Abstract
Detection of antigen-specific CD4+ T cells is central to the study of many human infectious diseases, vaccines, and autoimmune diseases. However, such cells are generally rare and heterogeneous in their cytokine profiles. Identification of antigen-specific germinal center (GC) T follicular helper (Tfh) cells by cytokine production has been particularly problematic. The function of a GC Tfh cell is to selectively help adjacent GC B cells via cognate interaction; thus, GC Tfh cells may be ‘stingy’ cytokine producers, fundamentally different than Th1 or Th17 cells in the quantities of cytokines produced. Conventional identification of antigen-specific cells by intracellular cytokine staining (ICS) relies on the ability of the CD4+ T cell to generate substantial amounts of cytokine. To address this problem, we have developed a cytokine-independent activation induced marker (AIM) methodology to identify antigen-specific GC Tfh cells in human lymphoid tissue. Whereas Group A Streptococcus (Strep)-specific GC Tfh cells produced minimal detectable cytokines by ICS, the AIM method identified 85-fold more antigen-specific GC Tfh cells. Intriguingly, these GC Tfh cells consistently expressed programmed death ligand 1 (PD-L1) upon activation. AIM also detected non-Tfh cells in lymphoid tissue. As such, we applied AIM for identification of rare antigen-specific CD4+ T cells in human peripheral blood. Dengue-, tuberculosis-, and pertussis-vaccine-specific CD4+ T cells were readily detectable by AIM. In sum, cytokine assays missed 98% of antigen-specific human GC Tfh cells, reflecting the biology of these cells, which could instead be sensitively identified by co-expression of TCR-dependent activation markers.
INTRODUCTION
Germinal center T follicular helper cells (GC Tfh) are key drivers needed to generate a germinal centers (GC) (1). Within the GC are resident GC B cells, which have the capacity to become memory B cells and plasma cells with proper instruction (2). GC Tfh cells instruct neighboring GC B cells to undergo class switch recombination and affinity maturation. These cells can then differentiate into memory B cells and plasma cells with the capacity to produce affinity matured class-switched immunoglobulins. The instruction received by the GC B cells arises from interactions with receptors on antigen-specific GC Tfh cells and cytokines produced by these cells. Receptors for cognate GC Tfh/GC B cell interactions include: PD-1/PD-L1, ICOS/ICOSL, CD40/CD40L, SLAM family receptors, and OX40/OX40L (3). IL-21, IL-4, and CXCL13 are the canonical secreted molecules of Tfh help to B cells(4-9).
Tfh cells have been associated with protective roles in human infectious disease (9, 10), vaccines (11, 12), and cancer (13, 14). Thus, quantifying and understanding these cells is important for biomedical research. In infections, antigen-specific GC Tfh cells are necessary to provide appropriate instruction to GC B cells for the development of T-dependent neutralizing or opsonizing antibodies. However, detection of antigen-specific GC Tfh cells has been very difficult (15). This appears to be related to GC Tfh cells producing little cytokine. This problem likely stems from the intrinsic biology of a GC Tfh cell, which is to instruct GC B cells in directly physical contact, therefore not requiring large amounts of cytokine production. Repeated and cyclical interaction with antigen-specific GC Tfh fuels the selection of GC B cells with affinity matured B cell receptors, but this evolutionary selection process can only occur if the GC Tfh cell help is selective, and thus a GC Tfh cell bathing an entire germinal center in cytokines would likely be counterproductive.
Germinal centers only exist in lymphoid tissues and tertiary lymphoid structures. GC B cells and GC Tfh cells are not present in peripheral blood. Accordingly, germinal center biology must be studied utilizing lymphoid tissue. Human tonsil serves as an accessible lymphoid tissue to study human Tfh and GC responses. We therefore explored approaches to identify human tonsillar antigen-specific GC Tfh cells. In doing so, we developed a cytokine independent method (AIM) for detection of Ag-specific GC Tfh cells. Using the AIM methodology, we determined that conventional cytokine staining missed 98% of human antigen-specific GC Tfh cells. We further determined that AIM is a highly sensitive technique valuable for detecting human CD4+ T cells specific for a range of viral and bacterial antigens.
MATERIALS AND METHODS
Human Samples
Fresh tonsils were obtained from pediatric donors undergoing tonsillectomy at Rady Children's Hospital or the Naval Medical Center. Informed consent was obtained from all donors under protocols approved by the institutional review boards (IRBs) of the University of California, San Diego, the La Jolla Institute for Allergy and Immunology (LJI), and the Naval Medical Center. Tonsillar mononuclear cells were obtained by homogenizing the tissue using a wire mesh, passage through a cell strainer, and isolation via Ficoll density gradient using Histopaque 1077 (Sigma-Aldrich, St. Louis, MO). For the dengue studies, peripheral blood was obtained from the National Blood Center, IRBs of both LJI and the Medical Faculty, University of Colombo (16). For the Mtb studies, healthy controls or individuals with latent TB were obtained from the University of California, San Diego Antiviral Research Center clinic. LTBI status was confirmed by a positive IFNγ release assay (IGRA; QuantiFERON-TB Gold In-Tube, Cellestis or T-SPOT.TB, Oxford Immunotec) and healthy controls all had a negative IGRA. None of the subjects had received a Bacillus Calmette-Guérin (BCG) vaccination. For the pertussis studies, individuals that were originally primed with either acellular Pertussis (aP) or whole cell Pertussis vaccine were from San Diego, California. A subset of these donors was boosted with aP within 3 months of donation. Informed consent was obtained from all donors and approved by the IRB at LJI. All individuals included in the EBV/CMV studies were assumed to have been exposed to one or both of these viruses.
PBMC isolation
PBMCs were isolated by density gradient centrifugation, according to manufacturer's instructions. Cells were suspended in fetal bovine serum containing 10% dimethyl sulfoxide, and cryopreserved in liquid nitrogen.
Serology
DENV seropositivity was determined by dengue IgG ELISA as previously described (17). Flow cytometry-based neutralization assays were performed for further characterization of seropositive donors, as previously described (18). Neutralization assays determined that all DENV donors included in this study have experienced infection with more than one serotype.
Peptides
Peptides were synthesized by A and A (San Diego) as crude material on a 1 mg scale. Individual peptides were resuspended in DMSO and equal amounts of each peptide were pooled to construct peptide pools. The pools used in this study were DENV (32 peptides, previously defined epitopes (16)), ESAT-6 (22 peptides, 15-mers overlapping by 10 and optimal epitopes (19)), CFP10 (21 peptides, 15-mers overlapping by 10 and optimal epitopes (19), EBV/CMV (122 previously defined epitopes (20)), and pertussis (132 previously defined epitopes, manuscript under review). ESAT-6 and CFP10 proteins are specific for the M. tuberculosis complex which includes M. bovis but not the BCG vaccine which has the region encoding ESAT-6 and CFP10 deleted (36). To minimize DMSO concentrations, the resulting peptide pools of more than 100 peptides were then lyophilized and resuspended in DMSO.
Intracellular Cytokine Staining
Cryopreserved tonsillar cells were thawed and cultured in serum free AIM-V media (Life Technologies, Grand Island, NY) overnight for 18 hours. Cells were stimulated with 10 μg/mL heat inactivated antibiotic-killed Group A Streptococcus, strain 5448. Four hours prior to staining, cells were incubated with 10 μg/mL brefeldin A. As a positive control, cells were stimulated with 25ng/mL PMA and 1 μg/mL ionomycin in the presence of brefeldin A for 4 hours.
Flow Cytometry
Cells were labeled with fixable viability dye eFluor 780 or eFluor 506 (eBioscience). FACS panels used for the AIM assay are detailed in Supplementary Table 1. Anti-human antibodies for surface staining are listed here, by company: eBiosciences, San Diego, CA: CD19 e780 (clone HIB19), CD14 e780 (clone 61D3), CD16 e780 (clone eBioCB16), CD8 e780 (clone RPA-T8), CD3 AF700 (clone UCHT1), CD4 APC, FITC or APCe780 (clone RPA-T4), CXCR5 APC (clone MU5UBEE), CD25 PE and PE-Cyanine 7 (clone BC96), PD-1 PE-Cyanine 7 (clone eBioJ105), CD45RA e450 (clone HI100); Biolegend, San Diego, CA: CD4 BV650 (clone OKT4), CD8a BV650 (clone RPA-T8), OX40 PECy7 (clone Ber-ACT35), CD45RA BV570 (clone HI100), PD-1 BV785 (clone EH12.2H7), PD-L1 PE (clone 29E.2A3), CCR7 PerCPCy5.5 (clone G043H7); BD Biosciences, Franklin Lake, NJ: CD8a V500 (clone RPA-T8), CD45RA PE-CF594 (clone HI100), CXCR5 BV421 (clone RF8B2), CD19 V500 (clone HIB19), CD14 V500 (clone M5E2), CD25 FITC (clone M-A251), (BD Biosciences, Franklin Lake, NJ). Intracellular cytokine antibodies used included: TNF AF488 (clone MAb11), CD40L PerCP-e710 (clone 24-31), IFNγ PE-Cyanine7 (clone 4S.B3), IL-21 PE (clone eBio3A3-N2), IL-10 PE (clone JES3-9D7), IL-4 PE-Cyanine7 (clone 8D4-8), IL-17F PerCP-eFluor 710 (clone SHLR17) (eBioscience) and IL-13 APC (clone JES10-5A2) (BioLegend). The tetramer used was DRB5*01:01 CFP1052-66 conjugated using PE-labeled streptavidin (Tetramer Core Laboratory, Benaroya Research Institute, Seattle WA). Cells were acquired on a BD Fortessa or BD LSRII and analyzed using FlowJo Software, version 9.8 to tonsils and FlowJo Software, version 10 for PBMCs.
