Abstract
Objective
Determine swallowing mechanics associated with the first and second epiglottic movements, that is, movement to horizontal and full inversion respectively, in order to provide a clinical interpretation of impaired epiglottic function.
Study Design
Retrospective cohort study.
Methods
A heterogeneous cohort of patients with swallowing difficulties was identified (n=92). Two speech-language pathologists reviewed 5ml thin and 5ml pudding videofluoroscopic swallow studies per subject, and assigned epiglottic component scores of 0=complete inversion, 1=partial inversion, and 2=no inversion forming three groups of videos for comparison. Coordinates mapping minimum and maximum excursion of the hyoid, pharynx, larynx, and tongue base during pharyngeal swallowing were recorded using ImageJ software. A canonical variate analysis with post-hoc discriminant function analysis of coordinates was performed using MorphoJ software to evaluate mechanical differences between groups. Eigenvectors characterizing swallowing mechanics underlying impaired epiglottic movements were visualized.
Results
Nineteen of 184 video-swallows were rejected for poor quality (n=165). A Goodman-Kruskal index of predictive association showed no correlation between epiglottic component scores and etiologies of dysphagia (λ=.04). A two-way analysis of variance by epiglottic component scores showed no significant interaction effects between sex and age (f=1.4, p=.25). Discriminant function analysis demonstrated statistically significant mechanical differences between epiglottic component scores: 1&2, representing the first epiglottic movement (Mahalanobis distance=1.13, p=.0007); and, 0&1, representing the second epiglottic movement (Mahalanobis distance=0.83, p=.003). Eigenvectors indicate that laryngeal elevation and tongue base retraction underlie both epiglottic movements.
Conclusion
Results suggest that reduced tongue base retraction and laryngeal elevation underlie impaired first and second epiglottic movements. The styloglossus, hyoglossus and long pharyngeal muscles are implicated as targets for rehabilitation in dysphagic patients with impaired epiglottic inversion.
Keywords: epiglottis, deglutition, dysphagia, morphometrics, swallowing mechanics Level of Evidence: 2b
Introduction
Epiglottic inversion is an element of airway protection during swallowing. While epiglottic dysfunction has been correlated with aspiration, the functional anatomy underlying epiglottic inversion remains unclear 1. When impaired epiglottic inversion is observed during an endoscopic exam in the clinic or during an MBS study, directed treatment efforts are well served if the underlying mechanism of epiglottic inversion is understood 2. Carroll and colleagues found that epiglottic inversion was maintained better in chemoradiation patients that participated in swallowing exercises 3. Many of these exercises (tongue-hold, tongue resistance, effortful swallow, and Mendelsohn maneuver) targeted muscle groups underlying hyoid movement, laryngeal elevation, and tongue base retraction. Focused rehabilitation on particular muscle groups known to invert the epiglottis may encourage patient compliance and improve swallowing safety in dysphagic patients. The purpose of this retrospective study was to use computational analysis of swallowing mechanics represented in modified barium swallow (MBS) imaging to determine the functional anatomy associated with impaired epiglottic inversion.
Two distinct movements of epiglottic inversion are observed in MBS studies 4-7. The first epiglottic movement (FEM) positions the epiglottis to a horizontal position with the second epiglottic movement (SEM) achieving full inversion. Reports disagree with respect to which elements of swallowing physiology are associated with each epiglottic movement. The first epiglottic movement is attributed to: hyoid movement and hyolaryngeal approximation by Fink and colleagues, and Ekberg and Sigurjónsson 4-5; elevation of the hyoid and larynx by Logemann and colleagues 6; and, tongue base retraction by Van Daele and colleagues 7. Logemann and colleagues report that tongue base retraction underlies the second epiglottic movement, whereas Van Daele and colleagues conclude that the anterior hyoid movement and hyolaryngeal approximation, aided by lateral hyoepiglottic ligaments, completes epiglottic inversion. Recently, Seo and colleagues utilized a cross-correlation analysis to find that hyoid and laryngeal movements correlate with epiglottic inversion in forty healthy subjects, but did not measure tongue base retraction 8.
