Abstract
Background
It has not been established to what extent oral health is associated with cognitive function in late middle–aged adults. In this study, which is part of the national Atherosclerosis Risk in Communities (ARIC) study, the authors investigated whether tooth loss and periodontitis are associated with lower cognitive function.
Methods
The authors analyzed ARIC data measuring cognitive function in 11,097 participants from 1996 through 1998 according to tests of delayed word recall, digit-symbol substitution (DSS) and word fluency; 9,874 participants answered dental screening questions. Of the 8,554 dentate participants, 5,942 received oral examinations. The authors used measures of dental status, number of teeth and periodontitis (classified according to the Biofilm-Gingival Interface Index) in multiple linear regression models to estimate these factors’ cross-sectional association with cognitive scores, adjusting for sociodemographic factors, cigarette smoking, alcohol use and diabetes.
Results
Approximately 13 percent of participants were edentulous. Of the dentate participants, 27.3 percent had fewer than 20 teeth and 12.4 percent had pocket depth of 4 millimeters or more with severe bleeding. Compared with dentate participants, edentulous participants had lower scores for all cognitive tests. Among the dentate participants, having fewer teeth and gingival bleeding were associated with lower DSS and word fluency test scores, although periodontal pocket depth was not.
Conclusions
In this cohort, edentulism was correlated with lower cognitive status. Tooth loss and gingival bleeding were markers of poorer executive function among dentate people.
Practical Implications
The association of lower cognitive scores with edentulism suggests that past oral diseases may be a risk indicator for cognitive decline, whereas the association with gingival inflammation indicates a possible effect of cognitive decline on oral health. Practitioners should be aware that both current and historical markers of oral disease might be associated with decline in cognitive function, even in adults of late middle age.
Keywords: Cognitive function, periodontitis, gingivitis, tooth loss
As populations age, cognitive impairment and dementia become increasingly challenging public health problems that adversely affect older adults’ quality of life and health care costs.1 Many studies, involving primarily older adults, have linked oral diseases, tooth loss or both with low cognitive performance and onset of dementia,2–14 although such associations could arise from various mechanisms. Whereas chronic poor oral health plausibly can influence cognitive decline, people with cognitive impairment have reduced ability to perform oral hygiene, which could lead to poor oral health.15–17
Cross-sectional studies, which have provided most of the evidence to date, typically are unable to establish the temporal sequence underlying causal associations. If the mechanism behind the association begins with cognitive impairment, an extended interval would be needed for the subsequent decline in oral health to translate into tooth loss and edentulism; thus, the association likely would not be observed in a cross-sectional study. A more likely pathway for the association is that poor oral health leads to cognitive decline. The results of several studies suggest that periodontal infection and increased inflammatory levels in early life could be responsible for later cognitive decline.6,7,11,14,18–20
Although investigators in cross-sectional studies14,20,21 and a prospective study19 found that biological markers for periodontal infection were associated with dementia or cognitive impairment, studies in which researchers have investigated these associations by using clinical signs of periodontal disease (for example, pocket depth, gingival bleeding or attachment loss) have shown mixed results.2,5,7,8,11,18,22,23 Possible reasons for this variability are that many of these studies had small numbers of participants,7,18 and some studies relied on measures of periodontal disease that are based on partial examinations8,22 or self-report.2 In addition, attachment loss, often used as an indicator of past periodontal disease, also may reflect noninflammatory processes such as gingival recession caused by physical injury resulting from tooth brushing or flossing.
The purpose of our study, therefore, was to examine the associations between cognitive performance in late middle–aged adults and periodontal disease as assessed by means of an index that reflects people’s current state of periodontal inflammation. To that end, we determined how three measures of cognitive performance related to three indicators of oral health—dental status, number of teeth and periodontal disease as classified according to the Biofilm-Gingival Interface (BGI) index24—among late middle–aged adults participating in the Atherosclerosis Risk in Communities (ARIC) study, a prospective epidemiologic study conducted in four U.S. communities under the sponsorship of the National Institutes of Health, Bethesda, Md. We hypothesized that lower cognitive performance was associated with greater occurrence of edentulism, tooth loss and severe periodontal disease.
METHODS
Design and study population
In this cross-sectional study, we analyzed data collected by research personnel working on the ARIC and Dental ARIC projects. Details regarding the study designs and protocols have been reported elsewhere.25–27 Briefly, ARIC is a prospective, community-based study of middle-aged adults (aged 45–64 years at the inception) followed from 1987 through 1989 (visit 1). Follow-up visits 2, 3 and 4 occurred at three-year intervals. Data from oral examinations were collected at the fourth visit (between 1996 and 1998) as part of Dental ARIC, an ancillary study of the parent ARIC study. We confined our analysis to African American and white participants who received a cognitive assessment and dental screening interview at visit 4. A subset of screened dentate participants also underwent comprehensive dental examinations, which included periodontal probing. Of 11,097 participants who completed cognitive battery tests at visit 4, 9,874 participants underwent dental screening interviews that asked about tooth loss. Of the 8,554 dentate respondents, 5,942 received a dental examination. (The process of selecting participants is shown in eFigure 1 in the supplemental data to the online version of this article [found at http://jada.ada.org/content/144/12/1362/suppl/DC1].)
Oral health measures
Exposures included dental status, number of teeth and clinical diagnosis of periodontal disease.
We computed a participant’s dental status from answers to the following two items on a self-administered questionnaire: “Do you have any natural teeth?” and “Do you have any dental implants?” We excluded from the study participants with only dental implants and no natural teeth (n = 21). During the dental examination, the number of teeth present was recorded by dental hygienists who were hired and trained to conduct oral examinations for the ARIC study.
