Abstract
The cell nucleus is becoming increasingly recognized as a mechanosensitive organelle. Most research on nuclear mechanosignaling focuses on the nuclear lamina and coupled actin structures. In this commentary, we discuss the possibility that the nuclear membrane senses and transduces mechanical signals similar to the plasma membrane. We briefly summarize possible (i) pathophysiological sources of nuclear membrane tension, (ii) features that render nuclear membranes particularly suited for mechanotransduction, and (iii) molecular sensing mechanisms.
Keywords: Nuclear envelope, Membrane tension, Mechanotransduction
Rapid nuclear deformations, such as stretching, squeezing or swelling, can give rise to increases of nuclear membrane tension. Such deformations may result from severe pathological perturbations of tissue homeostasis, such as ischemia, toxin exposure, trauma, or infection. For example, cell swelling and lysis, i.e., two of the most common hallmarks of tissue damage, both cause nuclear swelling. In case of cell swelling, nuclear swelling is due to cytoplasmic water influx that decreases the extranuclear oncotic pressure, which in turn results in water influx into the nucleus.14,15 In the case of cell lysis, the decrease of extranuclear oncotic pressure results from macromolecule efflux from cells. Ischemic cell swelling (‘cytotoxic edema’) occurs when the plasma membrane ion pumps that maintain the osmotic balance of a cell (e.g., Na+/K+-ATPase) run out of ATP.29 Cell swelling is a common denominator of programmed necrosis, including pyroptosis, necroptosis, etc., where it is caused by necrotic pore formation within the plasma membrane.3,30 Cell- and nuclear swelling not only occur during cell death, but also as a response to transient decreases of extracellular osmotic pressure:24 various internal epithelia of mammals are exposed to fluids of low osmolarity, such as saliva, which covers the linings of the mouth and esophagus. Epithelial damage allows this fluid to enter the tissue and to induce osmotic cell swelling. In freshwater vertebrates the entire outer epidermis is covered by hypotonic solution. Osmotic cell swelling after tail fin injury of zebrafish larvae is crucial for the recruitment of epithelial cells and leukocytes to the wound within minutes after injury.10,11,17,31 Further more, nuclear deformations occur during 3D migration of cells through confined spaces,7 e.g., when leukocytes squeeze their nuclei through narrow tissue channels on their way to injury or infection sites.16 On a much slower timescale (i.e., hours-to-days), cell spreading or cell growth during the cell cycle also induce nuclear expansion.13,35,38
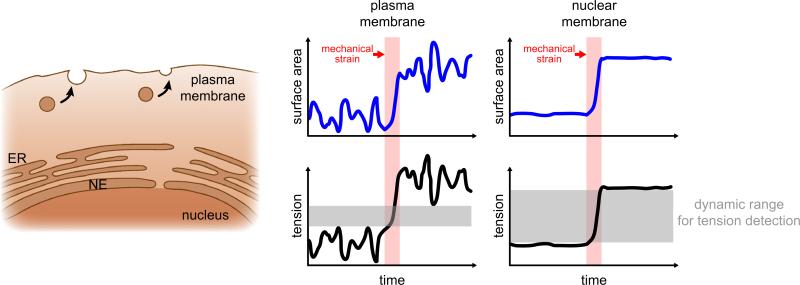
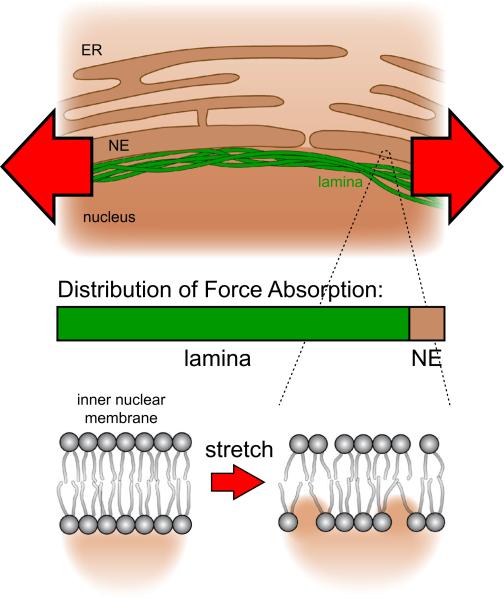
In contrast to the plasma membrane, membranes of the nuclear envelope do not participate in constitutive membrane trafficking; their surface area thus fluctuates less. This intrinsic quiescence should privilege them to function as low-noise detectors, to readily discriminate environmental perturbations from traffic-induced area/tension fluctuations (Fig. 1). Several further features make nuclear membranes well-suited for sensing external mechanical perturbations to transduce them into chemical signals: Low concentrations of cholesterol and a relative abundance of non-saturated, bulky fatty acids generate loosely packed nuclear membranes that are more fluid and easier to stretch than the plasma membrane.4,36 Nuclear membranes typically contain less charged lipids (e.g., phosphatidylserine) than the plasma membrane. Hence, protein binding to nuclear membranes tends to be less dominated by electrostatics, and more by hydrophobic interactions as compared to the plasma