Previous studies utilize MBS imaging of healthy subjects to document the association of epiglottic inversion with kinematic measurements of swallowing physiology such as hyoid movement, laryngeal elevation, and tongue base retraction. The present study adds to this knowledge in two important ways. Firstly, a quasi-experimental design is used to document the mechanics underlying the FEM and SEM by forming test groups of MBS video swallows of complete inversion, partial inversion, and no inversion of the epiglottis. Secondly, by anatomically mapping elements of swallowing mechanics using dynamic imaging, the action of covariant muscle groups can be visualized using vectors resulting from computational multivariate analysis of biomechanics associated with FEM and SEM 9,10.
In the present study, a multivariate computational analysis of swallowing mechanics was used to visualize and determine the functional anatomy associated with epiglottic impairment. To make this determination, a cohort of patients with heterogeneous etiologies of dysphagic complaints was selected. Groups were formed using the Modified Barium Swallow Impairment Profile (MBSImP™©) epiglottic component score (ECS), which is based on the two epiglottic movements described by Ekberg & Sigurjónsson 11. Coordinates of anatomical landmarks delineating muscle groups were recorded from lateral view MBS imaging 10(Fig. 1). These specific landmarks map the function of: the suprahyoid or floor of mouth muscles displacing the hyoid bone 12; the long pharyngeal muscles elevating the larynx 13-15; and the styloglossus and hyoglossus retracting the tongue base 16,17 (Fig. 2). Computational morphometric analysis of these coordinates enables comparison of multivariate swallowing mechanics associated with the three positions of the epiglottis during the pharyngeal phase of swallowing and produces vectors indicating hyoid movement, laryngeal elevation, pharyngeal shortening, and tongue base retraction underlying the FEM and SEM.
Figure 1.
Coordinates mapping anatomical landmarks are here used to characterize the actions of muscles underlying pharyngeal swallowing. Coordinates #1-5 map the interactions of the mandible, cranial base, and vertebrae as skeletal levers that suspend muscles of deglutition. The distal attachments of muscles that mobilize swallowing structures are mapped by coordinates 6-10 including: #6, upper esophageal sphincter; #7, posterior cricoid; #8, anterior cricoid; #9, hyoid; and, #10, pit of the valleculae.
Figure 2.

Coordinates approximate muscle attachments underlying hyolaryngeal excursion, pharyngeal shortening, and tongue base retraction. Coordinate 9 maps the function of the suprahyoid muscles including the geniohyoid (GH) and mylohyoid (MH). Coordinates 2&3, 6&7, map the proximal and distal attachments of the long pharyngeal muscles; the stylopharyngeus (SP), salpingopharyngeus, and palatopharyngeus (PP) underlie laryngeal elevation and pharyngeal shortening. Coordinates 3&10, map the function of the styloglossus (SG) and hyoglossus (HG) retracting the tongue base.
Materials and Methods
A retrospective cohort of MBS studies was selected from a voice and swallowing clinic database for study with ethics approval from institutional review boards of participant institutions. The sample of MBS studies included standardized barium consistencies of 5ml thin and 5ml pudding swallows (Varibar Thin Liquid [40% wt/vol]); Varibar Pudding [40% wt/vol, 30% wt/wt], EZ-EM Canada, Bracco Diagnostics Inc.) from 92 subjects including 59 males and 33 females with a mean age of 62.3±14.9 yo. The cohort represents a cross section of heterogeneous dysphagic patients referred to an otolaryngology clinic for an MBS. Etiologies or comorbidities of dysphagia included: head and neck cancer (n=26), neurological disorders (n=26), gastroesophageal reflux disease or globus sensation (n=12), respiratory diseases (n=7), and other (n=21).