Cellular Proliferation
Cryopreserved tonsillar cells were thawed and cultured in RPMI medium containing 10% Human AB serum (Gemini Bio-products, West Sacramento, CA). As a positive control, cells were stimulated with 100 ng/mL staphylococcal enterotoxin B (Toxin Technology, Sarasota, FL). For antigen-specific proliferation, cells were stimulated with Streptolysin O, which had been previously heat inactivated at 65°C for 20 minutes (Sigma, St. Louis, MO). Cells were labeled with Cell Trace Violet (Thermo Scientific, Waltham, MA) and cultured for 96 hours in medium supplemented with 4ng/mL IL-7 (Peprotech, Rocky Hill, NJ). Cells were labeled with fixable viability dye eFluor 780 (eBioscience). Tonsils were sorted using BD FACSAria III to isolate GC Tfh cells (Live CD19−, CD14−, CD16−, CD8a, CD4+CD45RA−CXCR5hiPD-1hi), mTfh cells (Live CD19−, CD14−, CD16−, CD8a, CD4+CD45RA−CXCR5+PD-1+), and non-Tfh cells (Live CD19−, CD14−, CD16−, CD8a, CD4+CD45RA−CXCR5−). Sorted cells were incubated at a 1 CD4+ T cell : 1 APC ratio with irradiated autologous lymphoblastoid cell lines created from each donor tonsil as APCs. Cells were cultured for 96 hours and acquired on BD Fortessa.
Activation Induced Marker Assay
Cryopreserved tonsillar cells were thawed and cultured in serum free AIM-V media (Life Technologies, Grand Island, NY) overnight for 18 hours. Cells were stimulated with 10 μg/mL heat inactivated antibiotic-killed Group A Streptococcus, strain 5448. As a positive control, cells were stimulated with 1 μg/mL staphylococcal enterotoxin B (Toxin Technology, Sarasota, FL). Cells were cultured for 18 hours and acquired on BD Fortessa. PBMCs were thawed and cultured in complete RPMI 1640 with 5% human AB serum (Gemini Bioproducts) for 24 hours. Cells were stimulated with 2 μg/mL peptide pools or 10 μg/mL PHA.
ELISPOT Assays
Ex vivo IFNγ ELISPOT assays was used for Mtb peptide pools as previously described (21). Responses were considered positive if the net spot-forming cells (SFC) per 106 were ≥20, the stimulation index ≥2, and p≤0.05 (Student's t-test, mean of triplicate values of the response against relevant pools vs. the DMSO control). For pertussis, PBMCs were cultured for in vitro expansion by incubating in RPMI 1640 supplemented with 5% human AB serum (Gemini Bioscience), GlutaMAX (Gibco), and penicillin/streptomycin (Omega Scientific) at 2 × 106 per mL in the presence of individual pertussis antigens; FHA (Reagent Proteins), PRN (Reagent Proteins), formaldehyde fixed pertussis toxin (‘PT’, Reagent Proteins), and Fim2/3 (List Biological Labs), each at 5 μg/mL. Every 3 days, 10 U/ml IL-2 in media were added to the cultures. After 14 days of culture, responses to peptides was measured by IFNγ and IL-5 dual ELISPOT as previously described (22). To be considered positive, response had to fulfill all three criteria described above.
Statistics
Comparisons between groups were made using the Mann-Whitney U test. Prism 5.0 (GraphPad) was used for all these calculations.
RESULTS
Detection of antigen-specific human GC Tfh cells by intracellular cytokine production
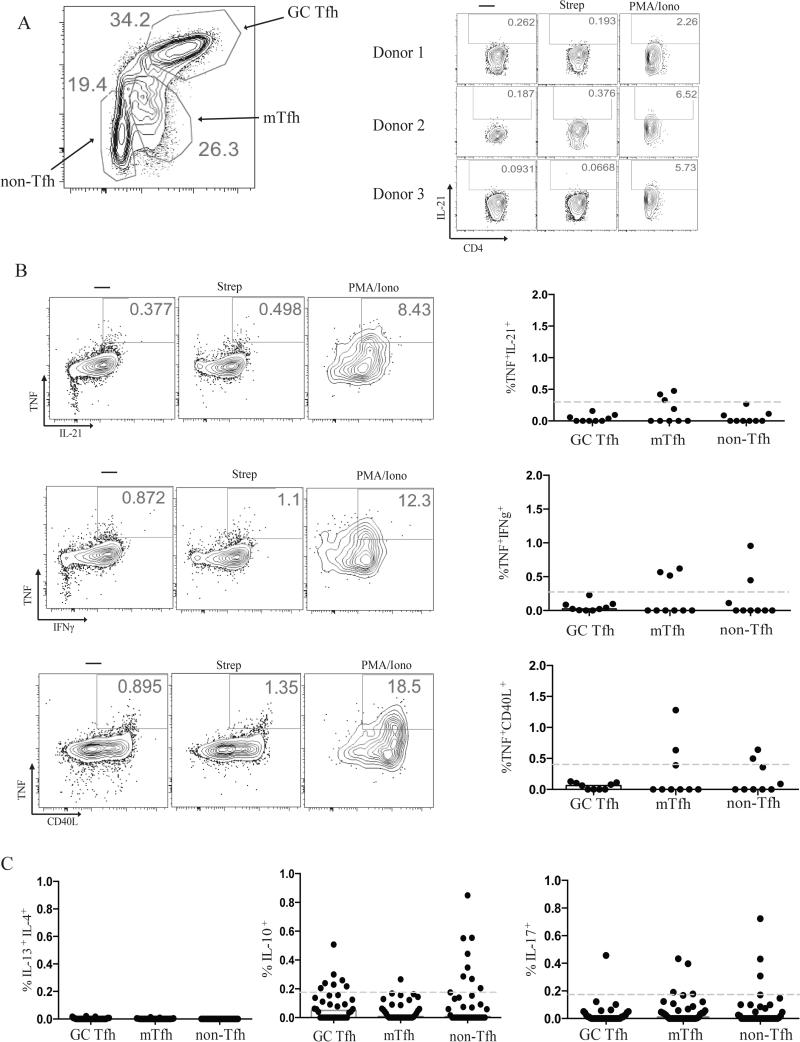
To identify antigen-specific GC Tfh cells within tonsils, we tested for expression of different cytokines by intracellular cytokine staining (ICS). Antigen-experienced CD4+ T cells within lymphoid tissue were categorized as GC Tfh (CD4+CD45RA−CXCR5hiPD-1hi), follicular mantle Tfh (mTfh. Also known as Tfh or pre-Tfh. CD4+CD45RA−CXCR5+PD-1+), and non-Tfh or effector cells (CD4+CD45RA−CXCR5−) (Fig. 1A). One ubiquitous pathogen is Group A Streptococcus (Strep), which is the etiologic agent for strep throat. Given the prevalence of strep throat within the pediatric population and about 10-15% asymptomatic carriage rate in healthy children, we reasoned that essentially all individuals have been exposed to this pathogen (23-26). We thus tested for production of IL-21 following stimulation with whole heat inactivated antibiotic-killed Group A Streptococcus (Strep). Very few cells produced detectable IL-21 following stimulation with Strep (median < 0.1%, Fig. 1A); and the IL-21 MFI was close to the limit of detection, which was insufficient to allow for confident identification of Strep-specific GC Tfh cells. This is similar to other studies in which antigen-specific GC Tfh responses have been difficult to identify via IL-21 production (15, 27). Strep-specific TCR stimulation did not increase IL-4 production (data not shown). We then tested other cytokines, including TNF, CD40L, IFNγ, IL-13, IL-17, and IL-10 (Fig. 1B and 1C). There was minimal IFNγ, IL-13, IL-17, and IL-10 expression by GC Tfh cells in response to antigen stimulation (Supplementary Fig. 1), consistent with previous observations that GC Tfh cells produced minimal Th1, Th2, or Th17 cytokines upon strong PMA/Ionomycin stimulation (8, 28, 29). Basal levels of CD40L were high and heterogenous from donor to donor, making identification of antigen-specific cells based on CD40L alone infeasible. Intracellular co-staining for TNF and CD40L upregulation identified Strep-specific GC Tfh cells better than any other cytokine or combination of cytokine plus intracellular CD40L (Fig. 1B). Nevertheless, only 0.053% of GC Tfh cells were Strep-specific based on TNF and CD40L upregulation. These data prompted us to conclude that cytokine production may significantly underestimate antigen-specific GC Tfh cell frequencies and prompted us to explore cytokine-independent approaches to identify antigen-specific human GC Tfh cells.
FIGURE 1.
Limited cytokine production by GC Tfh cells after antigen stimulation. (A) Representative flow cytometry plot of GC Tfh (CXCR5hiPD-1hiCD45RA−CD4+), mTfh (CXCR5+PD-1+CD45RA−CD4+), and non-Tfh (CXCR5−CD45RA−CD4+) cell gating with representative flow cytometry plots of IL-21 production by live GC Tfh cells from 3 different tonsils. (B) Median intracellular cytokine production of TNF+IL-21+, TNF+IFNγ+, and TNF+CD40L+ by Strep-specific GC Tfh, mTfh, and non-Tfh cells. Representative FACS plots following 18-hour culture of tonsil cells with 10ug/mL Strep or PMA/Ionomycin, as a positive control. Limit of detection denoted by the grey dotted line. Data are from 9 donors. The response by antigen-specific cells was background subtracted for each donor. (C) Median intracellular cytokine production of IL-4+IL-13+, IL-10+, and IL-17+ by Strep-specific GC Tfh, mTfh, and non-Tfh cells. Limit of detection denoted by the grey dotted line. Data are from 19 donors.