membrane.4 Loose lipid packing exposes the hydrophobic membrane core to solvent, making the bilayer more susceptible to hydrophobic protein interactions/insertions. Lipid packing can be modulated by membrane stretch, which has been demonstrated to regulate the function of associated proteins in vitro, likely through altering equilibrium lateral pressure.26 Experiments with artificial lipid bi- and monolayers have shown that the activity of various signaling enzymes, including protein kinase C, phospholipase C and A is principally sensitive to membrane stretch.5,28,40 A2 The relatively low mechanical stability of biological lipid bilayers, particularly those that are poor in cholesterol, such as the nuclear membrane, poses a challenge for mechanotransduction.4,37 To robustly function as a sensor, nuclear membranes require mechanisms that prevent stimulus-overload and damage. In case of the nucleus, a densely woven lamina provides force dampening and acts as a ‘molecular shock absorber’6 (Fig. 2). Furthermore, the nucleus also dynamically adjusts its stiffness within seconds to resist mechanically applied tension through the phosphorylation of emerin, a protein of the inner nuclear membrane.21 Possibly due to this protective feature, nuclear swelling behaves nonlinear and typically ceases before rupture occurs.15 Thus, the lamina and coupled structures (chromosomes, actin fibers, etc.), besides themselves being mechanotransducers, are likely to function as signal conditioners that permit and modulate mechanotransduction through the nuclear membrane, as extensively reviewed elsewhere.1,12,20,32 In addition to adaption to input signal strength, signal termination is another key feature of biological signaling. Signal termination in membrane tension based transduction mechanisms may occur through membrane relaxation, e.g., via mobilization of surplus membrane. During osmotic cell swelling, increases of plasma membrane tension promote exocytotic vesicle fusion, which relaxes the membrane.18,19 Remarkably, isolated nuclei can swell to ~2 times of their initial volume, corresponding to a ~1.6 times increase of membrane surface area.6 Lipid bilayers are little elastic and surface area expansion beyond ~2–4% causes rupture,22,23 thus a decrease of lipid packing density alone cannot explain this expansion. To which extent mobilization of surplus membrane reservoirs vs. e.g., regulation of nuclear pore size or number contribute to area expansion is unclear. Possible membrane reservoirs include nuclear invaginations or the endoplasmic reticulum (ER), which is contiguous with the outer nuclear membrane. Whether or how much of the ER is actually “devoured” by nuclear swelling is unclear. Regardless of precise source, it appears plausible that nuclear membranes, similar to the plasma membrane, signal their demand for surplus area through a tension based mechanism.
FIGURE 1.
Hypothesis: Nuclear membranes function as low-noise detectors of environmental perturbations. In contrast to the quiescent nuclear envelope (NE), constitutive membrane trafficking in the plasma membrane results in heavy surface area and tension fluctuations. Due to the higher signal-to-noise ratio, mechanical strain induced changes in tension are easier to discriminate in the nuclear membrane.
FIGURE 2.
Stretching force exerted on the nucleus is mainly absorbed by the lamina. The nuclear lamina acts as a signal conditioner by dampening the input force and protecting the nuclear envelope (NE) from stimulus overload. The remaining stretching force changes lipid packaging in the NE and exposes the hydrophobic membrane core to the nucleoplasm, which allows for novel hydrophobic protein interaction/insertions with the inner nuclear membrane.
