Abstract
In this work, we demonstrate that Eu(II) complex of DOTA-tetra(glycinate) has a higher reduction potential than most Eu(II) chelates reported so far. The reduced Eu(II) form acts as an efficient water proton T1 relaxation reagent while the Eu(III) form acts as a water-based CEST agent. The complex has extremely fast water exchange rate. Oxidation to the corresponding Eu(III) complex yields a well-defined signal from the paraCEST agent. The time course of oxidation was studied in vitro and in vivo by T1-weighted and CEST imaging.
Keywords: contrast agent, paraCEST, Europium, divalent lanthanide, T1- weighted
Graphical Abstract
A Eu(II) complex of DOTA-tetra(glycinate) has far less negative redox potential than most Eu(II) chelates reported so far. The reduced Eu(II) form acts as an efficient water proton T1 relaxation reagent while the Eu(III) form acts as a water-based CEST agent. The time course of oxidation was studied in vitro and in vivo by T1-weighted and CEST imaging.
Gadolinium complexes are commonly used as contrast agents in magnetic resonance imaging (MRI). They generate image contrast by shortening the longitudinal (T1) relaxation time of bulk water protons. The efficiency of a T1- agent is defined by r1 relaxivity, which is dependent on several parameters including the metal bound water exchange rate, rotational correlation time of the complex and the electronic relaxation time of the metal ion.[1] An alternative to the Gd3+-based contrast agents is the isoelectronic Eu2+ ion.[2] Both ions have a 4f7 electron configuration and a symmetric 8S7/2 ground state but Eu2+ complexes in general display much faster water exchange rates and faster electronic relaxation times.[2] Analogous complexes of Eu2+ and Gd3+ can produce similar relaxivity values at lower fields while at higher fields the Eu2+ complexes tend to be more efficient.[3]
The Eu2+ aqua ion is extremely sensitive to oxidation as demonstrated by its strongly negative reduction potential (−585 mV vs Ag+/AgCl). Eu2+ poly(amino carboxylate) chelates usually have lower reduction potential although some Eu2+-cryptates have been reported to be more stable towards oxidation.[4,5] Eu2+ complexes have been proposed as redox sensitive T1 agents,[3,5,6] because oxidation of Eu2+ leads to the formation of weakly paramagnetic Eu3+, which has little impact on water proton T1. The Eu3+ ion however, generates a moderately strong magnetic dipolar field that produces large hyperfine shifts of NMR signals of nearby ligand protons. While Eu3+ complexes are very poor T1-shortening agents, Eu3+ DOTA tetra(amides) (Figure 1) belong to a conceptually different class of MRI contrast agents, known as paraCEST agents that alter image contrast by transferring selectively saturated spins from a highly shifted small pool of proton spins (metal bound water) to the bulk water pool.[7] Chemical exchange saturation transfer (CEST) occurs when the proton exchange rate between the two pools (kex) is in the slow-to-intermediate exchange regime (kex ≤ Δω where Δω is the chemical shift difference between the two pools). A redox responsive liposomal Eu2+/Eu3+ system was recently reported that showed T1 shortening effect and lipoCEST effect (Δω ≈ 1ppm) originating from the exchange between the water protons inside the liposomes and the bulk water protons not associated with the liposomes. Upon oxidation, the T1 enhancement disappeared while the lipoCEST remained unaffected. The CEST effect in this system is not due to the formation of a Eu3+ complex.[8]
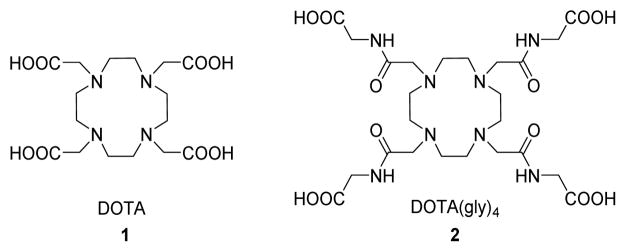
Figure 1.
Structure of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid 1 and 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid tetra(glycine amide) 2.