Detection of antigen-specific GC Tfh cells by cellular proliferation
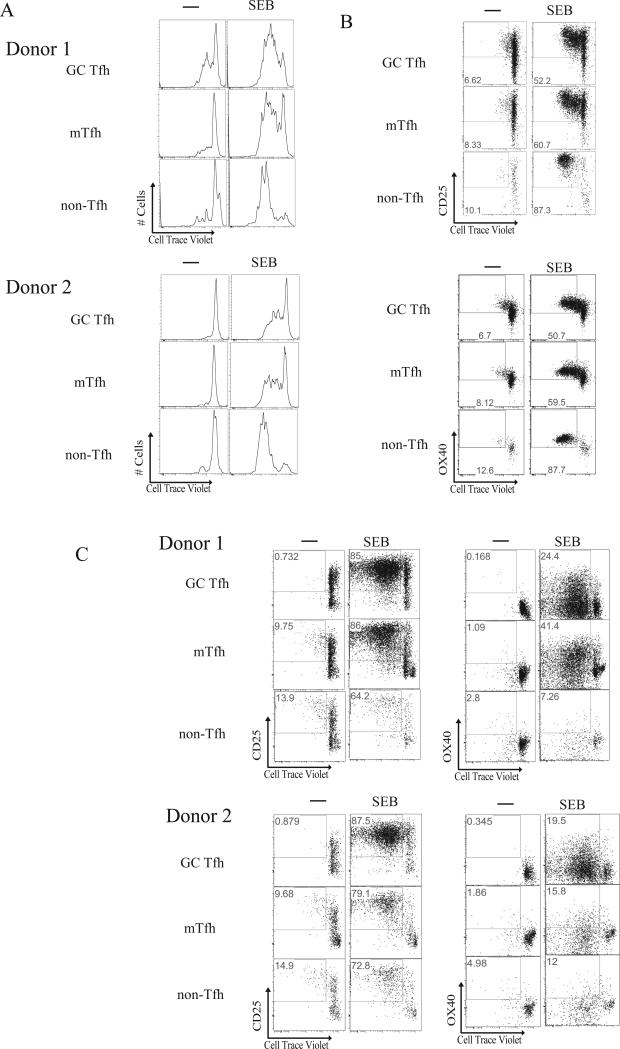
We next sought to identify antigen-specific GC Tfh cells by proliferation. GC Tfh cells are not end-stage differentiated cells. GC Tfh cells can migrate within and between germinal centers (30), proliferate (31-33), and become memory cells (1, 32, 34-36). Maintenance in the GC Tfh state requires TCR triggering by surrounding GC B cells as well as costimulation by other activation markers. We therefore assessed GC Tfh proliferation following a 96-hour culture of Cell Trace Violet (CTV) labeled tonsillar cells in the presence or absence of SEB. We were able to identify SEB-responsive cells based on proliferative responses within the GC Tfh (CD4+CD45RA−CXCR5hiPD-1hi), mTfh (CD4+CD45RA−CXCR5intPD-1int), and non-Tfh populations (CD4+CD45RA−CXCR5−) (Fig. 2A). Interestingly, proliferating SEB-responsive cells upregulated CD25 (IL-2Rα) and OX40 (Fig. 2B). OX40 participates in GC Tfh:GC B cell cognate interactions, and thus may be expected to be upregulated in response to antigen stimulation in GCs. However, CD25 upregulation was surprising because CD25 expression has been shown to be quite low on differentiating Tfh cells (37, 38) and IL-2 has been shown to inhibit Tfh differentiation both in mice and humans (39-42). Human GC Tfh cells express low amounts of CD25 ex vivo (Supplementary Figure 2). We speculate that either supraphysiological quantities of peptide:MHC complex in vitro drive CD25 expression in these restimulation conditions that would not be induced in vivo, or negative feedback loops exist in vivo to prevent high CD25 expression but those mechanisms are not present in vitro.
FIGURE 2.
Proliferation of GC Tfh cells upon restimulation. (A) Representative histograms of proliferating GC Tfh, mTfh, and non-Tfh cells from 2 different tonsils following stimulation of whole tonsil cells with 100ng/mL Staphylococcal enterotoxin B (SEB). (B) Representative flow cytometry plots of proliferating GC Tfh, mTfh, and non-Tfh cells from a donor following stimulation of whole tonsil cells with 100ng/mL SEB. Cells were gated on CTV+CD25+ or CTV+OX40+. Data are representative of a total of 11 samples from 2 independent experiments. (C) Representative flow cytometry plots of sorted GC Tfh (live CXCR5hiPD-1hiCD45RA−CD4+), mTfh (CXCR5+PD-1+CD45RA−CD4+), and non-Tfh cells (CXCR5−CD45RA−CD4+) from 3 different tonsils. Cells were co-cultured with autologous irradiated EBV-transformed lymphoblastoid cell lines for 96 hours, stimulated with 100ng/mL SEB, and analyzed for CTV+CD25+ or CTV+OX40+ expression. FACS plots show only 20% of collected events, for easier visualization. Data are representative of a total of 21 samples from 6 independent experiments.
Because CD4+ T cells could potentially change phenotype during the 18 hour in vitro culture, we sought to determine whether the proliferating SEB-responsive cells identified as CXCR5hiPD-1hi after proliferation were truly GC Tfh cells. To establish that we were observing proliferating GC Tfh cells after 96 hours in culture, separate GC Tfh, mTfh, and non-Tfh cell cultures were required. We created autologous lymphoblastoid cell lines (LCL) for each tonsil donor as antigen presenting cells. GC Tfh, mTfh, and non-Tfh cells were FACS sorted from donors and cultured with autologous LCL in the presence or absence of SEB. Proliferating GC Tfh cells highly upregulated both CD25 and OX40 in response to SEB, a potent TCR stimulus (Fig. 2C). CD25 and OX40 were also upregulated on SEB responsive mTfh and non-Tfh cells (Fig. 2C). Thus, antigen-specific GC Tfh could potentially be identified by proliferation in response to antigen. We observed extensive SEB-responsive proliferation in the GC Tfh sorted populations, as the LCLs had much improved antigen presenting capacity over primary B cells. Moreover, small quantities of IL-7 were added to enhance survival during culture, at a concentration which does not promote T cell proliferation. Subsequent CTV proliferation experiments with whole Strep, heat-inactivated Streptolysin O (a serodiagnostic marker for recent Strep infection), or diphtheria CRM197 were unable to consistently identify GC Tfh cells by proliferation due to variable low level nonspecific proliferation (data not shown).
Detection of antigen-specific GC Tfh cells by activation marker induction
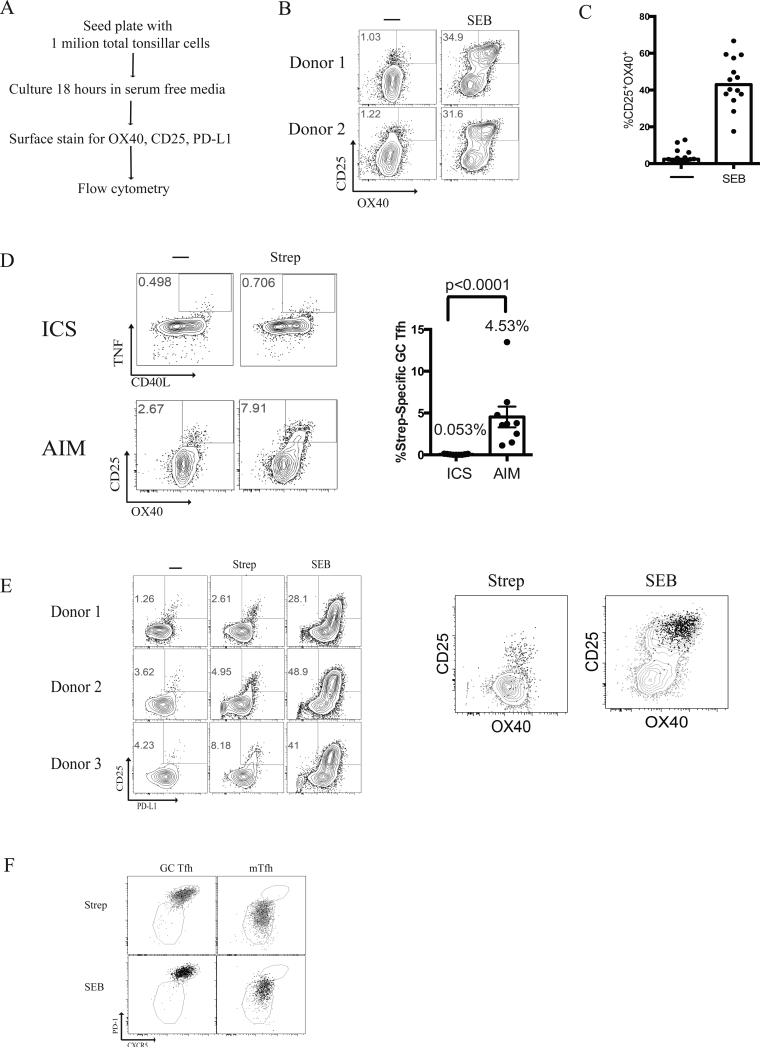
Tfh help is the primary positive selection step in a GC (1, 2). Tfh cells selectively provide help to the B cells with the most antigenic peptides, which are the high affinity B cells that have bound and endocytosed the most antigen (43, 44). The amount of help provided by the Tfh cell directly translates to the number of cell divisions and mutations a GC B cell will undergo in the dark zone in a single selection cycle (45, 46). One of the functional challenges for Tfh cells in GCs is that they are constantly exposed to antigen. They must therefore be able to distinguish between GC B cells with modest differences in their numbers of peptide:major histocompatibility complexes (p:MHC) and respond by providing help preferentially to the cognate B cells presenting more p:MHC complexes. Thus, we hypothesized that while GC Tfh cells retain sensitive TCR signaling, the outcome of the TCR detection of cognate antigen is not reflected by cytokine production, as GC Tfh cells may only produce infinitesimal quantities of cytokine necessary to signal the GC B cell in synaptic contact. We therefore explored whether changes in expression of proteins other than cytokines might be more consistent indicators of TCR signaling by GC Tfh cells. Based on our observation that proliferating GC Tfh cells upregulate OX40 and CD25 in vitro, we sought to determine if CD25 and OX40 were consistently upregulated on antigen-specific GC Tfh cells in response to TCR stimulation at more proximal time points. Following an 18-hour SEB stimulation, we observed a clear and robust population of CD25+OX40+ GC Tfh cells, consistent with the expected frequency of cells expressing SEB-reactive TCRβ chains (Fig. 3B). Each tonsil varied in the frequency of CD25+OX40+ GC Tfh cells observed after 18 hours in vitro in the absence of exogenous antigen, with a mean of 4.1%. We speculated that the CD25+OX40+ GC Tfh cells observed in the absence of exogenous antigen were responding to antigen presented by GC B cells and other APCs in the in vitro culture, as each tonsil contains antigen (Supplementary Fig. 3A). SEB stimulation of GC Tfh cells from 14 donors yielded a mean frequency of 44% CD25+OX40+ SEB-responsive GC Tfh cells (Fig 3C). (At a concentration of 1μg/mL, SEB stimulates proliferation of lower affinity TCR Vβ families (47).) Thus, CD25 and OX40 were reproducible indicators of GC Tfh cell TCR stimulation.
