Abstract
The cytoprotective effects of glycine against cell death have been recognized for over 28 years. They are expressed in multiple cell types and injury settings that lead to necrosis, but are still not widely appreciated or considered in the conceptualization of cell death pathways. In this paper, we review the available data on the expression of this phenomenon, its relationship to major pathophysiologic pathways that lead to cell death and immunomodulatory effects, the hypothesis that it involves suppression by glycine of the development of a hydrophilic death channel of molecular dimensions in the plasma membrane, and evidence for its impact on disease processes in vivo.
Keywords: Glycine, Cytoprotection, Cell death
Introduction
In 1987, it was first reported that the calcium-induced mitochondrial permeability transition (MPT), a central process in mitochondrial failure during injury states, could be blocked by cyclosporine A [1, 2]. This discovery ultimately led to the modern understanding of the molecular basis of that process [2–4]. It can reasonably be considered a critical early milestone in the path to the now widespread appreciation, as exemplified by this symposium, that cell death by necrosis is in fact a highly regulated process.
Initially reported that same year, 1987, but much less well known, was another observation highly relevant to understanding regulated necrosis, i.e., glycine cytoprotection [5]. The contexts, mechanisms, and relevance of glycine cytoprotection to necrotic cell death in vitro and in vivo have since been addressed in several hundred papers and reviews [6–15]. As we will cover here, this robust and widely replicated behavior that is expressed in multiple forms of necrotic cell damage to parenchymal, vascular, and inflammatory cells of diverse tissues has the potential to play a critical role in the development of immunogenic tissue injury and resulting disease processes. The fact that it targets a late downstream process common to necrosis elicited in so many different settings makes it of importance for understanding the fundamental pathobiology of that process. Additionally, it has led to the recognition that glycine can play an important role as an immunomodulator via effects on signaling in multiple inflammatory cells that are separate from the cytoprotection it provides but that can combine with cytoprotection, sometimes in the same cells, to suppress tissue damage during a variety of disease states.
Initial recognition of glycine cytoprotection and its relationship to intracellular glutathione metabolism
The discovery of glycine cytoprotection resulted from studies testing the role of glutathione in hypoxia/reoxygenation injury to freshly isolated proximal tubules [5]. These obligately aerobic and highly metabolically active cells are especially prone to hypoxic injury both in vivo and in vitro, because glycolytic pathways for preservation of ATP content in the absence of mitochondrial oxidative phosphorylation are either absent or only minimally expressed in healthy proximal tubule cells [16], so that in the absence of modifying interventions, oxygen deprivation of 15–30 min results in necrotic cell death to most cells as manifested by LDH release [17–19] and classical oncotic structural changes [18] that disrupt cellular structure and decrease the numbers of intact cells that can be recovered by centrifugation [5]. As a result of these events, the cells lose their ability to maintain integrated metabolic, energetic, and transport functions during reoxygenation as manifested by failed recovery of mitochondrial respiration and intracellular ATP and K+ levels. In the initial study that identified glycine cytoprotection, isolated tubules treated with exogenous glutathione remained intact and importantly recovered respiratory function and cell ATP and K+ levels after hypoxic periods that severely compromised these parameters in the absence of glutathione. Given that exogenous glutathione was known to be extensively metabolized by kidney tubules with subsequent uptake of its component amino acids followed by re-synthesis of glutathione [5, 6, 9, 20], the initial studies with glutathione simultaneously assessed the effect of each of its component amino acids, glycine, cysteine, and glutamate, with the surprising finding that only glycine conferred cytoprotection [5]. Work reported shortly thereafter then showed that even though exogenous glutathione produced the expected increases of intracellular glutathione [17, 21], glycine alone did not increase intracellular glutathione [17, 21], thus further dissociating its tubule cytoprotective effects from glutathione and the pathways it targets. Moreover, glycine retained the protective efficacy even when intracellular glutathione levels were lowered with the gamma glutamylcysteine synthetase inhibitor buthionine sulfoximine or the alkylating agent bis-chloroethylnitrosourea [17, 21–23].
The independence of glycine’s effects from those of glutathione is particularly notable given the important cytoprotective actions of glutathione via its antioxidant activity, which was well documented prior to the original glycine study and has been further been reinforced during the past several years with the recognition of the ferroptosis regulated necrosis pathway [24–26].
Thus, modification of glutathione metabolism is not a necessary component of glycine cytoprotection for kidney proximal tubules during injury from hypoxia-induced ATP depletion. Furthermore, glycine supplementation does not contribute to supporting glutathione levels in that context.
Involvement of other pathways for glycine metabolism
In addition to being a component of glutathione, glycine is the most abundant amino acid in the body [27] and is involved in multiple metabolic pathways [28]. Formation of acylglycines was considered as a possible mechanism of protection in the initial report of glycine cytoprotection [5], but this was subsequently found not to be the case [29].
Many of the metabolic reactions involving glycine occur over time frames and require conditions (e.g. ATP-dependent reactions) that are not available at the late point of injury during which cytoprotection occurs in the systems where protection is most cleanly expressed, so they cannot be an essential part of its mechanism. Consistent with this, no changes in total glycine levels were detected during its protection of isolated kidney tubules during hypoxia [5]. In work using 13C-glycine, the main products of glycine metabolism by proximal tubule cells were glutathione and serine [30], which is consistent with its major involvement in glutathione metabolism and in the folate cycle of one-carbon metabolism [31]. As covered below, other amino acids including l-alanine and d-alanine can share cytoprotective effects with glycine, albeit with less efficacy. Their metabolism was also not required for cytoprotection [18].
Although not involved in direct glycine cytoprotection, diverse pathways of glycine metabolism clearly have the potential to act earlier in the injury process and upstream from cytoprotection to contribute to its overall benefit against cell and tissue damage. These contribute to the large array of glycine-sensitive injury processes that have been identified in work that has been generated by recognition of glycine cytoprotection and are further considered in the context of those observations.
Cell types subject to glycine cytoprotection
The robustness of glycine cytoprotection against necrosis and its occurrence in multiple cell types have led to numerous studies addressed separately below that have identified the benefits of glycine for diverse forms of tissue injury in vivo, but many of these results derive from novel immunomodulatory, non-neuronal signaling effects of the amino acid that have come to light as a result of the work [11, 14] rather than from direct glycine cytoprotection against necrosis.
Table 1 summarizes cell types for which direct glycine cytoprotection against necrosis has been convincingly reported. Criteria for inclusion include use of models that actually produce necrosis, absence of effects on the many upstream pathways of injury listed in Table 2 that are not necessarily modified during glycine cytoprotection, and a compatible efficacy profile of other amino acids and related compounds (Table 3). The earliest studies clearly documented this for both fully differentiated kidney proximal tubules and kidney medullary thick ascending limb cells [5, 17, 32–39] and they were followed shortly thereafter by use of permanent tubule epithelial cell lines [40].
Table 1.
Types of cells and injury subject to glycine cytoprotection
| Cell types |
| Freshly isolated and primary cultured kidney proximal tubules [5, 17, 32–36, 90, 222, 259–262] |
| Medullary thick ascending limb of the isolated perfused kidney [37–39] |
| Permanent renal cell lines—MDCK, LLC-PK1, OK [40, 84, 85, 137] |
| Kidney mesangial cells (JMW unpublished data) |
| Endothelial—umbilical vein, aortic, hepatic sinusoidal [41–44, 263] |
| Hepatocytes—freshly isolated, primary culture, and cell lines [42, 45–49] |
| Peripheral macrophages—multiple types of primary cultures and cell lines [50–56, 161] |
| PC-12 cells [73] |
| Myocytes [71] |
| Injury types |
| Anoxia/hypoxia [5, 17, 33–35, 46, 48, 49, 259–261] |
| Mitochondrial respiratory chain inhibitors and uncouplers [34, 40, 45, 82, 260] |
| Calcium ionophores [40, 83, 84] |
| Oxidants |
| H2O2 [41, 85] |
| Menadione [86] |
| Cysteine conjugates [87] |
| Rewarming/reperfusion after cold storage [32, 47] |
| Agents/processes that alter plasma membrane permeability barriers |
| Pyroptosis due to intracellular bacterial infection and toxins [50–52, 54–56, 91] |
| Pore-forming peptides [44, 92, 168] |
| Activation of P2X7 receptors by ATP [53, 264] |
| Complement (JMW unpublished data) |
| Phosphate depletion [36] |
| Ouabain [23] |
| Cholesterol esterase [262] |
| Gabexate mesilate [263] |
References are limited to studies with isolated cell models or isolated tissue systems where direct glycine cytoprotection is likely. Additional references are in the text
Table 2.
Pathophysiologic processes not necessarily modified by glycine
| Glutathione depletion [5, 17, 21, 45, 82] |
| Decreased cell ATP levels [5, 17, 21, 29, 40, 45, 46, 82] |
| Prelethal increases of cytosolic free calcium [40, 41, 83, 84, 95, 100, 114, 117] and calpain activation [135] |
| Mitochondrial permeability transition [49, 265, 266] |
| Cytoskeletal disruption, actin depolymerization, blebbing [83, 96, 97, 101] |
| Disruption of monovalent cation homeostasis [5, 17, 23, 82], cell swelling [23], hypotonic lysis [113] |
| Nonesterified fatty acid accumulation [35, 84, 119] and fatty acid-mediated post-hypoxic mitochondrial energetic deficit [19, 118, 140, 246] |
| Intracellular acidification [122] |
| Iron-induced lipid peroxidation [86, 90] |
| Oxidative cross-linking of proteins [86] |
| Bax-mediated cytochrome c release and resulting DNA damage [131, 145] |
| Caspase-1 activation and resulting IL-1 β maturation and secretion processing [50–54, 91, 92] |
| Nitric oxide production [134] |
| Proteolysis [135, 136] |
Only selected references emphasizing initial and most informative observations and findings in diverse cell types are included due to space considerations
Table 3.
Ability of other amino acids and small molecules to reproduce glycine cytoprotection
| Strongly Protective |
| Glycine |
| L-Alanine [ 18, 33, 37, 39, 44, 45, 47 ] |
| β-Alanine [ 18, 37 ] |
| 1-Aminocyclopropane-1-carboxylate—agonist at glycine-sensitive site on NMDA receptor [18, 38] |
| Weak/variably protective |
| d-Alanine [ 18, 37, 44, 45 ] |
| 1-Aminocyclopropane-1-carboxylate—agonist at glycine-sensitive site on NMDA receptor [18, 38] |
| γ-Aminoisobutyric acid [18, 37, 39, 45] |
| l-Serine [ 18, 37 – 39, 47 ] |
| d-Serine [ 18, 38 ] |
| Consistently non-protective |
| l-Glutamate [ 5, 39 ] |
| l-Glutamine [ 33, 37, 39 ] |
| l-Cysteine [ 5, 39 ] |
| l-Taurine [ 18, 37, 38, 47 ] |
| l-Proline [ 18, 37, 44 ] |
| l-Valine [ 18, 44, 47 ] |
| Protective non-amino acids |
| Strychnine—glycine receptor antagonist [34, 46, 98, 104, 107, 145, 148] |
| Bicuculline—glycine and GABAA receptor antagonist [34, 145] |
| Norharmane—glycine receptor antagonist, benzodiazepine receptor agonist [34] |
| Avermectin B1a—GABAA receptor modulator and glycine receptor agonist [98] |
| Cyanotriphenylboron—GABAA and glycine receptor antagonist [98] |
| Muscimol—GABAA receptor agonist [111] |
| Allopregnanolone, pregnenolone sulfate, dehydroepiandrosterone sulfate—GABAA receptor modulators [111] |
| Chloride channel blockers—indanyloxyacetic acid, niflumic acid, N-phenylanthranilic acid, 5-nitro-(3-phenylpropylamino)benzoic acid, diphenylamine-2-carboxylate [46, 85, 116, 145, 267] |
Multiple direct comparisons of these compounds with each other and with other compounds that lack effects along with detailed concentration dependence considerations within the same models can be found in refs [18, 98, 111, 145]. References for glycine are not enumerated because virtually all studies include it. References cited are selected to be the earliest and most complete for multiple cell types where available
Subsequent work showed strong expression of glycine cytoprotection in multiple types of primary cultured endothelial cells [41–44] and in both freshly isolated and cultured hepatocytes [45–49]. Multiple peripheral macrophage cell lines as well as bone marrow-derived primary cultures are strongly protected against necrosis by glycine [50–56]. Kupffer cells, the specialized macrophages found in hepatic sinusoids, were the cell type in which the non-neuronal, immunomodulatory, anti-inflammatory signaling effects via peripheral glycine receptors were first recognized [57], but protection by glycine from necrosis has not been clearly shown for them. Microglia are another cell type whose activation can be significantly modified by glycine (in this case, via signaling effects resulting from glycine uptake-induced cell volume alterations), but for which there are no clear descriptions of glycine cytoprotection [58–61].
