Abstract
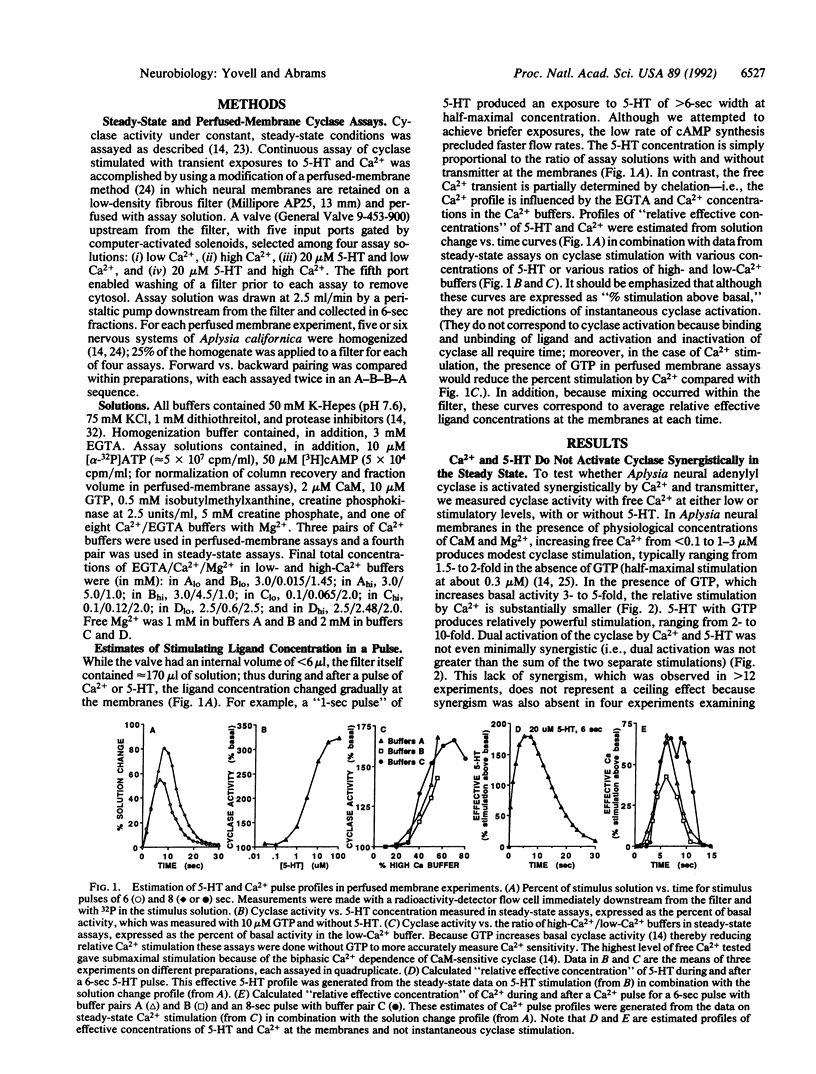
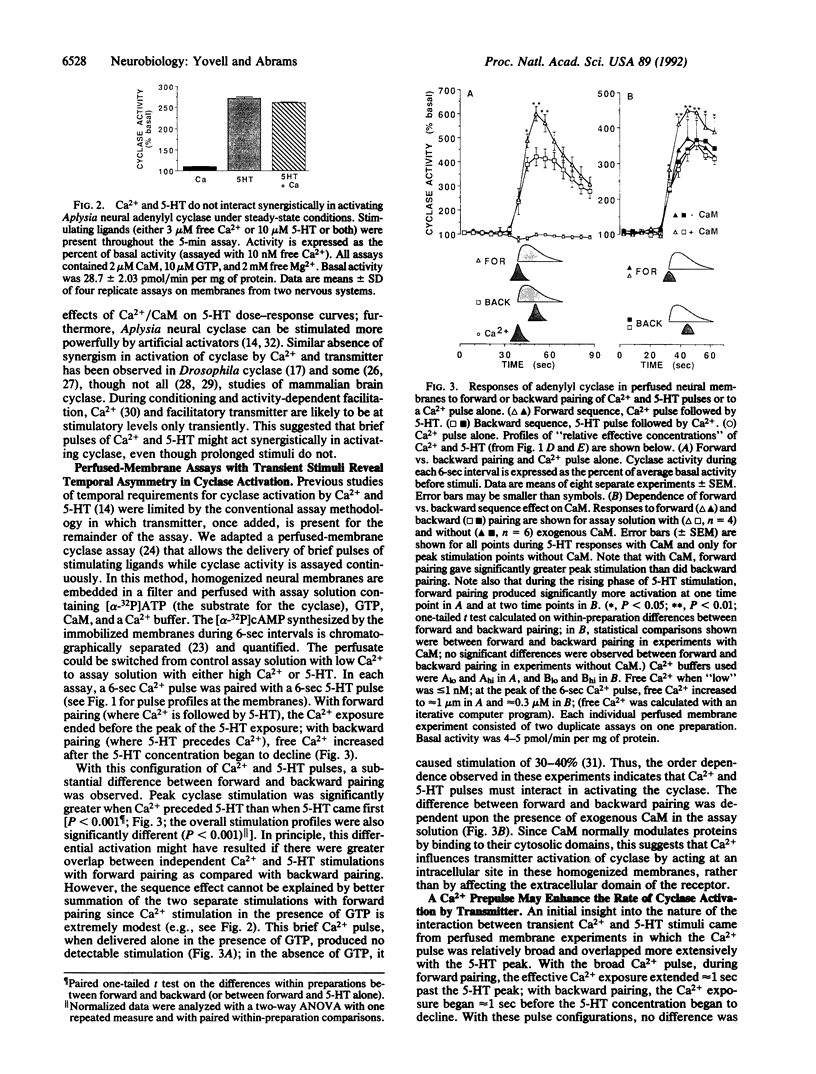
Cellular experiments have suggested that during classical conditioning of the gill and siphon withdrawal reflex of Aplysia, adenylyl cyclase may serve as a molecular site of convergence for Ca2+ and serotonin (5-hydroxytryptamine; 5-HT), the cellular representations of the conditioned and unconditioned stimuli (CS and US). We explored the possible molecular basis of the behavioral requirement that the CS and US be paired within a narrow time window and in the appropriate order. To examine the temporal interactions of brief pulses of Ca2+ and 5-HT in stimulating Aplysia neural cyclase, we used a perfused-membrane cyclase assay. When brief pulses of Ca2+ and 5-HT were paired, cyclase activation depended upon the sequence of the pulses: peak cyclase activation was greater when the Ca2+ pulse immediately preceded the 5-HT pulse. Examination of the rising phase of 5-HT stimulation suggested that a Ca2+ prepulse might accelerate the onset of activation by 5-HT, without affecting the final level of activation achieved with prolonged 5-HT exposure. The observed interactions between Ca2+ and transmitter in activating cyclase could contribute importantly to the temporal requirements of conditioning for CS-US pairing.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams T. W. Activity-dependent presynaptic facilitation: an associative mechanism in Aplysia. Cell Mol Neurobiol. 1985 Jun;5(1-2):123–145. doi: 10.1007/BF00711089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrams T. W., Kandel E. R. Is contiguity detection in classical conditioning a system or a cellular property? Learning in Aplysia suggests a possible molecular site. Trends Neurosci. 1988 Apr;11(4):128–135. doi: 10.1016/0166-2236(88)90137-3. [DOI] [PubMed] [Google Scholar]
- Abrams T. W., Karl K. A., Kandel E. R. Biochemical studies of stimulus convergence during classical conditioning in Aplysia: dual regulation of adenylate cyclase by Ca2+/calmodulin and transmitter. J Neurosci. 1991 Sep;11(9):2655–2665. doi: 10.1523/JNEUROSCI.11-09-02655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier L., Castellucci V. F., Kandel E. R., Schwartz J. H. Facilitatory transmitter causes a selective and prolonged increase in adenosine 3':5'-monophosphate in sensory neurons mediating the gill and siphon withdrawal reflex in Aplysia. J Neurosci. 1982 Dec;2(12):1682–1691. doi: 10.1523/JNEUROSCI.02-12-01682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H., Spira M. E., Kandel E. R., Siegelbaum S. A. Facilitatory and inhibitory transmitters modulate calcium influx during action potentials in aplysia sensory neurons. Neuron. 1990 Oct;5(4):487–499. doi: 10.1016/0896-6273(90)90088-w. [DOI] [PubMed] [Google Scholar]