Epiglottic movements were rated by two speech language pathologists (BMH and JB) who had been trained and met reliability criterion for use of the MBS Impairment Profile™© 11. An MBSImP™© epiglottic component score of zero indicates full epiglottic inversion, that is, the first and second epiglottic movements (FEM & SEM) are achieved. An ECS of one indicates only partial inversion, with only the FEM achieved. An ECS of two indicates that the FEM and SEM are absent.
Videos were reviewed to ensure suitability for coordinate mapping of swallowing mechanics, and were excluded from analysis if anatomical landmarks were not visible due to image quality, poor columniation, or patient positioning. Frequency data from the remaining videos were tabulated in a 3×3 table by ECS component score (0, 1, 2) and by etiology of dysphagia (neurogenic, cancer, and other). A Goodman-Kruskal index of predictive association was performed comparing frequency data of ECS groups with etiology groups to evaluate if a relationship exists between epiglottic inversion and etiology of swallowing impairment. Kano and colleagues documented increased calcification patterns in elderly male cadavers compared to younger groups 18. To evaluate if sex-linked age difference are a factor in this study, a two-way analysis of variance with post hoc Tukey HSD test of age-sex by ECS component score was performed. Both of these analyses were executed using Vassarstats, a statistical computation website (vassarstats.net).
A medical student (BT), who was blinded to the results of the ECS rating and met reliability criterion for coordinate mapping (inter-rater ICC>.95 for all coordinates), used a coordinate methodology described by Thompson and colleagues to map muscle groups underlying: 1.) hyoid excursion, 2.) laryngeal elevation, and 3.) pharyngeal shortening 10. Additionally, a coordinate in the pit of the valleculae was added to map tongue base retraction (Figure 1). Coordinates were mapped and recorded at minimum and maximum excursion of the hyoid, larynx, pharynx, and tongue base using ImageJ digital imaging software 19. Using this method, muscle function is inferred by the displacement of landmarks based on previous structural and functional studies 14,15 (Figure 2).
A multivariate morphometric analysis of coordinates was performed using MorphoJ to compare the functional anatomy associated with each group 9,20. First, a procrustean fit of 330 frames of ten coordinates (165 swallows at minimum and maximum hyolaryngeal excursion) was executed. Then, each frame was assigned appropriate categorical variables including: epiglottic component score (0=FEM & SEM, 1=FEM, 2=no epiglottic inversion); swallow position (minimum or maximum hyolaryngeal and tongue base excursion); bolus type (5ml thin liquid and 5ml pudding); and sex. A canonical variate analysis was performed with ECS, swallowing position, bolus type, and sex as variables of interest. By interrogation, it was determined that ECS defined the second canonical variate representing 9.17% of the variance of shape change. A series of pairwise post hoc discriminant function analyses of ECS scores was performed. By comparing ECS=2 (no inversion) vs. ECS=1 (partial inversion) statistical differences in mechanics associated with the FEM was determined; and, by comparing ECS=1 (partial inversion) vs. ECS=0 (full inversion) statistical differences in mechanics associated with the SEM was determined.
Mechanical changes are here defined as mean relative locations of coordinates mapping the hyoid, larynx, upper esophageal sphincter, and tongue base of one group in contrast to another. Where statistical differences between pairwise comparisons were determined, eigenvectors were produced to depict the specific mechanical changes in hyoid movement, laryngeal elevation, pharyngeal shortening, and tongue base retraction associated with the FEM and SEM. To control for potential differences in patient morphology that would skew the results, an additional post hoc discriminate function analysis comparing ECS groups at rest was performed with no significant statistically differences found between groups.