Given their structural and metabolic characteristics shared with renal epithelial cells and hepatocytes, it would seem that intestinal epithelial cells should be subject to glycine cytoprotection. There are multiple reports of benefit of glycine for intestinal insults in vivo and in vitro [14, 62–69]; however, convincing demonstration of direct glycine cytoprotection in isolated cell models is lacking. Cultured cell lines of intestinal epithelial cells were protected against oxidant injury from tert-buthylhydroperoxide (tBHP) [70], but this required pretreatment. Glycine only during the insult was not effective, which, as discussed below, is atypical for direct glycine cytoprotection.
There is only limited information available for myocytes [71, 72]. Although the data are consistent with glycine cytoprotection, the reported effect was weaker than typically seen and was suggested to be secondary to suppression of the mitochondrial permeability transition, which is not an effect of glycine that has been consistently observed. This issue is addressed further below.
A study with cultured PC-12 cells [73] indicates that they exhibit typical glycine cytoprotection. How that would be expressed in vivo in the central nervous system, however, is complex, because low micromolar concentrations of glycine that are well below cytoprotective levels are cofactors at N-methyl-d-aspartate (NMDA) receptors, which are responsible for much of neuronal damage during brain injury [74–77]. Moreover, the primary glycine effects as an inhibitory neurotransmitter come into play. As a result, the effects of glycine on neuronal injury are potentially complex and difficult to interpret [78, 79]. Glycine levels are kept low in the cerebrospinal fluid [80] and are tightly regulated by neuronal glycine transporters [81]. Microglial glycine uptake via sodium-coupled neutral amino acid transporters may help buffer these levels when glycine is released from neurons in injured areas, and, as noted above, this may affect microglial function [60], but that process is distinct from direct cytoprotection.
Types of injury subject to cytoprotection
ATP depletion-associated injury models produced by anoxia, severe hypoxia [5, 17, 33–35, 46, 48, 49], and mitochondrial respiratory chain inhibitors and uncouplers [34, 40, 45, 82] have been widely used to study glycine cytoprotection in multiple cell types (Table 1 and additional references therein). Necrosis that develops during rewarming after cold preservation of both kidney tubules and hepatocytes is alleviated by glycine [32, 47].
Calcium ionophore-induced injury has been very useful for studying glycine cytoprotection in kidney cells in conjunction with respiratory chain inhibitors and uncouplers, particularly in cultured cells where either type of maneuver by itself did not produce necrosis [40, 83, 84]. Glycine was not effective against calcium ionophore-induced killing of freshly isolated hepatocytes [47].
Protection by glycine has been reported for oxidant injury produced by both H2O2 [41, 85] and menadione [86]. Benefit for tBHP-induced injury, however, was mild or absent in both kidney tubule cells and hepatocytes [47, 86, 87]. There are reports of glycine protection of endothelial cells against iron-induced necrosis of endothelial cells [88] and of hepatocytes against iron-induced injury [89]. The hepatocyte effect was associated with decreased apoptosis rather than necrosis. In contrast to its strong effects against other insults to kidney proximal tubule cells, glycine does not protect against iron-induced necrosis of those cells [86, 90].
In 2000, it was first recognized that macrophage cell death induced by intracellular bacteria and mediated by caspase-1 during the process that would come to be known as pyroptosis was highly glycine sensitive [50]. Since then, there have been multiple studies demonstrating glycine cytoprotection during pyroptosis and related types of injury that target plasma membrane permeability barriers, including the pore-forming toxins maitotoxin and palytoxin as well as activation of P2X7 receptors by extracellular ATP [44, 50–53, 91, 92]. These models have provided new insights into the mechanism for glycine cytoprotection that are addressed further below.
Even where it was not the primary target, all of the aforementioned injury models are characterized by severe ATP depletion. However, glycine cytoprotection has also been reported in some settings where ATP is not severely depleted, i.e., low phosphate incubation [36] and ouabain treatment [23].
Effects of glycine on central injury-related pathogenic processes
The relevance of multiple common injury pathways and pathophysiologic processes to glycine cytoprotection have been assessed and none have been found to be essential, so Table 2 is titled based on that perspective. Nonetheless, the studies addressing them have been highly informative for understanding the nature of glycine cytoprotection, and, in turn glycine cytoprotection has been useful for clarifying their contribution to necrosis. As covered already, the role of glutathione in glycine cytoprotection was initially assessed in the early work that identified the process and it was clearly dissociated [5, 17, 21–23].
The initial recognition of glycine cytoprotection during hypoxia models in cells such as kidney proximal tubules [5] and hepatocytes [45] where the extent of ATP depletion is a critical determinant of progression to necrosis [33, 93, 94] was followed by multiple studies indicating that preservation of ATP is neither a primary effect of glycine nor necessary for glycine cytoprotection [5, 17, 21, 29, 40, 45, 46, 82]. Although increases of ATP may be seen secondarily due to retention of more intact cells under some conditions, it is clear that they are not a requirement for cytoprotection.
Early studies of glycine cytoprotection in kidney tubule cells made extensive use of calcium ionophore-induced injury under both high and low calcium conditions [40, 41, 83, 84, 95–97]. Glycine was highly protective in both settings indicating a target that was downstream of both very low and very high free intracellular calcium levels. As further detailed below, low calcium conditions were particularly useful for probing mechanistic processes specific for glycine cytoprotection, because they were not accompanied by the extensive biochemical and structural disruption induced by calcium that are not blocked by glycine even as it delays progression to necrosis [83, 84, 98, 99].
Terminal necrosis in the presence of normal extracellular free calcium of 1.25 mM results in a flood of Ca2+ into the cell where normal cytosolic free calcium (Caf) is <100 nM. Determining the extent to which progressive and pathogenic prelethal increases of Caf contribute to the events leading to necrosis was a major goal in studies of injury pathophysiology at the time glycine cytoprotection was first recognized. This was particularly difficult under cell/injury conditions such as ATP deletion of fully differentiated kidney proximal tubules where progression to necrosis was very rapid and accompanied by leakage of the fluorescent intracellular probes used to measure Caf. Use of glycine to delay necrosis allowed the first definitive studies in these systems, documenting the occurrence and extent of the prelethal changes of Caf [95, 100].
The cyclosporine-sensitive MPT is a major effector of cell injury that results from disturbances of cellular calcium homeostasis [2, 4], so is of considerable interest as a potential mediator of glycine cytoprotection, especially since glycine does not prevent increased Caf. Glycine protects calcium ionophore-treated kidney tubule cells under conditions of normal ambient Ca2+ despite large increases of Ca2+ in all cellular compartments including the mitochondrial matrix that invariably induce the MPT [40, 41, 83, 84, 95]. Therefore, the MPT cannot be the only or final target of glycine.
There are conflicting data as to whether glycine can modify MPT under milder (and more physiological) conditions than calcium ionophore treatment. Although glycine is less protective against calcium ionophore-induced injury in freshly isolated hepatocytes [47] than in the various fresh and cultured kidney tubule cell models [40, 41, 83, 84, 95], elegant imaging techniques and a model of pH-dependent reoxygenation injury to primary cultured hepatocytes were used to clearly dissociate the development of the cyclosporine A-sensitive MPT from glycine cytoprotection by showing that the MPT occurred within cells while they were still protected from necrosis by glycine [49]. We have also not found that glycine blocks the MPT in kidney proximal tubules (unpublished).
In contrast to the hepatocyte data [49], studies of a parallel model of glycine-suppressible, pH-dependent, reoxygenation injury to a cardiomyocyte cell line showed that parameters consistent with the calcium-induced MPT in mitochondria isolated from those cells were even more strongly suppressed by glycine than by cyclosporine A [71]. However, unlike the hepatocyte study [49], the cardiomyocyte paper [71] does not provide information about expression of MPT in the intact glycine-protected cells, which is needed to fully interpret those results. Given the importance of both the MPT and glycine cytoprotection to the development of necrosis, it would clearly be of interest to further investigate their interactions more completely in cardiomyocytes and other cell types.
Injury during the states of both low and high Caf is accompanied by pronounced cytoskeletal changes [83, 96, 97, 99]. Glycine did not modulate the cytoskeletal changes in any of these settings and its benefit was not modified by further disrupting the actin cytoskeleton with cytochalasin during anoxia [101].
ATP depletion states are accompanied by monovalent cation shifts and volume increases due to Na+ pump inhibition [102], opening of small membrane channels [103], and action of the sodium hydrogen exchanger (NHE) [104]. Measurements of cell K+ and volume in tubule models of ATP depletion, as well as injury induced by ouabain and incubation in high K+ medium, did not indicate modification by glycine of these processes during cytoprotection [5, 23, 82]. Moreover, incubation of tubules in hypotonic medium to aggravate volume changes during anoxia did not prevent glycine cytoprotection [105] and glycine remained protective during anoxia even in the presence of pore-forming agent, α-toxin. In a cultured tubule cell model of injury produced by Ca2+ ionophore under Ca2+-replete conditions, addition of sucrose as an osmoprotectant did enhance glycine cytoprotection even though sucrose alone was without effect [106].
For other cell types, the situation is more complicated. In hepatocytes, glycine cytoprotection is associated with decreased Na+ entry and limiting that entry is itself protective [46, 104]. However, the effect of glycine on Na+ entry may be an early signaling effect in which glycine-induced volume changes activate p38, which in turn suppresses NHE [107]. This process is likely distinct from glycine cytoprotection, even though it contributes to the benefit of glycine seen in those cells. One study of glycine-sensitive injury to endothelial cells has implicated volume increases that are blocked by glycine [43], but another did not [44].
Pyroptosis is another glycine-sensitive insult where volume changes likely mediated by ion entry play a role. When pyroptosis was induced by salmonella infection or anthrax toxin, protection of macrophages similar in degree to that produced by glycine was provided by oncotic support with addition to the medium of polyethylene glycols of 1450–2000 MW suggesting the involvement of 1.1–2.4 nm membrane pores [52, 91]. Benefit of the same-sized polyethylene glycols was also seen during glycine-sensitive killing of macrophages by Streptococcus pyogenes [54]. These observations contrast with the kidney tubule work showing that glycine still limits LDH release after addition of alpha-toxin to form plasma membrane pores in already anoxic proximal tubules [105]. The size of the α-toxin pores, 1.5 nm [108], is the same as the pores that are proposed to be involved in the protection provided by the polyethylene glycols during pyroptosis [52, 91].
The fact that the neuronal glycine receptor is a chloride channel [109] has led to investigations of the possibility that glycine acts on plasma membrane chloride channels in non-neuronal cells to limit their pathological opening during injury states and thereby decreases chloride entry that aggravates volume changes causing plasma membrane disruption and necrosis. This idea has been supported by evidence from several systems that chloride entry occurs during injury, is blocked by glycine, and contributes to progression of injury [110–112]. However, multiple other studies have failed to find any dependence of injury or glycine effects on chloride availability [43, 46, 53, 98], and the aforementioned work showing that protection by glycine is not modified by use of α-toxin to allow unhindered movements both cations and anions during anoxia definitively shows that movements of small ions such as chloride cannot be a necessary target of glycine cytoprotection [105]. Glycine also does not modify hypotonic swelling of erythrocytes [113]. Overall, the data from the diverse cell types and models indicate that volume changes associated with pore formation and chloride entry can aggravate progression to glycine-sensitive necrosis in a cell- and model-specific fashion, but glycine does not primarily target them.
The initial study of glycine cytoprotection using kidney tubules described strong post-hypoxic recovery of respiratory function, ATP levels, and K+ homeostasis [5], indicating that cytoprotection was not limited to preventing terminal necrosis, but instead could enable true integrated metabolic recovery necessary for long-term viability and subsequent reports showed similar behavior including prolonged viability [17, 44, 114–117]. However, metabolic and functional recovery may not occur after more severe conditions despite glycine cytoprotection [19, 32, 85]. In freshly isolated kidney tubules subjected to prolonged severe hypoxia, mitochondrial recovery can be absent or severely impaired [19]. This energetic deficit has been shown to result from mitochondrial uncoupling and de-energization resulting from accumulation of nonesterified fatty acids during hypoxia [118], which glycine does not prevent [35, 118, 119].
Decreases of pH during ATP depletion can both promote and prevent injury depending on the cell type and conditions [49, 104, 120, 121]. Glycine did not modify the behavior of intracellular pH during cytoprotection of kidney tubules [122].
As discussed above, the efficacy of glycine cytoprotection is less consistent for oxidant models than for those driven primarily by ATP depletion. Both H2O2- [123] and tBHP- [86] induced injury have strong components mediated by iron-driven lipid peroxidation, which is central to cell death by the newly described ferroptosis pathway [24]. Glycine does not protect against either iron-induced lipid peroxidation or oxidative protein cross-linking [86] under conditions where these processes are strongly blocked by ferroptosis inhibitors [25, 86].
The initiation of pyroptosis, whose terminal necrotic phase is strongly blocked by glycine [50–53, 91, 92], involves caspase-11 and/or caspase-1 activation followed by IL-1β maturation and secretion [124–130]. Neither of these processes is affected by glycine [50–54, 91, 92].
