Summary
Interleukin (IL)‐9 is a 28‐30 kDa monomeric glycosylated polypeptide belonging to the IL‐7/IL‐9 family of proteins that bind to a composite receptor consisting of the private receptor IL‐9R and the IL‐2 receptor, gamma (IL‐2RG), a common gamma subunit shared by the receptors of many different cytokines. The IL‐9R is expressed widely and IL‐9 impacts a number of effector cells, such as effector T cells, B cells, innate lymphoid cells, mast cells, polymorphonuclear cells, epithelial cells and smooth muscle cells, playing an important role in regulating inflammatory immunity. The critical role of IL‐9 in promoting cellular and humoral immune responses makes it an important focus of potential therapeutic interventions. Recently, a defined subset of T helper type cells, Th9 cells, has been identified by the potent production of IL‐9. The involvement of the Th9 cell subset has been described in many types of inflammatory diseases, namely atopic diseases, helminth infections, experimental autoimmune encephalomyelitis and ulcerative colitis. In this review, we summarize the IL‐9 biological activities, highlighting roles for IL‐9 and Th9 cells in rheumatoid and psoriatic arthritis, systemic vasculitis, systemic lupus erythematosus and systemic sclerosis.
Keywords: IL‐9, psoriatic arthritis, rheumatoid arthritis, SLE, systemic sclerosis, vasculitis, Th9 cells
Introduction
The role of CD4+ T cells in the pathogenesis of rheumatic diseases has been well established 1, 2. The differentiation of naive CD4(+) T cells into different cell subsets with distinct biological activities is determined largely by their interaction with dendritic cells (DCs) in lymphoid organs. In particular, the development of a particular subtype of functional T cells seems to be related to the interaction with the surrounding microenvironment, which include cellular interactions and the release of specific cytokines and transcription factors 3, 4. Different subsets of CD4+ T cells are defined usually by the specific expression of transcription factors, cytokine receptors and their released cytokines. T helper type 1 (Th1), Th2, Th17, T follicular helper and derived regulatory T cells are well‐known CD4+ T cell subsets 4, 5.
Interleukin (IL)‐9, a cytokine with pleiotropic functions in the immune system 6, 7, was identified originally as a T cell growth factor belonging to the common γ‐chain‐receptor cytokine family. IL‐9 signals through a heterodimeric receptor composed by a specific IL‐9 receptor chain (IL‐9Rα) and the common γ‐chain (also known as IL‐2Rγ) 8. IL‐9Rα is expressed on immune cells and its activation also promotes mast cell growth 8, 9 and accumulation in inflamed tissues, innate lymphoid cells (ILC) survival and B cell immunoglobulin (Ig)E class‐switch recombination 9, 10, 11 (Fig. 1 shows IL‐9R‐expressing cells). IL‐9Rα is also expressed on non‐haematopoietic cells such as airway and intestinal epithelial cells, smooth muscle cells and keratinocytes 12, 13.
Figure 1.
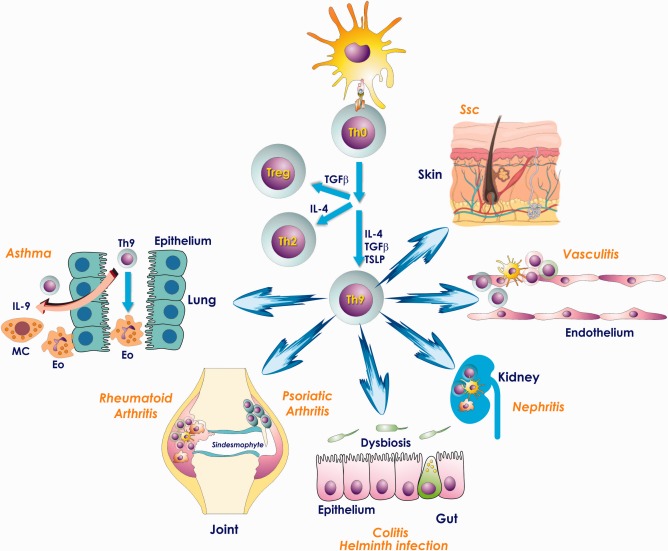
T helper type 9 (Th9) cells develop from naive T cells in the presence of transforming growth factor (TGF)‐β, interleukin (IL)‐4 and thymic stromal lymphopoietin (TSLP). Aberrant Th9 activation may cause the onset and progression of autoimmune and chronic inflammatory diseases such as asthma, rheumatoid and psoriatic arthritis, chronic gut inflammation, kidney diseases, systemic vasculitides and systemic sclerosis. MC = mast cells; Eo = eosinophils; Treg = regulatory T cells.
IL‐9 production was associated first with the Th2 phenotype and many of the preliminary functions of IL‐9 were studied in models of Th2‐associated immunity. Recently, a defined subset of T helper cells called Th9 has been identified by the potent production of IL‐9 14, 15, 16. Th9 cells develop from naive T cells in the presence of transforming growth factor (TGF)‐β, IL‐4 and thymic stromal lymphopoietin (TSLP) (Fig. 1) and require PU.1 and the interferon regulatory factor‐4 (IRF‐4) as specific transcription factors 7, 17.