Eu2+ complexes have several orders of magnitude faster water exchange rates in comparison to the corresponding Eu3+ complexes and with a suitable ligand system, may offer a unique opportunity in the design of redox responsive MR agent that shortens T1 in the reduced state and produces a CEST signal in the oxidized state. In the present work we show that Eu2+2 is an efficient T1 shortening agent because of the rapid water exchange of the Eu2+ bound water, but upon oxidation it turns into the well-known paraCEST agent, Eu3+2, which has slow water exchange kinetics. Merbach suggested in 2003 that the redox stability of Eu2+1 could be increased by substituting nitrogen containing donor groups for the carboxylate side-arms.[3,6] Here, we also show that Eu2+2 indeed has significantly improved redox stability compared to Eu2+1.
The CEST effect can be expressed as a decrease in total bulk water signal intensity, and assuming complete and instantaneous saturation of the bound water peak, the net magnetization of water protons at steady-state is given by the following equation:
| (1) |
where c is the concentration of the agent, q is the number of protons per agent, 111 represents the molar concentration of bulk water protons, T1 is the longitudinal (spin–lattice) relaxation time of bulk water, and τM is the lifetime of the exchanging proton (τM = 1/kex).[7] Thus, the magnitude of the CEST effect is dependent on both the agent concentration and the bulk water T1. Obviously, as Eu2+2 are oxidized, the T1 shortening effect of Eu2+ will diminish while the paraCEST agent concentration will increase over the course of the reaction. To study the dependence of CEST on T1, we designed a model experiment in which the paraCEST agent Eu3+2 were mixed with varying concentration of the T1 shortening agent Gd3+1 (Table S1). Figure 2 shows that the proton relaxation rate (R1 = 1/T1) of bulk water protons increases with increasing [Gd3+1] while the CEST signal from the paraCEST agent diminishes. At the two extremes of [Gd3+1], the images are dominated by either CEST (when [Gd3+1] = 0) or T1 (when [Gd3+1] = 4 mM) but there is a range of Gd3+ concentrations (samples 4, 5 and 6) where both CEST and T1 enhancement contribute to the signal. The CEST signal was <10% when R1 = 5 s−1. From the fitting of the T1 and CEST intensities to equation (1), a bound water residence lifetime (τM) of 410 ms was obtained for Eu3+2 at 19°C, in agreement with the τM value determined by other methods.[9] This same phenomenon, the sensitivity of CEST to water proton T1, formed the basis of a recently reported redox-sensitive paraCEST agent.
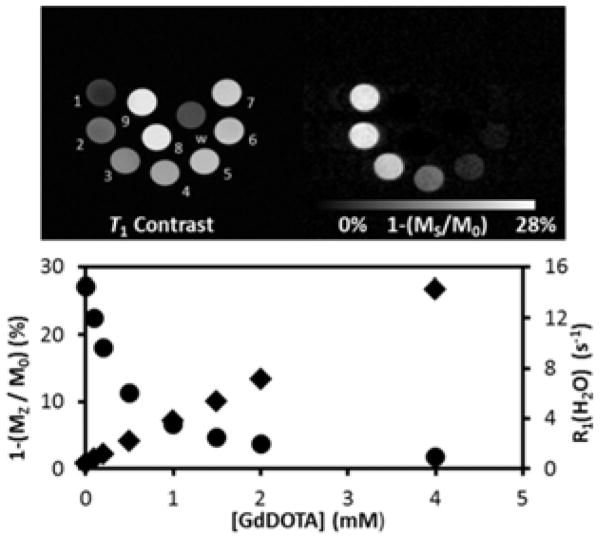
Figure 2.
(Top) Correlation between relaxation rates (circles) and the CEST effect (squares) in a mixture of Gd3+1 and Eu3+2. Each circle in the image represents a separate sample. [Eu3+2] in each sample was 10 mM while [Gd3+1] ranged from 0 to 4 mM. Vial 9 contained 8 mM Gd3+1 alone while vial w contained only water. (Bottom) Plots of CEST (1-(Ms/M0)) and R1 versus [Gd3+1]. Imaging parameters: B0=9.4 T, 20 °C; T1w: GEMS sequence, TR= 9.9 ms, TE= 5.0 ms; CEST: FSEMS sequence, sat time = 3 s, sat power = 10 μT, sat frq = 54 (on),-54 ppm (off-resonance).