Results
Of 184 potential video-swallows, 19 were excluded due to obscured anatomical landmarks either by poor image quality or columniation leaving 165 swallows analyzed with group distribution as follows: ECS=0 (control group or full epiglottic inversion) [n=88], ECS=1 (first epiglottic movement only)[n=43], and ECS=2 (no epiglottic inversion) [n=34]. A Goodman-Kruskal index of predictive association was used to determine that the etiology of dysphagias represented in this cohort were not predictive of the epiglottic component score (λ=.04). A two-way analysis of variance of each ECS group by age and sex showed no significant interaction effects between age and sex (f=1.4, p=.25).
Post hoc discriminant function analysis of epiglottic component scores indicated the following statistically significant differences: ECS=1 vs. ECS=2, representing the FEM (Mahalanobis distance=1.13, p =.0007); ECS=0 vs. ECS=1, representing the SEM (Mahalanobis distance=0.83, p =.003). Eigenvectors of coordinates documenting differences between ECS=1 vs. ECS=2, and ECS=0 vs. ECS=1 indicate that laryngeal elevation and tongue base retraction underlie both the FEM and SEM (Figures 3a-b). No significant differences between groups were noted in hyoid movement or pharyngeal shortening.
Figure 3a.
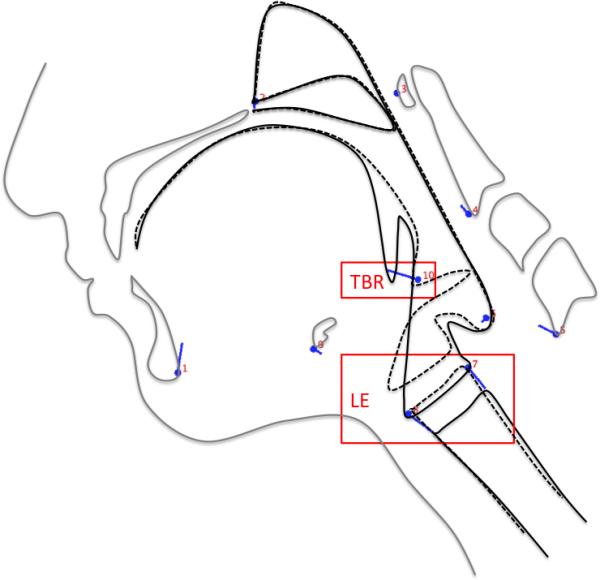
Eigenvectors of coordinates (blue lines) associated with ECS 2 (solid black lines) and ECS 1 (dashed black lines) indicate that reduced tongue base retraction (TBR) and laryngeal elevation (LE) underlie impairment of the first epiglottic movement (FEM).
Figure 3b.
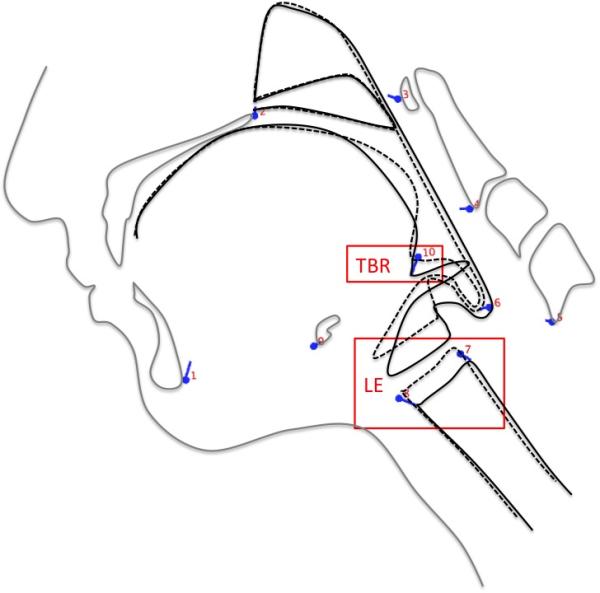
Eigenvectors of coordinates (blue lines) associated with ECS 0 (dashed black lines) and ECS 1 (solid black lines) indicate that reduced tongue base retraction (TBR) and laryngeal elevation (LE) also underlie impairment of the second epiglottic movement (SEM).