Although a role of the Th9 cell subset has been described recently in many types of inflammatory diseases (namely, atopic diseases, helminthic infections, experimental autoimmune encephalomyelitis and inflammatory bowel diseases) 18, 19, their role is still largely unknown in the pathogenesis of rheumatic disease. This paper aims to review the evidence indicating that Th9 cells may contribute to the pathogenesis of autoimmune‐related diseases, due to the recent demonstration of a possible role of Th9 T cells and of their related cytokine IL‐9 in rheumatoid arthritis (RA), psoriatic arthritis (PsA), systemic vasculitis, systemic lupus erythematosus (SLE) and systemic sclerosis (SSc) 20, 21, 22, 23, 24, 25, 26, 27, 28 (Fig. 1 shows the possible tissue target of Th9‐mediated inflammation).
IL‐9 signalling
IL‐9 is a protein of 144 amino acids with a secretory signal sequence of 18 amino acids. On target cells, IL‐9 binds to IL‐9R, a heterodimeric protein composed by IL‐9Rα belonging to the haematopoietin superfamily, and the IL‐2R‐γ, a common subunit shared by different cytokine receptors. Binding of IL‐9 with the cognate receptors leads to the activation of signal transducer and activator of transcription‐1 (STAT‐1), STAT‐3 and STAT‐5 29, 30. IL‐9R is expressed on effector T cells but not naive T cells. Th2, Th17 and Th9 display the highest IL‐9R expression. In asthmatic patients IL‐9R is also expressed on mast cells and polymorphonuclear cells 31. Several studies also suggest the expression of IL‐9R on lung and intestinal epithelial cells and on the surface of smooth muscle cells (Fig. 1) 31.
Th9 polarization
The differentiation of Th9 cells requires the activation of IL‐2/STAT‐5 and IL‐4/STAT‐6 signalling and TGF‐β, which acts by inducing redirection of naive T cells from a Th2 to Th9 cell differentiation pathway (in Fig. 1 it is summarized the differentiation pathway of Th9 cells) [7]. It has been demonstrated recently that, in primary cell cultures, the addition of TSLP led to an increase in IL‐9 production from human and mouse Th9 cells, and induced an increase in STAT‐5 activation and binding to the IL‐9 promoter 31. Th9 differentiation requires PU.1, IRF‐4 and the B cell activating transcription factor‐like (BATF), as specific transcription factors that bind to and activate the IL‐9 locus 7. Besides these cytokines, other signalling pathways seem to act by enhancing IL‐9 production by Th9 cells, such as IL‐25 and IL‐33, by activating nuclear factor kappa B (NF‐κB), type I interferons and IL‐1β by inducing STAT‐1/IRF‐1 expression 7. Furthermore, the interaction between T cells and antigen‐presenting cells (APC) through the T cell receptor (TCR)/peptide–major histocompatibility complex (MHC) class II, CD28/CD80, OX40/OX40L, NOTCH/DLL and Jagged may have an important role in Th9 differentiation 7.
IL‐9 and Th9 cells in RA
RA is a chronic autoimmune and inflammatory systemic disease that primarily affects synovial joints. It is characterized by the aberrant expression of several proinflammatory cytokines and recruitment of autoreactive lymphocytes into inflamed tissues 32. T and B cells are involved predominantly in the pathogenesis of RA, together with the orchestrated interaction of proinflammatory cytokines such as TNF‐α, IL‐6, IL‐1β and IL‐17 32.
IL‐9 has been demonstrated to be increased significantly in the sera of RA patients not related to disease duration, disease activity score (DAS28), health assessment questionnaire (HAQ), rheumatoid factor positivity or erosions on radiography 25. High levels of IL‐9 have been also detected in patients’ first‐degree relatives’ sera with RA or with asymptomatic RA‐related autoimmunity 26, 33. Furthermore, IL‐9, together with IL‐6, is the most responsible in the cytokine score calculated by summing all cytokine/chemokine levels, weighted by their regression coefficients for RA–autoantibody association 26. IL‐9 levels are also elevated significantly in the synovial fluid of RA patients compared to osteoarthritis (OA) and IL‐9 promotes proliferation and survival of synovial fluid CD3(+) T cells of RA patients, the proliferation of T cells being dependent upon the PI3K/Akt/mTOR signalling pathway 34.
IL‐9 has been reported to be over‐expressed significantly in synovial tissue of RA patients and correlated with the degree of tissue inflammation 24. IL‐9 expression has been found mainly among synovial fibroblasts and infiltrating mononuclear cells. Th9 cells were demonstrated to be the main source of IL‐9 in both synovial tissues (Fig. 1) and peripheral blood of RA, given the predominant co‐expression of IL‐9 with PU.1 24. Th9 polarization in RA synovium was accompanied by a significant up‐regulation of IL‐4, TGF‐β and TSLP correlated directly with the number of IL‐9‐positive cells. Interestingly, Th17 also produced IL‐9 in RA synovium, although to a lesser degree 24.