Eu2+ complexes of 1 and 2 were conveniently prepared by directly reacting the ligands with commercially available EuCl2 under oxygen free conditions.[10] The relaxivity of Eu2+2 was measured as 3.2 mM−1 s−1 at 9.4T and 1 T (Figures S1 and S2). The Eu2+ bound water exchange rate as estimated by fits of variable temperature 17O NMR water linewidth data to theory was kex = 0.21 × 109 s−1 for Eu2+2 and kex = 0.63 × 109 s−1 for Eu2+1 in 20% dioxane − 80% water solutions (Figure S3, S4 and Table S2–S4), which are in the range of previously reported values.[3,6,11,12] It is worth noting that the water exchange for Eu2+2 is nearly the same as that of Eu2+1. This indicates that the glycinate amide side-chains in Eu2+2 do not affect the water exchange rate in comparison to the corresponding Eu3+ complexes where the difference between carboxylate and amide donor ligands is typically 3 orders of magnitude.[1,2] Unlike Gd3+ complexes, the r1 value of Eu2+2 did not decrease significantly at high field, in agreement with previously published data for other Eu2+ complexes. The redox stability of Eu2+2 was studied by cyclic voltammetry. The reduction potential measured for the Eu2+2/Eu3+2 redox couple was found to be −226 mV vs. Ag+/AgCl electrode (Figure S5), which is far less negative than that of Eu2+1 (−1135 mV), or than the Eu2+ aqua ion (−585 mV against Ag+/AgCl electrode).[3] The rates of conversion of Eu2+2 and Eu2+1 to their respective Eu3+ complexes were also investigated by NMR by measuring the decay of the relaxation rate of the bulk water in a sealed NMR tube under N2 atmosphere at 9.4T (Figures S6 and S7). The measured half lives (t1/2), 19 and 7 days respectively, also show that Eu2+2 is the most stable cyclen-based Eu2+ complex reported so far. Similar stabilizing effect of the charge neutral amide group was reported for Eu2+ complexes of 1,10-diaza-18-crown-6 derivatives in which picolinamide pendant arms were substituted for picolinate groups.[13]
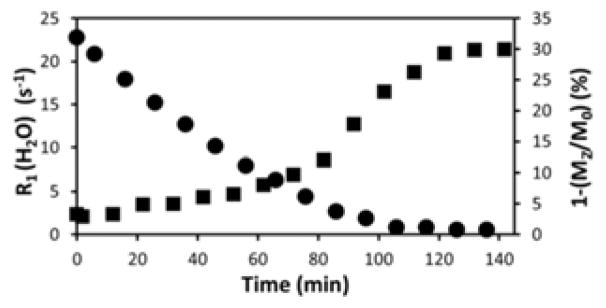
Next, a fresh solution of Eu2+2 was prepared in a 6 mm tall vessel and exposed to air while both T1 and CEST were measured as a function of time by imaging a slice 4 mm below the surface of the sample (Figure 3). As shown, the bulk water R1 decreased steadily for about 100 minutes in this slice reflecting oxidation of Eu2+ to Eu3+. The CEST signal increased following a sigmoid curve with an inflection point at around 100 min reaching 10% at around 6 s−1 bulk water relaxation rate in agreement with the results shown in Figure 2. These data reveal that relaxation of bulk water protons limits the intensity of the CEST signal and at ~80 min, the T1 relaxation is slow enough for CEST to become efficient. As expected, the rate of oxidation depends heavily on the slice selected. For example, the T1w enhancement in a slice just below the surface disappeared just after 10 minutes (Figure S8).
Figure 3.
Plots of relaxation rate (R1) (circles) and CEST (squares) for a solution initially containing 10 mM Eu2+2 versus time. The data were collected from images in a single slice 4 mm away from the surface. Imaging parameters: B0=9.4 T, 20 °C, T1w: FSEMS sequence, TR = 2 s, TE = 3.0 ms; CEST: FSEMS sequence, sat time = 3 s, sat power = 10 μT, sat frq = 54 (on-resonance), -54 ppm (off-resonance).
