and all seedlings were grown in growth chambers with a temperature of 25/20 °C (day/night), a 14-h photoperiod, photon flux density of 400 μmol m−2 s−1 and a relative humidity of 65%. Four days after transplanting (10 d after sowing), seedlings were used for the following treatments in the same growth chambers.
Treatments and Experimental Design
For aluminium toxicity treatments, seedlings were treated with ½-strength Hoagland’s solution (pH 4.5) supplemented with 0 (-Al) or 100 μM AlCl3 (Al) (Supplementary Fig. 1) for 1, 3 and 10 d, and then plant samples were collected for further analysis. For root tip (1–10 mm) collection, both the main root tips and the secondary root tips were collected. For IAA treatments, plants exposed to 0 (-Al) or 100 μM AlCl3 (Al) were foliar sprayed with 2 ml of 6 mg L−1 IAA (Sigma, MO, USA) every two days, which comprised the -Al + IAA and Al + IAA treatments, and the control plants were sprayed with 2 ml water (pH 6.0). The 6 mg L−1 IAA concentration used for foliar spraying was selected from a preliminary experiment showing positive effects on mitigating Al3+ stress50. After 1, 3 and 10 d of IAA treatment, plant samples were collected for further analysis.
For microscopy study and immunohistochemical analysis of IAA in root tips, the alfalfa seeds were germinated in ½-strength Hoagland’s nutrient solution for 60 h and then supplemented with 0 (-Al) or 100 μM AlCl3 (Al) for 1, 3, and 10 days; the root tips (0–8 mm) were then cut for the microscopy study and immunohistochemical analysis.
In the apical buds removal experiment, to analyze the effect of Al3+ stress on IAA synthesis in apical buds, seedlings were divided into two groups: apical bud intact and apical bud removed. Apical buds of seedlings in the latter group were removed before being treated with aluminium and IAA. Each group was treated with aluminium and IAA using the method previously described for 24 h and was then sampled for IAA measurement.
To determine whether the deficiency of Zn2+ and Mg2+ in Al3+-stressed alfalfa influenced auxin synthesis, we designed the following treatments. After the first leaf of seedlings emerged under the growth condition of ½-strength Hoagland’s nutrient solution, roots of the seedlings were washed with deionized water and grown for 12 h in deionized water. Seedlings were then grown for 24 h in 0.5 mM Ca2+ solution (pH 4.5) with 0 (-Al) or 100 μM AlCl3 (Al). Among the Al3+ treatments, some of the plants received 25 μM or 50 μM MgCl2 for the Mg2+ treatment, and 25 μM or 50 μM ZnCl2 for the Zn2+ treatment.
Physiological analysis
After 1, 3 and 10 d of treatment with aluminium and IAA, 10 plants from each container were separated into shoots and roots. The fresh weight of shoots was measured. Roots were blotted dry with paper towels and then weighed to determine the root fresh weight. Root length was measured from the root tip to the base of the main root. Membrane lipid peroxidation, expressed as malondialdehyde (MDA) content, was determined using the thiobarbituric acid (TBA) protocol51. Chlorophyll content was estimated according to the procedure described by Porra et al.52. Root activity was measured using the triphenyltetrazolium chloride (TTC) reduction method as described by Islam et al.53.
Microscopy investigation
The collected root tips were immediately fixed in 2.5% (v/v) glutaraldehyde and 4% paraformaldehyde (Electron Microscopy Sciences) in PBS, pH 7.2, for 48 h at 4 °C. Samples were then washed with PBS, dehydrated in a graded ethanol series, and embedded in LR White resin (Ted Pella Inc.). Transverse and longitudinal sections (1 μm) were cut with a diamond knife on a Leica EM UC6 ultramicrotome (Leica Mikrosysteme). For Toluidine Blue O staining, 1-μm semi-thin sections were placed onto glass slides, stained with 1% (w/v) Toluidine Blue O (with 1% [w/v] sodium borate) for 5 min, and observed under a microscope (Olympus IX71, Olympus Optical Co. Ltd, Tokyo, Japan). The meristem length of the root tips was measured from the quiescent centre to the cortical cells, which are rapidly elongated, as described by Blilou et al.41. The meristem width was measured in the middle of the meristematic zone. The results presented are average values of more than 30 seedlings per treatment from three independent experiments.