RA synovial tissue may display a different topographical distribution of the inflammatory infiltrates: diffuse infiltrates of inflammatory cells without aggregates of specific microstructures, T and B cell aggregates without germinal centre (GC) organization and aggregation of inflammatory cells in secondary follicles with GC formation 32. IL‐9 and IL‐9R expression has been proved to be correlated directly with the degree of inflammatory infiltrate and lymphoid organization in RA patients 24. The strong connection between IL‐9/IL‐9R axis activation and the induction of autoimmune responses has been highlighted recently due to the potential role of IL‐9 in T cell‐dependent B cell differentiation, expansion and antibody production 10, as well as by the evidence that IL‐9R is expressed in GC B cells where its stimulation induces STAT‐5 activation 35. The correlation between IL‐9/IL‐9R and lymphoid organization could confirm the importance of IL‐9‐ and IL‐9‐producing cells in the induction of autoimmunity in RA.
Autoimmunity in RA is characterized by aberrant immune responses to autoantigens that have been modified post‐translationally by citrullination 36, 37. Citrullinated arthritogenic aggrecan peptide has been considered to be a biomarker of RA, having been identified in the peripheral blood of RA patients, and has shown to stimulate a specific CD4 response 38, 39, 40. It is considered a candidate autoantigen for RA, being one of the principle proteoglycans for cartilage extracellular matrix functioning in cushioning synovial joints, self‐antigens that share a common sequence at position 70–74 of the human leucocyte antigen D‐related (HLA‐DR) β‐chain in 90% of RA patients 38, 41. In RA patients, stimulation with the arthritogenic aggrecan peptide resulted in a strong and significant expansion of Th9 cells. Together, these results suggest the existence of an association between the emergence of autoreactive Th9 cells and the occurrence of synovial inflammation and citrullination process 24.
IL‐9 and Th9 cells in psoriatic arthritis
PsA is a chronic inflammatory disease affecting the spine or peripheral joints of patients with psoriasis or with a positive personal or familial history of skin manifestations 42. PsA has long been considered as a Th1‐mediated disease, with interferon (IFN)‐γ and IL‐12 as signature cytokines 43. Recently, however, it has been shown that both innate and adaptive immunity, leading to the activation of the IL‐23/Th17 axis, may contribute to the initiation of tissue inflammation 44, 45, 46. More recently, IL‐9 has been also demonstrated to be involved in the pathogenesis of psoriasis 47. In particular, Th9 cells that are increased in the skin lesions of psoriasis seem to have a specific tropism for the skin and their aberrant activation may contribute to skin inflammatory diseases 48.
Psoriasis and PsA have been demonstrated to be linked strongly to gut inflammation 49. Several studies have highlighted the importance of subclinical gut inflammation in spondyloarthropathy pathogenesis, indicating the inflamed gut as the site where autoreactive and proinflammatory cells are activated and from which they are released in the systemic circulation, with consequent localization in extra‐intestinal inflamed sites 50. Few studies, however, have addressed specifically the immune responses in the gut of PsA patients.
PsA subclinical gut inflammation is characterized histologically by a higher number of infiltrating inflammatory cells, which are organized frequently in lymphoid follicles. Immunologically, the ileum of PsA patients shows a clear and defined IL‐23/Th17 response, as shown by both IL‐23 and IL‐17 strong up‐regulation, with a defective Th1 polarization 50. However, IL‐9 seems to mark gut inflammation in PsA specifically, given the impressive IL‐9 expression observed in the gut of PsA patients 27. IL‐9 is produced particularly by inflammatory cells and epithelial cells that were demonstrated to be Paneth cells (PC), as co‐expression of IL‐9 and α‐defensin 5 (a specific marker of PC) has been observed. In PsA gut, PC also express the IL‐9R and after in‐vitro stimulation with recombinant IL‐9, produce high levels of specific peptides such as α‐defensin 5 and cytokines such as IL‐23, indicating the occurrence of an autocrine loop involving IL‐9 27. PC are highly specialized small intestine epithelial cells; they are located precisely at the bottom of Lieberkühn crypts and are involved in innate immune responses and anti‐microbial host defence, thus contributing to the maintenance of the gastrointestinal barrier 51. A specific microbiome signature has been demonstrated recently in PsA patients, indicating a role for dysbiosis in the pathogenesis of PsA 52. The specific release of IL‐9 by PsA PC could be relevant in this context, as it may represent a significant immune link between innate and adaptive responses.
Th9 polarization also characterizes the synovium (Fig. 1) and the peripheral blood of PsA patients, and the percentage of circulating Th9 cells is correlated significantly with disease activity 27. Moreover, Th9 cells, isolated from both synovium and peripheral blood of PsA patients, express the intestinal homing receptor α4β7, indicating that these cells are probably activated in the gut and recirculate into sites of inflammation 27. Interestingly, we have recorded that circulating Th9 cells in PsA decreased after anti‐TNF and ustekinumab treatment, leading us to speculate that the clinical improvement observed in PsA patients treated with these classes of drugs could be, at least in part, referred to the modulation of Th9 response 27. Together these findings indicate that PsA may be characterized by IL‐9 and Th9 polarization, suggesting that these cells may represent a future therapeutic target.