FIGURE 3.
Activation Induced Marker induction by GC Tfh cells. (A) Experimental design for human tonsillar AIM assay (B) Representative flow cytometry plots of CD25+OX40+ upregulation by live GC Tfh cells (CXCR5hiPD-1hiCD45RA−CD4+) from 3 different tonsils following 18 hours stimulation with 1μg/mL Staphylococcal enterotoxin B (SEB). (C) Median CD25+OX40+ expression by live GC Tfh cells following stimulation with SEB. Data are from 14 samples from 2 independent experiments. The response by antigen-specific cells was background subtracted for each donor. (D) Comparison of Strep-specific GC Tfh by ICS (TNF+CD40L+) and AIM (CD25+OX40+). The % Strep-specific GC Tfh responses were background subtracted. Data are from 9 samples from 2 independent experiments. The response by antigen-specific cells was background subtracted for each donor. (E) CD25+OX40+ GC Tfh cells are also PD-L1+. Representative FACS plots of CD25+PD-L1+ co-expression following stimulation with either Strep or SEB. Strep-specific PD-L1+CD4+ GC Tfh cells (black contour plot) were overlaid onto Strep-specific CD25+OX40+ GC Tfh cells (grey dots). SEB responsive PD-L1+CD4+ GC Tfh cells (black contour plot) were overlaid onto SEB responsive CD25+OX40+ GC Tfh cells (grey dots). Data are from 14 samples from 2 independent experiments. (F). Representative flow cytometry overlay plots demonstrating CD25+OX40+ cells (black dots) within each sorted population (grey dots) for each condition (Strep-stimulated, and SEB-stimulated). This is representative of 3 independent experiments consisting of 7 donors.
We next tested whether Strep-specific GC Tfh cells could be detected by CD25 and OX40 expression after stimulation with Strep antigen. Upon stimulation, we observed a robust Strep-specific GC Tfh cell population based on CD25+OX40+ co-expression (Fig. 3D). This activation induced marker assay (AIM) detected Strep-specific GC Tfh cells at a mean frequency of 4.53% (Fig 3D). In contrast, ICS yielded a mean frequency of only 0.053% Strep-specific GC Tfh cells (TNF+CD40L+, Fig. 3D. Background signal present in unstimulated samples were subtracted in all cases.) The CXCR5hiPD-1hi cells detected by AIM were GC Tfh cells, as sorting of GC Tfh cells and mTfh cells followed by Strep stimulations showed that the CXCR5 and PD-1 expression profiles of each cell type were maintained after activation (Fig 3F). The AIM method provided, on average, an 85-fold improvement in sensitivity for quantifying Strep-specific cells GC Tfh cells. Thus, cytokine-dependent identification missed > 98% of antigen-specific GC Tfh cells.
In a separate study, RNAseq analysis of rhesus macaque (Macaca mulatta) lymph node GC Tfh cells revealed that programmed death 1 molecule ligand (PD-L1) was robustly induced upon TCR stimulation (48). We therefore examined whether PD-L1 was upregulated on antigen-specific GC Tfh cells upon Strep antigen stimulation or SEB stimulation. Similar to CD25+OX40+ co-expression, we observed a population of CD25+PD-L1+ GC Tfh cells after either Strep or SEB stimulation (Fig. 3E). PD-L1 expression was present on 95.7% of Strep-specific GC Tfh cells expressing CD25+OX40+ (Fig. 3E). PD-L1 thus functions as a TCR signaling output following antigen-specific GC Tfh cells stimulation; and PD-L1, OX40, and CD25 are interchangeable for identification of responding GC Tfh cells.
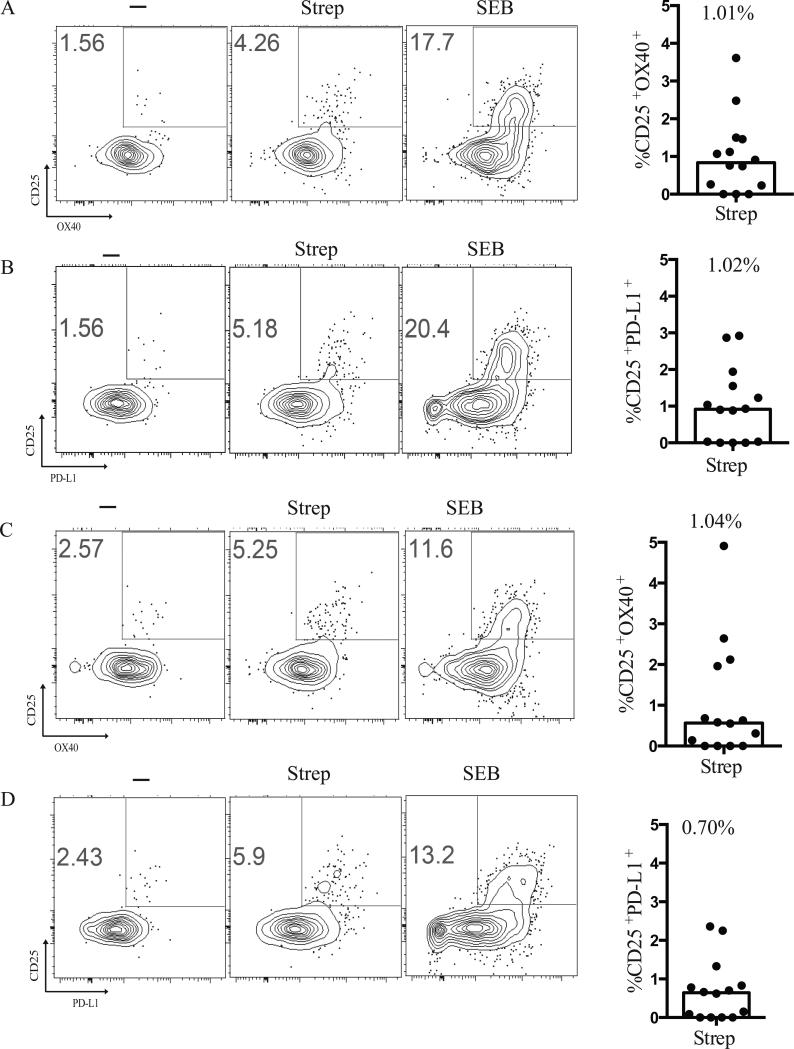
After establishing a highly sensitive method to detect antigen-specific GC Tfh cells, we next wanted to assess the ability of the AIM assay to identify Strep-specific mTfh and non-Tfh. As with GC Tfh cells, we found CD25, OX40, and PD-L1 expression on Strep-specific mTfh and non-Tfh cells upon stimulation with Strep antigen (Fig. 4). Strep-specific non-Tfh cells or effector cells (CD4+CD45RA−CXCR5−) could be identified by the AIM assay as either CD25+OX40+ (Fig. 4A and C) or CD25+PD-L1+ (Fig. 4B and D). Thus, the cell surface proteins CD25, OX40, and PD-L1 appear to be activation markers expressed by antigen-specific human CD4+ T cells, independent of whether the cells were GC Tfh cells. Therefore, the AIM assay may be of practical utility for identifying antigen-specific CD4+ T cells in lymphoid tissue, independent of abundant production of a given cytokine.
FIGURE 4.
Activation Induced Marker induction by mTfh and non-Tfh cells. (A) Representative flow cytometry plots of CD25+OX40+ upregulation by live mTfh (CXCR5+PD-1+CD45RA−CD4+) following stimulation with Strep or 1μg/mL SEB. Median CD25+OX40+ expression by live mTfh following stimulation with Strep. Responses were background subtracted. Data are from 14 samples from 2 independent experiments. The response by antigen-specific cells was background subtracted for each donor. (B) Representative flow cytometry plots of and CD25+PD-L1+ upregulation by live mTfh (CXCR5+PD-1+CD45RA−CD4+) following stimulation with Strep or 1μg/mL SEB. Median CD25+PD-L1+ expression by live mTfh following stimulation with Strep. Responses were background subtracted. Data are from 14 samples from 2 independent experiments. The response by antigen-specific cells was background subtracted for each donor. (C) Representative flow cytometry plots of CD25+OX40+ upregulation by non-Tfh cells (CXCR5−CD45RA−CD4+). Median CD25+OX40+ expression by live non-Tfh following stimulation with Strep. Responses were background subtracted. Data are from 14 samples from 2 independent experiments. The response by antigen-specific cells was background subtracted for each donor. (D). Representative flow cytometry plots of CD25+PDL1+ upregulation by non-Tfh cells (CXCR5−CD45RA−CD4+). Median CD25+PD-L1+ expression by live non-Tfh following stimulation with Strep. Responses were background subtracted. Data are from 14 samples from 2 independent experiments. The response by antigen-specific cells was background subtracted for each donor.