Immunohistochemical Analysis
Auxin immunolocalization was performed as described by Nishimura et al.54 and Watanabe et al.55. Excised root tips were immediately fixed in 4% 1-ethyl-3-(dimethylaminopropyl)-carbodiimide hydrochloride (EDAC) and 4% paraformaldehyde for 24 h at 4 °C. Samples were then dehydrated in a graded ethanol series, infiltrated with xylene, and embedded in paraffin wax using conventional methods. Material sections were cut to thicknesses of 10μm and pasted onto poly-lysine slides. The sections were then treated in a blocking solution consisting of 10 mM PBS/Tween-20/glycine/bovine serum albumin (BSA) (93.5/0.1/1.5/5, v/v/w/w) for 45 min, rinsed in regular-salt rinse solution (RSR) containing 10 mM PBS/Tween-20/BSA/NaCl (98/0.1/0.8/0.88, v/v/w/w) for 5 min, and treated with an additional solution containing 10 mM PBS/Triton X-100/BSA (99/0.8/0.1, v/v/w). Subsequently, the tissue sections were incubated in the primary rabbit polyclonal antibody to IAA (Abcam, UK) in the dark at 37◦C for 30 min. They were then washed in a high-salt rinse solution consisting of 10 mM PBS/Tween-20/BSA/NaCl (97/0.1/0.1/2.9, v/v/w/w). After several rinses in RSR and PBS, the tissue sections were incubated with the secondary antibody (anti-rabbit IgG Alexa Fluor 488-conjugate; Protein tech Group, Chicago, IL, USA) and were detected immune signals at 495 nm. Fluorescence was detected with a laser-scanning confocal microscope (Leica TCS SP5II).
Indole-3-acetic acid was extracted and purified according to the method described by Yan et al.56 with some modifications. A 0.5-g fresh sample was ground in liquid nitrogen, and then 5 ml pre-cooled (−20 °C) 80% methanol (containing 10 mg L−1 BHT, w/v) was added to the sample. After overnight extraction at −20 °C, solids were separated by centrifugation (20,000 g, 15 min) and re-extracted for 30 min in an additional 5 ml of the same extraction solution. Then, the supernatants were concentrated to 2.0 ml and were passed through a Sep-Pak Plus C18 cartridge (SepPak Plus, Waters, USA). After washing with 3 ml 20% methanol containing 1% (v/v) acetic acid, the cartridges were eluted with 1 ml pure methanol for HPLC measurement. The determination was repeated at least three times. Analyses were carried out on a Shimadzu LC-10A HPLC (Shimadzu, Kyoto, Japan) system equipped with an SPD-10Avp detector.
Callose content was measured according to the method described by Jones et al.36
Gene expression analysis
Three genes related to auxin transport were examined with quantitative PCR (Q-PCR): auxin transporter-like protein (AUX1), and auxin efflux carrier components 1 and 2 (PIN1 and PIN2). Total RNA was isolated using Trizol (Invitrogen). The first-strand cDNA was synthesized with a PrimeScript RT reagent kit (Takara) using oligo (dT) primers. For real-time RT-PCR analysis, 1 μl 10-fold-diluted cDNA was used for quantitative analysis of gene expression performed with SYBR Premix ExTaq (Takara) with the specific primers (Table 2).