- Carew T. J., Walters E. T., Kandel E. R. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981 Dec;1(12):1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V. F., Nairn A., Greengard P., Schwartz J. H., Kandel E. R. Inhibitor of adenosine 3':5'-monophosphate-dependent protein kinase blocks presynaptic facilitation in Aplysia. J Neurosci. 1982 Dec;2(12):1673–1681. doi: 10.1523/JNEUROSCI.02-12-01673.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Dash P. K., Karl K. A., Colicos M. A., Prywes R., Kandel E. R. cAMP response element-binding protein is activated by Ca2+/calmodulin- as well as cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5061–5065. doi: 10.1073/pnas.88.11.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Zvi S. Adenylate cyclase in the Drosophila memory mutant rutabaga displays an altered Ca2+ sensitivity. Neurosci Lett. 1984 Jun 15;47(2):119–124. doi: 10.1016/0304-3940(84)90416-6. [DOI] [PubMed] [Google Scholar]
- Eliot L. S., Dudai Y., Kandel E. R., Abrams T. W. Ca2+/calmodulin sensitivity may be common to all forms of neural adenylate cyclase. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9564–9568. doi: 10.1073/pnas.86.23.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanzman D. L., Mackey S. L., Hawkins R. D., Dyke A. M., Lloyd P. E., Kandel E. R. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989 Dec;9(12):4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith B. A., Abrams T. W. Reversal of synaptic depression by serotonin at Aplysia sensory neuron synapses involves activation of adenylyl cyclase. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9021–9025. doi: 10.1073/pnas.88.20.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. Physiological mechanisms underlying long-term potentiation. Trends Neurosci. 1988 Apr;11(4):156–162. doi: 10.1016/0166-2236(88)90142-7. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Carew T. J., Kandel E. R. Effects of interstimulus interval and contingency on classical conditioning of the Aplysia siphon withdrawal reflex. J Neurosci. 1986 Jun;6(6):1695–1701. doi: 10.1523/JNEUROSCI.06-06-01695.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. D., Schacher S. Identified facilitator neurons L29 and L28 are excited by cutaneous stimuli used in dishabituation, sensitization, and classical conditioning of Aplysia. J Neurosci. 1989 Dec;9(12):4236–4245. doi: 10.1523/JNEUROSCI.09-12-04236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. R., Levy W. B. Insights into associative long-term potentiation from computational models of NMDA receptor-mediated calcium influx and intracellular calcium concentration changes. J Neurophysiol. 1990 May;63(5):1148–1168. doi: 10.1152/jn.1990.63.5.1148. [DOI] [PubMed] [Google Scholar]