Periodontal probing depth (PPD) and bleeding on probing (BOP) were assessed at six sites per tooth on all remaining teeth by trained examiners. We computed the score on the BGI index by using measures of PPD (≤ 3 millimeters, no periodontal pocket; ≥ 4 mm, periodontal pocket present) and the extent of BOP (< 10 percent, low; 10–50 percent, moderate; ≥ 50 percent, severe). The BGI index has been developed on the basis of a concept that clinical disease classification should reflect an underlying biological process of periodontal disease in which there is a complex interaction of the microorganisms with host inflammatory and immune responses. We used the BGI index to classify five levels of periodontitis. We classified study participants with PPD of 3 mm or less as being periodontally healthy if the extent of their BOP was less than 10 percent or as having gingivitis if the extent of their BOP was 10 percent or greater. We classified study participants with one or more PPD of 4 mm or higher as having periodontitis with either low bleeding (LB), moderate bleeding (MB) or severe bleeding (SB).24 As secondary endpoints, we computed the case classifications as defined by Page and Eke.28 (Additional information on this is available in eTables 3 and 4 in the supplemental data to the online version of this article [found at http://jada.ada.org/content/144/12/1362/suppl/DC1].)
Cognitive function
The outcomes of interest were scores from the following cognitive tests administered by ARIC interviewers: delayed word recall (DWR), digit-symbol substitution (DSS) and word fluency (WF). The DWR tested study participants’ verbal learning and recent memory, producing scores ranging from 0 (low cognitive function) to 10 (high cognitive function). Study participants were asked to recall a list of 10 common nouns, one at a time. After a five-minute delay, participants were asked to recall these words by composing a sentence containing each one. The WF test evaluates expressive language, and the DSS test is a test of concentration and psychomotor speed. Both are used to assess executive function. For the WF test, participants were asked to generate words beginning with F, A and S, not including proper names or places, within 60 seconds for each trial. For the DSS test, participants were required to translate numbers to symbols within 90 seconds by using a key. The total number of correct translations determined the study participant’s score (ranging from 0 [low cognitive function] to 93 [high cognitive function]). Higher scores on each of the three tests indicate better cognitive ability. Cognitive test protocols from ARIC have been reported elsewhere.26
Covariates
Covariates included sociodemographic factors (age, race, sex, educational level, income and study sites), cardiovascular risk factors, apolipoprotein E (APOE) genotype, stroke and coronary heart disease (CHD). We classified educational levels as less than high school, completion of high school and postsecondary education. We coded household income (in 1996–1998 U.S. dollars) as less than $25,000, $25,000 to $50,000, greater than $50,000 and not reported. The four ARIC communities are Forsyth County, N.C.; Jackson, Miss.; the northwest suburbs of Minneapolis; and Washington County, Md. We created a variable representing race and ARIC field centers to control for the racial, regional and examiner differences in the ARIC cohort as the following: Forsyth/white, Forsyth/black, Jackson/black, Minnesota/white and Washington/white. Cardiovascular risk factors included cigarette smoking and alcohol use (each recorded as never, former or current), diabetes, hypertension, hyperlipidemia and body mass index (BMI). We dichotomized APOE genotype as the presence or absence of the APOE ɛ4 allele. (More information on the methods is provided in the appendix, which can be found in the supplemental data to the online version of this article [found at http://jada.ada.org/content/144/12/1362/suppl/DC1].)
Statistical analyses
We used bivariate analyses to assess associations of covariates with cognitive scores and oral health measures. (See eTable 2 in the supplemental data to the online version of this article [found at http://jada.ada.org/content/144/12/1362/suppl/DC1].) We analyzed cognitive scores as continuous measures by using multiple linear regression models. We used directed acyclic graphs (DAGs) and change-in-estimate procedures to select the adjustment variables in this study. We considered CHD and stroke to be mediators of the exposure-outcome association and therefore did not include them in the regression models. We defined “reduced models” as those with a minimally sufficient set of covariates, as identified in the DAGs; they included age, race, sex, study site, education, income, cigarette smoking, alcohol use and diabetes. We defined fully adjusted models as those comprising variables from the reduced model as well as BMI, hyperlipidemia, hypertension and APOE ɛ4. We compared the regression coefficient for the oral health measure (that is, dental status, number of teeth or BGI index score) from the DAG model with that from the fully adjusted model. If the estimate changed by less than 10 percent or ± 0.1, we selected the more parsimonious (reduced) model for the primary analysis. We used statistical software (SAS Version 9.3, SAS Institute, Cary, N.C.) for all analyses. The University of North Carolina at Chapel Hill institutional review board determined that this study, which involved secondary analysis of de-identified data, was of minimal risk and therefore was exempt from continuing review.
RESULTS
Characteristics of study participants
The majority of the study sample (56 percent) were women. About 80 percent of all participants were white, but all Jackson participants and about 9 percent of Forsyth participants were black. About one-half of the participants were current drinkers and 14 percent were current smokers. Compared with white participants, black participants had less education, lower incomes, more medical problems and lower scores on all cognitive tests.
Of 9,874 participants who underwent dental screening, 13.4 percent (1,320) were edentulous. Among the dentate participants (n = 8,554), those who did not receive a comprehensive dental examination (n = 2,612) were more likely than the examined dentate participants to be black and female. Participants who did not receive a dental examination also tended to have less education, low income and compromised systemic conditions (data not shown). Mean DWR scores did not differ meaningfully between participants who received examinations and those who did not (mean [standard deviation {SD}] = 6.6 [1.6] for participants without examinations versus 6.7 [1.6] for participants with examinations), although mean DSS and WF test scores were slightly lower among those who did not receive examinations (43.6 [13.6] versus 45.7 [12.5] for DSS and 33.9 [12.4] versus 34.8 [12.3] for WF). More than two-thirds of participants who received a comprehensive dental examination had periodontal disease (that is, PPD ≥ 4 mm), and approximately 12 percent were classified as having severe bleeding or severe periodontitis (Table 1). Participants with severe periodontitis had the greatest extent of attachment loss. (See eFigure 2 in the supplemental data to the online version of this article [found at http://jada.ada.org/content/144/12/1362/suppl/DC1].)
TABLE 1.