Table 2. Primer sequences used for Q-PCR.
| Gene | Forward sequence | Reverse sequence | GenBank accession |
|---|---|---|---|
| Elongation factor 1-α (EF1-α) | 5′-GCACCAGTGCTCGATTGC-3′ | 5′-TCGCCTGTCAATCTTGGTAACAA-3′ | XM_003618727 |
| Auxin transporter-like protein (AUX1) | 5′-GACTACATACACTGCTTGGT-3′ | 5′-AGTGGCAGAAGGAATCGTTA-3′ | XM_003623180 |
| Auxin efflux carrier components 2 (PIN2) | 5′-GATGCTGGTCTTGGAATGGC-3′ | 5′-ATTGCTATTGAGGTTGCCGC-3′ | XM_003609978 |
| Auxin efflux carrier components 1 (PIN1) | 5′-GCTTCACCTGTCTCTGATGTG-3′ | 5′-CCACCTTTCTCACCTCCTTC-3′ | XM_003619781 |
Reactions were run on an ABI PRISM 5700 Sequence Detector (Applied Biosystems). Elongation factor 1-α (EF1-α) was selected as an endogenous control. Each gene was examined three times. All PCR efficiencies were above 95%. Sequence Detection Software (version 1.3, Applied Biosystems) results were exported as tab-delimited text files and imported into Microsoft Excel (Redmond, WA, USA) for further analysis. The median coefficient of variation (based on calculated quantities) of duplicated samples was 6%.
Statistical analysis
The data were subjected to analysis of variance (ANOVA), and the least significant difference (LSD) test was employed to determine differences among the treatments at P = 0.05 levels.
Additional Information
How to cite this article: Wang, S. et al. Aluminium-induced reduction of plant growth in alfalfa (Medicago sativa) is mediated by interrupting auxin transport and accumulation in roots. Sci. Rep. 6, 30079; doi: 10.1038/srep30079 (2016).
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31572451 and No. 31272198), the Science and Technology Commission of Shanghai (No. 13JC1403200 and 15391912400), and Committee of Shanghai agriculture (No. 2013-5-10).
Footnotes
Author Contributions S.W. conceived, designed and performed all experiments, analyzed the data and wrote the manuscript. X. R. helped in preparing Figures 4–6. G.W. helped in preparing Figure 2. B.H. revised the manuscript. Y.A. and P.Z. monitored the experimental work and critically commented on the manuscript. All authors read and approved the final manuscript.
References
- Kochian L. V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Biol. 46, 237–260 (1995). [Google Scholar]
- Kochian L. V., Hoekenga O. A. & Piñeros M. A. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 55, 459–493 (2004). [DOI] [PubMed] [Google Scholar]
- Ryan P. R., Ditomaso J. M. & Kochian L. V. Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J. Exp. Bot. 44, 437–446 (1993). [Google Scholar]
- Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. Int. Rev.Cytol. 200, 1–46 (2000). [DOI] [PubMed] [Google Scholar]
- Sivaguru M., Baluška F., Volkmann D., Felle H. H. & Horst W. J. Impacts of aluminum on the cytoskeleton of the maize root apex. Short-term effects on the distal part of the transition zone. Plant Physiol. 119, 1073–1082 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst W. J., Wang Y. & Eticha D. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann. Bot-London 106, 185–197 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmeier M., Felle H. H. & Horst W. J. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum? Plant Physiol. 122, 945–956 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaguru M. et al. Aluminum-induced 1→ 3-β-d-glucan inhibits cell-to-cell trafficking of molecules through plasmodesmata. A new mechanism of aluminum toxicity in plants. Plant Physiol. 124, 991–1006 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y. Q. et al. Elevation of NO production increases Fe immobilization in the Fe-deficiency roots apoplast by decreasing pectin methylation of cell wall. Sci. Rep. 5, 10746 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S. & Friml J. Auxin: a trigger for change in plant development. Cell 136, 1005–1016 (2009). [DOI] [PubMed] [Google Scholar]
- Yu Y. et al. Inhibition of ethylene production by putrescine alleviates aluminium-induced root inhibition in wheat plants. Sci. Rep. 6, 18888 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. et al. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant soil 326, 321–330 (2010). [Google Scholar]