IL‐9 and Th9 cells in large‐vessel vasculitis
Large‐vessel vasculitides (LVV) are characterized by the autoimmune inflammation of medium and large arteries leading to occlusion of the lumen with ischaemic damage of dependent organs 53. Several effector cytokines involved in both innate and adaptive immunity have been identified in vasculitic lesions 54. However, among the different immune pathways characterizing immune responses, the IL‐6–IL‐17 axis and the IL‐12–IFN‐γ axis seem to play a fundamental role in LVV pathogenesis 54.
Giant cell arteritis (GCA) is the prototype of LVV and is characterized by a range of histological patterns of vascular wall injury 54. Beyond the classic transmural inflammation (with or without giant cells), two other different histological aspects of GCA have been described 55: small vessel vasculitis (SVV) defined as inflammation of the small vessels external to the temporal artery adventitia and vasa vasorum vasculitis (VVV), defined as isolated inflammation of temporal artery vasa vasorum 55.
Th1 and Th17 subsets of effector T cells have been demonstrated clearly to participate in the pathogenesis of GCA 54, 56. Although Th2‐derived cytokines have been demonstrated previously to be consistently absent 54, the IL‐33 pathway seems to be over‐expressed in the inflamed arteries of GCA patients and accompanied by a strong M2 macrophage polarization 57. Beyond the role in promoting the Th2 response, IL‐33 has also been associated recently with the secretion of IL‐9 by human CD4+ T cells isolated from peripheral blood 58, 59, 60, apparently indicating a potential role of Th9 cells in the pathogenesis of GCA.
Analysis of IL‐9 and IL‐17 expression in different histological subsets of GCA has demonstrated that a different immunological polarization characterizes different histological patterns of GCA 20. The different expression of cytokines in different patterns of tissue pathology may be of immunological relevance. Multiple effector T cell subsets (such as Th17 and Th9) may induce GCA independently of each other, probably by overlapping but with distinct mechanisms. IL‐9 and Th9 cells, in fact, immunologically mark the inflamed arteries of GCA patients with inflammation restricted to the peri‐adventitial small vessels. Conversely, a more intense Th17 polarization, with weak IL‐9 expression, predominates in the GCA arteries with inflammation restricted to the vasa vasorum. Interestingly, a concomitant Th9 and Th17 response is observed in arteries displaying transmural inflammation and especially in those arteries with granulomatous reaction 20.
Inflammatory cells infiltrating the artery wall, giant cells, endothelial cells of vessels scattered through the inflammatory infiltrates and among vascular smooth muscle cells showed intense positivity for IL‐9 20. IL‐9 tissue expression was also correlated significantly with the intensity of the systemic inflammatory response, including erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP) 20. Infiltrating inflammatory cells producing IL‐9 were demonstrated to be Th9 cells, considering the significant co‐localization observed between IL‐9 and PU.1 (the specific transcription factor for Th9 cells), suggesting the prevalent Th9 phenotype of IL‐9 mononuclear‐producing cells (Fig. 1). Notably, when present, giant cells scattered in the inflamed arteries showed an intense co‐expression of IL‐9 and IL‐17 20.
Th9 cells are a distinct subpopulation of CD4+ effector T cells that require TGF‐β, IL‐4 and TSLP for their differentiation 7. GCA arteries with higher expression of IL‐9 (transmural inflammation with granulomatous reaction and SVV) displayed intense over‐expression of TGF‐β, IL‐4 and TSLP, suggesting that the upstream Th9 cytokine network is also up‐regulated in GCA 20. The biological effects of IL‐9 are mediated by the functional IL‐9 receptor complex, that is composed of this protein as well as the IL‐2 receptor gamma (IL‐2RG), a common gamma subunit shared by the receptors of many different cytokines 7. The activation of this receptor leads to the activation of various Janus kinases (JAK) and STAT proteins 8 which connect to different biological responses 6. In GCA, IL‐9R positivity was observed among endothelial cells of vessels distributed in the inflammatory infiltrates and neutrophils 20. Functional expression of IL‐9R receptor by human neutrophils has been demonstrated in asthmatic patients, with an important role in IL‐8 release 61. IL‐9/IL‐9R over‐expression in GCA was accompanied by a significantly high expression of IL‐8 and IL‐8R, especially in patients with transmural inflammation, providing a functional immunological link between IL‐9R expression and neutrophil activity, thus highlighting the importance of neutrophils in the pathogenesis of GCA 20.
Studies conducted on small series have shown previously that IL‐17A‐expressing cells are reduced dramatically in specimens obtained from glucocorticoid‐treated patients 56. Differently from IL‐17, IL‐9 was modified only marginally by glucocorticoids. This apparent IL‐9 low sensitivity to glucocorticoid inhibition may indicate a potential role for Th9 cells in steroid‐resistant GCA 20.