Race- and sex-specific characteristics of the study participants, as determined during ARIC visit 4.*
| CHARACTERISTIC | PARTICIPANTS
|
||||
|---|---|---|---|---|---|
| African American | White | All (N = 9,874) | |||
|
| |||||
| Female (n = 1,301) | Male (n = 693) | Female (n = 4,209) | Male (n = 3,671) | ||
|
| |||||
| Age at Visit 4, Mean (Standard Deviation [SD]) | 61.6 (5.6) | 61.7 (5.8) | 62.7 (5.6) | 63.5 (5.6) | 62.8 (5.7) |
|
| |||||
| Study Sites, % | |||||
| Forsyth County, N.C. | 11.7 | 12.1 | 28.8 | 28.7 | 25.4 |
| Jackson, Miss. | 88.3 | 87.9 | 0 | 0 | 17.8 |
| Minneapolis | 0 | 0 | 36.1 | 37.0 | 29.1 |
| Washington County, Md. | 0 | 0 | 35.1 | 34.3 | 27.7 |
|
| |||||
| Education, % | |||||
| Less than high school | 32.4 | 32.0 | 13.4 | 14.6 | 17.6 |
| Completion of high school | 31.6 | 27.9 | 51.3 | 39.5 | 42.7 |
| Postsecondary education | 36.0 | 40.1 | 35.3 | 45.9 | 39.7 |
|
| |||||
| Income, % | |||||
| Not reported | 2.5 | 2.7 | 2.1 | 2.1 | 2.2 |
| Less than $25,000 | 64.1 | 44.6 | 28.0 | 17.9 | 30.2 |
| $25,000 to less than $50,000 | 22.9 | 27.4 | 37.4 | 38.0 | 35.0 |
| $50,000 or more | 22.9 | 27.4 | 37.4 | 38.0 | 35.0 |
|
| |||||
| Cigarette Smoking, % | |||||
| Current | 13.5 | 22.2 | 13.9 | 13.9 | 14.4 |
| Former | 28.9 | 49.9 | 35.2 | 58.2 | 43.9 |
| Never | 57.6 | 27.9 | 50.9 | 27.9 | 41.6 |
|
| |||||
| Alcohol Use, % | |||||
| Current | 18.2 | 40.4 | 51.6 | 62.2 | 50.4 |
| Former | 36.9 | 43.6 | 25.5 | 28.8 | 29.5 |
| Never | 44.9 | 16.0 | 22.9 | 9.0 | 20.1 |
|
| |||||
| Medical History | |||||
| Diabetes mellitus, % | 26.6 | 23.9 | 11.1 | 15.7 | 15.8 |
| Hypertension, % | 70.1 | 59.9 | 41.0 | 42.3 | 46.6 |
| Coronary heart disease, % | 4.5 | 9.5 | 3.8 | 14.7 | 8.4 |
| Stroke, % | 2.2 | 4.5 | 1.4 | 2.3 | 2.1 |
| Hyperlipidemia, % | 35.7 | 36.9 | 41.1 | 40.8 | 40.0 |
| Body mass index (kilograms per square meter), mean (SD) | 31.7 (6.7) | 28.7 (4.9) | 28.2 (5.9) | 28.4 (4.3) | 28.7 (5.6) |
| Presence of APOE† ɛ4, % | 39.0 | 42.1 | 27.2 | 27.4 | 29.9 |
|
| |||||
| Oral Health Conditions | |||||
| Edentulism, % | 22.5 | 14.0 | 10.9 | 12.8 | 13.4 |
| Number of teeth,‡ mean (SD) | 17.2 (7.4) | 18.3 (7.8) | 23.0 (6.4) | 22.8 (6.8) | 21.9 (7.1) |
| Periodontal disease (BGI§),‡ % | |||||
| Had periodontal pockets | |||||
| Severe bleeding | 14.4 | 31.0 | 8.2 | 13.3 | 12.3 |
| Moderate bleeding | 21.9 | 27.4 | 39.6 | 47.4 | 39.5 |
| Low bleeding | 8.7 | 12.4 | 19.9 | 20.5 | 18.5 |
| No periodontal pockets | |||||
| Had gingivitis | 22.8 | 16.0 | 16.0 | 11.4 | 14.9 |
| Healthy | 32.2 | 13.2 | 16.3 | 7.4 | 14.4 |
|
| |||||
| Cognitive Functions | |||||
| Delayed word recall, mean score (SD)¶ | 6.3 (1.6) | 5.6 (1.7) | 7.0 (1.5) | 6.3 (1.5) | 6.6 (1.6) |
| Digit-symbol substitution, mean score (SD)# | 32.3 (13.2) | 28.9 (13.2) | 49.7 (11.0) | 43.9 (10.7) | 43.8 (13.3) |
| Word fluency, mean score (SD)** | 29.1 (12.7) | 27.4 (14.1) | 36.1 (11.6) | 33.5 (12.3) | 33.6 (12.5) |
ARIC: Atherosclerosis Risk in Communities, a study being conducted by the National Institutes of Health, Bethesda, Md. At visit 4 (1996–1998) of the ARIC study, participants were invited to participate in the Dental ARIC study. Participants underwent dental screening interviews, and a subset of screened dentate participants received comprehensive dental examinations.
APOE: Apolipoprotein E.
Only among dentate participants who received periodontal examinations (n = 5,942).
BGI: Biofilm-Gingival Interface (Offenbacher and colleagues24). In the BGI index, periodontal disease is classified on the basis of probing pocket depth and the extent of bleeding on probing.
Possible range: 0 (low cognitive function) to 10 (high cognitive function).
Possible range: 0 (low cognitive function) to 93 (high cognitive function).
The minimum score is 0; however, there is no maximum value. The score is the total number of words beginning with the letters F, A, and S—not including proper names or places—that a participant generates within 60 seconds for each trial.