AIM detects antigen-specific CD4 T cells in human peripheral blood
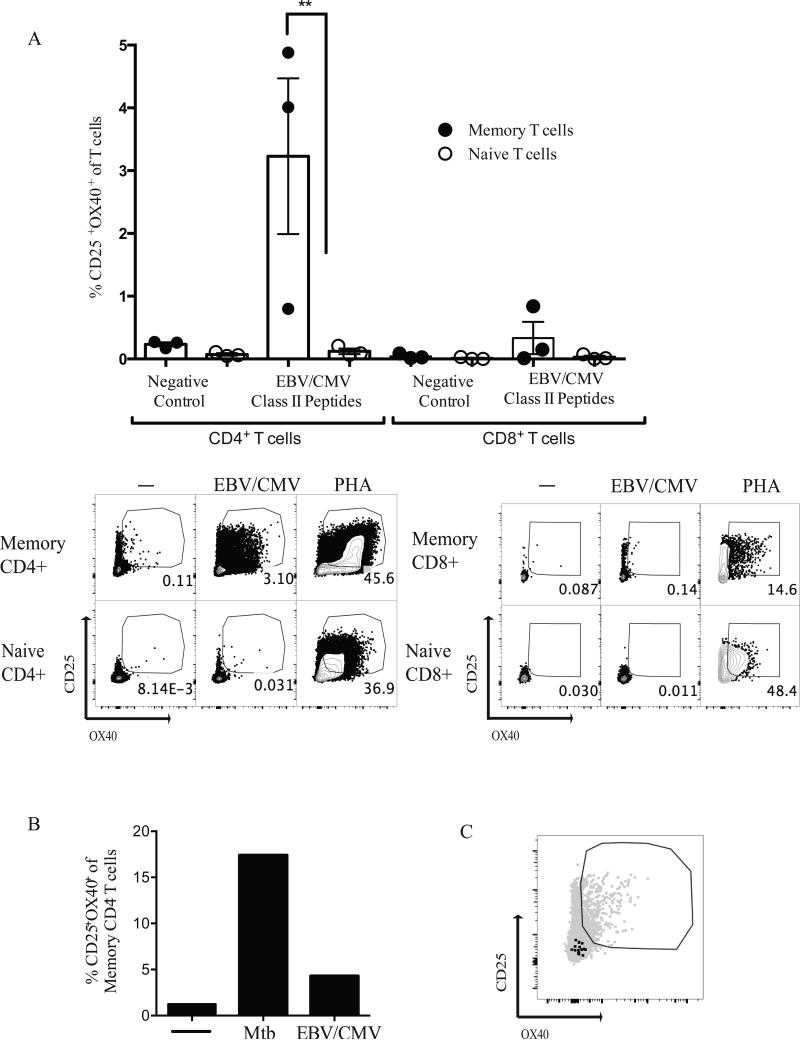
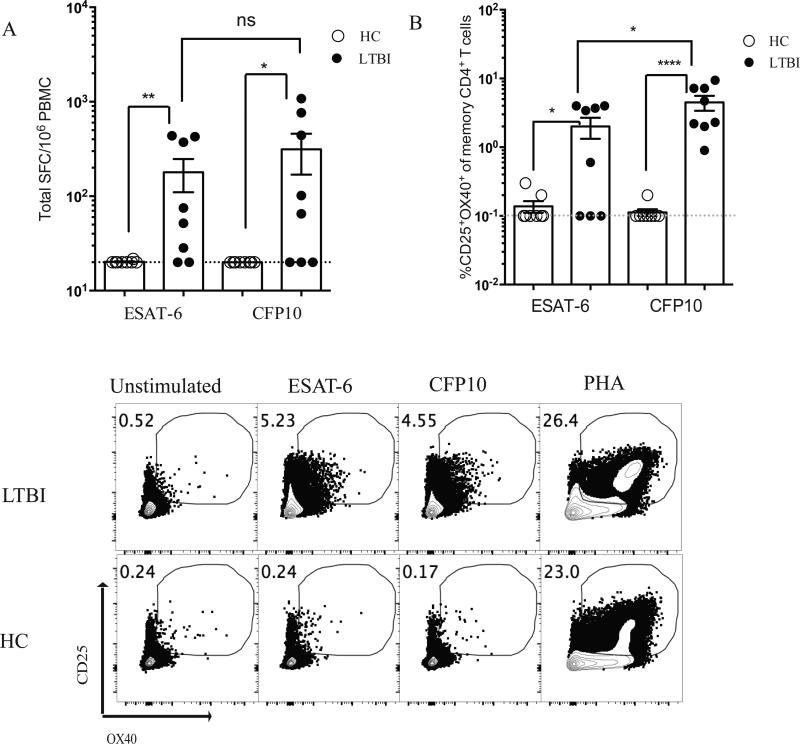
We identified CD25, OX40, and PD-L1 as TCR activation-dependent markers of antigen-specific human GC Tfh cells. Independently, CD25+OX40+ co-expression was explored for detection of memory CD4 T cells to EBV/CMV and Mycobacterium tuberculosis (Mtb) in human peripheral blood (49, 50). Those studies frequently used ~48 hour stimulation periods, which may allow sufficient time for significant T cell proliferation or death, thus skewing the quantitation of responding T cells. Additionally, 50% of latent Mtb (LTBI) cases, which are known to respond to Mtb antigens in the QuantiFERON-TB Gold assay, failed to be detected upon stimulation with IGRA antigens in one study (49). Here, using AIM, we identified EBV/CMV-specific CD4+ T cells in PBMCs from healthy individuals presumed seropositive for either virus using previously defined EBV and CMV Class II epitopes (16) (Fig. 5A). We assessed the ability of AIM to detect tuberculosis-specific CD4+ T cells using M. tuberculosis (Mtb)-specific proteins CFP10 and ESAT-6. ESAT-6 and CFP10 are Mtb-specific proteins utilized in the QuantiFERON-TB Gold clinical test to determine previous exposure to Mtb (35). Healthy controls (QuantiFERON-TB Gold negative) were compared to individuals with latent tuberculosis (LTBI) who are by definition QuantiFERON-TB Gold positive. In an IFNγ ELISPOT assay, PBMCs from most LTBI individuals produced IFNγ in response to both ESAT-6 and CFP10 (Fig. 6A). In the AIM assay, PBMCs from LTBI individuals had a 14.5-fold increase in the % CD25+OX40+ memory CD4+ T cells after stimulation with ESAT-6, and a nearly 40-fold increase after stimulation with CFP10 compared to healthy controls, (p = 0.027 and p < 0.0001, respectively)(Fig. 6B). Of interest, 100% of LTBI donors were detected as positive by AIM using CFP10, in contrast to ELISPOT (Fig. 6B). Identification of Mtb-specific and EBV/CMV-specific CD4+ T cells by AIM was specific for antigen-experienced CD4+ T cells (Fig 5A and 6B). Notably, background noise in the AIM assay was very low when using PBMCs (Fig. 5A, 6B), in contrast to antigen-containing tonsillar tissue (Fig. 2B, 3B), consistent with the ‘background signal’ in tonsils being due to an antigen-specific CD4+ T cells responding to antigens in tonsil, given that tonsils are a sentinel tissue. There was also undetectable bystander activation in the PBMCs, as there was no upregulation of CD25+OX40+ on CD8+ T cells or naive CD4+ T cells (Fig. 5A, 7, and 8A).
FIGURE 5.
Detection of EBV/CMV-specific CD4+ T cells in peripheral blood. (A) Three individuals were tested by AIM for response to a Class II EBV/CMV peptide pool. AIM+ (CD25+OX40+) memory CD4+ T cells (CD45RO+CD4+), naïve CD4+ T cells (CD45RO−CD4+), memory CD8+ T cells (CD45RO+CD8+), and naïve CD8+ T cells (CD45RO−CD8+) were quantified. (**, p=0.0079). (B) An LTBI DRB5*01:01 donor with defined DRB5*01:01 Mtb epitope was stimulated with Mtb and EBV/CMV peptide pool. AIM+ (CD25+OX40+) memory CD4+ T cells were quantified. (C) Representative FACS plot demonstrates that EBV/CMV-specific CD4+ T cells (CD25+OX40+) were not positive for Mtb-tetramer (black dots).
FIGURE 6.
Detection of Mtb-specific CD4+ T cells in peripheral blood. Eight QuantiFERON-TB Gold Positive patients with latent tuberculosis (LTBI) and eight QuantiFERON-TB Gold negative healthy controls (HC) were tested for response to a pool of ESAT-6 or CFP10 epitopes. (A) IFNγ ELISPOT of PBMCs from LTBI patients and healthy controls (*, p=0.0035 for ESAT-6, *, p=0.013 for CFP10). Limit of detection denoted by the grey dotted line. (B) AIM assay (CD25+OX40+) of PBMCs from LTBI patients and healthy controls similarly had higher CD25+OX40+ expression by AIM assay (*, p=0.027 for ESAT-6, ****, p<0.0001 for CFP10). Limit of detection denoted by the grey dotted line. Representative flow cytometry plots of CD25+OX40+ by an LTBI patient and a HC patient.
FIGURE 7.
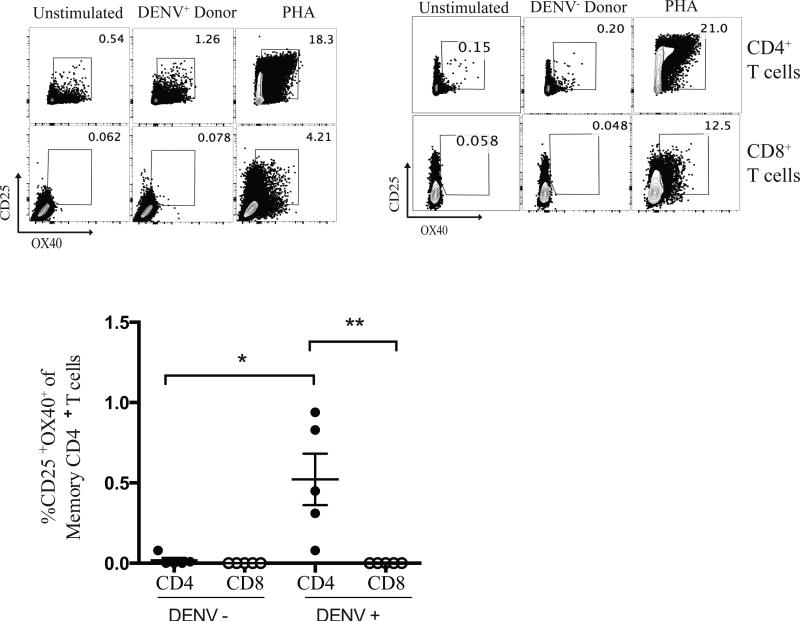
Detection of dengue-specific CD4+ T cells in peripheral blood. Representative FACS plots of CD4+ and CD8+ T cells following stimulation with dengue peptides or PHA, as a positive control. Cumulative data from five dengue seropositive patients (DENV+) and five dengue seronegative patients (DENV−) with known expression of the HLA DRB1*0401 molecule. PBMCs were stimulated with dengue DRB1*0401 restricted peptide for 24 hours. (*, p=0.016). (**, p=0.0079).
FIGURE 8.