IL‐9 and Th9 cells in anti‐neutrophil cytoplasmic antibodies (ANCA)‐associated vasculitis
Granulomatosis with polyangiitis (GPA) is a systemic necrotizing vasculitis that affects small‐ and medium‐sized vessels in many organs associated with granulomatous inflammation and the presence of ANCAs directed against proteinase 3 (PR3) 62, 63. PR3 has been demonstrated to be expressed at the plasma membrane of neutrophils and may act by interfering with induction of anti‐inflammatory mechanisms following phagocytosis of these cells by macrophages and/or by facilitating anti‐PR3 ANCA binding, leading to neutrophil activation and consequent tissue inflammation 64. Interestingly, in different murine models, membrane‐associated PR3 on apoptotic cells triggered secretion of inflammatory cytokines and chemokines, thus inducing a specific microenvironment instructing plasmocytoid dendritic cells (pDC) to promote a specific effector T cell subset. In particular, pDCs exposed to apoptotic cells expressing membrane PR3 in a TGF‐β‐rich environment induce the generation of Th9 cells. Th9 and Th2 cell polarization 65. Interestingly, PBMCs obtained from active, untreated systemic GPA patients also displayed skewed Th9 and Th17 responses, revealing a GPA‐specific mechanism of immune polarization 65.
Th9 cells in systemic lupus erythematosus
SLE is a disorder of generalized autoimmunity characterized by the dysregulation of cellular and humoral immune responses, the break in immune tolerance to self‐antigens, polyclonal B cell activation and the production of autoantibodies. Autoantibody deposition and inflammatory cell infiltration in target organs such as kidneys and brain characterizes SLE. It has been reported that aberrant T lymphocyte activation and altered cytokine production are important contributors to SLE pathogenesis. However, so far the exact mechanisms that lead to the development of SLE remain undefined.
Only few studies have been carried out on Th9 cells and their related cytokine, IL‐9, in human SLE. These studies have shown an increase in the Th9 cell subset and IL‐9 serum levels on active SLE. In lupus‐prone mice Th9 cells accumulated in the spleen and in the kidneys (Fig. 1) and their number is related closely to the presence of germinal centres 23. In addition, in‐vivo treatment with neutralizing anti‐IL‐9 antibodies was able to reverse serum anti‐dsDNA antibody levels 23.
IL‐9 can be produced by different T cell subsets that include CD8+ cells, invariant natural killer T (NK T) cells, γδ T cells, Th17 cells and innate lymphoid cell group 2 (ILC2) 50, 51, 52, 53. IL‐9 is also known to induce TH17 differentiation and IL‐17 production, and these cytokines may work together synergistically in promoting SLE pathogenesis. Despite these observations, the role of Th9 and IL‐9 in SLE patients remains unclear.
IL‐9 and Th9 cells in systemic sclerosis
Expression of IL‐4, TGF‐β and TSLP has been implicated in fibrosis and remodelling in SSc 66, 67, 68. Furthermore, Th9 and IL‐9 seem to increase tissue fibrosis, as demonstrated in the liver, where IL‐9 induces hepatic fibrosis and an exacerbated disease end‐point 69. Serum IL‐9 level has been demonstrated to be increased in patients with SSc and associated with a lower frequency and severity of pulmonary fibrosis, indicating IL‐9 as a protective factor in SSc 28. However, the presence of high IL‐9 levels and the strong expression of Th9 polarizing cytokines in SSc raise the question of whether Th9 cells might participate in SSc pathogenesis. In this regard, strong expression of IL‐9 and IL‐9R has been demonstrated recently in skin tissue of patients with diffuse SSc and correlated with the modified Rodnan skin score and the presence of pulmonary fibrosis 70. In particular, IL‐9 expression was observed mainly in the context of infiltrating mononuclear cells and in the keratinizing squamous epithelium of skin from SSc patients 70. The majority of IL‐9‐producing cells in the skin were identified as Th9 and Th17 cells. Among peripheral blood mononuclear cells, Th9 cells were the major source of IL‐9, being expanded significantly in SSc patients compared to controls 70. The participation of IL‐9 and Th9 in the process of inflammation and fibrosis action in SSc, however, is still not clear, and further studies are required to better clarify their role in modulating tissue fibrosis.
Conclusions
IL‐9 and Th9 cells seem to be involved in the immunopathology of several human diseases. In systemic rheumatic diseases this axis is activated and involved potentially in the triggering and/or maintaining inflammation. The targeting of IL‐9/Th9 cells could be considered a productive strategy in treating systemic autoimmune diseases. However, further studies are required to understand more clearly the specific contribution of this axis in the pathogenesis of different systemic autoimmune diseases.
Disclosure
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by a grant from Ministero dell'Istruzione, della Università e della ricerca scientifica from Italy.