About 70 percent of edentulous and dentate participants reported past tooth loss due to caries. Edentulous participants were twice as likely as dentate participants (32.5 percent versus 11.1 percent) to report past tooth loss due to periodontal disease (“gum disease” was the response on the questionnaire) and 42.1 percent of edentulous participants had had an initial diagnosis of “gum disease” more than 30 years before (Table 2, page 1367).
TABLE 2.
Comparisons of self-reported causes of tooth loss, prosthesis use and periodontal disease among study participants, according to dental status.
| SELF-REPORTED ITEMS | DENTAL STATUS
|
|
|---|---|---|
| Edentulous (n = 1,320) | Dentate (n = 8,554) | |
|
| ||
| Causes of Tooth Loss, % | ||
| Cavities* | 73.6 | 71.8 |
| Unknown | 1.9 | 3.4 |
| Data missing | 0.08 | 0.01 |
| Gum disease* | 32.5 | 11.1 |
| Unknown | 3.3 | 2.0 |
| Data missing | 0.08 | 0.01 |
| Wisdom teeth* | 75.9 | 75.1 |
| Unknown | 2.6 | 3.4 |
| Data missing | 0.08 | 0.05 |
| Overcrowding* | 9.2 | 18.6 |
| Unknown | 2.0 | 2.2 |
| Data missing | 0.08 | 0.01 |
| Other reasons* | 18.6 | 14.8 |
| Unknown | 1.2 | 0.6 |
| Data missing | 0.2 | 0.1 |
|
| ||
| Prosthesis Use | ||
| Had false teeth,* % | 97.8 | 50.5 |
| Data missing | 0.2 | 0.05 |
| Age at receipt of first false tooth,† mean (standard deviation [SD]) | 36.0 (13.7) | 38.6 (14.6) |
|
| ||
| Gum Disease | ||
| Ever noticed any loose teeth, % | 26.1 | 15.7 |
| Unknown | 1.8 | 0.2 |
| Data missing | 2.2 | 1.0 |
| Had gum disease, % | 22.0 | 22.3 |
| Unknown | 0.3 | 0.5 |
| Data missing | 0 | 0.06 |
| Duration of gum disease‡ | ||
| Mean (SD) | 24.3 (12.9) | 11.1 (10.1) |
| Less than 10 years, % | 10.7 | 51.0 |
| 10 to less than 20 years, % | 23.1 | 27.0 |
| 20 to less than 30 years, % | 24.1 | 14.8 |
| 30 years or more, % | 42.1 | 7.2 |
Of 1,290 edentulous and 7,698 dentate participants who reported history of tooth loss.
Of 1,261 edentulous and 3,886 dentate participants who had prostheses, 1,202 edentulous and 3,683 dentate participants reported the age at which they received their first false tooth.
Of 291 edentulous and 1,907 dentate participants who had periodontal disease, 290 edentulous and 1,985 dentate participants reported duration since they were first diagnosed.
In the multiple regression analyses, hypertension, BMI, hyperlipidemia and APOE ɛ4 were not confounding variables for any of the associations between oral health measures and cognitive scores. Therefore, we present results from solely the DAG models in Table 3 (page 1368).
TABLE 3.
Regression coefficients for the associations between oral health measures and cognitive scores at ARIC visit 4.*†‡
| ORAL HEALTH MEASURE | NO. | TEST
|
|||||
|---|---|---|---|---|---|---|---|
| Delayed Word Recall | Digit-Symbol Substitution | Word Fluency | |||||
|
| |||||||
| b§ (SE¶) | P Value | b (SE) | P Value | b (SE) | P Value | ||
|
| |||||||
| Dental Status | 9,874 | ||||||
| Edentulous | 1,320 | −0.16 (0.046) | .0004 | −2.18 (0.30) | < .0001 | −1.87 (0.35) | < .0001 |
| Dentate | 8,554 | Reference | Reference | Reference | |||
|
| |||||||
| Periodontal Disease (BGI#) | 5,942 | ||||||
| Had periodontal pockets | |||||||
| Severe bleeding | 733 | 0.047 (0.076) | .4493 | −0.44 (0.48) | .0464 | −0.78 (0.59) | .0015 |
| Moderate bleeding | 2,374 | 0.069 (0.060) | 0.26 (0.38) | 0.12 (0.46) | |||
| Low bleeding | 1,097 | 0.047 (0.069) | 0.21 (0.44) | 1.31 (0.53) | |||
| No periodontal pockets | |||||||
| Gingivitis | 884 | 0.13 (0.071) | −0.84(0.45) | −0.78 (0.55) | |||
| Healthy | 854 | Reference | Reference | Reference | |||
|
| |||||||
| Number of Teeth | |||||||
| One-tooth increase | 5,942 | 0.0024 (0.003) | .4252 | 0.069 (0.019) | .0003 | 0.086 (0.023) | .0002 |
ARIC: Atherosclerosis Risk in Communities, a study being conducted by the National Institutes of Health, Bethesda, Md. At visit 4 (1996–1998) of the ARIC study, participants were invited to participate in the Dental ARIC study. Participants underwent dental screening interviews, and a subset of screened dentate participants received comprehensive dental examinations.
Type III P value tested the overall effect of periodontal disease.
Covariates in models included age, sex, race/study center, education, income, smoking, alcohol use and diabetes.
b: Regression coefficient.
SE: Standard error of the mean.
BGI: Biofilm-Gingival Interface (Offenbacher and colleagues24). In the BGI index, periodontal disease is classified on the basis of probing pocket depth and the extent of bleeding on probing.
Tooth loss and cognitive function
Complete tooth loss was significantly associated with lower DWR, DSS and WF scores (Table 3, Figure 1 [page 1369]). Regression coefficients for complete tooth loss were attenuated greatly after we adjusted for sociodemographic factors. The associations remained significant after we controlled for cigarette smoking, alcohol use and diabetes. Among the dentate participants, a larger number of teeth present was associated with higher cognitive scores for all tests. However, the number of teeth was no longer associated with DWR scores after we adjusted for sociodemographic factors. The adjusted associations with DSS (b = 0.069 per tooth, = .0003) and WF (b = 0.086, P = .0002) were significant in the final models (Table 3).