- Levin L. R., Han P. L., Hwang P. M., Feinstein P. G., Davis R. L., Reed R. R. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992 Feb 7;68(3):479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S. Genetic dissection of Drosophila adenylate cyclase. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5992–5996. doi: 10.1073/pnas.82.17.5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M. S., Sziber P. P., Quinn W. G. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984 May;37(1):205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Mackey S. L., Kandel E. R., Hawkins R. D. Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci. 1989 Dec;9(12):4227–4235. doi: 10.1523/JNEUROSCI.09-12-04227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsukari N., Hanai H., Matsunaga T., Fujita M. Synergistic activation of brain adenylate cyclase by calmodulin, and either GTP or catecholamines including dopamine. Brain Res. 1990 Nov 26;534(1-2):170–176. doi: 10.1016/0006-8993(90)90126-v. [DOI] [PubMed] [Google Scholar]
- Ocorr K. A., Walters E. T., Byrne J. H. Associative conditioning analog selectively increases cAMP levels of tail sensory neurons in Aplysia. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2548–2552. doi: 10.1073/pnas.82.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y. Adenylate cyclase assay. Adv Cyclic Nucleotide Res. 1979;10:35–55. [PubMed] [Google Scholar]
- Salter R. S., Krinks M. H., Klee C. B., Neer E. J. Calmodulin activates the isolated catalytic unit of brain adenylate cyclase. J Biol Chem. 1981 Oct 10;256(19):9830–9833. [PubMed] [Google Scholar]
- Treisman G. J., Bagley S., Gnegy M. E. Calmodulin-sensitive and calmodulin-insensitive components of adenylate cyclase activity in rat striatum have differential responsiveness to guanyl nucleotides. J Neurochem. 1983 Nov;41(5):1398–1406. doi: 10.1111/j.1471-4159.1983.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983 Jan 28;219(4583):405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Slow depolarization produced by associative conditioning of Aplysia sensory neurons may enhance Ca2+ entry. Brain Res. 1983 Nov 28;280(1):165–168. doi: 10.1016/0006-8993(83)91186-1. [DOI] [PubMed] [Google Scholar]
- Xia Z. G., Refsdal C. D., Merchant K. M., Dorsa D. M., Storm D. R. Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron. 1991 Mar;6(3):431–443. doi: 10.1016/0896-6273(91)90251-t. [DOI] [PubMed] [Google Scholar]
- Yovell Y., Kandel E. R., Dudai Y., Abrams T. W. Biochemical correlates of short-term sensitization in Aplysia: temporal analysis of adenylate cyclase stimulation in a perfused-membrane preparation. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9285–9289. doi: 10.1073/pnas.84.24.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]