Discussion
Eigenvectors of the present study indicate that reduced laryngeal elevation and reduced tongue base retraction, but not differences in hyoid movement, underlie impaired epiglottic inversion in both the first and second epiglottic movements. This is the first study to show that non-floor of mouth muscle groups underlie impairment of both epiglottic movements.
Vectors characterizing the multivariate biomechanics in this study showed minimal observable differences in hyoid displacement between control and test groups. Seo and colleagues correlate anterior hyoid movement and laryngeal elevation with epiglottic inversion, as do other reports as previously noted. Our data show that while hyoid movement may correlate with epiglottic movement, the movement of the hyoid alone by floor of mouth muscles does not produce the FEM or SEM when tongue base retraction or laryngeal elevation is impaired. Whether or not the FEM or SEM can be produced by tongue base retraction or laryngeal elevation without hyoid movement needs further investigation.
The present findings suggest that the long pharyngeal muscles and styloglossus with hyoglossus are likely more important to the airway protection provided by the epiglottis than the suprahyoid or floor of mouth muscles. Previous studies often assume that hyolaryngeal approximation (or laryngeal elevation) is achieved by the unaccompanied action of the thyrohyoid. Recent structural and functional evidence indicates that the long pharyngeal muscles (stylopharyngeus, palatopharyngeus and salpingopharyngeus) function as a posterior muscular sling to elevate the larynx; these have been found to contribute more significantly to hyolaryngeal approximation than the thyrohyoid in normal swallowing 13-15. Tongue base retraction has been attributed to the covariant action of the styloglossus and hyoglossus 16,17. Eigenvectors in the present study approximating the mechanics of the styloglossus and hyoglossus functioning to retract the tongue base support this hypothesis.
Previous studies propose that mechanisms underlying the FEM and SEM are distinct. For example Logemann and colleagues report that hyoid movement and laryngeal elevation are associated with the FEM and tongue base retraction with SEM, whereas Van Daele and colleagues reports the inverse, that is the FEM is attributed to tongue base retraction and the SEM to hyoid movement and laryngeal elevation. The present multivariate findings may reconcile this discrepancy by suggesting that the difference between the FEM and SEM is not found in two different mechanisms, but simply an increase in the covariant functional anatomy underlying laryngeal elevation and tongue base retraction.
An additional limitation of the present study is that the contribution of the pharyngeal constrictors was not included. It appears that epiglottic inversion correlates with the action of the pharyngeal constrictor muscles as well as laryngeal elevation and tongue base retraction. This may be attributable to the glossopharyngeal part of the superior pharyngeal constrictor facilitating tongue base retraction. It is also possible that the approximation of the posterior pharyngeal wall and passing bolus against the tip of the epiglottis may contribute to the SEM, though epiglottic inversion is observed in the absence any bolus as with a saliva swallow. Future studies should consider the contribution of the active or passive contribution of the posterior pharyngeal wall muscles to the SEM.
Ekberg and Sigurjónsson proposed that muscles such as the thyroepiglottis and aryepiglottis achieve the SEM. Fink and colleagues argued this is unlikely based on muscle architecture and force data. Evidence from the current study does not exclude the possible contribution of these diminutive muscles.
How muscle forces are translated to achieve a distinct SEM also remains unclear. Van Dale and colleagues provide evidence that the internal architecture of the epiglottis proposed by Fink and colleagues is insufficient and proposed that lateral hyoepiglottic ligament produces the SEM. Reidenbach reports that the lateral hyoepiglottic ligament is variable, and proposed that the periepiglottic space, which includes the hyoepiglottic fat pad, compresses the epiglottis to achieve epiglottic inversion 21. In the opinion of the authors, the SEM is likely multifactorial and what matters in terms of the clinical benefit is to focus on the anatomy that can be rehabilitated in order to improve function.