Figure 1.
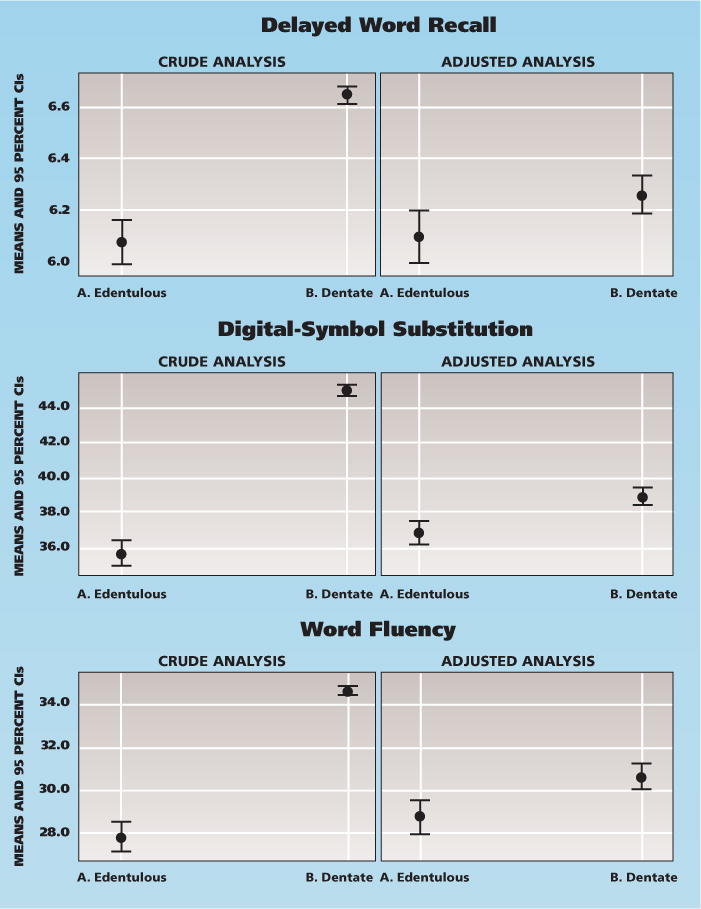
Crude and adjusted mean with 95 percent confidence intervals (CIs) of three cognitive scores, comparing edentulous participants with dentate participants (N = 9,874). Covariates in the adjusted models included age, sex, race/study center, education, income, cigarette smoking, alcohol use and diabetes.
Periodontal disease and cognitive function
In the crude analyses, periodontal disease was associated with all cognitive test results, and the group with severe periodontitis had the lowest cognitive scores. As with the analysis for the number of teeth, the association with DWR scores was not evident after adjustment for sociodemographic factors. In the final models, BGI was associated marginally with DSS (P = .05) and significantly with WF (P = .001); however, there was no clear trend of the relationship across the five levels of the BGI index. The lowest cognitive scores were found in participants with gingivitis or severe periodontitis (Table 3, Figure 2 [page 1370]).
Figure 2.
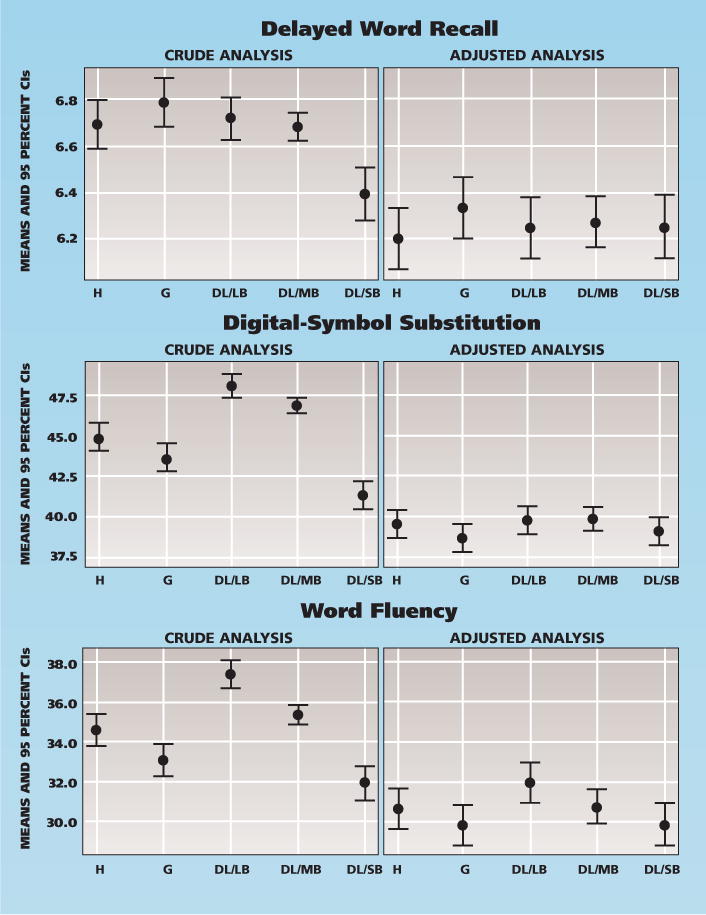
Crude and adjusted mean with 95 percent confidence intervals (CIs) of three cognitive scores, comparing five levels of periodontal conditions (n = 5,942). Covariates in the adjusted models included age, sex, race/study center, education, income, cigarette smoking, alcohol use and diabetes. DL/LB: Deep lesion/low bleeding. DL/MB: Deep lesion/moderate bleeding. DL/SB: Deep lesion/severe bleeding. G: Gingivitis. H: Healthy.
DISCUSSION
In this cohort of late middle–aged adults, participants with no teeth had cognitive scores lower than those of dentate particpants on all three tests. The other two indicators of poor oral health—decrease in number of teeth and presence of periodontal disease—were associated with lower DSS and WF scores, but not with lower DWR scores. We did not observe a clear dose-response relationship among five levels of periodontal disease created by the BGI index. Instead, cognitive scores were related to the extent of BOP.