Glycine does not affect either cytochrome c release during apoptosis or the characteristic DNA laddering [131, 132], and there are no reports that it blocks any of the intermediate apoptotic pathways. In fact, the initial demonstration of apoptosis resulting from hypoxia was enabled by the use of glycine to prevent the cells from undergoing necrosis instead [131, 132]. In this regard, it is of note that both DNA laddering and TUNEL-positive nuclei can be seen in necrotic cells (reviewed in [133]). Using streptolysin-O to permeabilize glycine-protected cells, it was shown that necrosis-associated DNA laddering can result from post-lethal serine protease mediated, caspase-independent endonuclease activation [106]. Although the effect of glycine to shift necrotic to apoptotic cell death has not been studied in multiple cell types, it is likely an important generalized phenomenon that impacts on the course of tissue injury by favoring nonimmunogenic cell death via apoptosis rather than proinflammatory, immunogenic necrosis.
Excess nitric acid production, particularly by inducible nitric oxide synthetase, can contribute to cell injury and death. Glycine does not block that process in kidney tubules [134].
An early study of glycine cytoprotection in hepatocytes proposed a role for inhibition of proteolysis [45]. However, subsequent work has suggested that the effects on proteolysis are due to limitation by glycine of postlethal enhancement rather than being a prelethal effect that contributes to primary cytoprotection [135, 136].
Glycine and related compounds that appear to induce the same cytoprotection have been reported to modify other injury modifiers including hsp70 [137], heme oxygenase [138], ERK [89, 139], and p38MAPK [107], but they have not been rigorously established as being necessary for true glycine cytoprotection. Some of these changes may reflect actions of glycine on signaling pathways that certainly contribute to the evolution of injury but that are not central to the unique cytoprotection provided by glycine.
Timing of the glycine effect
In the study that originally identified glycine cytoprotection using a hypoxia/reoxygenation model [5], it was shown that the protective effect of glycine required its presence at the time of the lethal event, which was during the hypoxic period in that experimental setting. Pretreatment with glycine followed by removing it from the medium was not effective, nor was addition during reoxygenation, which was predictable since most lethal injury in that model occurred during hypoxia. Subsequently, it was shown using chemical hypoxia ATP depletion models of both kidney tubule cells [87] and hepatocytes [45] that glycine could be added after the ATP depletion period was well underway and still prevent subsequent LDH release. These examples of efficacy without any pretreatment resulting from the addition during periods when the injury-inducing insult suppresses most ATP-dependent metabolic pathways emphasize the dissociation between glycine cytoprotection and metabolic pathways requiring active metabolism. Another timing consideration highly relevant to understanding an essential element of glycine cytoprotection derives from observations on withdrawal of glycine during insults. These were initially reported using tubules injured by a halogenated hydrocarbon [87]. Subsequently, this behavior was demonstrated in a more common disease-relevant context using a hypoxia/reoxygenation model where, as discussed above, the tubules develop a severe energetic deficit during reoxygenation mediated by persistently high levels of nonesterified fatty acids (NEFA) [118, 140]. If glycine is withdrawn before the energetic deficit is corrected, necrosis rapidly occurs. If, on the other hand, the energetic deficit is corrected in the presence of glycine by maneuvers that lower the NEFA burden and restore ATP, glycine can then be withdrawn without development of necrosis [140]. Glycine cytoprotection was also shown to be lost when glycine was withdrawn from endothelial cells subjected to chemical hypoxia [43] or maitotoxin treatment [44]. These observations emphasize that glycine must be present at the time of the lethal event and that the glycine-suppressible process rapidly proceeds if glycine is removed.
Relationship of cytoprotection to neuronal glycine receptors
The inhibitory neuronal glycine receptor (GlyR) is a pentameric gated chloride channel composed of alpha and beta subunits [109, 141]. It is a member of the nicotinoid receptor, cysteine-loop superfamily that also includes the excitatory nicotinic acetylcholine receptor, the inhibitory γ-aminobutyric acid, Type A receptors, and the cation permeable serotonin type 3 receptor. Neuronal glycine receptors are found in spinal cord, brain stem, caudal brain, and retina. In humans, there are four types of alpha subunits. The most common GlyR consists of three α1 48 kDa and two β 58 kDa subunits along with a 98 kDa cytoplasmic anchoring protein, gephyrin, which binds to the β subunit to anchor it to the subsynaptic membrane. The presence of alpha subunits is sufficient to confer receptor function, which has been widely studied in HEK 293 cells expressing α1 homomers [142, 143]. Classical primary agonists for GlyR besides glycine are β-alanine and taurine with a potency order glycine > β-alanine > taurine (EC50s of 18, 52, and 153 μΜ, respectively, in HEK cells expressing homomeric α1 receptors [143]: 90, 100, and 500 μΜ, respectively, in isolated hypothalamic neurons [144]). l-Alanine and d-alanine show similar activity less than that of taurine and greater than l-serine. d-Serine is without significant activity [144]. Glycine is antagonized by low micromolar concentrations of strychnine, which binds to the α subunit [109, 141]. In addition to being the primary agonist at the neuronal glycine receptor, glycine is a coagonist at the NMDA receptor [74].
The data reviewed in the preceding sections showing independence of glycine cytoprotection from its major metabolic pathways, absence of modification of multiple metabolic and structural changes that contribute to cell death, and rapid ‘on–off’ behavior at its target are all consistent with the involvement in cytoprotection of a ligand–receptor type of action. Even before most of these latter observations were made, the possibility that glycine cytoprotection was mediated by a non-neuronal glycine receptor was addressed using a pharmacological approach testing the efficacy of known analogs for the neuronal glycine receptor [18]. The results of this study and of subsequent work by multiple laboratories that extended the observations using additional compounds, injury models, and cell types are summarized in Table 3.
In the initial study of the molecular pharmacology of glycine cytoprotection in kidney proximal tubules, 4 of 45 compounds tested, glycine, l-alanine, β-alanine, and 1-aminocyclopropane-1-carboxylate were found to be highly protective [18] and these results have been confirmed in other reports testing them as summarized by the citations in Table 3. The EC50 for glycine in this study [18] and the original report of its cytoprotective effect [5] was ~0.5–0.75 mM. Similar EC50s have been reported in multiple other systems [34, 44, 45, 145]. Maximal effects, usually complete cytoprotection, are generally reached by 2 mM. l-Alanine had a substantially higher EC50, ~2.5 mM, in the studies with sufficient data to estimate it [17, 18], but in all reports was as protective as glycine when its concentration was increased to 5 mM [18, 33, 37, 39, 44, 45, 47]. Sufficient data to estimate EC50s for β-alanine and 1-aminocyclopropane-1-carboxylate are not available, but based on smaller effects than glycine at 2 mM and incomplete protection at 5 mM, they were substantially less effective than glycine and l-alanine [18].
In the original study [18], d-alanine had a weak cytoprotective effect, but α-aminoisobutyric acid, l-serine and d-serine were all without activity. Other reports (summarized in Table 3) have described variable results for those compounds. Multiple other amino acids importantly including taurine, which is an invariant agonist at neuronal glycine receptors [143, 144], and glutamate, which is the natural primary agonist at the NMDA receptor [74], have not exhibited cytoprotective activity like that of glycine in multiple studies that have compared them with glycine ([18] and others summarized in Table 3). 1-Aminocyclopropane-1-carboxylate, which had some cytoprotective activity, is, like glycine, a coagonist at the NMDA receptor. However, d-serine, the other main physiological coagonist at that receptor [74], does not have consistent cytoprotective effects [18, 38] and two antagonists at the glycine-modulatory site, kynurenic acid [146] and cycloleucine [147], had no effect on their own and did not alter the effects of glycine [18]. Cytoprotection in the tubule system required that both amino and carboxyl groups be present and unmodified [18]. Neither taurine, as already covered, cysteamine, sarcosine, propargylamine, betaine, aminoacetonitrile, ethanolamine, or alaninol, all of which have variations in structure relative to glycine and alanine that are limited to either their amino or carboxyl groups, had any activity, nor did propionic and acetic acid, which lack amino groups [18].
Strychnine is the classical antagonist at the GlyR and the first purification of GlyR was via a strychnine affinity column. It is a high-affinity competitive antagonist that binds to the 48 kDa α subunit, inhibits all known GlyR isoforms, and is considered to be the definitive pharmacological tool for identifying glycinergic synaptic currents [141] with an IC50 for inhibition of glycine-induced chloride currents in hippocampal neurons of 20 nM and a maximal effect at 1 μM [144]. True glycine cytoprotection (as distinct from glycine effects on signaling in nonneuronal cells that will be addressed below) has never been shown to be antagonized by strychnine. Rather, multiple laboratories have demonstrated that strychnine has similar cytoprotective effects to those of glycine at concentrations similar to those at which glycine is effective [34, 46, 98, 104, 107, 145, 148]. Strychnine has behavior similar to glycine with respect to timing, in that it can be added late just before the lethal event and cytoprotection is rapidly lost when it is removed [87]. Labeled strychnine showed specific binding to isolated kidney proximal tubules with an IC50 of 0.87 mM [149]. Unlike neuronal binding [150], the proximal tubule binding was not displaced by excess glycine. However, strong support for the specificity of strychnine effects on a surface target has been provided by studies with MDCK cells using novel, water-soluble, cell impermeant, fluoresceinated strychnines, which allowed verification of their localization [145]. In this work, a fluoresceinated derivative in which the strychnine moiety was not altered at any of its critical active sites provided cytoprotection without entering the cells. A similar derivative modified by methylation of the nitrogen atom at position N22, which is near the region involved in receptor binding, was not cytoprotective.
Further studies summarized in Table 3 have compared the cytoprotective efficacy of glycine with that of other agents active at the GlyR and other nicotinoid receptors as well as chloride channel blockers and have identified multiple additional cytoprotective compounds, some of which had substantially higher potency than glycine, particularly when injury was produced under Ca2+-limited conditions [98]. These include the GlyR and GABAA receptor antagonists avermectin B1A and cyanotriphenylboron [98], the neurosteroid GABAA receptor modulators allopregnanolone, pregnenolone sulfate, and dehydroepiandrosterone sulfate [111], and the chloride channel blockers indanyloxyacetic acid and niflumic acid [85, 98].
Thus, molecular pharmacology studies of glycine cytoprotection from multiple laboratories indicate substantial overlap with agents active at neuronal receptors for which glycine is an agonist or coagonist and indicate highly constrained steric and conformational requirements for the interaction, which, along with the rapid on–off timing of the effects, is consistent with the involvement of reversible ligand binding site interactions. Even though, as covered previously, chloride is not necessarily involved in glycine cytoprotection, the facts that GlyR is a chloride channel and the chloride channel blockers have cytoprotective efficacy, including cyanotriphenylboron and niflumic acid that bind to glycine receptor M2 channel domains, also strongly support the concept that a protein with chloride channel properties is involved in cytoprotection [98]. However, the profile of cytoprotective activity does not correspond to either of the known neuronal receptor types targeted by glycine or that of other nicotinoid receptors.
The results of studies assessing the presence of the GlyR in cells showing strong glycine cytoprotection have been equivocal. Immunoblotting of rat hepatocyte membrane extracts for the α1 subunit was negative on the same blots where rat spinal cord and rat liver Kupffer cell (see below) membrane extracts were positive [151]. Evidence for expression of the GlyR β-subunit has been reported for endothelial cells where, as covered below, glycine-gated chloride channel signaling is seen, but that effect of glycine is blocked by micromolar levels of strychnine [152], which is not a characteristic of nonneuronal glycine cytoprotection. For kidney proximal tubules, there are short reports providing immunoblots suggesting the presence of the β-subunit and gephyrin, but negative for the α1-subunit [153], PCR screening of human and rat kidney cortex cDNA libraries that were positive for β but not α1 subunits [154], and immunofluorescence positive for the β-subunit and gephryin, but not the alpha subunit [154]. In the latter studies, the β-subunit bands overlap with the IgG heavy chain making it difficult to interpret the immunoblots shown [153] and the immunofluorescence was not clearly membrane localized [154]. There is no reported work testing the effects on cytoprotection of manipulating availability of the β-subunit or gephyrin and whether these molecules alone could confer functional GlyR activity is not clear. We have queried a recently reported RNA-Seq transcriptosome data set from microdissected rat nephron segments [155] for the presence of GlyR α1- and β-subunits and gephryin and did not detect any expression (unpublished).