References
- 1. Gizinski AM, Fox DA. T cell subsets and their role in the pathogenesis of rheumatic disease. Curr Opin Rheumatol 2014; 26:204–10. [DOI] [PubMed] [Google Scholar]
- 2. Cope AP. T cells in rheumatoid arthritis. Arthritis Res Ther 2008; 10:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmitt N, Ueno H. Regulation of human helper T cell subset differentiation by cytokines. Curr Opin Immunol 2015; 34:130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010; 28:445–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caza T, Landas S. Functional and phenotypic plasticity of CD4 T cell subsets. Biomed Res Int 2015; 2015:521957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goswami R, Kaplan MH. A brief history of IL‐9. J Immunol 2011; 186:3283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol 2015; 15:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Smedt M, Verhasselt B, Kerre T et al Signals from the IL‐9 receptor are critical for the early stages of human intrathymic T cell development. J Immunol 2000; 164:1761–7. [DOI] [PubMed] [Google Scholar]
- 9. Turner JE, Morrison PJ, Wilhelm C et al IL‐9‐mediated survival of type 2 innate lymphoid cells promotes damage control in helminth‐induced lung inflammation. J Exp Med 2013; 210:2951–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knoops L, Louahed J, Renauld JC. IL‐9‐induced expansion of B‐1b cells restores numbers but not function of B‐1 lymphocytes in xid mice. J Immunol 2004; 172:6101–6. [DOI] [PubMed] [Google Scholar]
- 11. Jeannin P, Delneste Y, Lecoanet‐Henchoz S, Gretener D, Bonnefoy JY. Interleukin‐7 (IL‐7) enhances class switching to IgE and IgG4 in the presence of T cells via IL‐9 and sCD23. Blood 1998; 91:1355–61. [PubMed] [Google Scholar]
- 12. Renauld JC, Kermouni A, Vink A, Louahed J, Van Snick J. Interleukin‐9 and its receptor: involvement in mast cell differentiation and T cell oncogenesis. J Leukoc Biol 1995; 57:353–60. [DOI] [PubMed] [Google Scholar]
- 13. Renauld JC, Houssiau F, Uyttenhove C, Vink A, Van Snick J. Interleukin‐9: a T‐cell growth factor with a potential oncogenic activity. Cancer Invest 1993; 11:635–40. [DOI] [PubMed] [Google Scholar]
- 14. Veldhoen M, Uyttenhove C, van Snick J et al Transforming growth factor‐beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9‐producing subset. Nat Immunol 2008; 9:1341–6. [DOI] [PubMed] [Google Scholar]
- 15. Ramming A, Druzd D, Leipe J, Schulze‐Koops H, Skapenko A. Maturation‐related histone modifications in the PU.1 promoter regulate Th9‐cell development. Blood 2012; 119:4665–74. [DOI] [PubMed] [Google Scholar]
- 16. Staudt V, Bothur E, Klein M et al Interferon‐regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 2010; 33:192–202. [DOI] [PubMed] [Google Scholar]
- 17. Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev 2013; 252:104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kerzerho J, Maazi H, Speak AO et al Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol 2013; 131:1048–57, 57 e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nalleweg N, Chiriac MT, Podstawa E et al IL‐9 and its receptor are predominantly involved in the pathogenesis of UC. Gut 2015; 64:743–55. [DOI] [PubMed] [Google Scholar]
- 20. Ciccia F, Rizzo A, Guggino G et al Difference in the expression of IL‐9 and IL‐17 correlates with different histological pattern of vascular wall injury in giant cell arteritis. Rheumatology (Oxf) 2015; 54:1596–604. [DOI] [PubMed] [Google Scholar]
- 21. Leng RX, Pan HF, Ye DQ, Xu Y. Potential roles of IL‐9 in the pathogenesis of systemic lupus erythematosus. Am J Clin Exp Immunol 2012; 1:28–32. [PMC free article] [PubMed] [Google Scholar]
- 22. Ouyang H, Shi Y, Liu Z et al Increased interleukin‐9 and CD4+IL‐9+ T cells in patients with systemic lupus erythematosus. Mol Med Rep 2013; 7:1031–7. [DOI] [PubMed] [Google Scholar]
- 23. Yang J, Li Q, Yang X, Li M. Interleukin‐9 is associated with elevated anti‐double‐stranded DNA antibodies in lupus‐prone mice. Mol Med 2015; 21:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciccia F, Guggino G, Rizzo A et al Potential involvement of IL‐9 and Th9 cells in the pathogenesis of rheumatoid arthritis. Rheumatology (Oxf) 2015; 54:2264–72. [DOI] [PubMed] [Google Scholar]