Strengths of this study include its large population-based sample and high quality of periodontal and cognitive function assessments. To our knowledge, our study is the first examination of the association between periodontal disease, as classified by using indicators of disease at the biofilm-gingival interface, and cognitive performance. A primary advantage of using the BGI index was that it enabled us to investigate the association between natural gradients of periodontal disease severity and cognitive function. The five levels of the BGI differ in their underlying biological process, which include the microbial, inflammatory and acquired immune responses. For example, participants with gingivitis (BGI-G), deep lesions and moderate BOP (BGI-DL/MB) and deep lesions and severe BOP (BGI-DL/SB) were more likely to have increased immunoglobulin G (IgG) titers specific for Campylobacter rectus. The two latter groups were more likely to show elevated IgG titers of Prophyromonas gingivalis.27
A fundamental limitation of our study is that our data are cross-sectional, so associations could reflect effects of poor oral health on cognitive decline or the reverse. Furthermore, oral examinations in ARIC were limited to study participants who had no contraindication to periodontal probing (that is, no requirement for antibiotic prophylaxis). If those who require antibiotic prophylaxis have medical conditions that are associated with severe periodontal disease, this exclusion could lead to an underestimation of the association with cognitive function. Another limitation is that our cognitive tests covered only two cognitive domains, memory (via the DWR test) and executive function (via the DSS and WF tests).
The cross-sectional nature of our study complicates interpretation of the associations we examined. One difficulty is that associations between oral health and cognitive scores could reflect influences in either direction, as mentioned previously. Another difficulty is that cross-sectional associations are more vulnerable to confounding by important covariables—notably, educational attainment—than are analyses of changes in cognitive function.29 In a separate publication, our research group30 investigated changes in cognitive function between visit 2 and visit 4 as a predictor of oral health status at visit 4. In that analysis, in which we also controlled for study participants’ education, most oral health measures—including periodontal disease—were not significantly associated with change in cognitive function, although edentulism was. The consistency of findings regarding complete tooth loss in that study and this one provide some reassurance that residual confounding was not a serious problem. Last, it has been reported that poor oral health and cognitive deficits also are related to compromised systemic health, unhealthy behaviors (such as cigarette smoking) or psychosocial factors (such as stress).9–11,22 Although we included all measured confounding variables and modifiers in the analyses, biased estimates due to unmeasured confounders still are possible.
Although research personnel measured tooth loss and cognitive function concurrently, we noted that study participants’ loss of multiple teeth typically occurred earlier in adulthood. In addition, edentulous participants were more likely to report tooth loss due to periodontal disease than were dentate participants (Table 2), suggesting that past periodontal disease may be a risk indicator for low cognitive function in midlife. On the other hand, at least part of the positive association between complete tooth loss and cognitive function may be attributable to sociodemographic factors. The characteristic of having no teeth retained statistical significance in the multiple regression models for low cognitive function that included terms for health behaviors and medical conditions, although residual confounding by socioeconomic status is a distinct possibility. In contrast, the authors of a large cross-sectional study that included middle-aged adults (45 years and older) suggested that an association they observed between tooth loss and word recall test scores likely was due to confounding because it disappeared after they controlled for socioeconomic status.31
Our results are consistent with those from studies that suggest that loss of multiple teeth early in life increases the risk of experiencing cognitive decline.2,3,5,7,10 For instance, a case-control study involving monozygotic twins reported that only history of tooth loss before the age of 35 years was a significant risk factor for Alzheimer disease.10 A cross-sectional study in Japanese older adults reported an association between having an extended edentulous period (15 years or longer) and increased risk of having low cognitive scores.3 However, both of these studies were retrospective and relied on self-reports of tooth loss.
Two possible biological pathways whereby tooth loss could accelerate cognitive decline have been proposed: systemic infection and resultant inflammation7,32 and nutritional deficiency.4,33 Indirect evidence for the second pathway comes from a Japanese study of older adults in which having few teeth and no dentures increased the risk of experiencing the onset of dementia (hazard ratio, 1.85; 95 percent confidence interval, 1.04–3.31).4 However, in this study, we found that edentulous study participants had BMIs higher than those of dentate participants (data not shown), contradicting the idea that tooth loss might have contributed to nutritional deficiency. We also found that most edentulous study participants (98 percent) had dentures, which probably compensated for nutritional deficiencies caused by tooth loss. Overall, our study has insufficient direct evidence to support or refute the proposed pathway mediated by nutritional deficiency.
Among the dentate participants in our study, both number of teeth and presence of periodontal disease were associated with diminished executive cognitive function (as gauged by the results of the DSS and WF tests), but these associations were relatively weak. Examination of cognitive scores in relation to each component of the BGI index indicated that lower cognitive function was related primarily to the extent of gingival bleeding rather than to periodontal pocket depth. (See eTable 4 in the supplemental data to the online version of this article at http://jada.ada.org/content/144/12/1362/suppl/DC1.) Our finding was consistent with the findings of a previous cross-sectional study analyzing data from the 1988–1994 National Health and Nutrition Examination Survey, in which the extent of gingival bleeding was associated with low scores on two cognitive tests in young and middle-aged adults (20–59 years).22
The BGI index reflects both the current and the cumulative burden of periodontal disease. Thus, the group that had periodontitis with severe bleeding also had a greater extent of attachment loss.24 We conducted a supplementary analysis, which showed that low cognitive scores also were correlated with greater extent of attachment loss. (More details are shown in eTable 4, available as supplemental data to the online version of this article at http://jada.ada.org/content/144/12/1362/suppl/DC1.) In theory, if chronic periodontal disease and inflammatory burden truly contribute to early onset of cognitive decline, participants with severe periodontitis should have the lowest cognitive scores. However, a relationship might not be seen if periodontal disease exposure is underestimated owing to tooth loss or if the induction period for cognitive decline is long. In a longitudinal study in older men, investigators found that additional tooth loss with progression of alveolar bone loss or progression of pocket depth per decade predicted low cognitive function after a 32-year follow-up interval.5 Participants who had gingivitis with active inflammation (that is, a greater extent of bleeding) could represent people with early lesions of periodontal disease or people whose periodontal disease has been treated. In our study sample, about one-half of dentate participants reported less than 10 years of having periodontal disease, and about 11 percent have lost teeth because of periodontal disease (Table 2).