Message and protein for GlyRα1 were detected in MDCK cells [156]. In this study, siRNA knockdown of GlyRα1 decreased cytoprotection by glycine in proportion to the efficacy of the siRNA knockdown, transfection of GlyRa1 into HEK-293 cells conferred protective effects of both glycine and strychnine that were not present in wild-type HEK-293, and the protective effects were abrogated by mutating Tyr202 of GlyR to phenylalanine or leucine, which impair glycine and strychnine binding [156]. Although these observations would seem to be strong evidence for involvement of GlyRα1 in cytoprotection, the same laboratory has subsequently reported data suggesting that the effects of GlyRα1 in both cell systems are mediated by ERK1/2 and AKT activation [139]. Since there are multiple experimental models reviewed in the preceding sections where ERK1/2 and AKT activation cannot occur and/or cannot explain the timing of glycine’s effects relative to injury, those pathways cannot be an essential or generalized feature of glycine cytoprotection. These authors have also reported strong glycine cytoprotection of primary neuron cultures subjected to oxygen and glucose deprivation (OGD) that is convincingly blocked by anti-GlyR and antibodies or GlyRα1 knockdown [157]. Injury in that system was also strongly decreased by the NMDA receptor antagonist, MK-801, although its effect was independent of GlyR. Since NMDA receptor antagonists do not generally reproduce glycine cytoprotection [18], the relationship between these neuronal GlyR-mediated effects in OGD injury and nonneuronal glycine cytoprotection remains to be clarified.
Although the findings reviewed thus far have not shown that either the presence of GlyR or modification of chloride movements is essential for glycine cytoprotection, studies further investigating them have led to recognition of previously unsuspected and widely expressed immunomodulatory effects of glycine that do appear to utilize those pathways [11, 14]. This concept originated from work investigating the effects of glycine on whole liver models of ischemia/reperfusion and transplantation in which strong benefit was observed [42, 158, 159]. Analysis of the mechanisms for the whole liver effects provided evidence that they involved glycine suppression of inflammatory Kupffer cell activation resulting from glycine-induced chloride uptake leading to hyperpolarization and suppression of calcium influx via voltage-gated calcium channels and subsequent signaling similar to the neuronal effects of glycine [57].
Immunomodulatory effects were then found by these investigators and others in multiple types of inflammatory cells in addition to Kupffer cells including peripheral macrophages, alveolar macrophages, T cells, polymorphonuclear leukocytes, as well as endothelial cells [152, 160–163]. Moreover, evidence for the presence of GlyR was provided for several of these cell types where GlyRα1 was detected [151]. The pharmacology of the Kupffer cell effects was also consistent with the effects being mediated by true neuronal-type receptors in that they were fully reproduced by the other strong classical glycine receptor agonists, β-alanine and taurine [57]. As reviewed previously, β-alanine consistently has cytoprotective effects like glycine, but taurine does not (Table 3). EC50s for these glycine effects on inflammatory cell signaling were in the range 0.3–0.5 mM with maximal effects at 1 mM [152, 160, 162, 163], which is slightly lower than for glycine cytoprotection, but higher than for neuronal glycine receptors. Similar signaling effects have also been reported for cardiomyocytes [164]. The effects of glycine on each type of inflammatory cell were maximally blocked by 1 μM strychnine [152, 160, 162, 163]. Interestingly, raising the strychnine concentration to 1 mM with neutrophils and alveolar macrophages appeared to change it from a glycine antagonist to an agonist [160, 163].
Studies assessing whether activation of microglial cells is also modified by glycine in this fashion instead demonstrated a separate pathway of glycine-induced immunomodulation, where glycine uptake via the sodium-linked neutral amino acid transporter induces cell Na+ and volume increases that in turn affect signaling and function. [58, 60, 61]. Distinctly higher glycine concentrations were required for these effects with initial changes seen at 1 mM and consistently maximal effects at 10 mM. Glycine similarly modulated peritoneal macrophage function via its uptake by neutral amino acid transporters in peritoneal macrophages even though those cells expressed the GlyR α1 subunit and gephyrin [59]. In addition to the effects of glycine on neutrophils that were apparently mediated by strychnine-sensitive signaling, it has also been reported that both N-formyl methionyl leucyl phenylalanine peptide and phorbol myristate acetate-induced reactive oxygen species production in neutrophils can be inhibited by glycine with EC50s of 0.5–1.5 mM by a strychnine-insensitive mechanism that is unrelated to changes of Caf [165]. None of the studies of inflammatory cells displaying immunomodulatory effects of glycine and implicating GlyR in them extended the models to assess glycine modulation of necrosis in those cells, which would clearly be of interest in view of the work that has shown strong protection by glycine against pyroptosis of macrophages [50–54, 91, 92].
Nature of protection
Necrosis/oncosis is usually defined and assessed by changes in the plasma membrane permeability barrier, which, at the point of widespread cellular disruption, allows leakage of large intracellular proteins such as lactate dehydrogenase (136 kDa). Prevention of LDH leakage has been used since the earliest studies of glycine cytoprotection [17, 21] to assess it and is the most common way of quantifying the process. Glycine also prevents pathological permeability increases to small probes that are used to monitor cell death as initially shown for propidium and fluorescein in cultured kidney tubule cells [40] and then subsequently for them and others in multiple cell types (Table 4).
Table 4.
Fluorescent probe studies of plasma membrane permeabilization in relation to glycine cytoprotection
| Cell type | Injury model | Pore probes | Behavior without glycine | Behavior with glycine |
|---|---|---|---|---|
| MDCK and LLC-PK1 (renal epithelial) [40] and human umbilical vein endothelial [41] | Uncoupler [carbonyl cyanide m-chlorophenylhydrazone (CCCP)] + ionomycin |
Propidium (416 Da) Fluorescein (332 Da) |
Nearly all cells develop LDH release, fluorescein release, and propidium uptake | LDH release, fluorescein release, and propidium uptake all prevented |
| MDCK [99] | Uncoupler (CCCP) + ionomycin in 100 nM Ca2+ medium |
Propidium (416 Da) Fluorescent dextrans—4 kDa (2–3 nm molecular diameter), 70 kDa (10–15 nm), 2000 kDa (54 nm) |
Majority of cells leak LDH and become progressively positive for propidium over 120 min and similarly permeable to 4 kDa dextran. Heterogeneous loss of permeability to 70 kDa dextrans with many propidium-positive cells that completely exclude 70 kDa dextran. All cells exclude 2000 kDa dextran | LDH leak blocked. Rare propidium-positive cells. All dextrans including 4 kDa excluded |
| MDCK [145] | Uncoupler (CCCP) + ionomycin in 100 nM Ca2+ medium |
Calcein (623 Da) Ethidium homodimer (717) |
Majority of cells had leaked LDH and calcein and taken up ethidium homodimer at 2 h | LDH and calcein leaks and ethidium homodimer uptake blocked |
| LLC-PK1 [85] | H2O2 | Trypan blue (961 Da) | Cells display LDH release and Trypan blue uptake at 2 h | LDH release suppressed, but no change in failure of Trypan blue uptake |
| Hepatic sinusoidal endothelial in primary culture [43] | Chemical hypoxia (cyanide) |
Propidium (416 Da) Calcein (623 Da) Lucifer Yellow (443 Da) Fluorescent dextrans (40–2000 kDa) |
Cells become permeable to calcein and start forming blebs at 135–180 min, followed after 30–60 min by uptake of propidium and 40 kDa dextran and, to a lesser extent, 2000 kDa dextran. Uptake of propidium and all sizes of dextrans is simultaneous | Slows but does not prevent the calcein and Lucifer yellow uptake. Prevents uptake of propidium and 40 and 2000 kDa dextran |
| Freshly isolated kidney proximal tubules [167] | Hypoxia |
Propidium (416 Da) Phallacidin (846 Da) Fluorescent dextrans (3 and 70 kDa) Trypan blue (961 Da) |
Rapid (5–15′) uptake of 20 μM propidium followed after 15′ by LDH release. Phallacidin uptake delayed relative to propidium At 30 min, when LDH release was 50 %, all cells were permeable to 3 kDa dextran, but only a few were permeable to 70 kDa dextran |
No effect on propidium uptake, but all cells remain impermeable to 3 and 70 kDa dextran |
| Bovine aortic endothelial cells [44, 169] | Maitotoxin |
Ethidium (359 Da) Propidium (416 Da) Transfected GFP concatemers (27–162 kDa) |
Initial Ca2+ increase-dependent induction of slow ethidium uptake followed by accelerated ethidium uptake coinciding with LDH release, propidium uptake, and release of GFP concatemers without detectable size dependence in latency to release or rate of release | No effect on Ca2+ increase or initial slow phase of ethidium uptake. Prevents the rapid phase of ethidium uptake, propidium uptake, and release of LDH and GFP concatemers |
| Bovine aortic endothelial cells [168] | Palytoxin |
Ethidium (359 Da) Yo-Pro-1 (377 Da) Propidium (416 Da) Transfected GFP (27 kDa) |
Initial ouabain-sensitive Ca2+ increase and slow ethidium uptake, but no uptake of Yo-Pro-1 or propidium. After variable delay, rapid ethidium uptake coincides with Yo-Pro-1 and propidium uptake and GFP loss | No effect on initial slow ethidium uptake, but blocks the subsequent rapid phase of ethidium uptake and GFP loss |
| BAC1.2F5 macrophages [92] | Maitotoxin | Yo-Pro-1 (377 Da) | Early Ca2+-dependent induction of slow Yo-Pro uptake followed by accelerated Yo-Pro uptake coinciding with LDH release | No effect on initial slow phase of Yo-Pro uptake. Prevents the rapid phase of Yo-Pro and release of LDH |
|
J774A.1 macrophages [52] Bone marrow-derived macrophages, dendritic cells [91] |
S. typhimurium and anthrax lethal toxin-induced pyroptosis |
Ethidium (359 Da) Ethidium homodimer-2 (793 Da) |
2.5 h of infection induces caspase-1-dependent permeability to ethidium, but not to ethidium homodimer-2; cell swelling and LDH release follow | Prevents swelling and LDH release, but not the initial ethidium uptake. PEG 1450 (2.4 nm molecular diameter) and PEG 2000 (2.8 nm), but not PEG 200 (1.12 nm), also limit LDH release |
Several studies with more detailed investigations of the progressive nature of the plasma membrane alterations that underlie these permeabilities in the context of glycine cytoprotection have provided insight into both that process and the underlying pathophysiology of necrosis/oncosis in general. In the first reported study of this type [99], the permeability of dying MDCK cells to propidium was compared to 4 kDa, 70, and 2000 kDa fluorescent dextrans using a low Ca2+ injury model. The majority of cells became progressively permeable to propidium (416 Da) over 120 min and were similarly permeable to 4 kDa dextran (3 nm molecular diameter [166]) In contrast, development of permeability to 70 kDa dextran (10–15 nm molecular diameter [166]) was delayed and more heterogeneous with many propidium-positive cells that still excluded dextran for the full duration. 2000 kDa dextran (54 nm molecular diameter [166]) was entirely excluded, although as pointed out subsequently [43], volume exclusion by intracellular membranous and cytoskeletal elements could have contributed for that very large molecule. The changes of MDCK cell permeability occurred without overt disruption of the plasma membrane. In the model, cells became uniformly swollen (essentially single large blebs), but had continuous membranes based on fluorescent visualization of biotinylated surface proteins and displayed osmotic shrinkage when suspended in medium with osmotically active concentrations of large dextrans [99]. This membrane continuity and suppression by glycine of progression from small to large membrane defects was also seen in hypoxic, hypotonically swollen proximal tubules that were deliberately permeabilized to small molecules (eosin and propidium) by α-toxin, yet did not leak LDH as long as glycine was present [105]. Additionally, the MDCK cell study demonstrated that the permeability defects in the absence of glycine were suppressible by an impermeant homobifunctional ‘nearest neighbor’ cross-linking reagent, further supporting plasma membrane protein involvement [99].
The second study of this type subjected primary cultures of hepatic sinusoidal endothelial cells to ATP depletion injury in Ca2+-containing medium and tested permeation of two small anionic probes, calcein (623 Da) and Lucifer yellow (443 Da), of propidium, which is cationic, and of 40 and 2000 kDa dextran [43]. In the absence of glycine, the first change in these endothelial cells was permeability to calcein and Lucifer yellow simultaneously with the start of bleb formation. 30–60 min later, they became permeable to propidium and all the dextrans tested without evident differences between the differently sized dextrans other than weaker uptake of the 2000 kDa dextran that was attributed to volume exclusion by intracellular membranous and cytoskeletal components. Glycine slowed, but did not stop the calcein and Lucifer yellow entry. It completely prevented entry of propidium and the dextrans. Subsequent withdrawal of glycine allowed entry of propidium and the dextrans within 20 min [43].
In the third related study [167], anoxic freshly isolated proximal tubules were incubated with propidium, phallacidin, Trypan blue, and 4 and 70 kDa fluorescent dextran as membrane permeability probes. These cells showed early permeability to propidium even in the presence of glycine, but propidium here was used at the very high concentration of 20 μM (3 μM was typical in the other studies) and the early propidium-positive cells were impermeable to phallacidin and Trypan blue and retained mitochondrial function, so failure to exclude propidium here was not indicative of cell death. The fluorescent dextrans, however, behaved similarly to the MDCK cell study with much greater permeability in the absence of glycine to the 4 kDa molecule than to the 70 kDa molecule and glycine blocked the development of permeability to both. This fresh tubule study [167] also tested bifunctional cross-linkers and they were found to protect against LDH release (without impairing LDH activity), but, in contrast to the MDCK cell work with cross-linkers [99], only permeant cross-linkers were protective for the fresh tubules.