Finally, controlling confounding variables such as the etiology of dysphagia or age of subjects is difficult in a retrospective study of human subjects. However, the Goodman-Kruskal index of predictive association shows almost zero correlation between etiologies of dysphagia and epiglottic component scores. Furthermore, concerning possible age- sex related differences in the epiglottic cartilage, a two-way analysis of variance indicates no statistical differences between test groups in our sample. While Kano and colleagues concluded from a cadaver study that the lower part of the epiglottic cartilage might be problematic in male patients due to sex-related differences in calcification patterns, clinical reports of calcification of the epiglottis preventing inversion during swallowing are exceedingly rare 22,23.
In sum, impaired epiglottic inversion may be attributed to reduced laryngeal elevation and tongue base retraction. Therapies that target the long pharyngeal muscles, styloglossus and hyoglossus, or treatment options that preserve the same, may improve or preserve airway protection in swallowing.
Acknowledgments
Financial Disclosures: The 2014 Sushruta-Guha Award in Clinical Anatomy provided by the American Association of Clinical Anatomists supported the dissemination of this study. Imaging data included in this study were acquired through the following grants: NIH/NIDCD 1K24DC1280.
Footnotes
Work completed by institution: Videofluoroscopic and MBS impairment profile epiglottic component score data collected at Medical University of South Carolina; and, imaging and computational analysis performed at the Medical College of Georgia.
Conflicts of Interest: One of the authors (BMH) is the investigator who established the MBS Impairment profile (MBSImP™©). Dr. Martin-Harris trains other SLPs in the use of this methodology supported by North Speech Services.
This data in this manuscript was originally presented at American Association of Clinical Anatomists in a presentation entitled “The Functional Anatomy Underlying Epiglottic Inversion” in Orlando, FL on July 2014.
Contributor Information
William G. Pearson, Jr., Department of Cellular Biology and Anatomy, Department of Otolaryngology, Medical College of Georgia at Georgia Regents University, Augusta, GA, USA
Brandon K Taylor, Medical College of Georgia at Georgia Regents University, Augusta, GA, USA.
Julie Blair, Department of Otolaryngology Head and Neck Surgery, Medical University of South Carolina, Charleston, SC, USA.
Bonnie Martin-Harris, Department of Otolaryngology Head and Neck Surgery, Medical University of South Carolina, Charleston, SC, USA.
References
- 1.Garon BR, Huang Z, Hommeyer M, Eckmann D, Stern GA, Ormiston C. Epiglottic dysfunction: abnormal epiglottic movement patterns. Dysphagia. 2002;17(1):64. doi: 10.1007/s00455-001-0102-8. [DOI] [PubMed] [Google Scholar]
- 2.Amin MR, Postma GN. Office evaluation of swallowing. Ear Nose Throat J. 2004;83(7 Suppl 2):13–6. [PubMed] [Google Scholar]
- 3.Carroll WR, Locher JL, Canon CL, Bohannon IA, McColloch NL, Magnuson JS. Pretreatment swallowing exercises improve swallow function after chemoradiation. The Laryngoscope. 2008;118:39–43. doi: 10.1097/MLG.0b013e31815659b0. [DOI] [PubMed] [Google Scholar]
- 4.Fink BR, Martin RW, Rohrmann CA. Biomechanics of the human epiglottis. Acta Otolaryngol (Stockh) 1979;87:554–559. doi: 10.3109/00016487909126464. [DOI] [PubMed] [Google Scholar]
- 5.Ekberg O, Sigurjónsson SV. Movement of the epiglottis during deglutition. Gastrointest Radiol. 1982;7:101–107. doi: 10.1007/BF01887619. [DOI] [PubMed] [Google Scholar]