Alternatively, a reverse causal process may be possible. An early decline in the executive function domain may alter a person’s cognitive processes (for example, planning, reasoning, initiation or making decisions), resulting in inadequate oral hygiene. Our previous study results revealed that six-year changes in executive function (between the 1990–1992 and 1996–1998 periods) were associated with infrequent tooth brushing, gingival inflammation or greater plaque deposits.31 This finding may explain why participants with gingivitis also had lower DSS and WF scores when compared with the others. Similar to tooth loss, periodontal disease was associated strongly with sociodemographic factors in the ARIC study. Therefore, potential residual confounding by sociodemographic factors is the most obvious alternative explanation for our study results.
CONCLUSIONS
Our study findings add to the evidence that complete tooth loss, low number of teeth and the inflammatory stage of periodontal disease are associated with lower cognitive performance. Given that severe periodontitis and edentulism likely occurred many years before participants were examined in this study, we believe that the process through which these conditions developed preceded, rather than followed, age-related cognitive decline. The association of poorer cognitive function with gingivitis could reflect an effect of cognitive decline, however. Thus, the association between oral health and cognitive function could reflect causal processes in either direction—or both directions.
Supplementary Material
Acknowledgments
The Atherosclerosis Risk in Communities study was carried out as a collaborative study supported by the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, Md. (contracts N01-HC55015, N01-HC 55016, N01-HC 55018, N01-HC 55019, N01-HC 55020, N01-HC 55021 and N01-HC 55022). The collection and analysis of dental data were supported by the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, Md. (grants DE 13807-01A1 and DE1 1551).
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
ABBREVIATION KEY
- APOE
Apolipoprotein E
- ARIC
Atherosclerosis Risk in Communities
- BGI
Biofilm-Gingival Interface
- BMI
Body mass index
- BOP
Bleeding on probing
- CHD
Coronary heart disease
- DAG
Directed acyclic graphs
- DL
Deep lesion
- DSS
Digit-symbol substitution
- DWR
Delayed word recall
- G
Gingivitis
- H
Healthy
- IgG
Immunoglobulin G
- LB
Low bleeding
- MB
Moderate bleeding
- PPD
Periodontal probing depth
- SB
Severe bleeding
- WF
Word fluency
Footnotes
Disclosure. None of the authors reported any disclosures.
Contributor Information
Dr. Supawadee Naorungroj, Research assistant, Department of Dental Ecology, School of Dentistry, University of North Carolina at Chapel Hill.
Dr. Victor J. Schoenbach, Associate professor, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill.
Dr. James Beck, Professor, Department of Dental Ecology, School of Dentistry, University of North Carolina at Chapel Hill.
Dr. Thomas H. Mosley, Professor, Division of Geriatrics and Gerontology, Department of Medicine, University of Mississippi Medical Center, Jackson.
Dr. Rebecca F. Gottesman, Associate professor of neurology, Cerebrovascular Center, School of Medicine, The Johns Hopkins University, Baltimore.
Dr. Alvaro Alonso, Associate professor, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis.
Dr. Gerardo Heiss, Professor, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill.
Dr. Gary D. Slade, Professor, Department of Dental Ecology, School of Dentistry, University of North Carolina at Chapel Hill, Koury Oral Health Sciences Building, Room 4501E, CB#7455, Chapel Hill, N.C., 27599-7455.
References
- 1.Alzheimer’s Association. 2012 Alzheimer’s facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Batty GD, Li Q, Huxley R, et al. VANCE Collaborative Group Oral disease in relation to future risk of dementia and cognitive decline: prospective cohort study based on the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation (ADVANCE) trial. Eur Psychiatry. 2013;28(1):49–52. doi: 10.1016/j.eurpsy.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okamoto N, Morikawa M, Okamoto K, et al. Relationship of tooth loss to mild memory impairment and cognitive impairment: findings from the Fujiwara-kyo study. Behav Brain Funct. 2010;6:77. doi: 10.1186/1744-9081-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto T, Kondo K, Hirai H, Nakade M, Aida J, Hirata Y. Association between self-reported dental health status and onset of dementia: a 4-year prospective cohort study of older Japanese adults from the Aichi Gerontological Evaluation Study (AGES) project. Psychosom Med. 2012;74(3):241–248. doi: 10.1097/PSY.0b013e318246dffb. [DOI] [PubMed] [Google Scholar]
- 5.Kaye EK, Valencia A, Baba N, Spiro A, 3rd, Dietrich T, Garcia RI. Tooth loss and periodontal disease predict poor cognitive function in older men. J Am Geriatr Soc. 2010;58(4):713–718. doi: 10.1111/j.1532-5415.2010.02788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein PS, Kryscio RJ, Desrosiers M, Donegan SJ, Gibbs MB. Tooth loss, apolipoprotein E, and decline in delayed word recall. J Dent Res. 2010;89(5):473–477. doi: 10.1177/0022034509357881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein PS, Desrosiers M, Donegan SJ, Yepes JF, Kryscio RJ. Tooth loss, dementia and neuropathology in the Nun study. JADA. 2007;138(10):1314–1322. doi: 10.14219/jada.archive.2007.0046. [DOI] [PubMed] [Google Scholar]
- 8.Arrivé E, Letenneur L, Matharan F, et al. Oral health condition of French elderly and risk of dementia: a longitudinal cohort study. Community Dent Oral Epidemiol. 2012;40(3):230–238. doi: 10.1111/j.1600-0528.2011.00650.x. [DOI] [PubMed] [Google Scholar]