- Hu Y. F. et al. Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J. Plant Physiol. 170, 965–975 (2013). [DOI] [PubMed] [Google Scholar]
- Yuan H. M. & Huang X. Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signaling in Arabidopsis. Plant cell environ. 39, 120–135 (2015). [DOI] [PubMed] [Google Scholar]
- Krishnamurthy A. & Rathinasabapathi B. Auxin and its transport play a role in plant tolerance to arsenite-induced oxidative stress in Arabidopsis thaliana. Plant cell environ. 36, 1838–1849 (2013). [DOI] [PubMed] [Google Scholar]
- Yuan H.-M., Xu H.-H., Liu W.-C. & Lu Y.-T. Copper regulates primary root elongation through PIN1-mediated auxin redistribution. Plant Cell Physiol. 54, 766–778 (2013). [DOI] [PubMed] [Google Scholar]
- Nomura T. et al. Copper mediates auxin signalling to control cell differentiation in the copper moss Scopelophila cataractae. J. Exp. Bot. 66, 1205–1213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartel P. & Bouton J. Rhizobium meliloti inoculation of alfalfa selected for tolerance to acid, aluminum-rich soils. Plant soil 116, 283–285 (1989). [Google Scholar]
- Narasimhamoorthy B., Bouton J., Olsen K. & Sledge M. Quantitative trait loci and candidate gene mapping of aluminum tolerance in diploid alfalfa. Theor. Appl. Genet (TAG). 114, 901–913 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang F., Ren X., Huang B. & An Y. Phytotoxicity of aluminum on root growth and indole-3-acetic acid accumulation and transport in alfalfa roots. Environ. Exp. Bot. 104, 1–8 (2014). [Google Scholar]
- Peleg Z. & Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Curr.Opin.Plant Biol. 14, 290–295 (2011). [DOI] [PubMed] [Google Scholar]
- Versees W., Spaepen S., Vanderleyden J. & Steyaert J. The crystal structure of phenylpyruvate decarboxylase from Azospirillum brasilense at 1.5 Å resolution. FEBS J. 274, 2363–2375 (2007). [DOI] [PubMed] [Google Scholar]
- Bybordi A. & Shabanov J. A. Effects of the foliar application of magnesium and zinc on the yield and quality of three grape cultivars grown in the calcareous soils of iran. Not. Bot. Horti. Agrobo. 2, 81–86 (2010). [Google Scholar]
- Silva S. et al. Differential aluminium changes on nutrient accumulation and root differentiation in an Al sensitive vs. tolerant wheat. Environ. Exp. Bot. 68, 91–98 (2010). [Google Scholar]
- An Y., Zhou P., Xiao Q. & Shi D. Effects of foliar application of organic acids on alleviation of aluminum toxicity in alfalfa. J. Plant Nutr. Soil Sc. 177, 421–430 (2014). [Google Scholar]
- Bose J., Babourina O. & Rengel Z. Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 62, 2251–2264 (2011). [DOI] [PubMed] [Google Scholar]
- Deng W. et al. Overexpression of an Arabidopsis magnesium transport gene, AtMGT1, in Nicotiana benthamiana confers Al tolerance. J. Exp. Bot. 57, 4235–4243 (2006). [DOI] [PubMed] [Google Scholar]
- Ahn S. J., Sivaguru M., Osawa H., Chung G. C. & Matsumoto H. Aluminum inhibits the H+-ATPase activity by permanently altering the plasma membrane surface potentials in squash roots. Plant physiol. 126, 1381–1390 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band L. R. et al. Systems analysis of auxin transport in the arabidopsis root apex. Plant Cell 26, 862–875 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk N. M., Jiang K. N. & Feldman L. J. Auxin metabolism in the root apical meristem. Plant physiol. 122, 925–932 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce G., Barlow P. W., Feldman L. J. & Cassab G. I. Auxin and ethylene interactions control mitotic activity of the quiescent centre, root cap size, and pattern of cap cell differentiation in maize. Plant cell environ. 28, 719–732 (2005). [DOI] [PubMed] [Google Scholar]
- Čiamporová M. Morphological and structural responses of plant roots to aluminium at organ, tissue, and cellular levels. Biol. Plantarum 45, 161–171 (2002). [Google Scholar]
- Gunsé B., Garzón T. & Barceló J. Study of aluminum toxicity by means of vital staining profiles in four cultivars of Phaseolus vulgaris L. J. Plant Physiol. 160, 1447–1450 (2003). [DOI] [PubMed] [Google Scholar]