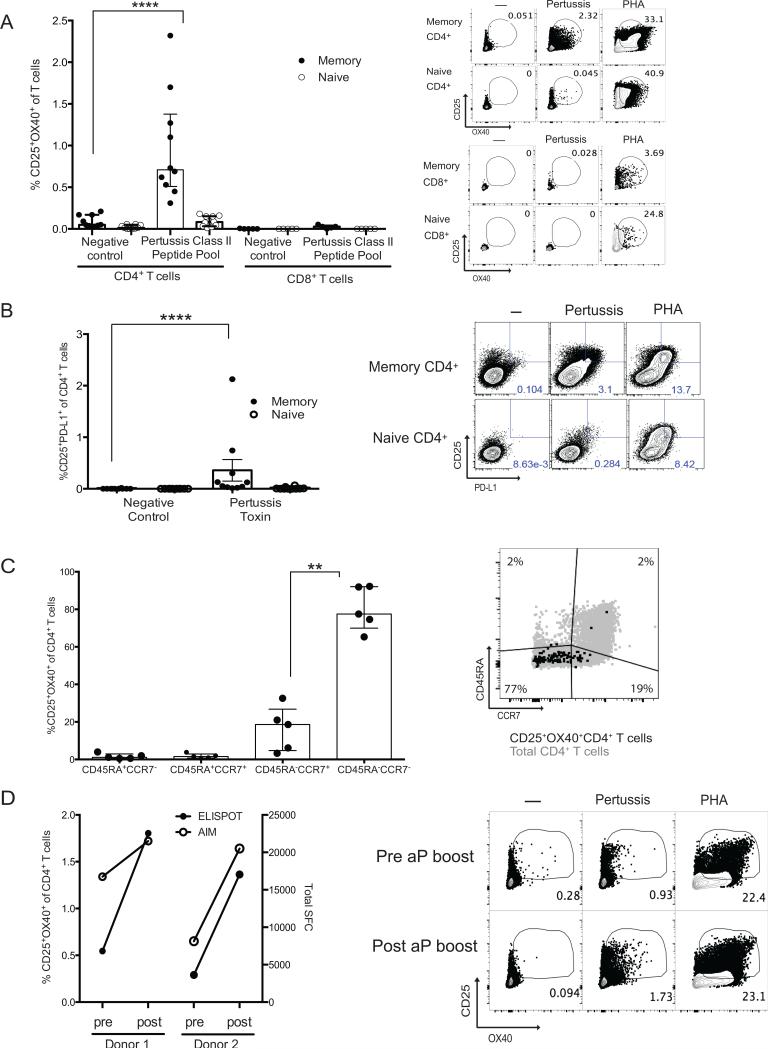
Detection of pertussis-specific CD4+ T cells in peripheral blood using a pertussis peptide megapool. (A). AIM+ (CD25+OX40+) memory CD4+ T cells (CD45RA−CCR7+CD4+) naïve CD4+ T cells (CD45RA+CCR7+CD4+), memory CD8+ T cells (CD45RA−CCR7+CD8+), and naïve CD8+ T cells (CD45RA+CCR7+CD8+) were quantified. (****, p<0.0001). (B). CD25+PD-L1+ memory CD4+ T cells were quantified (****, p<0.0001) in ten individuals. (C). Quantification of AIM+ (CD25+OX40+) pertussis-specific within CD4+ T cell subsets. Pertussis-specific cells were predominantly in the CD45RA−CCR7− (**, p=0.0079). (D). Comparison of AIM+ (CD25+OX40+) pertussis-specific and ELISPOT pertussis-specific responses in individuals pre and post acellular pertussis booster vaccination.
We performed a test of AIM specificity utilizing an Mtb-specific Class II tetramer (HLA DRB5*01:01 CFP1052-66 tetramer). The HLA DRB5*01:01 latent tuberculosis donor selected was found to have a very strong Mtb-specific CD4+ T cell response by AIM (17%; Fig. 6B), and was also EBV/CMV+ (4% EBV/CMV+ CD4+ T cells, Fig 5A). When PBMC from the HLA DRB5*01:01 Mtb+EBV/CMV+ donor were stimulated with the EBV/CMV peptide pool and then stimulated with the Mtb tetramer, the Mtb-tetramer specific CD4+ T cells showed no evidence of bystander activation (Fig. 5B and 5C). Thus, the AIM method specifically detects antigen-specific CD4+ T cells.
We then sought to assess the applicability of AIM for identifying dengue-specific CD4+ T cells or rare pertussis vaccine specific memory CD4+ T cells in peripheral blood. We first focused on identifying dengue virus-specific CD4+ T cell responses using PBMCs from healthy individuals from a highly endemic area who were either seropositive (DENV+) or seronegative (DENV−) for prior dengue viral infection. High frequencies of dengue-specific CD4+ T cells have been correlated with an decreased likelihood of developing dengue hemorrhagic fever (16, 51). We were able to discern dengue-specific memory CD4+ T cell responses using the AIM assay (Fig. 7). Using PBMCs from five DENV+ individuals bearing the HLA DRB1*04:01 allele, we were able to detect 0.08-0.94% dengue-specific memory CD4+ T cells following stimulation with dengue DRB1*04:01 restricted epitopes after 24 hours in culture, p=0.016 (16). CD8+ T cells did not respond in the AIM assay to the dengue Class II peptides, as expected (Fig. 7).
Pertussis-specific CD4+ T cells have only been detected in most individuals after 14 day expansion of cells in vitro followed by cytokine ELISPOT. We examined whether pertussis-specific CD4+ T cells could be detected directly ex vivo by the AIM assay, as a stringent test of assay sensitivity. Ten donors were tested, and pertussis-specific CD4+ T cells in all 10 donors were detected by AIM (CD25+OX40+ mean = 0.97, range 0.45-2.32%, Fig. 8A. CD25+PD-L1+ mean =0.36, range 0.0134-2.13%, Fig. 8B). These donors were healthy individuals who had no recent pertussis immunizations. Pertussis-specific CD4+ T cells were memory cells (Fig. 8C) and were found to predominantly (80%) have an effector memory phenotype (CD45RA−CCR7−)(Fig. 8C). Two donors received an acellular pertussis vaccine boost within a three-month period from their pre-acellular pertussis boost PBMC specimen. The AIM assay detected an increase in pertussis-specific CD4+ T cell responses after the acellular pertussis vaccination (Fig. 8D). In comparison to a 14-day re-stimulation ELISPOT assay, the 24 hour AIM assay followed the same data trends as the 14-day re-stimulation ELISPOT assay (Fig. 8D). The AIM assay was simpler and shorter, and allowed for extensive phenotypic cellular characterization at the single cell level by multiparameter flow cytometry.
DISCUSSION
We have shown that it is extremely difficult to quantify antigen-specific GC Tfh cells within human tonsils using the traditional ICS method, as GC Tfh cells are inherently stingy cytokine producers and make little to no detectable cytokine upon antigen stimulation. However, we determined that Strep-specific GC Tfh cells do retain sensitive TCR activation in response to antigen, resulting in upregulation of CD25, OX40, and PD-L1. We exploited this biology to identify antigen-specific GC Tfh cells with 85-fold greater sensitivity than ICS. Furthermore, we observed that the utility of this approach is not limited to GC Tfh cells but can be extrapolated to other CD4+ T cells within human lymphoid tissue and peripheral blood.
Upon TCR triggering of an antigen-experienced CD4+ T cell, co-stimulatory molecules and co-inhibitory molecules are upregulated (52). The interplay of co-stimulatory and co-inhibitory molecules and which signals dominate are part of antigen-specific CD4+ T cell development (53). OX40 is one such receptor, upregulated on antigen-experienced CD4+ T cells as early 1-4 hours upon TCR stimulation, depending on the type of CD4+ T cell and the strength of the peptide-MHC II complex (3). OX40 on antigen-specific CD4+ T cells interacts with OX40L on APCs to promote CD4+ T cell survival (3). Similarly, CD25 is another activation marker quickly upregulated upon TCR stimulation and sustained following expression of IL-2 in an autocrine or paracrine manner (54). For GC Tfh cells, OX40 upregulation upon antigen stimulation by GC B cells in the follicle was not unexpected as OX40:OX40L constitutes part of the GC Tfh: GC B cell cognate interaction (1). However, CD25 upregulation on GC Tfh cells within the GC follicle is counterintuitive, as IL-2 is known to inhibit Tfh differentiation (39, 40). GC Tfh cells expressed low amounts of CD25 directly ex vivo (Supplementary Fig. 2C), even though most GC Tfh cells experience frequent TCR stimulation in germinal centers (55, 56). Thus, a negative feedback mechanism to limit CD25 expression appears to be engaged in vivo but not in vitro. An alternative explanation is that stimulation with small amount of antigen in vitro is supraphysiologic and does not recapitulate what is seen in vivo, permitting CD25 upregulation. Many receptors that inhibit GC Tfh cells are expressed by GC Tfh cells (57, 58), pointing to a central role of inhibitory pathways in maintaining appropriate Tfh biology in germinal centers.
PD-L1 upregulation on Strep-specific GC Tfh was also unexpected. A role of PD-L1 on GC Tfh cells has not been described. PD-L1 expression is not restricted to myeloid cells and non-hematopoetic cells; PD-L1 expression has previously been observed on both CD4+ and CD8+ T cells in certain conditions (59). PD-L1 has been found on activated CD4+ T cells in rheumatoid arthritis patients (60, 61). For GC Tfh cells, interpretation of the function of PD-L1 is complicated, as these cells also express PD-1. The PD-1/PD-L1 axis is involved in GC Tfh interactions with GC B cells, with PD-1 highly expressed by GC Tfh and PD-L1 expressed by GC B cells (62). High PD-1 expression prevents GC Tfh cell proliferation, allowing the GC to function properly, as the purpose of the GC is to drive GC B cell proliferation and selection while maintaining a relatively constant number of GC Tfh cells (33, 57, 63). Expression of PDL1 by an activated GC Tfh cell may inhibit neighboring PD-1hi GC Tfh cells in a bystander manner, as an additional mechanism to limit excessive GC Tfh cell activation and/or proliferation.