- 9.Shimazaki Y, Soh I, Saito T, et al. Influence of dentition status on physical disability, mental impairment, and mortality in institutionalized elderly people. J Dent Res. 2001;80(1):340–345. doi: 10.1177/00220345010800010801. [DOI] [PubMed] [Google Scholar]
- 10.Gatz M, Mortimer JA, Fratiglioni L, et al. Potentially modifiable risk factors for dementia in identical twins. Alzheimers Dement. 2006;2(2):110–117. doi: 10.1016/j.jalz.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Stewart R, Weyant RJ, Garcia ME, et al. Adverse oral health and cognitive decline: the health, aging and body composition study. J Am Geriatr Soc. 2013;61(2):177–184. doi: 10.1111/jgs.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stewart R, Hirani V. Dental health and cognitive impairment in an English national survey population. J Am Geriatr Soc. 2007;55(9):1410–1414. doi: 10.1111/j.1532-5415.2007.01298.x. [DOI] [PubMed] [Google Scholar]
- 13.Lexomboon D, Trulsson M, Wardh I, Parker MG. Chewing ability and tooth loss: association with cognitive impairment in an elderly population study. J Am Geriatr Soc. 2012;60(10):1951–1956. doi: 10.1111/j.1532-5415.2012.04154.x. [DOI] [PubMed] [Google Scholar]
- 14.Noble JM, Borrell LN, Papapanou PN, Elkind MSV, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry. 2009;80(11):1206–1211. doi: 10.1136/jnnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syrjälä AM, Ylöstalo P, Ruoppi P, et al. Dementia and oral health among subjects aged 75 years or older. Gerodontology. 2012;29(1):36–42. doi: 10.1111/j.1741-2358.2010.00396.x. [DOI] [PubMed] [Google Scholar]
- 16.Chalmers JM, Carter KD, Spencer AJ. Caries incidence and increments in community-living older adults with and without dementia. Gerodontology. 2002;19(2):80–94. doi: 10.1111/j.1741-2358.2002.00080.x. [DOI] [PubMed] [Google Scholar]
- 17.Avlund K, Holm-Pedersen P, Morse DE, Viitanen M, Winblad B. Tooth loss and caries prevalence in very old Swedish people: the relationship to cognitive function and functional ability. Gerodontology. 2004;21(1):17–26. doi: 10.1046/j.1741-2358.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 18.Kamer AR, Morse DE, Holm-Pedersen P, Mortensen EL, Avlund K. Periodontal inflammation in relation to cognitive function in an older adult Danish population. J Alzheimers Dis. 2012;28(3):613–624. doi: 10.3233/JAD-2011-102004. [DOI] [PubMed] [Google Scholar]
- 19.Sparks Stein P, Steffen MJ, Smith C, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimer’s and Dementia. 2012;8(3):196–203. doi: 10.1016/j.jalz.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cockburn AF, Dehlin JM, Ngan T, et al. High throughput DNA sequencing to detect differences in the subgingival plaque microbiome in elderly subjects with and without dementia. Investig Genet. 2012;3(1):19. doi: 10.1186/2041-2223-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rai B, Kaur J, Anand SC. Possible relationship between periodontitis and dementia in a North Indian old age population: a pilot study. Gerodontology. 2012;29(2):e200–e205. doi: 10.1111/j.1741-2358.2010.00441.x. [DOI] [PubMed] [Google Scholar]
- 22.Stewart R, Sabbah W, Tsakos G, D’Aiuto F, Watt RG. Oral health and cognitive function in the Third National Health and Nutrition Examination Survey (NHANES III) Psychosom Med. 2008;70(8):936–941. doi: 10.1097/PSY.0b013e3181870aec. [DOI] [PubMed] [Google Scholar]
- 23.Sabbah W, Watt RG, Sheiham A, Tsakos G. The role of cognitive ability in socio-economic inequalities in oral health. J Dent Res. 2009;88(4):351–355. doi: 10.1177/0022034509334155. [DOI] [PubMed] [Google Scholar]
- 24.Offenbacher S, Barros SP, Singer RE, Moss K, Williams RC, Beck JD. Periodontal disease at the biofilm-gingival interface. J Periodontol. 2007;78(10):1911–1125. doi: 10.1902/jop.2007.060465. [DOI] [PubMed] [Google Scholar]
- 25.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 26.Cerhan JR, Folsom AR, Mortimer JA, et al. Correlates of cognitive function in middle-aged adults. Gerontology. 1998;44(2):95–105. doi: 10.1159/000021991. [DOI] [PubMed] [Google Scholar]
- 27.Elter JR, Offenbacher S, Toole JF, Beck JD. Relationship of periodontal disease and edentulism to stroke/TIA. J Dent Res. 2003;82(12):998–1001. doi: 10.1177/154405910308201212. [DOI] [PubMed] [Google Scholar]
- 28.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7 suppl):1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 29.Schneider AL, Sharrett AR, Patel MD, et al. Education and cognitive change over 15 years: the Atherosclerosis Risk in Communities study. J Am Geriatr Soc. 2012;60(10):1847–1853. doi: 10.1111/j.1532-5415.2012.04164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naorungroj S, Slade GD, Beck JD, et al. Cognitive decline and oral health in middle-aged adults: the ARIC study. J Dent Res. 2013;92(9):795–801. doi: 10.1177/0022034513497960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthews JC, You Z, Wadley VG, Cushman M, Howard G. The association between self-reported tooth loss and cognitive function in the REasons for Geographic And Racial Differences in Stroke study: an assessment of potential pathways. JADA. 2011;142(4):379–390. doi: 10.14219/jada.archive.2011.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement. 2008;4(4):242–250. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Kim JM, Stewart R, Prince M, et al. Dental health, nutritional status and recent-onset dementia in a Korean community population. Int J Geriatr Psychiatry. 2007;22(9):850–855. doi: 10.1002/gps.1750. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