- 6.Logemann JA, Kahrilas PJ, Cheng J, et al. Closure mechanisms of laryngeal vestibule during swallow. Am J Physiol Gastrointest Liver Physiol. 1992;262:G338–344. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- 7.Van Daele DJ, Perlman AL, Cassell MD. Intrinsic fibre architecture and attachments of the human epiglottis and their contributions to the mechanism of deglutition. J Anat. 1995;186:1. [PMC free article] [PubMed] [Google Scholar]
- 8.Seo HG, Oh B-M, Leigh J-H, Han TR. Correlation Varies with Different Time Lags Between the Motions of the Hyoid Bone, Epiglottis, and Larynx during Swallowing. Dysphagia. 2014;29:591–602. doi: 10.1007/s00455-014-9550-9. [DOI] [PubMed] [Google Scholar]
- 9.Pearson WG, Zumwalt AC. Visualising Hyolaryngeal Mechanics in Swallowing Using Dynamic MRI. Comput Methods Biome: Imaging & Visualization. 2014;2:208–216. doi: 10.1080/21681163.2013.846231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson ZT, Obeidin F, Davidoff AA, et al. Coordinate Mapping of Hyolaryngeal Mechanics in Swallowing JoVE. 2014:87. doi: 10.3791/51476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment, MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearson W, Langmore S, Zumwalt A. Evaluating the Structural Properties of Suprahyoid Muscles and their Potential for Moving the Hyoid. Dysphagia. 2011;26:345–351. doi: 10.1007/s00455-010-9315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi D-Y, Bae J-H, Youn K-H, Kim H-J, Hu K-S. Anatomical Considerations of the Longitudinal Pharyngeal Muscles in Relation to their Function on the Internal Surface of Pharynx. Dysphagia. 2014:1–9. doi: 10.1007/s00455-014-9568-z. [DOI] [PubMed] [Google Scholar]
- 14.Pearson WG, Hindson DF, Langmore SE, Zumwalt AC. Evaluating Swallowing Muscles Essential for Hyolaryngeal Elevation by Using Muscle Functional Magnetic Resonance Imaging. Int J Radiat Oncol. 2013;85:735–740. doi: 10.1016/j.ijrobp.2012.07.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson WG, Langmore SE, Yu LB, Zumwalt AC. Structural Analysis of Muscles Elevating the Hyolaryngeal Complex. Dysphagia. 2012;27:445–451. doi: 10.1007/s00455-011-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito H, Itoh I. The three-dimensional architecture of the human styloglossus especially its posterior muscle bundles. Annals of Anatomy-Anatomischer Anzeiger. 2007;189:261–267. doi: 10.1016/j.aanat.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Sawczuk A, Mosier K. Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med. 2001;12:18–37. doi: 10.1177/10454411010120010101. [DOI] [PubMed] [Google Scholar]
- 18.Kano M, Shimizu Y, Okayama K, Igari T, Kikuchi M. A morphometric study of age-related changes in adult human epiglottis using quantitative digital analysis of cartilage calcification. Cells Tissues Organs. 2005;180:126–137. doi: 10.1159/000086753. [DOI] [PubMed] [Google Scholar]
- 19.Rasband W. U. S. National Institutes of Health. Bethesda, Maryland, USA: 2012. [Google Scholar]
- 20.Klingenberg CP. MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 2011;11:353–357. doi: 10.1111/j.1755-0998.2010.02924.x. [DOI] [PubMed] [Google Scholar]
- 21.Reidenbach MM. The periepiglottic space: topographic relations and histological organisation. J Anat. 1996;188:173. [PMC free article] [PubMed] [Google Scholar]
- 22.Günbey HP, Günbey E, Sayit AT. A Rare Cause of Abnormal Epiglottic Mobility and Dyspagia: Calcification of the Epiglottis. J Craniofac Surg. 2014;25:e519–e521. doi: 10.1097/SCS.0000000000001025. [DOI] [PubMed] [Google Scholar]
- 23.Ardran G. Calcification of the epiglottis. Br J Radiol. 1965;38:592–595. doi: 10.1259/0007-1285-38-452-592. [DOI] [PubMed] [Google Scholar]