The AIM method exhibited greatly increased sensitivity for detecting Strep-specific GC Tfh cells in comparison to ICS. IL-21 protein production was rarely detected by ICS of GC Tfh cells after antigen stimulation. Detection of IL-21 protein from antigen-specific human GC Tfh cells within lymphoid tissue has been difficult, with perhaps only one reported success (15). In macaque studies, there have been little to no detection by ICS of IL-21 protein induction in antigen-specific GC Tfh cells (27, 64). IL-21 RNA can be detected in stimulated human GC Tfh cells, and an IL-21 fluorescent reporter mouse detects shifts in fluorescent reporter protein expression after antigen-stimulation in vivo (55), consistent with Il21 mRNA induction. Thus, it appears that GC Tfh cells are intrinsically ‘stingy’ producers of IL-21 protein. Note that this cytokine biology of GC Tfh cells within lymphoid tissue does not extend to circulating resting memory Tfh cells or circulating recently activated Tfh cells in blood (9, 11, 65, 66), which are not GC Tfh cells. GC Tfh cells are not in peripheral blood. CD4+ T cells in peripheral blood much more readily produce cytokine than do bona fide GC Tfh cells in lymphoid tissues. Peripheral Tfh cells consist of multiple populations, but the vast majority ~99% (9) can be categorized as resting central memory Tfh cells (based on their resting phenotype), and a small population (~1-5%) can be characterized as recently activated Tfh-like cells, based on their activated phenotype (ICOS+PD-1hiKi67+, but no detectable Bcl6 protein, or Maf, or CD200). A significant fraction of the resting central memory Tfh cells circulating through blood readily produce cytokines, including IL-21, as shown by multiple labs. GC Tfh cells are quite different, as they are more differentiated cells, in a very different environment, and have much more restricted cytokine production.
As with ICS, the AIM technique is also subject to the potential concern of possible bystander activation. We have demonstrated a lack of bystander activation by several independent tests. Class II peptide pools dengue-specific for HLA DRB1*04:01 did not induce CD25+OX40+ expression on CD8+ T cells. Second, minimal background was observed in PBMCs. Finally, we demonstrated a lack of Mtb-tetramer positive cells among the CD25+OX40+ CD4+ T cells stimulated with an unrelated antigen.
While identification of antigen-specific GC Tfh cells by CD25, OX40, and PD-L1 co-expression is novel, CD25+OX40+ co-expression has previously been studied in peripheral blood (49, 50, 67, 68). We show here that dengue-specific and pertussis-specific memory CD4+ T cells are sensitively detected in peripheral blood by this approach, and we show controls for the specificity of the methodology. For dengue virus infections, the AIM assay could potentially be useful in detecting antigen specific responses irrespective of the functional cytokine specificity. Indeed it has been reported that severe DENV infection associated pathology is linked to complex patterns of cytokine production, and the AIM assay will ensure detection regardless of the effector specificity of the DENV-specific T cells (51). Additionally, given the resurgence of pertussis in the United States, the AIM assay could potentially identify children with waning vaccine responses who may warrant more frequent booster vaccinations via ex vivo analysis of their pertussis-specific CD4+ T cell responses (69-71).
In summary, antigen-specific GC Tfh, mTfh, and non-Tfh cells can be detected with great sensitivity within secondary lymphoid tissue using AIM. This method will likely be valuable for understanding germinal center biology as it applies to infections, cancer, and autoimmune disease. The wide applicability of this assay also makes this ideal for detecting rare ex vivo human antigen-specific CD4+ T cell responses. Moreover, since AIM is a live cell assay it is well suited in combination for downstream applications.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ericka Anderson and Victor Nizet (UCSD) for providing the initial Group A Strep stock, and the members of the Flow Cytometry core at La Jolla Institute for Allergy and Immunology for technical support. We thank Daniel Kaufman (University of Montreal) for valuable discussions.
1 Financial Support: We thank the Thrasher Research Fund for an Early Career Award to support this research, as well as funding by CHAVI-ID 1UM1AI100663, LJI Institutional Funds, and NIH training grants 5T32AI007036-35 and 5T32AI007384-25.
REFERENCES
- 1.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Victora GD, Nussenzweig MC. Germinal centers. Annual review of immunology. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 3.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134). Annual review of immunology. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havenar-Daughton C, Lindqvist M, Heit A, Wu JE, Reiss SM, Kendric K, Belanger S, Kasturi SP, Landais E, Akondy RS, McGuire HM, Bothwell M, Vagefi PA, Scully E, Investigators IPCP, Tomaras GD, Davis MM, Poignard P, Ahmed R, Walker BD, Pulendran B, McElrath MJ, Kaufmann DE, Crotty S. CXCL13 is a plasma biomarker of germinal center activity. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:2702–2707. doi: 10.1073/pnas.1520112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. The Journal of experimental medicine. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). Journal of immunology. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinhardt RL, Liang HE, Locksley RM. Cytokine-secreting follicular T cells shape the antibody repertoire. Nature immunology. 2009;10:385–393. doi: 10.1038/ni.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma CS, Suryani S, Avery DT, Chan A, Nanan R, Santner-Nanan B, Deenick EK, Tangye SG. Early commitment of naive human CD4(+) T cells to the T follicular helper (T(FH)) cell lineage is induced by IL-12. Immunology and cell biology. 2009;87:590–600. doi: 10.1038/icb.2009.64. [DOI] [PubMed] [Google Scholar]
- 9.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK, International AVIPCPI, Poignard P, Crotty S. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity. 2013;39:758–769. doi: 10.1016/j.immuni.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obeng-Adjei N, Portugal S, Tran TM, Yazew TB, Skinner J, Li S, Jain A, Felgner PL, Doumbo OK, Kayentao K, Ongoiba A, Traore B, Crompton PD. Circulating Th1-Cell-type Tfh Cells that Exhibit Impaired B Cell Help Are Preferentially Activated during Acute Malaria in Children. Cell reports. 2015;13:425–439. doi: 10.1016/j.celrep.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, Xu H, Pascual V, Banchereau J, Garcia-Sastre A, Palucka AK, Ramilo O, Ueno H. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Science translational medicine. 2013;5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vargas-Inchaustegui DA, Demers A, Shaw JM, Kang G, Ball D, Tuero I, Musich T, Mohanram V, Demberg T, Karpova TS, Li Q, Robert-Guroff M. Vaccine Induction of Lymph Node-Resident Simian Immunodeficiency Virus Env-Specific T Follicular Helper Cells in Rhesus Macaques. Journal of immunology. 2016;196:1700–1710. doi: 10.4049/jimmunol.1502137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothe F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. The Journal of clinical investigation. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pages F, Speicher MR, Trajanoski Z, Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze zur Wiesch J, Streeck H. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. The Journal of clinical investigation. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM, Crotty S, Peters B, Sette A. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E4256–4263. doi: 10.1073/pnas.1505956112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanakaratne N, Wahala WM, Messer WB, Tissera HA, Shahani A, Abeysinghe N, de-Silva AM, Gunasekera M. Severe dengue epidemics in Sri Lanka, 2003-2006. Emerging infectious diseases. 2009;15:192–199. doi: 10.3201/eid1502.080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kraus AA, Messer W, Haymore LB, de Silva AM. Comparison of plaque- and flow cytometry-based methods for measuring dengue virus neutralization. Journal of clinical microbiology. 2007;45:3777–3780. doi: 10.1128/JCM.00827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arlehamn CS, Sidney J, Henderson R, Greenbaum JA, James EA, Moutaftsi M, Coler R, McKinney DM, Park D, Taplitz R, Kwok WW, Grey H, Peters B, Sette A. Dissecting mechanisms of immunodominance to the common tuberculosis antigens ESAT-6, CFP10, Rv2031c (hspX), Rv2654c (TB7.7), and Rv1038c (EsxJ). Journal of immunology. 2012;188:5020–5031. doi: 10.4049/jimmunol.1103556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrasco Pro S, Sidney J, Paul S, Lindestam Arlehamn C, Weiskopf D, Peters B, Sette A. Automatic Generation of Validated Specific Epitope Sets. Journal of immunology research. 2015;2015:763461. doi: 10.1155/2015/763461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carpenter C, Sidney J, Kolla R, Nayak K, Tomiyama H, Tomiyama C, Padilla OA, Rozot V, Ahamed SF, Ponte C, Rolla V, Antas PR, Chandele A, Kenneth J, Laxmi S, Makgotlho E, Vanini V, Ippolito G, Kazanova AS, Panteleev AV, Hanekom W, Mayanja-Kizza H, Lewinsohn D, Saito M, McElrath MJ, Boom WH, Goletti D, Gilman R, Lyadova IV, Scriba TJ, Kallas EG, Murali-Krishna K, Sette A, Lindestam Arlehamn CS. A side-by-side comparison of T cell reactivity to fifty-nine Mycobacterium tuberculosis antigens in diverse populations from five continents. Tuberculosis. 2015;95:713–721. doi: 10.1016/j.tube.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oseroff C, Sidney J, Vita R, Tripple V, McKinney DM, Southwood S, Brodie TM, Sallusto F, Grey H, Alam R, Broide D, Greenbaum JA, Kolla R, Peters B, Sette A. T cell responses to known allergen proteins are differently polarized and account for a variable fraction of total response to allergen extracts. Journal of immunology. 2012;189:1800–1811. doi: 10.4049/jimmunol.1200850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts AL, Connolly KL, Kirse DJ, Evans AK, Poehling KA, Peters TR, Reid SD. Detection of group A Streptococcus in tonsils from pediatric patients reveals high rate of asymptomatic streptococcal carriage. BMC pediatrics. 2012;12:3. doi: 10.1186/1471-2431-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebell MH, Smith MA, Barry HC, Ives K, Carey M. The rational clinical examination. Does this patient have strep throat? Jama. 2000;284:2912–2918. doi: 10.1001/jama.284.22.2912. [DOI] [PubMed] [Google Scholar]
- 25.Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta-analysis. Pediatrics. 2010;126:e557–564. doi: 10.1542/peds.2009-2648. [DOI] [PubMed] [Google Scholar]