To demonstrate the feasibility of using Eu2+2 as redox sensitive probe we studied its reaction with H2O2. A closed phantom containing Eu2+2 solution (1 mL, 10 mM) was constructed and a small volume (20 μL) of hydrogen peroxide solution (3%) was injected into the container. T1w and CEST images were recorded consecutively over a period of 1 h. As anticipated, the complex reacted rapidly with H2O2 and the mixing and diffusion of H2O2 in the solution could sequentially be observed by both T1w and CEST imaging. These images also demonstrate that the complex is stable in both oxidation states and throughout the process of oxidation (Figure 4 and S9, S10).
Figure 4.
Sequential T1w (top) and CEST (bottom) images of a phantom containing Eu2+2 (1 mL, 10 mM, left) and H2O (right) after the injection of H2O2 (3%, 20 μL). Imaging parameters: B0=9.4 T, 20°C T1w: FSEMS sequence, TR = 2 s, TE = 5 ms; PARACEST: FSEMS sequence, sat time = 3 s, sat power = 10 μT, sat frq = 54 (on-resonance),-54 ppm (off-resonance).
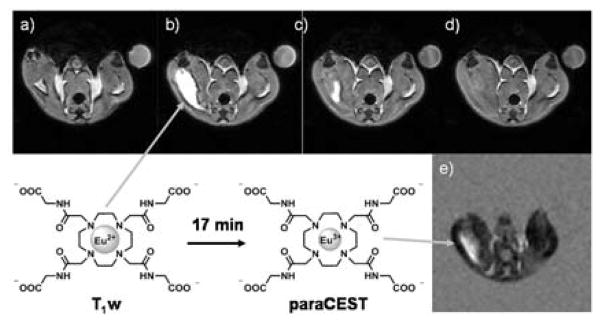
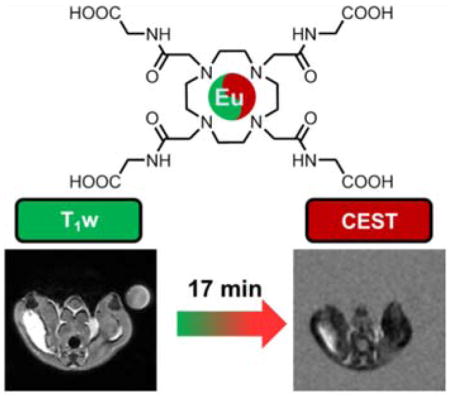
Encouraged by the enhanced stability of Eu2+2 we tested the agent in vivo as well. The complex was injected into the thigh muscle of healthy female mice at a dose of 0.05 mmol/kg. Oxidation of the agent at the injection site could be observed by both T1w and CEST imaging (Figure 5). Given that diffusion of small molecules away from the injection site and into the vascular bed occurs relatively slowly, the relatively rapid decrease in water proton enhancement seen in T1w images at the injection site over ~15 min presumably reflects oxidation of Eu2+ to Eu3+. To validate this assumption, after T1w enhancement of the signal vanished, CEST images were collected to verify that Eu3+2 was indeed present. The CEST signal detected near the injection site (Figure 5e) remained evident over a period of ~20 min before disappearing (Figure S11).
Figure 5.
T1w and CEST imaging of an intramuscular injection of Eu2+2 (10 mM, 100 μl) into the thigh muscle of a healthy female C57/blk6 mouse at B0=9.4 T. T1w images at a) pre-injection, b) 5 min, c) 12 min, d) 16 min and e) the CEST image at 17 min. Selected imaging parameters: T1w: ge3D sequence, TR=3.6 ms, TE=1.8 ms. T1w images have been normalized to muscle tissue; PARACEST: FSEMS sequence, sat time = 3 s, sat power = 10 μT, sat frq = 42 (on-resonance),-42 (off-resonance) ppm.
Since free Eu3+ does not produce a CEST effect, the detection of the agent by CEST imaging after the T1w enhancement indicates that the complex remained intact in vivo throughout the oxidation process. All of the mice recovered after imaging and no evidence of toxicity was apparent after injection of Eu2+2.