The final detailed permeability testing of this type was done using injury induced by the pore-forming toxins, maitotoxin [44] and palytoxin [168], in bovine aortic endothelial cells and employed small chemical fluorescent probes along with transfected green fluorescent protein (GFP) as permeability markers. The maitotoxin study [44] importantly included concatemers of the 27 kDa GFP up to 162 kDa in size. Both maitotoxin and palytoxin induce rapid large increases of Caf due to extracellular influx, which are necessary for cell killing [44, 168, 169]. For maitotoxin, the agent itself is thought to form a channel; for palytoxin, the channel appears to involve alterations of the configuration of Na,K-ATPase because it is ouabain sensitive [44, 168, 169]. Both toxins induced biphasic uptake of ethidium (359 Da) characterized by an initial slow rate than an abrupt acceleration. Uptake of propidium after maitotoxin [44] and of either propidium or Yo-Pro-1 after palytoxin [168] coincided with the accelerated phase of ethidium uptake. Loss of transfected GFP, which was followed in multiple single cells, similarly coincided with the accelerated phase of ethidium uptake [44, 168, 169]. There were no correlations between the sizes of GFP concatemers and the time to start of their release or the rate of release once it was underway. Glycine prevented the rapid phase of ethidium uptake, propidium uptake, and release of LDH and GFP concatemers. Similarly to the endothelial cells subjected to metabolic inhibition [43], withdrawal of glycine led to GFP release after about 20 min [44]. The single cell analysis used for the maitotoxin and palytoxin studies included high-resolution visualization of the time course of bleb formation during injury [44, 168]. As was the case for the MDCK [99] and proximal tubule [167] studies, there was no evidence for bleb rupture or loss of gross membrane continuity during the permeability changes. In fact, blebs were noted to continue to increase in size after GFP loss [44, 168]. Maitotoxin potently elicits inflammasome formation and pyroptosis in macrophages [127]. Although size dependence of the permeability changes during pyroptosis has not been studied in same detail as in endothelial cells, the biphasic uptake of small fluorescent probes has been described during glycine-sensitive pyroptosis induced by both maitotoxin [92] and bacterial products [52, 91].
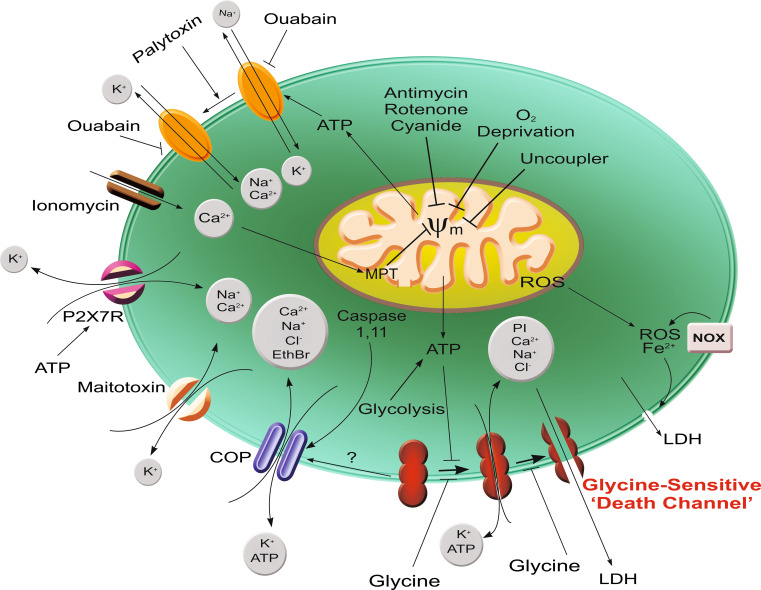
Overall, these fluorescent probe data using multiple forms of injury in several cell types are consistent with the conclusion that glycine prevents the development of relatively large, but size-specific hydrophilic membrane pores of molecular dimensions. There may be charge selectivity of the earliest manifestation of these pores [43], but that has only been studied in one system. There is good evidence for progression of pore size over time in renal tubules [99, 167], but not in endothelial cells [43, 44, 168]. Although glycine prevention of membrane rupture related to inhibition of large ion fluxes has been hypothesized in two of the these experimental systems [43, 52, 91], models in which membrane continuity was assessed in detail have not provided evidence that membrane rupture is a necessary or consistent feature of the glycine-sensitive permeability alteration [44, 99, 101, 167, 168]. Figure 1 summarizes the major pathways that have been identified as leading to glycine-sensitive injury and the pore formation that accounts for it.
Fig. 1.
Pathways for development of glycine-sensitive cell death. Summarized here are pathways of injury discussed in the text for which glycine protection is well documented and a scheme for ‘death channel’ development that incorporates data from studies with differently sized fluorescent probes. Primary insults include multiple maneuvers that impair mitochondrial ATP generation (oxygen deprivation, electron transport inhibitors, uncouplers, Ca2+-induced development of the mitochondrial permeability transition), maneuvers that produce transmembrane cation shifts prominently including increased Ca2+ entry (ionomycin, maitotoxin, palytoxin-induced modification of Na, K, ATPase, and P2X7 receptor activation), and activation of caspase 1 and/or 11. The glycine-insensitive ‘cytolytic oncotic pore’ mediating ethidium bromide uptake, which develops after P2X7 receptor activation, maitotoxin and likely caspase activation promotes the cation shifts and can also lead to loss of ATP. This further enhances the ATP depletion. Development of the glycine-sensitive ‘death channel’ is depicted as a sequential process of pore enlargement that is normally suppressed by ATP, because ATP depletion is a common factor in most processes and other major injury mediators such as Ca2+ increases are not necessary for it to occur. It is possible that the cytolytic oncotic pore rather than being a separate process as depicted is a stage in the development of the glycine-sensitive channel. Cell death associated with ROS and Fe2+-mediated lipid peroxidation directly targeting the lipid phase of the membrane is shown as a separate pathway, since it is relatively glycine insensitive. Other early injury-associated membrane permeability changes such as activation of connexin [268] and TRPM [103] channels almost certainly feed into the pathways shown, but are not illustrated because they have not been specifically studied with glycine
Glycine effects on disease models in vivo
Circulating glycine levels in humans, [170, 171], rats and mice [172–174], cats [80], and dogs [175] range from 0.2 to 0.4 mM. In rabbits [176, 177] and pigs [28], they are 1–1.5 mM. Intracellular concentrations are much higher, particularly in renal proximal tubules, which reabsorb filtered glycine and reach 4–10× circulating levels depending on whether they are being actively perfused [178, 179]. The high intracellular levels are in excess of the 2 mM typically needed for maximal glycine cytoprotection and are reflected in whole cortex measurements [30, 172, 180]. Intracellular levels are high in other tissues as well [27, 170, 181]. The dynamics of glycine movements during injury conditions have been studied in detail using freshly isolated kidney tubules [30]. Those cells became severely depleted of glycine during their isolation. During incubation with 0.25–2 mM glycine in the medium, they actively concentrated it to 4×, the medium level under control conditions, but then rapidly lost it if they were metabolically inhibited and transferred to glycine-free medium. If glycine was added to ATP-depleted or sodium pump-inhibited cells, it reached levels similar to those in the medium, but was not concentrated. Thus, cellular glycine is labile and its ability to maintain cytoprotection under in vivo conditions could vary depending on the injury state and where it acts. If, as reviewed above, glycine acts primarily at the outer surface of the plasma membrane, leakage of glycine from the large intracellular pools during a low flow ischemic state could bring extracellular concentrations to cytoprotective levels and thus favor tissue resistance. In fact, microdialysis measurements of tissue extracellular glycine showed increases during back table processing of transplanted livers [182], although they were not large and remained well below fully protective levels. During reflow states, restoration of blood flow could remove any leaked protective extracellular glycine while intracellular glycine remains depleted, thus promoting injury as seen when glycine is withdrawn from isolated cells that have not metabolically recovered [140]. There is evidence for persistent depletion of glycine after acute kidney injury in vivo [172, 180], but its precise timing relative to development of cell death has not been studied and it is possible that those changes seen simply reflect loss of healthy cells that can maximally concentrate it.
In considering modification of injury at the whole organ level (i.e., isolated organs such as those used for transplantation and in vivo), interpretation of the effects is complicated by the presence of multiple cell types and tissue compartments. In vivo, the contributions of circulating cells and cross talk between organ systems via cytokine generation are additional factors. As covered in the preceding sections, studies of glycine cytoprotection have led to the recognition that glycine acts on multiple types of parenchymal and inflammatory cells and can do so via effects on both upstream signaling pathways as well as its membrane cytoprotective action to delay the lethal membrane damage that results in necrosis and pyroptosis. Moreover, by limiting the necrosis, secondary inflammatory effects will be prevented. Since the recognition of its cytoprotective effects, multiple studies have investigated the effects of glycine in disease models in vivo. Protective effects in multiple disease models in vivo have been reported. These include such diverse processes as aortic allograft rejection [183], arthritis [184, 185], distension-induced bladder wall damage [67], diabetic complications [186], hypertension and the metabolic syndrome [187–197], and angiogenesis in tumors [198–200]. The majority of these are due to primary signaling or anti-inflammatory effects [11, 14] not associated with necrosis and cannot be covered in detail within the confines of this review. Additional references to studies of glycine effects during diverse disease processes can be found in other reviews [10, 11, 13–15]. However, a number of effects in which cytoprotection could be a primary target or during which necrotic cell damage is a major component merit more detailed consideration here.
Although glycine is considered a nonessential amino acid, its availability may, in fact, be limiting for all of its normal metabolic roles [201]. Manipulating glycine levels in vivo to potentially elicit protective effects is feasible because circulating glycine levels in multiple species, including humans and rodents, are at or below the EC50s (0.5–0.75) required for both its cytoprotective and anti-inflammatory effects. Serum glycine levels can be readily increased by either oral feeding [28, 171, 202–206] or parenteral administration [207–209], and maximal cytoprotection is seen at 2–5 mM concentrations that are well below the 16–36 mM levels associated with acute toxicity [210]. Although not an issue for acute processes, it is notable that glycine and products of its metabolism can promote tumor growth [31, 211–214], which is potentially a limiting factor for long-term administration.
Effects of glycine on ischemia/perfusion-induced acute kidney injury in vivo have been tested in several studies. A single report has described benefit accruing from combined oral and parenteral glycine supplementation for an ischemia/reperfusion-induced AKI [206]. However, despite its cytoprotective and anti-inflammatory effects and the additional action of glycine (like other amino acids) to increase renal blood flow and glomerular filtration rate [215–217], most studies have either shown no benefit of parenteral glycine administration [207] or aggravation of structural and functional changes [216, 218]. These findings are similar to earlier work with amino acid mixtures [219]. In these models, it has been hypothesized that increased renal metabolic demand produced by glycine through its effects on renal blood flow and glomerular filtration rate could have aggravated tissue hypoxia after the insults [216]. Consistent with this possibility, worsening of function by glycine was prevented by antagonizing NMDA receptors [218], which have been implicated in amino acid-induced vasodilation [217]. Additionally, glycine has been reported to lower blood pressure in an NMDA-sensitive fashion [220]. Blood pressure is rarely measured in rodent AKI models, but can play a major role in the process and effects of administered metabolites on it [221].
Glycine has been tested in several nephrotoxic models of acute kidney injury (AKI) in vivo. Oral glycine feeding doubled both serum and tissue glycine levels and strongly protected against maleate-induced proximal tubule dysfunction (Fanconi syndrome) and proximal tubule cell necrosis without modifying associated glutathione oxidation and depletion [202]. Since tissue ATP was preserved by the glycine treatment in this study, it was possible that glycine was in some way primarily alleviating the metabolic effect of maleate to inhibit tricarboxylic acid cycle metabolism. However, subsequent work testing direct maleate toxicity to isolated proximal tubules showed that glycine strongly protected against maleate-induced necrosis without preserving their ATP levels [222]. Glycine ameliorated cisplatin-induced nephrotoxicity, but this was associated with reduced early cisplatin delivery to the tubule epithelium [223]. It also ameliorated subacute cyclosporine-induced nephrotoxicity, probably by modifying drug-induced vasoconstriction [224, 225].
Glycine administration is beneficial in multiple experimental models of liver injury in vivo and transplantation ([42, 159, 226–233] and reviewed in [11, 12, 14, 234, 235]) and has been shown to ameliorate transaminase increases after transplantation of human livers [234]. As previously discussed, efficacy in liver models led to recognition that glycine suppresses Kupffer cell activation during injury and that suppression of Kupffer cell activation plays a major role in the observed liver effects [14, 57, 158, 236–239].
The protective effects of glycine have been reported in several types of acute bowel injury in vivo [62, 66, 68, 69] and in chemical-induced inflammatory bowel disease [67]. It is likely that inflammatory cells in the intestinal mucosa are a major target. Whether protection involves suppression of activation, inhibition of pyroptosis, or both is not known.