- 26.DeMuri GP, Wald ER. The Group A Streptococcal Carrier State Reviewed: Still an Enigma. Journal of the Pediatric Infectious Diseases Society. 2014;3:336–342. doi: 10.1093/jpids/piu030. [DOI] [PubMed] [Google Scholar]
- 27.Hessell AJ, Malherbe DC, Pissani F, McBurney S, Krebs SJ, Gomes M, Pandey S, Sutton WF, Burwitz BJ, Gray M, Robins H, Park BS, Sacha JB, LaBranche CC, Fuller DH, Montefiori DC, Stamatatos L, Sather DN, Haigwood NL. Achieving Potent Autologous Neutralizing Antibody Responses against Tier 2 HIV-1 Viruses by Strategic Selection of Envelope Immunogens. Journal of immunology. 2016;196:3064–3078. doi: 10.4049/jimmunol.1500527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroenke MA, Eto D, Locci M, Cho M, Davidson T, Haddad EK, Crotty S. Bcl6 and Maf cooperate to instruct human follicular helper CD4 T cell differentiation. Journal of immunology. 2012;188:3734–3744. doi: 10.4049/jimmunol.1103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Shulman Z, Gitlin AD, Targ S, Jankovic M, Pasqual G, Nussenzweig MC, Victora GD. T follicular helper cell dynamics in germinal centers. Science. 2013;341:673–677. doi: 10.1126/science.1241680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, Connick E, Meditz A, Freeman GJ, Abesada-Terk G, Jr., Jacobson JM, Brooks AD, Crotty S, Estes JD, Pantaleo G, Lederman MM, Haddad EK. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nature medicine. 2013;19:494–499. doi: 10.1038/nm.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luthje K, Kallies A, Shimohakamada Y, Belz GT, Light A, Tarlinton DM, Nutt SL. The development and fate of follicular helper T cells defined by an IL-21 reporter mouse. Nature immunology. 2012;13:491–498. doi: 10.1038/ni.2261. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Hillsamer P, Kim CH. Phenotype, effector function, and tissue localization of PD-1-expressing human follicular helper T cell subsets. BMC immunology. 2011;12:53. doi: 10.1186/1471-2172-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitano M, Moriyama S, Ando Y, Hikida M, Mori Y, Kurosaki T, Okada T. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity. 2011;34:961–972. doi: 10.1016/j.immuni.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tubo NJ, Fife BT, Pagan AJ, Kotov DI, Goldberg MF, Jenkins MK. Most microbe-specific naive CD4(+) T cells produce memory cells during infection. Science. 2016;351:511–514. doi: 10.1126/science.aad0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pepper M, Pagan AJ, Igyarto BZ, Taylor JJ, Jenkins MK. Opposing signals from the Bcl6 transcription factor and the interleukin-2 receptor generate T helper 1 central and effector memory cells. Immunity. 2011;35:583–595. doi: 10.1016/j.immuni.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, Crotty S. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity. 2011;34:932–946. doi: 10.1016/j.immuni.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. The Journal of experimental medicine. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kenefeck R, Wang CJ, Kapadi T, Wardzinski L, Attridge K, Clough LE, Heuts F, Kogimtzis A, Patel S, Rosenthal M, Ono M, Sansom DM, Narendran P, Walker LS. Follicular helper T cell signature in type 1 diabetes. The Journal of clinical investigation. 2015;125:292–303. doi: 10.1172/JCI76238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cubas R, van Grevenynghe J, Wills S, Kardava L, Santich BH, Buckner CM, Muir R, Tardif V, Nichols C, Procopio F, He Z, Metcalf T, Ghneim K, Locci M, Ancuta P, Routy JP, Trautmann L, Li Y, McDermott AB, Koup RA, Petrovas C, Migueles SA, Connors M, Tomaras GD, Moir S, Crotty S, Haddad EK. Reversible Reprogramming of Circulating Memory T Follicular Helper Cell Function during Chronic HIV Infection. Journal of immunology. 2015;195:5625–5636. doi: 10.4049/jimmunol.1501524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tangye SG, Ma CS, Brink R, Deenick EK. The good, the bad and the ugly - TFH cells in human health and disease. Nature reviews. Immunology. 2013;13:412–426. doi: 10.1038/nri3447. [DOI] [PubMed] [Google Scholar]
- 44.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nature reviews. Immunology. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509:637–640. doi: 10.1038/nature13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gitlin AD, Mayer CT, Oliveira TY, Shulman Z, Jones MJ, Koren A, Nussenzweig MC. HUMORAL IMMUNITY. T cell help controls the speed of the cell cycle in germinal center B cells. Science. 2015;349:643–646. doi: 10.1126/science.aac4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Llewelyn M, Sriskandan S, Terrazzini N, Cohen J, Altmann DM. The TCR Vbeta signature of bacterial superantigens spreads with stimulus strength. International immunology. 2006;18:1433–1441. doi: 10.1093/intimm/dxl076. [DOI] [PubMed] [Google Scholar]
- 48.Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, de la Pena AT, Kasturi SP, Dan JM, Bothwell M, Sanders RW, Pulendran B, Silvestri G, Crotty S. Cytokine-independent detection of antigen-specific germinal center T follicular helper (Tfh) cells in immunized non-human primates using a live cell Activation Induced Marker (AIM) technique. doi: 10.4049/jimmunol.1600320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Escalante P, Peikert T, Van Keulen VP, Erskine CL, Bornhorst CL, Andrist BR, McCoy K, Pease LR, Abraham RS, Knutson KL, Kita H, Schrum AG, Limper AH. Combinatorial Immunoprofiling in Latent Tuberculosis Infection. Toward Better Risk Stratification. American journal of respiratory and critical care medicine. 2015;192:605–617. doi: 10.1164/rccm.201412-2141OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaunders JJ, Munier ML, Seddiki N, Pett S, Ip S, Bailey M, Xu Y, Brown K, Dyer WB, Kim M, de Rose R, Kent SJ, Jiang L, Breit SN, Emery S, Cunningham AL, Cooper DA, Kelleher AD. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). Journal of immunology. 2009;183:2827–2836. doi: 10.4049/jimmunol.0803548. [DOI] [PubMed] [Google Scholar]
- 51.Weiskopf D, Sette A. T-cell immunity to infection with dengue virus in humans. Frontiers in immunology. 2014;5:93. doi: 10.3389/fimmu.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature reviews. Immunology. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu Y, Yao S, Chen L. Cell surface signaling molecules in the control of immune responses: a tide model. Immunity. 2011;34:466–478. doi: 10.1016/j.immuni.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shulman Z, Gitlin AD, Weinstein JS, Lainez B, Esplugues E, Flavell RA, Craft JE, Nussenzweig MC. Dynamic signaling by T follicular helper cells during germinal center B cell selection. Science. 2014;345:1058–1062. doi: 10.1126/science.1257861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, Luo D, Qi H. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015;517:214–218. doi: 10.1038/nature13803. [DOI] [PubMed] [Google Scholar]
- 57.Crotty S. Follicular helper CD4 T cells (TFH). Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 58.Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, Rigoni R, Radaelli E, Caprioli F, Preziuso S, Brannetti B, Thelen M, McCoy KD, Slack E, Traggiai E, Grassi F. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer's patches to promote host-microbiota mutualism. Immunity. 2014;41:789–801. doi: 10.1016/j.immuni.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Rosignoli G, Lim CH, Bower M, Gotch F, Imami N. Programmed death (PD)-1 molecule and its ligand PD-L1 distribution among memory CD4 and CD8 T cell subsets in human immunodeficiency virus-1-infected individuals. Clinical and experimental immunology. 2009;157:90–97. doi: 10.1111/j.1365-2249.2009.03960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature medicine. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 61.Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, Tamura H, Driscoll CL, Chen L. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. The Journal of clinical investigation. 2003;111:363–370. doi: 10.1172/JCI16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. The Journal of experimental medicine. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hams E, McCarron MJ, Amu S, Yagita H, Azuma M, Chen L, Fallon PG. Blockade of B7-H1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. Journal of immunology. 2011;186:5648–5655. doi: 10.4049/jimmunol.1003161. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto T, Lynch RM, Gautam R, Matus-Nicodemos R, Schmidt SD, Boswell KL, Darko S, Wong P, Sheng Z, Petrovas C, McDermott AB, Seder RA, Keele BF, Shapiro L, Douek DC, Nishimura Y, Mascola JR, Martin MA, Koup RA. Quality and quantity of TFH cells are critical for broad antibody development in SHIVAD8 infection. Science translational medicine. 2015;7:298ra120. doi: 10.1126/scitranslmed.aab3964. [DOI] [PubMed] [Google Scholar]
- 65.Schultz BT, Teigler JE, Pissani F, Oster AF, Kranias G, Alter G, Marovich M, Eller MA, Dittmer U, Robb ML, Kim JH, Michael NL, Bolton D, Streeck H. Circulating HIV-Specific Interleukin-21(+)CD4(+) T Cells Represent Peripheral Tfh Cells with Antigen-Dependent Helper Functions. Immunity. 2016;44:167–178. doi: 10.1016/j.immuni.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 66.Chahroudi A, Silvestri G. HIV and Tfh Cells: Circulating New Ideas to Identify and Protect. Immunity. 2016;44:16–18. doi: 10.1016/j.immuni.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 67.Keoshkerian E, Helbig K, Beard M, Zaunders J, Seddiki N, Kelleher A, Hampartzoumian T, Zekry A, Lloyd AR. A novel assay for detection of hepatitis C virus-specific effector CD4(+) T cells via co-expression of CD25 and CD134. Journal of immunological methods. 2012;375:148–158. doi: 10.1016/j.jim.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 68.Sadler R, Bateman EA, Heath V, Patel SY, Schwingshackl PP, Cullinane AC, Ayers L, Ferry BL. Establishment of a healthy human range for the whole blood “OX40” assay for the detection of antigen-specific CD4+ T cells by flow cytometry. Cytometry. Part B, Clinical cytometry. 2014;86:350–361. doi: 10.1002/cyto.b.21165. [DOI] [PubMed] [Google Scholar]
- 69.Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, Zell E, Martin S, Messonnier NE, Clark TA, Skoff TH. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics. 2013;131:e1047–1052. doi: 10.1542/peds.2012-1928. [DOI] [PubMed] [Google Scholar]
- 70.Quinn HE, Snelling TL, Macartney KK, McIntyre PB. Duration of protection after first dose of acellular pertussis vaccine in infants. Pediatrics. 2014;133:e513–519. doi: 10.1542/peds.2013-3181. [DOI] [PubMed] [Google Scholar]
- 71.Matthias J, Pritchard PS, Martin SW, Dusek C, Cathey E, D'Alessio R, Kirsch M. Sustained Transmission of Pertussis in Vaccinated, 1-5-Year-Old Children in a Preschool, Florida, USA. Emerging infectious diseases. 2016;22:242–246. doi: 10.3201/eid2202.150325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.