How may the nuclear membrane convert tension into biochemical signals? Not much is known. Mechanosensitive ion channels have been identified on the outer nuclear membrane, and reported to mediate store-release of Ca2+ in response to nuclear envelope/ER stretch caused by cell spreading.25,34 This mechanism has been implicated in transcriptional regulation involving calmodulin-dependent kinase IV mediated phosphorylation of MEF2.25 Another interesting possibility is that nuclear membrane tension may directly regulate the activity of peripheral membrane enzymes. As mentioned above, some kinases and phospholipases are principally sensitive to the biophysical properties of their target membranes. Yet it is unknown whether this serves any regulatory purpose in vivo, e.g., on nuclear membranes. Interestingly, the nuclear envelope functions as an activation scaffold for a variety of important peripheral membrane proteins, including two key members of the ‘eicosanoid’ cascade, i.e., cytosolic phospholipase A2 (cPLA2) and 5-lipoxygenase (5-LOX).33 The latter two enzymes are part of a central inflammatory pathway that mediates production of highly chemotactic lipid mediators (incl. leukotrienes, oxo-eicosanoids) on the nuclear envelope. The physiological reasons for the nuclear association of this paracrine pathway remain little understood. In zebrafish, osmotic cell swelling after epithelial damage triggers the production of chemotactic eicosanoids that attract leukocytes to the injury site.10 Furthermore, accumulation of eicosanoid pathway metabolites characterizes a variety of pathological conditions that imply cell swelling and thus nuclear swelling, e.g., ischemia and macrophage pyroptosis.2,41 Given the previous findings that PLA2-type phospholipases (and other membrane enzymes) are principally sensitive to membrane lipid packing density, this raises the intriguing possibility that the nuclear association of this central inflammatory pathway permits its mechanical regulation through nuclear membrane tension.11,28
Whereas cytoskeletal mechanics and its role in nuclear mechanotransduction have become a thriving line of research, the field of nuclear membrane mechanics and its pathophysiological implications remains almost entirely untouched. Seizing its opportunities will require an interdisciplinary approach that combines methods of enzymology, lipid biochemistry, biophysics and signaling. Breaking this field open for mainstream biomedical research will be accelerated by the development of biosensors of membrane stretch that allow non-invasive measurements of tension on intracellular organelles. Current techniques of biophysical membrane tension measurement, such as atomic force microscopy or micropipette aspiration assess the combined mechanical properties of membranes and underlying cytoskeleton (i.e., ‘apparent membrane tension’).27 It can be challenging to deconvolute actual membrane tension from these experiments.8 Also, such direct mechanical probing techniques are unsuited for non-invasive in vivo measurements inside intact cells. As eluded to above, membrane tension can be measured by assessing the hydration status of a membrane as its hydrophobic core becomes exposed to solvent upon stretch.42 Latter can be measured by generalized polarization dyes, such as laurdan, which integrate into the bilayer and change their fluorescence according to the polarity of their environment.42 The application of many of these dyes is still limited by their poor membrane permeability, but improved versions are being developed.9,39 In order to target biosensors to specific organelles, such as the nucleus, within specific cell types and inside live animals, genetic approaches are required. Systematically assessing the stretch sensing abilities of membrane binding domains should be a first step towards the development of genetically encoded membrane tension sensors. This calls for a considerable research investment, but the reward promises to be exciting.
ACKNOWLEDGMENTS
The authors would like to thank Kris Noel Dahl for helpful discussions. The authors are supported by the National Institutes of Health Grant GM099970 to P.N.
Footnotes
CONFLICT OF INTEREST
Balázs Enyedi and Philipp Niethammer declare that they have no conflicts of interest.
ETHICAL STANDARDS
No human studies were carried out by the authors for this article. No animal studies were carried out by the authors for this article.