There are only limited observations of the effects of glycine during myocardial and skeletal muscle injury. As discussed previously, reduction of necrosis by glycine in an isolated perfused heart model of ischemia was attributed to suppression of MPT [71] and there is a report of attenuation of I/R injury in vivo [240]. Glycine supplementation had beneficial cardiac effects in a burn model [241]. Infusion of glycine at the end of 6 h of skeletal muscle ischemia and then during the first hour of reperfusion decreased necrosis and increased metabolic and functional recovery [242]. Glycine suppression of reactive oxygen species production by neutrophils [165] may have played a major role in this skeletal muscle effect [243].
Both cold and warm I/R injury to the lung was ameliorated by glycine [244, 245]. This benefit was attributed to mitochondrial protection, but given the lack of benefit of glycine for mitochondrial function in rigorously studied models [19, 49, 118, 140, 246], the mitochondrial function effects in these systems were likely secondary.
Interpretation of CNS effects is complicated by the role of glycine as a coagonist of NMDA-glutamate receptors, which typically promote injury [74–77], and its effects on microglia [58–61]. As a result, diverse effects have been reported in brain ischemia models [77, 157, 247–251].
Protective effects of glycine have been reported for several sepsis models [209, 252–255] and hemorrhagic shock [256], but not after gunshot trauma [257]. Glycine was beneficial early after cecal ligation and puncture [255], but not late [209]. It is not known to what extent suppression of pyroptosis [258] in addition to the effects of glycine on signaling and neutrophil function was involved in these effects.
Conclusions
Glycine cytoprotection is a robust and widely expressed biological phenomenon that confers resistance to multiple forms of necrotic cell death as well as pyroptosis and can reasonably be considered the ultimate downstream regulator of necrosis in multiple injury settings. The specific molecular target of glycine remains undefined 29 years after the effect was first discovered, but it is likely to involve suppression of the reconfiguration of plasma membrane proteins to form hydrophilic channels with molecular dimensions. Glycine’s effects can be reproduced by other small molecules that are active at neuronal glycine and GABAA receptors and other non-receptor chloride channels, but the pharmacology of protection does not conform to any established receptor and chloride itself is not required for the effect. That glycine cytoprotection is seen in so many forms of necrotic cell death and different cell types indicates that the targeted process is a final common pathway for necrotic cell death. The normally high tissue levels of glycine provide an endogenous reservoir of glycine to protect during ischemic conditions when intracellular glycine leaks into the static extracellular space. Glycine cytoprotection suppresses inflammation by preventing the immunogenic effects of necrosis and directly inhibiting pyroptosis. Moreover, efforts to understand the basis for glycine cytoprotection have led to the discovery of novel upstream immunomodulatory effects of glycine to block primary activation of multiple types of inflammatory cells that, unlike glycine cytoprotection, appear in many cases to involve previously unsuspected nonneuronal glycine receptors. The EC50s for both glycine cytoprotection and its immunomodulatory effects are close to the usual circulating concentrations of the amino acid, allowing for manipulation in vivo with benefit for multiple disease processes in experimental models.
Acknowledgments
Preparation of this manuscript was supported by Merit Review # I01 BX002367 (JMW) from the United States (US) Department of Veterans Affairs. Original investigations by the authors cited here were supported by NIH Grants, DK-34275 (JMW), DK-37139, and DK-48417 (MAV), the Office of Naval Research (JMW), the Department of Veterans Affairs (JMW), and the Dr. Werner Jackstaedt-Foundation (AB). The contents do not represent the views of the US Department of Veterans Affairs or the United States Government.
Abbreviations
- CCCP
carbonylcyanide m-chlorophenylhydrazone
- COP
Cytolytic oncotic pore
- ∆ψm
Mitochondrial membrane potential
- EthBr
Ethidium bromide
- LDH
Lactate dehydrogenase
- MPT
Mitochondrial permeability transition
- NHE
Sodium-hydrogen exchanger
- P2X7R
P2X7 purinergic receptor
References
- 1.Fournier N, Ducet G, Crevat A. Action of cyclosporine on mitochondrial calcium fluxes. J Bioenerg Biomembr. 1987;19(3):297–303. doi: 10.1007/BF00762419. [DOI] [PubMed] [Google Scholar]
- 2.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258(5):C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 3.Halestrap AP, Davidson AM. Inhibition of Ca2+-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268:153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardi P, Rasola A, Forte M, Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol Rev. 2015;95(4):1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinberg JM, Davis JA, Abarzua M, Rajan T. Cytoprotective effects of glycine and glutathione against hypoxic injury to renal tubules. J Clin Invest. 1987;80(5):1446–1454. doi: 10.1172/JCI113224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg JM. The effect of amino acids on ischemic and toxic injury to the kidney. Semin Nephrol. 1990;10(5):491–500. [PubMed] [Google Scholar]
- 7.Weinberg JM. The cell biology of ischemic renal injury. Kidney Int. 1991;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- 8.Bonventre JV, Weinberg JM. Kidney preservation ex vivo for transplantation. Annu Rev Med. 1992;43:523–553. doi: 10.1146/annurev.me.43.020192.002515. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg JM. Glutathione and glycine in acute renal failure. Ren Fail. 1992;14(3):311–319. doi: 10.3109/08860229209106635. [DOI] [PubMed] [Google Scholar]
- 10.Hall JC. Glycine. JPEN J Parenter Enteral Nutr. 1998;22(6):393–398. doi: 10.1177/0148607198022006393. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler MD, Ikejema K, Enomoto N, Stacklewitz RF, Seabra V, Zhong Z, Yin M, Schemmer P, Rose ML, Rusyn I, Bradford B, Thurman RG. Glycine: a new anti-inflammatory immunonutrient. Cell Mol Life Sci. 1999;56(9–10):843–856. doi: 10.1007/s000180050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habib MM, Hodgson HJ, Davidson BR. The role of glycine in hepatic ischemia-reperfusion injury. Curr Pharm Des. 2006;12(23):2953–2967. doi: 10.2174/138161206777947605. [DOI] [PubMed] [Google Scholar]
- 13.Gundersen RY, Vaagenes P, Breivik T, Fonnum F, Opstad PK. Glycine–an important neurotransmitter and cytoprotective agent. Acta Anaesthesiol Scand. 2005;49(8):1108–1116. doi: 10.1111/j.1399-6576.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ. l-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6(2):229–240. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Petrat F, Boengler K, Schulz R, de Groot H. Glycine, a simple physiological compound protecting by yet puzzling mechanism(s) against ischaemia-reperfusion injury: current knowledge. Br J Pharmacol. 2012;165(7):2059–2072. doi: 10.1111/j.1476-5381.2011.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberg JM, Molitoris BA. Illuminating mitochondrial function and dysfunction using multiphoton technology. J Am Soc Nephrol. 2009;20(6):1164–1166. doi: 10.1681/ASN.2009040419. [DOI] [PubMed] [Google Scholar]
- 17.Mandel LJ, Schnellmann RG, Jacobs WR. Intracellular glutathione in the protection from anoxic injury in renal proximal tubules. J Clin Invest. 1990;85(2):316–324. doi: 10.1172/JCI114440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinberg JM, Venkatachalam MA, Garzo-Quintero R, Roeser NF, Davis JA. Structural requirements for protection by small amino acids against hypoxic injury in kidney proximal tubules. FASEB J. 1990;4(15):3347–3354. doi: 10.1096/fasebj.4.15.2253849. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA. Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int. 1997;52(1):140–151. doi: 10.1038/ki.1997.313. [DOI] [PubMed] [Google Scholar]
- 20.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 21.Weinberg JM, Davis JA, Abarzua M, Kiani T. Relationship between cell ATP and glutathione content and protection by glycine against hypoxic proximal tubule cell injury. J Lab Clin Med. 1989;113:612–623. [PubMed] [Google Scholar]
- 22.Weinberg JM, Davis JA, Abarzua M, Kiani T, Kunkel R. Protection by glycine of proximal tubules from injury due to inhibitors of mitochondrial Atp production. Am J Physiol. 1990;258(6):C1127–C1140. doi: 10.1152/ajpcell.1990.258.6.C1127. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg JM, Davis JA, Abarzua M, Smith RK, Kunkel R. Ouabain-induced lethal proximal tubule cell injury is prevented by glycine. Am J Physiol. 1990;258(2):F346–F355. doi: 10.1152/ajprenal.1990.258.2.F346. [DOI] [PubMed] [Google Scholar]
- 24.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136(12):4551–4556. doi: 10.1021/ja411006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Brasen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA. 2014;111(47):16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu GY, Wu ZL, Dai ZL, Yang Y, Wang WW, Liu C, Wang B, Wang JJ, Yin YL. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids. 2013;44(4):1107–1113. doi: 10.1007/s00726-012-1444-2. [DOI] [PubMed] [Google Scholar]
- 28.Wang W, Wu Z, Dai Z, Yang Y, Wang J, Wu G. Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids. 2013;45(3):463–477. doi: 10.1007/s00726-013-1493-1. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg JM, Buchanan DN, Davis JA, Abarzua M. Metabolic aspects of protection by glycine against hypoxic injury to isolated proximal tubules. J Am Soc Nephrol. 1991;1(7):949–958. doi: 10.1681/ASN.V17949. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg JM, Nissim I, Roeser NF, Davis JA, Schultz S, Nissim I. Relationships between intracellular amino-acid levels and protection against injury to isolated proximal tubules. Am J Physiol. 1991;260(3):F410–F419. doi: 10.1152/ajprenal.1991.260.3.F410. [DOI] [PubMed] [Google Scholar]
- 31.Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13(8):572–583. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saunder A, Ametani MS, Belzer FO, Southard JH. Cytoprotective effect of glycine in cold stored canine renal tubules. Cryobiology. 1993;30(3):243–249. doi: 10.1006/cryo.1993.1022. [DOI] [PubMed] [Google Scholar]
- 33.Garza-Quintero R, Ortega-Lopez J, Stein JH, Venkatachalam MA. Alanine protects rabbit proximal tubules against anoxic injury in vitro. Am J Physiol. 1990;258(4 Pt 2):F1075–F1083. doi: 10.1152/ajprenal.1990.258.4.F1075. [DOI] [PubMed] [Google Scholar]
- 34.Aleo MD, Schnellmann RG. The neurotoxicants strychnine and bicuculline protect renal proximal tubules from mitochondrial inhibitor-induced cell death. Life Sci. 1992;51(23):1783–1787. doi: 10.1016/0024-3205(92)90048-T. [DOI] [PubMed] [Google Scholar]
- 35.Wetzels JFM, Wang X, Gengaro PE, Nemenoff RA, Burke TJ, Schrier RW. Glycine protection against hypoxic but not phospholipase A2-induced injury in rat proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol. 1993;264:F94–F99. doi: 10.1152/ajprenal.1993.264.1.F94. [DOI] [PubMed] [Google Scholar]
- 36.Almeida AR, Wetzels JF, Bunnachak D, Burke TJ, Chaimovitz C, Hammond WS, Schrier RW. Acute phosphate depletion and in vitro rat proximal tubule injury: protection by glycine and acidosis. Kidney Int. 1992;41(6):1494–1500. doi: 10.1038/ki.1992.218. [DOI] [PubMed] [Google Scholar]
- 37.Baines AD, Shaikh N, Ho P. Mechanisms of perfused kidney cytoprotection by alanine and glycine. Am J Physiol. 1990;259(1 Pt 2):F80–F87. doi: 10.1152/ajprenal.1990.259.1.F80. [DOI] [PubMed] [Google Scholar]