- 25. Dantas AT, Marques CD, da Rocha Junior LF et al Increased serum interleukin‐9 levels in rheumatoid arthritis and systemic lupus erythematosus: pathogenic role or just an epiphenomenon? Dis Markers 2015; 2015:519638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hughes‐Austin JM, Deane KD, Derber LA et al Multiple cytokines and chemokines are associated with rheumatoid arthritis‐related autoimmunity in first‐degree relatives without rheumatoid arthritis: studies of the aetiology of rheumatoid arthritis (SERA). Ann Rheum Dis 2013; 72:901–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ciccia F, Guggino G, Ferrante A et al IL‐9 over‐expression and Th9 polarization characterize the inflamed gut, the synovial tissue and the peripheral blood of patients with psoriatic arthritis. Arthritis Rheumatol 2016. doi: 10.1002/art.39649. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28. Yanaba K, Yoshizaki A, Asano Y, Kadono T, Sato S. Serum interleukin 9 levels are increased in patients with systemic sclerosis: association with lower frequency and severity of pulmonary fibrosis. J Rheumatol 2011; 38:2193–7. [DOI] [PubMed] [Google Scholar]
- 29. Demoulin JB, Louahed J, Dumoutier L, Stevens M, Renauld JC. MAP kinase activation by interleukin‐9 in lymphoid and mast cell lines. Oncogene 2003; 22:1763–70. [DOI] [PubMed] [Google Scholar]
- 30. Bauer JH, Liu KD, You Y, Lai SY, Goldsmith MA. Heteromerization of the gammac chain with the interleukin‐9 receptor alpha subunit leads to STAT activation and prevention of apoptosis. J Biol Chem 1998; 273:9255–60. [DOI] [PubMed] [Google Scholar]
- 31. Yao W, Zhang Y, Jabeen R et al Interleukin‐9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity 2013; 38:360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van de Sande MG, Baeten DL. Immunopathology of synovitis: from histology to molecular pathways. Rheumatology (Oxf) 2016; 55:599–606. [DOI] [PubMed] [Google Scholar]
- 33. Barra L, Summers K, Bell D, Cairns E. Serum cytokine profile of unaffected first‐degree relatives of patients with rheumatoid arthritis. J Rheumatol 2014; 41:280–5. [DOI] [PubMed] [Google Scholar]
- 34. Kundu‐Raychaudhuri S, Abria C, Raychaudhuri SP. IL‐9, a local growth factor for synovial T cells in inflammatory arthritis. Cytokine 2016; 79:45–51. [DOI] [PubMed] [Google Scholar]
- 35. Fawaz LM, Sharif‐Askari E, Hajoui O, Soussi‐Gounni A, Hamid Q, Mazer BD. Expression of IL‐9 receptor alpha chain on human germinal center B cells modulates IgE secretion. J Allergy Clin Immunol 2007; 120:1208–15. [DOI] [PubMed] [Google Scholar]
- 36. Clancy KW, Weerapana E, Thompson PR. Detection and identification of protein citrullination in complex biological systems. Curr Opin Chem Biol 2015; 30:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. Citrullination and autoimmunity. Autoimmun Rev 2015; 14:490–7. [DOI] [PubMed] [Google Scholar]
- 38. Wilson CL, Hine DW, Pradipta A, Pearson JP, van Eden W, Robinson JH, Knight AM. Presentation of the candidate rheumatoid arthritis autoantigen aggrecan by antigen‐specific B cells induces enhanced CD4(+) T helper type 1 subset differentiation. Immunology 2012; 135:344–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. von Delwig A, Locke J, Robinson JH, Ng WF. Response of Th17 cells to a citrullinated arthritogenic aggrecan peptide in patients with rheumatoid arthritis. Arthritis Rheum 2010; 62:143–9. [DOI] [PubMed] [Google Scholar]
- 40. Glant TT, Radacs M, Nagyeri G et al Proteoglycan‐induced arthritis and recombinant human proteoglycan aggrecan G1 domain‐induced arthritis in BALB/c mice resembling two subtypes of rheumatoid arthritis. Arthritis Rheum 2011; 63:1312–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scally SW, Petersen J, Law SC et al A molecular basis for the association of the HLA‐DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med 2013; 210:2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olivieri I, D'Angelo S, Palazzi C, Padula A. Advances in the management of psoriatic arthritis. Nat Rev Rheumatol 2014; 10:531–42. [DOI] [PubMed] [Google Scholar]
- 43. Barnas JL, Ritchlin CT. Etiology and pathogenesis of psoriatic arthritis. Rheum Dis Clin North Am 2015; 41:643–63. [DOI] [PubMed] [Google Scholar]
- 44. Raychaudhuri SK, Saxena A, Raychaudhuri SP. Role of IL‐17 in the pathogenesis of psoriatic arthritis and axial spondyloarthritis. Clin Rheumatol 2015; 34:1019–23. [DOI] [PubMed] [Google Scholar]
- 45. Mitra A, Raychaudhuri SK, Raychaudhuri SP. IL‐17 and IL‐17R: an auspicious therapeutic target for psoriatic disease. Actas Dermosifiliogr 2014; 105:21–33.] [DOI] [PubMed] [Google Scholar]