In conclusion, we have shown that ligand 2 forms a complex with Eu2+ that is surprisingly stable to oxidation. The Eu2+-bound water exchange rate for this complex (kex = 0.21 × 109 s−1) was found to be extremely fast, indicating that the amide sidearms do not have a significant decelerating effect on the water exchange. The agent has different contrast enhancing properties depending on the oxidation state of the metal. In its divalent form it is an efficient T1 shortening agent with an r1 relaxivity comparable to Gd-based contrast agents. Oxidation converts it into Eu3+2, which is a commonly used paramagnetic chemical exchange saturation (paraCEST) agent. The oxidation of Eu2+2 by air or H2O2 could be followed by both T1w and CEST imaging. The improved oxidative stability of Eu2+2 When injected intramuscularly into healthy mice the complex generated strong T1 enhanchement that gradually diminished over several minutes after which strong CEST effect could be observed at the injection site. This complex could serve as a design platform for a novel class of redox sensitive bimodal MR contrast agents in which the redox potential of the Eu2+ may be fine-tuned by the nature of the peripheral amide groups.
Supplementary Material
Acknowledgments
Financial support from the NIH (R01-CA115531, P41-EB015908 and 1P30-CA142543) and the Robert A. Welch Foundation (AT-584) is acknowledged. The authors thank Prof Kayla Green (Texas Christian University) for help with cyclic voltammetry.
Footnotes
Experimental Section
Detailed experimental procedures are given in the Supporting Information.
Contributor Information
Dr. Alexander M. Funk, Advanced Imaging Research Center, UT Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, Texas, 75390 (USA)
Dr. Veronica Clavijo Jordan, Advanced Imaging Research Center, UT Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, Texas, 75390 (USA)
Prof. A. Dean Sherry, Advanced Imaging Research Center, UT Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, Texas, 75390 (USA). Department of Chemistry, University of Texas, Dallas, 800 West Campbell Road, Richardson, Texas, 75080 (USA)
Dr. S. James Ratnakar, Email: james.ratnakar@utsouthwestern.edu, Advanced Imaging Research Center, UT Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, Texas, 75390 (USA)
Prof. Zoltan Kovacs, Email: zoltan.kovacs@utsouthwestrn.edu, Advanced Imaging Research Center, UT Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, Texas, 75390 (USA)
References
- 1.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 2.Tóth É, Burai L, Merbach AE. Coord Chem Rev. 2001;216–217:363–382. [Google Scholar]
- 3.Burai L, Tóth É, Moreau G, Sour A, Scopelliti R, Merbach AE. Chem – Eur J. 2003;9:1394–1404. doi: 10.1002/chem.200390159. [DOI] [PubMed] [Google Scholar]
- 4.Gamage NDH, Mei Y, Garcia J, Allen MJ. Angew Chem Int Ed. 2010;49:8923–8925. doi: 10.1002/anie.201002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekanger LA, Polin LA, Shen Y, Haacke EM, Martin PD, Allen MJ. Angew Chem Int Ed. 2015;54:14398–14401. doi: 10.1002/anie.201507227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caravan P, Merbach AE. Chem Commun. 1997:2147–2148. [Google Scholar]
- 7.Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Chem Rev. 2010;110:2960–3018. doi: 10.1021/cr900284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekanger LA, Ali MM, Allen MJ. Chem Commun. 2014;50:14835–14838. doi: 10.1039/c4cc07027e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon WT, Ren J, Lubag AJM, Ratnakar J, Vinogradov E, Hancu I, Lenkinski RE, Sherry AD. Magn Reson Med. 2010;63:625–632. doi: 10.1002/mrm.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zucchi G, Thuéry P, Rivière E, Ephritikhine M. Chem Commun. 2010;46:9143–9145. doi: 10.1039/c0cc02539a. [DOI] [PubMed] [Google Scholar]
- 11.Moreau G, Burai L, Helm L, Purans J, Merbach AE. J Phys Chem A. 2003;107:758–769. [Google Scholar]
- 12.Seibig S, Tóth É, Merbach AE. J Am Chem Soc. 2000;122:5822–5830. [Google Scholar]
- 13.Regueiro-Figueroa M, Barriada JL, Pallier A, Esteban-Gómez D, de Blas A, Rodríguez-Blas T, Tóth É, Platas-Iglesias C. Inorg Chem. 2015;54:4940–4952. doi: 10.1021/acs.inorgchem.5b00548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.