- 38.Heyman S, Spokes K, Rosen S, Epstein FH. Mechanism of glycine protection in hypoxic injury: analogies with glycine receptor. Kidney Int. 1992;42(1):41–45. doi: 10.1038/ki.1992.258. [DOI] [PubMed] [Google Scholar]
- 39.Silva P, Rosen S, Spokes K, Epstein FH. Effect of glycine on medullary thick ascending limb injury in perfused kidneys. Kidney Int. 1991;39(4):653–658. doi: 10.1038/ki.1991.78. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg JM, Venkatachalam MA, Roeser NF, Davis JA, Varani J, Johnson KJ. Amino acid protection of cultured kidney tubule cells against calcium ionophore-induced lethal cell injury. Lab Invest. 1991;65(6):671–678. [PubMed] [Google Scholar]
- 41.Weinberg JM, Varani J, Johnson KJ, Roeser NF, Dame MK, Davis JA, Venkatachalam MA. Protection of human umbilical vein endothelial cells by glycine and structurally similar amino acids against calcium and hydrogen peroxide-induced lethal cell injury. Am J Pathol. 1992;140(2):457–471. [PMC free article] [PubMed] [Google Scholar]
- 42.Currin RT, Caldwell-Kenkel JC, Lichtman SN, Bachmann S, Takei Y, Kawano S, Thurman RG, Lemasters JJ. Protection by Carolina rinse solution, acidotic pH, and glycine against lethal reperfusion injury to sinusoidal endothelial cells of rat livers stored for transplantation. Transplantation. 1996;62(11):1549–1558. doi: 10.1097/00007890-199612150-00004. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura Y, Lemasters JJ. Glycine blocks opening of a death channel in cultured hepatic sinusoidal endothelial cells during chemical hypoxia. Cell Death Differ. 2001;8(8):850–858. doi: 10.1038/sj.cdd.4400877. [DOI] [PubMed] [Google Scholar]
- 44.Estacion M, Weinberg JS, Sinkins WG, Schilling WP. Blockade of maitotoxin-induced endothelial cell lysis by glycine and l-alanine. Am J Physiol Cell Physiol. 2003;284(4):C1006–C1020. doi: 10.1152/ajpcell.00258.2002. [DOI] [PubMed] [Google Scholar]
- 45.Dickson RC, Bronk SF, Gores GJ. Glycine cytoprotection during lethal hepatocellular injury from adenosine triphosphate depletion. Gastroenterology. 1992;102(6):2098–2107. doi: 10.1016/0016-5085(92)90338-y. [DOI] [PubMed] [Google Scholar]
- 46.Frank A, Rauen U, de Groot H. Protection by glycine against hypoxic injury of rat hepatocytes: inhibition of ion fluxes through nonspecific leaks. J Hepatol. 2000;32(1):58–66. doi: 10.1016/S0168-8278(00)80190-7. [DOI] [PubMed] [Google Scholar]
- 47.Marsh DC, Vreugdenhil PK, Mack VE, Belzer FO, Southard JH. Glycine protects hepatocytes from injury caused by anoxia, cold ischemia and mitochondrial inhibitors, but not injury caused by calcium ionophores or oxidative stress. Hepatology. 1993;17(1):91–98. doi: 10.1002/hep.1840170117. [DOI] [PubMed] [Google Scholar]
- 48.Nyberg SL, Hardin JA, Matos LE, Rivera DJ, Misra SP, Gores GJ. Cytoprotective influence of ZVAD-fmk and glycine on gel-entrapped rat hepatocytes in a bioartificial liver. Surgery. 2000;127(4):447–455. doi: 10.1067/msy.2000.103162. [DOI] [PubMed] [Google Scholar]
- 49.Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997;273(6 Pt 1):C1783–C1792. doi: 10.1152/ajpcell.1997.273.6.C1783. [DOI] [PubMed] [Google Scholar]
- 50.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol. 2000;38(1):31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 51.Stockbauer KE, Foreman-Wykert AK, Miller JF. Bordetella type III secretion induces caspase 1-independent necrosis. Cell Microbiol. 2003;5(2):123–132. doi: 10.1046/j.1462-5822.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 52.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8(11):1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 53.Verhoef PA, Kertesy SB, Lundberg K, Kahlenberg JM, Dubyak GR. Inhibitory effects of chloride on the activation of caspase-1, IL-1 beta secretion, and cytolysis by the P2X7 receptor. J Immunol. 2005;175(11):7623–7634. doi: 10.4049/jimmunol.175.11.7623. [DOI] [PubMed] [Google Scholar]
- 54.Goldmann O, Sastalla I, Wos-Oxley M, Rohde M, Medina E. Streptococcus pyogenes induces oncosis in macrophages through the activation of an inflammatory programmed cell death pathway. Cell Microbiol. 2009;11(1):138–155. doi: 10.1111/j.1462-5822.2008.01245.x. [DOI] [PubMed] [Google Scholar]
- 55.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):U1136–U1194. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wellington M, Koselny K, Sutterwala FS, Krysan DJ. Candida albicans triggers NLRP3-mediated pyroptosis in macrophages. Eukaryot Cell. 2014;13(2):329–340. doi: 10.1128/EC.00336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikejima K, Qu W, Stachlewitz RF, Thurman RG. Kupffer cells contain a glycine-gated chloride channel. Am J Physiol. 1997;272(6 Pt 1):G1581–G1586. doi: 10.1152/ajpgi.1997.272.6.G1581. [DOI] [PubMed] [Google Scholar]
- 58.Schilling T, Eder C. A novel physiological mechanism of glycine-induced immunomodulation: Na+-coupled amino acid transporter currents in cultured brain macrophages. J Physiol. 2004;559(Pt 1):35–40. doi: 10.1113/jphysiol.2004.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carmans S, Hendriks JJ, Thewissen K, Van den Eynden J, Stinissen P, Rigo JM, Hellings N. The inhibitory neurotransmitter glycine modulates macrophage activity by activation of neutral amino acid transporters. J Neurosci Res. 2010;88(11):2420–2430. doi: 10.1002/jnr.22395. [DOI] [PubMed] [Google Scholar]
- 60.Van den Eynden J, Notelaers K, Brone B, Janssen D, Nelissen K, Sahebali S, Smolders I, Hellings N, Steels P, Rigo JM. Glycine enhances microglial intracellular calcium signaling. A role for sodium-coupled neutral amino acid transporters. Pflugers Arch. 2011;461(4):481–491. doi: 10.1007/s00424-011-0939-0. [DOI] [PubMed] [Google Scholar]
- 61.Komm B, Beyreis M, Kittl M, Jakab M, Ritter M, Kerschbaum HH. Glycine modulates membrane potential, cell volume, and phagocytosis in murine microglia. Amino Acids. 2014;46(8):1907–1917. doi: 10.1007/s00726-014-1745-8. [DOI] [PubMed] [Google Scholar]
- 62.Mangino JE, Kotadia B, Mangino MJ. Characterization of hypothermic intestinal ischemia-reperfusion injury in dogs. Effects of glycine. Transplantation. 1996;62(2):173–178. doi: 10.1097/00007890-199607270-00005. [DOI] [PubMed] [Google Scholar]
- 63.Iijima S, Shou J, Naama H, Calvano SE, Daly JM. Beneficial effect of enteral glycine in intestinal ischemia/reperfusion injury. J Gastrointest Surg. 1997;1(1):61–67. doi: 10.1007/s11605-006-0011-0. [DOI] [PubMed] [Google Scholar]
- 64.Kallakuri S, Ascher E, Pagala M, Gade P, Hingorani A, Scheinman M, Mehraein K, Jacob T. Protective effect of glycine in mesenteric ischemia and reperfusion injury in a rat model. J Vasc Surg. 2003;38(5):1113–1120. doi: 10.1016/S0741-5214(03)00939-X. [DOI] [PubMed] [Google Scholar]
- 65.Lee MA, McCauley RD, Kong SE, Hall JC. Influence of glycine on intestinal ischemia-reperfusion injury. JPEN J Parenter Enteral Nutr. 2002;26(2):130–135. doi: 10.1177/0148607102026002130. [DOI] [PubMed] [Google Scholar]
- 66.Diestel CF, Marques RG, Lopes-Paulo F, Paiva D, Horst NL, Caetano CE, Portela MC. Role of l-glutamine and glycine supplementation on irradiated colonic wall. Int J Colorectal Dis. 2007;22(12):1523–1529. doi: 10.1007/s00384-007-0341-8. [DOI] [PubMed] [Google Scholar]
- 67.Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y, Sato N. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology. 2003;125(3):775–785. doi: 10.1016/S0016-5085(03)01067-9. [DOI] [PubMed] [Google Scholar]
- 68.Stoffels B, Turler A, Schmidt J, Nazir A, Tsukamoto T, Moore BA, Schnurr C, Kalff JC, Bauer AJ. Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol Motil. 2011;23(1):76–87. doi: 10.1111/j.1365-2982.2010.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Petrat F, Drowatzky J, Boengler K, Finckh B, Schmitz KJ, Schulz R, de Groot H. Protection from glycine at low doses in ischemia-reperfusion injury of the rat small intestine. Eur Surg Res. 2011;46(4):180–187. doi: 10.1159/000324393. [DOI] [PubMed] [Google Scholar]
- 70.Howard A, Tahir I, Javed S, Waring SM, Ford D, Hirst BH. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J Physiol Lond. 2010;588(6):995–1009. doi: 10.1113/jphysiol.2009.186262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruiz-Meana M, Pina P, Garcia-Dorado D, Rodriguez-Sinovas A, Barba I, Miro-Casas E, Mirabet M, Soler-Soler J. Glycine protects cardiomyocytes against lethal reoxygenation injury by inhibiting mitochondrial permeability transition. J Physiol. 2004;558(Pt 3):873–882. doi: 10.1113/jphysiol.2004.068320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodriguez-Sinovas A, Garcia-Dorado D, Pina P, Ruiz-Meana M, Soler-Soler J. Effect of sarcolemmal rupture on myocardial electrical impedance during oxygen deprivation. Am J Physiol Heart Circ Physiol. 2005;288(3):H1396–H1403. doi: 10.1152/ajpheart.00768.2004. [DOI] [PubMed] [Google Scholar]
- 73.Zhang K, Weinberg JM, Venkatachalam MA, Dong Z. Glycine protection of PC-12 cells against injury by ATP-depletion. Neurochem Res. 2003;28(6):893–901. doi: 10.1023/A:1023275426637. [DOI] [PubMed] [Google Scholar]
- 74.Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150(3):633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 75.Newell DW, Barth A, Ricciardi TN, Malouf AT. Glycine causes increased excitability and neurotoxicity by activation of NMDA receptors in the hippocampus. Exp Neurol. 1997;145(1):235–244. doi: 10.1006/exnr.1997.6463. [DOI] [PubMed] [Google Scholar]
- 76.Patel J, Zinkand WC, Thompson C, Keith R, Salama A. Role of glycine in the N-methyl-d-aspartate-mediated neuronal cytotoxicity. J Neurochem. 1990;54(3):849–854. doi: 10.1111/j.1471-4159.1990.tb02329.x. [DOI] [PubMed] [Google Scholar]
- 77.Yao W, Ji F, Chen Z, Zhang N, Ren SQ, Zhang XY, Liu SY, Lu W. Glycine exerts dual roles in ischemic injury through distinct mechanisms. Stroke. 2012;43(8):2212–2220. doi: 10.1161/STROKEAHA.111.645994. [DOI] [PubMed] [Google Scholar]
- 78.Tonshin AA, Lobysheva NV, Yaguzhinsky LS, Bezgina EN, Moshkov DA, Nartsissov YR. Effect of the inhibitory neurotransmitter glycine on slow destructive processes in brain cortex slices under anoxic conditions. Biochemistry (Mosc) 2007;72(5):509–517. doi: 10.1134/S0006297907050070. [DOI] [PubMed] [Google Scholar]
- 79.Zhao P, Qian H, Xia Y. GABA and glycine are protective to mature but toxic to immature rat cortical neurons under hypoxia. Eur J Neurosci. 2005;22(2):289–300. doi: 10.1111/j.1460-9568.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 80.Aprison MH, Werman R. The distribution of glycine in cat spinal cord and roots. Life Sci. 1965;4(21):2075–2083. doi: 10.1016/0024-3205(65)90325-5. [DOI] [PubMed] [Google Scholar]
- 81.Eulenburg V, Armsen W, Betz H, Gomeza J. Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci. 2005;30(6):325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 82.Weinberg JM, Davis JA, Abarzua M, Kiani T. Glycine-dependent protection of proximal tubules against lethal cell injury due to inhibitors of mitochondrial ATP production. Am J Physiol. 1990;258:C1127–C1140. doi: 10.1152/ajpcell.1990.258.6.C1127. [DOI] [PubMed] [Google Scholar]
- 83.Garza-Quintero R, Weinberg JM, Ortega-Lopez J, Davis JA, Venkatachalam MA. Conservation of structure in ATP-depleted proximal tubules: role of calcium, polyphosphoinositides, and glycine. Am J Physiol. 1993;265(5 Pt 2):F605–F623. doi: 10.1152/ajprenal.1993.265.5.F605. [DOI] [PubMed] [Google Scholar]
- 84.Venkatachalam MA, Weinberg JM, Patel Y, Hussong U, Davis JA. Effects of Ca++ and glycine on lipid breakdown and death of ATP-depleted MDCK cells. Kidney Int. 1995;48(1):118–128. doi: 10.1038/ki.1995.275. [DOI] [PubMed] [Google Scholar]
- 85.Meng X, Reeves WB. Effects of chloride channel inhibitors on H(2)O(2)-induced renal epithelial cell injury. Am J Physiol Renal Physiol. 2000;278(1):F83–F90. doi: 10.1152/ajprenal.2000.278.1.F83. [DOI] [PubMed] [Google Scholar]