REFERENCES
- 1.Alam S, Lovett DB, Dickinson RB, Roux KJ, Lele TP. Nuclear forces and cell mechanosensing. 1st ed. Prog. Mol. Biol. Transl. Sci. 2014 doi: 10.1016/B978-0-12-394624-9.00008-7. http://dx.doi.org/10.1016/B978-0-12-394624-9.00008-7. [DOI] [PMC free article] [PubMed]
- 2.Bazán NG. Effects of ischemia and electroconvulsive shock on free fatty acid pool in the brain. Biochim. Biophys. Acta. 1970;218:1–10. doi: 10.1016/0005-2760(70)90086-x. [DOI] [PubMed] [Google Scholar]
- 3.Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. http://www.nature.com/doifinder/10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 4.Bigay J, Antonny B. Curvature, lipid packing, and electrostatics of membrane organelles: defining cellular territories in determining specificity. Dev. Cell. 2012;23:886–895. doi: 10.1016/j.devcel.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Boguslavsky V, Rebecchi M, Morris AJ, Jhon DY, Rhee SG, McLaughlin S. Effect of monolayer surface pressure on the activities of phosphoinositide-specific phospholipase C-beta 1, -gamma 1, and -delta 1. Biochemistry. 1994;33:3032–3037. doi: 10.1021/bi00176a036. http://www.ncbi.nlm.nih.gov/pubmed/8130216. [DOI] [PubMed] [Google Scholar]
- 6.Dahl KN, Kahn SM, Wilson KL, Discher DE. The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 2004;117:4779–86. doi: 10.1242/jcs.01357. http://www.ncbi.nlm.nih.gov/pubmed/15331638. [DOI] [PubMed] [Google Scholar]
- 7.Davidson PM, Sliz J, Isermann P, Denais C, Lammerding J. Design of a microfluidic device to quantify dynamic intra-nuclear deformation during cell migration through confining environments. Integr. Biol. (Camb) 2015;7:1534–1546. doi: 10.1039/c5ib00200a. http://www.ncbi.nlm.nih.gov/pubmed/26549481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diz-Muñoz A, Fletcher D. a, Weiner OD. Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 2013;23:47–53. doi: 10.1016/j.tcb.2012.09.006. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3558607&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodes Traian MM, González Flecha FL, Levi V. Imaging lipid lateral organization in membranes with C-laurdan in a confocal microscope. J. Lipid Res. 2012;53:609–616. doi: 10.1194/jlr.D021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enyedi B, Kala S, Nikolich-Zugich T, Niethammer P. Tissue damage detection by osmotic surveillance. Nat. Cell Biol. 2013 doi: 10.1038/ncb2818. http://www.ncbi.nlm.nih.gov/pubmed/23934216. [DOI] [PMC free article] [PubMed]
- 11.Enyedi B, Niethammer P. Mechanisms of epithelial wound detection. Trends Cell Biol. 2015;25:398–407. doi: 10.1016/j.tcb.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedorchak GR, Kaminski A, Lammerding J. Cellular mechanosensing: getting to the nucleus of it all. Prog. Biophys. Mol. Biol. 2014 doi: 10.1016/j.pbiomolbio.2014.06.009. http://linkinghub.elsevier.com/retrieve/pii/S0079610714000510. [DOI] [PMC free article] [PubMed]
- 13.Fidorra J, Mielke T, Booz J, Feinendegen LE. Cellular and nuclear volume of human cells during the cell cycle. Radiat. Environ. Biophys. 1981;19:205–214. doi: 10.1007/BF01324188. [DOI] [PubMed] [Google Scholar]
- 14.Finan JD, Guilak F. The effects of osmotic stress on the structure and function of the cell nucleus. J. Cell. Biochem. 2010;109:460–467. doi: 10.1002/jcb.22437. http://www.pubmedcentral.nih.gov/articleren der.fcgi?artid=3616882&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finan JD, Chalut KJ, Wax A, Guilak F. Nonlinear osmotic properties of the cell nucleus. Ann. Biomed. Eng. 2009;37:477–491. doi: 10.1007/s10439-008-9618-5. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2749482&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr. Opin. Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. http://linkinghub.elsevier.com/retrieve/pii/S0955067410001869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gault WJ, Enyedi B, Niethammer P. Osmotic surveillance mediates rapid wound closure through nucleotide release. J. Cell Biol. 2014;207:767–782. doi: 10.1083/jcb.201408049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gauthier NC, Fardin MA, Roca-Cusachs P, Sheetz MP. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc. Natl. Acad. Sci. USA. 2011;108:14467–14472. doi: 10.1073/pnas.1105845108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauthier NC, Masters TA, Sheetz MP. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 2012;22:527–535. doi: 10.1016/j.tcb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Guilluy C, Burridge K. Nuclear mechanotransduction: forcing the nucleus to respond. Nucleus. 