Abstract
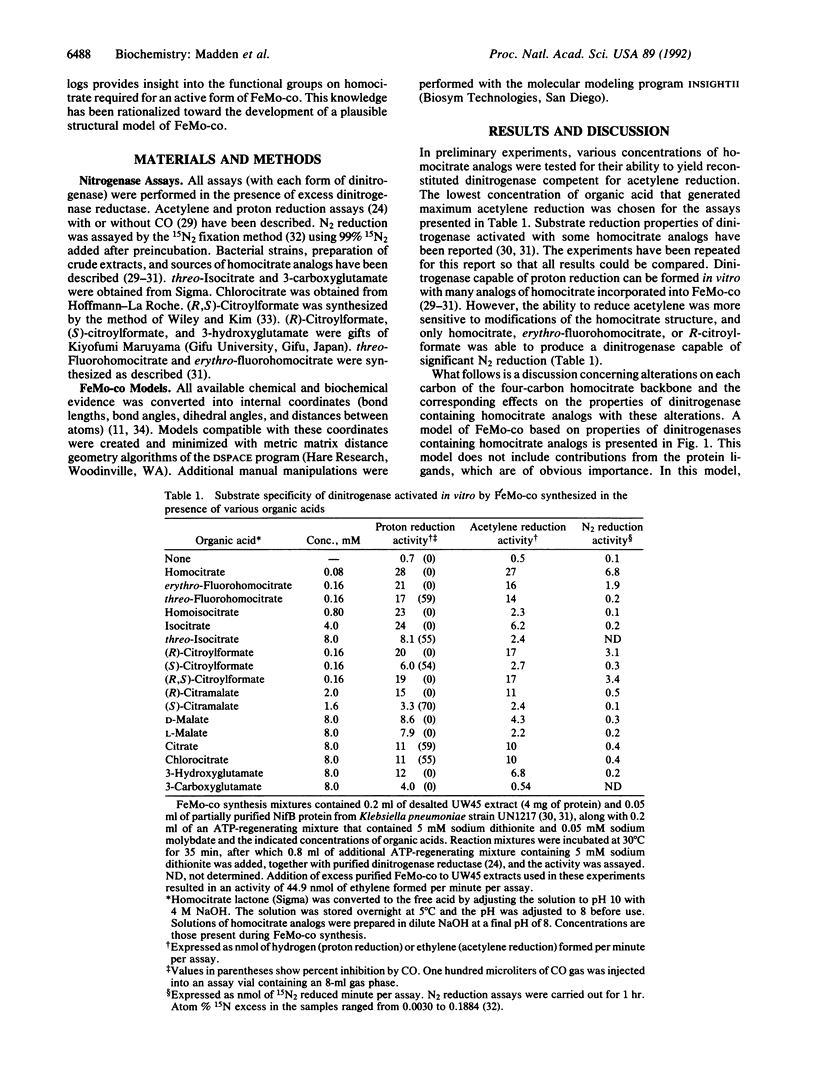
A plausible structure of the iron-molybdenum cofactor of nitrogenase [reduced ferredoxin:dinitrogen oxidoreductase (ATP-hydrolyzing), EC 1.18.6.1] is presented based on altered substrate reduction properties of dinitrogenase containing homocitrate analogs within the cofactor. Alterations on each carbon of the four-carbon homocitrate backbone were correlated with altered substrate reduction properties of dinitrogenase containing these analogs. Altered substrate reduction properties are the basis for a model in which homocitrate is oriented about two cubane metal clusters.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bulen W. A., LeComte J. R. The nitrogenase system from Azotobacter: two-enzyme requirement for N2 reduction, ATP-dependent H2 evolution, and ATP hydrolysis. Proc Natl Acad Sci U S A. 1966 Sep;56(3):979–986. doi: 10.1073/pnas.56.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Nitrogenase and nitrogenase reductase associate and dissociate with each catalytic cycle. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2699–2702. doi: 10.1073/pnas.75.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes T. R., McLean P. A., Smith B. E. Nitrogenase from nifV mutants of Klebsiella pneumoniae contains an altered form of the iron-molybdenum cofactor. Biochem J. 1984 Jan 1;217(1):317–321. doi: 10.1042/bj2170317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover T. R., Imperial J., Liang J. H., Ludden P. W., Shah V. K. Dinitrogenase with altered substrate specificity results from the use of homocitrate analogues for in vitro synthesis of the iron-molybdenum cofactor. Biochemistry. 1988 May 17;27(10):3647–3652. doi: 10.1021/bi00410a019. [DOI] [PubMed] [Google Scholar]
- Hoover T. R., Imperial J., Ludden P. W., Shah V. K. Homocitrate is a component of the iron-molybdenum cofactor of nitrogenase. Biochemistry. 1989 Apr 4;28(7):2768–2771. doi: 10.1021/bi00433a004. [DOI] [PubMed] [Google Scholar]
- Hoover T. R., Robertson A. D., Cerny R. L., Hayes R. N., Imperial J., Shah V. K., Ludden P. W. Identification of the V factor needed for synthesis of the iron-molybdenum cofactor of nitrogenase as homocitrate. 1987 Oct 29-Nov 4Nature. 329(6142):855–857. doi: 10.1038/329855a0. [DOI] [PubMed] [Google Scholar]
- Huynh B. H., Münck E., Orme-Johnson W. H. Nitrogenase XI: Mössbauer studies on the cofactor centers of the MoFe protein from Azotobacter vinelandii OP. Biochim Biophys Acta. 1979 Jan 25;576(1):192–203. doi: 10.1016/0005-2795(79)90497-5. [DOI] [PubMed] [Google Scholar]
- Imperial J., Hoover T. R., Madden M. S., Ludden P. W., Shah V. K. Substrate reduction properties of dinitrogenase activated in vitro are dependent upon the presence of homocitrate or its analogues during iron-molybdenum cofactor synthesis. Biochemistry. 1989 Sep 19;28(19):7796–7799. doi: 10.1021/bi00445a040. [DOI] [PubMed] [Google Scholar]
- Ljones T., Burris R. H. Nitrogenase: the reaction between the Fe protein and bathophenanthrolinedisulfonate as a probe for interactions with MgATP. Biochemistry. 1978 May 16;17(10):1866–1872. doi: 10.1021/bi00603a010. [DOI] [PubMed] [Google Scholar]
- Madden M. S., Kindon N. D., Ludden P. W., Shah V. K. Diastereomer-dependent substrate reduction properties of a dinitrogenase containing 1-fluorohomocitrate in the iron-molybdenum cofactor. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6517–6521. doi: 10.1073/pnas.87.17.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Levy M. A., Orme-Johnson W. H. Metal and sulfur composition of iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci U S A. 1983 Jan;80(1):147–150. doi: 10.1073/pnas.80.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings J., Shah V. K., Chisnell J. R., Brill W. J., Zimmermann R., Münck E., Orme-Johnson W. H. Novel metal cluster in the iron-molybdenum cofactor of nitrogenase. Spectroscopic evidence. J Biol Chem. 1978 Feb 25;253(4):1001–1004. [PubMed] [Google Scholar]
- Robinson A. C., Dean D. R., Burgess B. K. Iron-molybdenum cofactor biosynthesis in Azotobacter vinelandii requires the iron protein of nitrogenase. J Biol Chem. 1987 Oct 15;262(29):14327–14332. [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Imperial J., Ugalde R. A., Ludden P. W., Brill W. J. In vitro synthesis of the iron-molybdenum cofactor of nitrogenase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1636–1640. doi: 10.1073/pnas.83.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]