- Bakos F. et al. A cytological study on aluminium-treated wheat anther cultures resulting in plants with increased Al tolerance. Plant breeding 127, 235–240 (2008). [Google Scholar]
- Arroyave C., Barceló J., Poschenrieder C. & Tolrà R. Aluminium-induced changes in root epidermal cell patterning, a distinctive feature of hyperresistance to Al in Brachiaria decumbens. J. Inorg. Biochem. 105, 1477–1483 (2011). [DOI] [PubMed] [Google Scholar]
- Jones D. L., Blancaflor E. B., Kochian L. V. & Gilroy S. Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant cell environ. 29, 1309–1318 (2006). [DOI] [PubMed] [Google Scholar]
- Kochian L. V., Piñeros M. A., Liu J. & Magalhaes J. V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 66, 571–598 (2015). [DOI] [PubMed] [Google Scholar]
- Čiamporová M. Diverse responses of root cell structure to aluminium stress. Plant Soil 226, 113–116 (2000). [Google Scholar]
- Doncheva S., Amenós M., Poschenrieder C. & Barceló J. Root cell patterning: a primary target for aluminium toxicity in maize. J. Exp. Bot. 56, 1213–1220 (2005). [DOI] [PubMed] [Google Scholar]
- Sun C. et al. Decreasing methylation of pectin caused by nitric oxide leads to higher aluminium binding in cell walls and greater aluminium sensitivity of wheat roots. J. Exp. Bot. 67, 979–989 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blilou I. et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44 (2005). [DOI] [PubMed] [Google Scholar]
- Habets M. E. & Offringa R. PIN-driven polar auxin transport in plant developmental plasticity: a key target for environmental and endogenous signals. New Phytol. 203, 362–377 (2014). [DOI] [PubMed] [Google Scholar]
- Sun P., Tian Q.-Y., Chen J. & Zhang W.-H. Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J. Exp. Bot. 61, 347–356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illéš P. et al. Aluminium toxicity in plants: internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J. Exp. Bot. 57, 4201–4213 (2006). [DOI] [PubMed] [Google Scholar]
- Yang Z.-B. et al. TAA1-Regulated Local Auxin Biosynthesis in the Root-Apex Transition Zone Mediates the Aluminum-Induced Inhibition of Root Growth in Arabidopsis. Plant Cell 114, 2889–2904 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. W. et al. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 154, 810–819 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Rejano E. M. et al. Auxin and ethylene are involved in the responses of root system architecture to low boron supply in Arabidopsis seedlings. Physiol. Plantarum 142, 170–178 (2011). [DOI] [PubMed] [Google Scholar]
- Zhu X. F. et al. Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J. Hazard. Mater. 263, 398–403 (2013). [DOI] [PubMed] [Google Scholar]
- Pan X.-B., Zhu C. & Cheng C. Assessment of techniques for screening alfalfa cultivars for aluminum tolerance. Euphytica 164, 541–549 (2008). [Google Scholar]
- Ren X. Y., Zhou P. & An Y. Effects of exogenous application of IAA on alleviate aluminum toxicity in alfalfa Seedlings. Pratacultural science 7, 1323–1329 (2013). [Google Scholar]
- Peever T. L. & Higgins V. J. Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol. 90, 867–875 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R., Thompson W. & Kriedemann P. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. (BBA)-Bioenergetics 975, 384–394 (1989). [Google Scholar]
- Islam E. et al. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 147, 806–816 (2007). [DOI] [PubMed] [Google Scholar]
- Nishimura T. et al. Immunohistochemical observation of indole-3-acetic acid at the IAA synthetic maize coleoptile tips. Plant Signal. Behav. 6, 2013–2022 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe C. et al. Gravistimulation changes the accumulation pattern of the CsPIN1 auxin efflux facilitator in the endodermis of the transition zone in cucumber seedlings. Plant Physiol. 158, 239–251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H., Wang F., Han D. & Yang G. Simultaneous determination of four plant hormones in bananas by molecularly imprinted solid-phase extraction coupled with high performance liquid chromatography. Analyst 137, 2884–2890 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.