2015;6:19–22. doi: 10.1080/19491034.2014.1001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guilluy C, et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 2014;16:376–381. doi: 10.1038/ncb2927. http://www.ncbi.nlm.nih.gov/pubmed/24609268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 23.Hategan A, Law R, Kahn S, Discher DE. Adhesively-tensed cell membranes: lysis kinetics and atomic force microscopy probing. Biophys. 2003;85:2746–2759. doi: 10.1016/s0006-3495(03)74697-9. http://dx.doi.org/10.1016/S0006-3495(03)74697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irianto J, et al. Osmotic challenge drives rapid and reversible chromatin condensation in chondrocytes. Biophys. J. Biophys. Soc. 2013;104:759–769. doi: 10.1016/j.bpj.2013.01.006. http://dx.doi.org/10.1016/j.bpj.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itano N, Okamoto S, Zhang D, Lipton SA, Ruoslahti E. Cell spreading controls endoplasmic and nuclear calcium: a physical gene regulation pathway from the cell surface to the nucleus. Proc. Natl. Acad. Sci. USA. 2003;100:5181–5186. doi: 10.1073/pnas.0531397100. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=154319&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janmey PA, Kinnunen PKJ. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006;16:538–546. doi: 10.1016/j.tcb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Lammerding J, Dahl KN, Discher DE, Kamm RD. Nuclear mechanics and methods. Methods Cell Biol. 2007;83:269–294. doi: 10.1016/S0091-679X(07)83011-1. http://www.ncbi.nlm.nih.gov/pubmed/17613312. [DOI] [PubMed] [Google Scholar]
- 28.Lehtonen JY, Kinnunen PK. Phospholipase A2 as a mechanosensor. Biophys. J. 1995;68:1888–1894. doi: 10.1016/S0006-3495(95)80366-8. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1282092&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang D, Bhatta S, Gerzanich V, Simard JM. Cytotoxic edema: mechanisms of pathological cell swelling. Neurosurg. Focus. 2007;22:E2. doi: 10.3171/foc.2007.22.5.3. http://www.ncbi.nlm.nih.gov/pubmed/1761323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995;146:3–15. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1870771&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 31.Niethammer P. Healed by our inner fish? Oncotarget. 2015;6:15732–15733. doi: 10.18632/oncotarget.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osmanagic-myers S, Dechat T, Foisner R. Lamins at the crossroads of mechanosignaling. Genes Dev. 2015;29:225–237. doi: 10.1101/gad.255968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters-Golden M, Brock TG. Intracellular compartmentalization of leukotriene synthesis: unexpected nuclear secrets. FEBS Lett. 2001;487:323–326. doi: 10.1016/s0014-5793(00)02374-7. [DOI] [PubMed] [Google Scholar]
- 34.Prat AG, Cantiello HF. Nuclear ion channel activity is regulated by actin filaments. Am. J. Physiol. 1996;270:C1532–C1543. doi: 10.1152/ajpcell.1996.270.5.C1532. [DOI] [PubMed] [Google Scholar]
- 35.Prescott DM. Relation between cell growth and cell division. III. Changes in nuclear volume and growth rate and prevention of cell division in Amoeba proteus resulting from cytoplasmic amputations. Exp. Cell Res. 1956;11:94–98. doi: 10.1016/0014-4827(56)90193-8. [DOI] [PubMed] [Google Scholar]
- 36.Raffy S, Teissié J. Control of lipid membrane stability by cholesterol content. Biophys. J. 1999;76:2072–2080. doi: 10.1016/S0006-3495(99)77363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redondo-Morata L, Giannotti MI, Sanz F. Influence of cholesterol on the phase transition of lipid bilayers: a temperature-controlled force spectroscopy study. Langmuir. 2012;28:12851–12860. doi: 10.1021/la302620t. [DOI] [PubMed] [Google Scholar]
- 38.Roca-Cusachs P, Alcaraz J, Sunyer R, Samitier J, Farré R, Navajas D. Micropatterning of single endothelial cell shape reveals a tight coupling between nuclear volume in G1 and proliferation. Biophys. J. 2008;94:4984–4995. doi: 10.1529/biophysj.107.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sezgin E, Sadowski T, Simons K. Measuring lipid packing of model and cellular membranes with environment sensitive probes. Langmuir. 2014;30:8160–8166. doi: 10.1021/la501226v. [DOI] [PubMed] [Google Scholar]
- 40.Souvignet C, Pelosin JM, Daniel S, Chambaz EM, Ransac S, Verger R. Activation of protein kinase C in lipid monolayers. J. Biol. Chem. 1991;266:40–44. [PubMed] [Google Scholar]
- 41.von Moltke J, et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature. 2012;490:107–111. doi: 10.1038/nature11351. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3465483&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y-L, Frangos JA, Chachisvilis M. Laurdan fluorescence senses mechanical strain in the lipid bilayer membrane. Biochem. Biophys. Res. Commun. 2006;347:838–841. doi: 10.1016/j.bbrc.2006.06.152. [DOI] [PubMed] [Google Scholar]