- 46. Maeda S, Hayami Y, Naniwa T, Ueda R. The Th17/IL‐23 axis and natural immunity in psoriatic arthritis. Int J Rheumatol 2012; 2012:539683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Singh TP, Schon MP, Wallbrecht K, Gruber‐Wackernagel A, Wang XJ, Wolf P. Involvement of IL‐9 in Th17‐associated inflammation and angiogenesis of psoriasis. PLOS ONE 2013; 8:e51752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schlapbach C, Gehad A, Yang C et al Human TH9 cells are skin‐tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med 2014; 6:219ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Praet L, Van den Bosch FE, Jacques P et al Microscopic gut inflammation in axial spondyloarthritis: a multiparametric predictive model. Ann Rheum Dis 2013; 72:414–7. [DOI] [PubMed] [Google Scholar]
- 50. Ciccia F, Rizzo A, Triolo G. Subclinical gut inflammation in ankylosing spondylitis. Curr Opin Rheumatol 2016; 28:89–96. [DOI] [PubMed] [Google Scholar]
- 51. Keshav S. Paneth cells: leukocyte‐like mediators of innate immunity in the intestine. J Leukoc Biol 2006; 80:500–8. [DOI] [PubMed] [Google Scholar]
- 52. Scher JU, Ubeda C, Artacho A et al Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol 2015; 67:128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weyand CM, Goronzy JJ. Medium‐ and large‐vessel vasculitis. N Engl J Med 2003; 349:160–9. [DOI] [PubMed] [Google Scholar]
- 54. Weyand CM, Goronzy JJ. Immune mechanisms in medium and large‐vessel vasculitis. Nat Rev Rheumatol 2013; 9:731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Restuccia G, Cavazza A, Boiardi L et al Small‐vessel vasculitis surrounding an uninflamed temporal artery and isolated vasa vasorum vasculitis of the temporal artery: two subsets of giant cell arteritis. Arthritis Rheum 2012; 64:549–56. [DOI] [PubMed] [Google Scholar]
- 56. Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T‐cell responses in giant cell arteritis. Circulation 2010; 121:906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ciccia F, Alessandro R, Rizzo A et al IL‐33 is overexpressed in the inflamed arteries of patients with giant cell arteritis. Ann Rheum Dis 2013; 72:258–64. [DOI] [PubMed] [Google Scholar]
- 58. Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL‐33‐activated dendritic cells induce an atypical TH2‐type response. J Allergy Clin Immunol 2009; 123:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kroeger KM, Sullivan BM, Locksley RM. IL‐18 and IL‐33 elicit Th2 cytokines from basophils via a MyD88‐ and p38alpha‐dependent pathway. J Leukoc Biol 2009; 86:769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gerlach K, Hwang Y, Nikolaev A et al TH9 cells that express the transcription factor PU.1 drive T cell‐mediated colitis via IL‐9 receptor signaling in intestinal epithelial cells. Nat Immunol 2014; 15:676–86. [DOI] [PubMed] [Google Scholar]
- 61. Abdelilah S, Latifa K, Esra N et al Functional expression of IL‐9 receptor by human neutrophils from asthmatic donors: role in IL‐8 release. J Immunol 2001; 166:2768–74. [DOI] [PubMed] [Google Scholar]
- 62. Thai LH, Charles P, Resche‐Rigon M, Desseaux K, Guillevin L. Are anti‐proteinase‐3 ANCA a useful marker of granulomatosis with polyangiitis (Wegener's) relapses? Results of a retrospective study on 126 patients. Autoimmun Rev 2014; 13:313–8. [DOI] [PubMed] [Google Scholar]
- 63. Kallenberg CG. Key advances in the clinical approach to ANCA‐associated vasculitis. Nat Rev Rheumatol 2014; 10:484–93. [DOI] [PubMed] [Google Scholar]
- 64. Gabillet J, Millet A, Pederzoli‐Ribeil M et al Proteinase 3, the autoantigen in granulomatosis with polyangiitis, associates with calreticulin on apoptotic neutrophils, impairs macrophage phagocytosis, and promotes inflammation. J Immunol 2012; 189:2574–83. [DOI] [PubMed] [Google Scholar]
- 65. Millet A, Martin KR, Bonnefoy F et al Proteinase 3 on apoptotic cells disrupts immune silencing in autoimmune vasculitis. J Clin Invest 2015; 125:4107–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huang XL, Wang YJ, Yan JW et al Role of anti‐inflammatory cytokines IL‐4 and IL‐13 in systemic sclerosis. Inflamm Res 2015; 64:151–9. [DOI] [PubMed] [Google Scholar]
- 67. Lafyatis R. Transforming growth factor β at the centre of systemic sclerosis. Nat Rev Rheumatol 2014; 10:706–19. [DOI] [PubMed] [Google Scholar]
- 68. Usategui A, Criado G, Izquierdo E et al A profibrotic role for thymic stromal lymphopoietin in systemic sclerosis. Ann Rheum Dis 2013; 72:2018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shan‐yu Q, Dong‐hong L, Xiao‐yun G et al A deleterious role for Th9/IL‐9 in hepatic brogenesis. Sci Rep 2016; 6:18694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guggino G, Ciccia F, Cipriani P et al IL-9 over-expression and Th9 polarization characterize SSc patients. Abstract from the 4th Systemic Sclerosis World Congress. J Scleroderm Relat Dis 2016. [Google Scholar]