- 86.Sogabe K, Roeser NF, Venkatachalam MA, Weinberg JM. Differential cytoprotection by glycine against oxidant damage to proximal tubule cells. Kidney Int. 1996;50(3):845–854. doi: 10.1038/ki.1996.384. [DOI] [PubMed] [Google Scholar]
- 87.Miller GW, Lock EA, Schnellmann RG. Strychnine and glycine protect renal proximal tubules from various nephrotoxicants and act in the late-phase of necrotic cell injury. Toxicol Appl Pharmacol. 1994;125(2):192–197. doi: 10.1006/taap.1994.1064. [DOI] [PubMed] [Google Scholar]
- 88.Zager RA, Johnson AC, Hanson SY, Wasse H. Parenteral iron formulations: a comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis. 2002;40(1):90–103. doi: 10.1053/ajkd.2002.33917. [DOI] [PubMed] [Google Scholar]
- 89.Bhattacharyya S, Ghosh J, Sil PC. Iron induces hepatocytes death via MAPK activation and mitochondria-dependent apoptotic pathway: beneficial role of glycine. Free Radic Res. 2012;46(10):1296–1307. doi: 10.3109/10715762.2012.712690. [DOI] [PubMed] [Google Scholar]
- 90.Zager RA, Burkhart KM, Conrad DS, Gmur DJ. Iron, heme oxygenase, and glutathione: effects on myohemoglobinuric proximal tubular injury. Kidney Int. 1995;48(5):1624–1634. doi: 10.1038/ki.1995.457. [DOI] [PubMed] [Google Scholar]
- 91.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc Natl Acad Sci USA. 2008;105(11):4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verhoef PA, Kertesy SB, Estacion M, Schilling WP, Dubyak GR. Maitotoxin induces biphasic interleukin-1 beta secretion and membrane blebbing in murine macrophages. Mol Pharmacol. 2004;66(4):909–920. doi: 10.1124/mol.66.4.. [DOI] [PubMed] [Google Scholar]
- 93.Venkatachalam MA, Patel YJ, Kreisberg JI, Weinberg JM. Energy thresholds that determine membrane integrity and injury in a renal epithelial-cell line (Llc-Pk1)—relationships to phospholipid degradation and unesterified fatty-acid accumulation. J Clin Investig. 1988;81(3):745–758. doi: 10.1172/JCI113380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anundi I, de Groot H. Hypoxic liver cell death: critical Po2 and dependence of viability on glycolysis. Am J Physiol. 1989;257(1 Pt 1):G58–G64. doi: 10.1152/ajpgi.1989.257.1.G58. [DOI] [PubMed] [Google Scholar]
- 95.Weinberg JM, Davis JA, Roeser NF, Venkatachalam MA. Role of increased cytosolic free calcium in the pathogenesis of rabbit proximal tubule cell injury and protection by glycine or acidosis. J Clin Invest. 1991;87(2):581–590. doi: 10.1172/JCI115033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nurko S, Sogabe K, Davis JA, Roeser NF, Defrain M, Chien A, Hinshaw D, Athey B, Meixner W, Venkatachalam MA, Weinberg JM. Contribution of actin cytoskeletal alterations to ATP depletion and calcium-induced proximal tubule cell injury. Am J Physiol. 1996;270(1 Pt 2):F39–F52. doi: 10.1152/ajprenal.1996.270.1.F39. [DOI] [PubMed] [Google Scholar]
- 97.Sogabe K, Roeser NF, Davis JA, Nurko S, Venkatachalam MA, Weinberg JM. Calcium dependence of integrity of the actin cytoskeleton of proximal tubule cell microvilli. Am J Physiol. 1996;271(2 Pt 2):F292–F303. doi: 10.1152/ajprenal.1996.271.2.F292. [DOI] [PubMed] [Google Scholar]
- 98.Venkatachalam MA, Weinberg JM, Patel Y, Saikumar P, Dong Z. Cytoprotection of kidney epithelial cells by compounds that target amino acid gated chloride channels. Kidney Int. 1996;49(2):449–460. doi: 10.1038/ki.1996.64. [DOI] [PubMed] [Google Scholar]
- 99.Dong Z, Patel Y, Saikumar P, Weinberg JM, Venkatachalam MA. Development of porous defects in plasma membranes of ATP-depleted Madin-Darby canine kidney cells and its inhibition by glycine. Lab Invest. 1998;78:657–668. [PubMed] [Google Scholar]
- 100.Weinberg JM, Davis JA, Venkatachalam MA. Cytosolic-free calcium increases to greater than 100 micromolar in ATP-depleted proximal tubules. J Clin Invest. 1997;100(3):713–722. doi: 10.1172/JCI119584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen J, Dai JW, Grant RL, Doctor RB, Sheetz MP, Mandel LJ. Loss of cytoskeletal support is not sufficient for anoxic plasma membrane disruption in renal cells. Am J Physiol Cell Physiol. 1997;272:C1319–C1328. doi: 10.1152/ajpcell.1997.272.4.C1319. [DOI] [PubMed] [Google Scholar]
- 102.Soltoff SP, Mandel LJ. Active ion transport in the renal proximal tubule. III. The ATP dependence of the Na pump. J Gen Physiol. 1984;84(4):643–662. doi: 10.1085/jgp.84.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gao GF, Wang WW, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB. TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. J Clin Investig. 2014;124(11):4989–5001. doi: 10.1172/JCI76042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carini R, Bellomo G, Grazia De Cesaris M, Albano E. Glycine protects against hepatocyte killing by KCN or hypoxia by preventing intracellular Na+ overload in the rat. Hepatology. 1997;26(1):107–112. doi: 10.1002/hep.510260114. [DOI] [PubMed] [Google Scholar]
- 105.Chen J, Mandel LJ. Role of water and electrolyte influxes in anoxic plasma membrane disruption. Am J Physiol. 1997;273(4 Pt 1):C1341–C1348. doi: 10.1152/ajpcell.1997.273.4.C1341. [DOI] [PubMed] [Google Scholar]
- 106.Dong Z, Saikumar P, Weinberg JM, Venkatachalam MA. Internucleosomal DNA cleavage triggered by plasma membrane damage during necrotic cell death—involvement of serine but not cysteine proteases. Am J Pathol. 1997;151(5):1205–1213. [PMC free article] [PubMed] [Google Scholar]
- 107.Carini R, Alchera E, Baldanzi G, Piranda D, Splendore R, Grazia De Cesaris M, Caraceni P, Graziani A, Albano E. Role of p38 map kinase in glycine-induced hepatocyte resistance to hypoxic injury. J Hepatol. 2007;46(4):692–699. doi: 10.1016/j.jhep.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 108.Stefureac R, Long YT, Kraatz HB, Howard P, Lee JS. Transport of alpha-helical peptides through alpha-hemolysin and aerolysin pores. Biochemistry. 2006;45(30):9172–9179. doi: 10.1021/bi0604835. [DOI] [PubMed] [Google Scholar]
- 109.Cascio M. Structure and function of the glycine receptor and related nicotinicoid receptors. J Biol Chem. 2004;279(19):19383–19386. doi: 10.1074/jbc.R300035200. [DOI] [PubMed] [Google Scholar]
- 110.Miller GW, Schnellmann RG. Cytoprotection by inhibition of chloride channels: the mechanism of action of glycine and strychnine. Life Sci. 1993;53(15):1211–1215. doi: 10.1016/0024-3205(93)90539-F. [DOI] [PubMed] [Google Scholar]
- 111.Waters SL, Miller GW, Aleo MD, Schnellmann RG. Neurosteroid inhibition of cell death. Am J Physiol. 1997;273(6 Pt 2):F869–F876. doi: 10.1152/ajprenal.1997.273.6.F869. [DOI] [PubMed] [Google Scholar]
- 112.Miller GW, Schnellmann RG. Inhibitors of renal chloride transport do not block toxicant- induced chloride influx in the proximal tubule. Toxicol Lett. 1995;76:179–184. doi: 10.1016/0378-4274(94)03224-U. [DOI] [PubMed] [Google Scholar]
- 113.Peters SM, de Jong MD, Bindels RJ, van Os CH, Wetzels JF. Effects of renal cytoprotective agents on erythrocyte membrane stability. Life Sci. 1998;63(11):975–983. doi: 10.1016/S0024-3205(98)00355-5. [DOI] [PubMed] [Google Scholar]
- 114.Kribben A, Wieder ED, Wetzels JF, Yu L, Gengaro PE, Burke TJ, Schrier RW. Evidence for role of cytosolic free calcium in hypoxia-induced proximal tubule injury. J Clin Invest. 1994;93(5):1922–1929. doi: 10.1172/JCI117183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gronow G, Klause N, Malyusz M. Restriction of hypoxic membrane defect by glycine improves mitochondrial and cellular function in reoxygenation renal tubules. In: Hogan MC, Mathieu-Costello O, Poole DC, Wagner PD, editors. Oxygen transport to tissues. Advances in experimental medicine and biology. New York: Plenum Press Div Plenum Publishing Corp; 1994. pp. 585–589. [DOI] [PubMed] [Google Scholar]
- 116.Moran JH, Schnellmann RG. Diverse cytoprotectants prevent cell lysis and promote recovery of respiration and ion transport. Biochem Biophys Res Commun. 1997;234(1):275–277. doi: 10.1006/bbrc.1997.6625. [DOI] [PubMed] [Google Scholar]
- 117.Dong Z, Saikumar P, Griess GA, Weinberg JM, Venkatachalam MA. Intracellular Ca2+ thresholds that determine survival or death of energy-deprived cells. Am J Pathol. 1998;152(1):231–240. [PMC free article] [PubMed] [Google Scholar]
- 118.Feldkamp T, Kribben A, Roeser NF, Senter RA, Weinberg JM. Accumulation of nonesterified fatty acids causes the sustained energetic deficit in kidney proximal tubules after hypoxia–reoxygenation. Am J Physiol Renal Physiol. 2006;290(2):F465–F477. doi: 10.1152/ajprenal.00305.2005. [DOI] [PubMed] [Google Scholar]
- 119.Weinberg JM, Venkatachalam MA, Goldberg H, Roeser NF, Davis JA. Modulation by Gly, Ca, and acidosis of injury-associated unesterified fatty acid accumulation in proximal tubule cells. Am J Physiol. 1995;268(1 Pt 2):F110–F121. doi: 10.1152/ajprenal.1995.268.1.F110. [DOI] [PubMed] [Google Scholar]
- 120.Weinberg JM. Oxygen deprivation-induced injury to isolated rabbit kidney tubules. J Clin Invest. 1985;76:1193–1208. doi: 10.1172/JCI112075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zager RA, Schimpf BA, Gmur DJ. Physiological pH: effects on posthypoxic proximal tubular injury. Circ Res. 1993;72:837–846. doi: 10.1161/01.RES.72.4.837. [DOI] [PubMed] [Google Scholar]
- 122.Weinberg JM, Davis JA, Roeser NF, Venkatachalam MA. Role of intracellular pH during cytoprotection of proximal tubule cells by glycine or acidosis. J Am Soc Nephrol. 1994;5(6):1314–1323. doi: 10.1681/ASN.V561314. [DOI] [PubMed] [Google Scholar]
- 123.Jansova H, Machacek M, Wang Q, Haskova P, Jirkovska A, Potuckova E, Kielar F, Franz KJ, Simunek T. Comparison of various iron chelators and prochelators as protective agents against cardiomyocyte oxidative injury. Free Radic Biol Med. 2014;74:210–221. doi: 10.1016/j.freeradbiomed.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7(2):99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 126.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490(7419):288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 128.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 129.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 130.Man SM, Kanneganti TD. Gasdermin D: the long-awaited executioner of pyroptosis. Cell Res. 2015;25(11):1183–1184. doi: 10.1038/cr.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, Weinberg JM, Venkatachalam MA. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene. 1998;17(26):3401–3415. doi: 10.1038/sj.onc.1202590. [DOI] [PubMed] [Google Scholar]
- 132.Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene. 1998;17(25):3341–3349. doi: 10.1038/sj.onc.1202579. [DOI] [PubMed] [Google Scholar]
- 133.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yaqoob M, Edelstein CL, Wieder ED, Alkhunaizi AM, Gengaro PE, Nemenoff RA, Schrier RW. Nitric oxide kinetics during hypoxia in proximal tubules: effects of acidosis and glycine. Kidney Int. 1996;49(5):1314–1319. doi: 10.1038/ki.1996.187. [DOI] [PubMed] [Google Scholar]
- 135.Edelstein CL, Ling H, Gengaro PE, Nemenoff RA, Bahr BA, Schrier RW. Effect of glycine on prelethal and postlethal increases in calpain activity in rat renal proximal tubules. Kidney Int. 1997;52(5):1271–1278. doi: 10.1038/ki.1997.452. [DOI] [PubMed] [Google Scholar]
- 136.Tijsen MJ, Peters SM, Bindels RJ, van Os CH, Wetzels JF. Glycine protection against hypoxic injury in isolated rat proximal tubules: the role of proteases. Nephrol Dial Transplant. 1997;12(12):2549–2556. doi: 10.1093/ndt/12.12.2549. [DOI] [PubMed] [Google Scholar]
- 137.Nissim I, Hardy M, Pleasure J, Nissim I, States B. A mechanism of glycine and alanine cytoprotective action: stimulation of stress-induced HSP70 mRNA. Kidney Int. 1992;42(3):775–782. doi: 10.1038/ki.1992.347. [DOI] [PubMed] [Google Scholar]
- 138.Grosser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schroder H. Antioxidant action of l-alanine: heme oxygenase-1 and ferritin as possible mediators. Biochem Biophys Res Commun. 2004;314(2):351–355. doi: 10.1016/j.bbrc.2003.12.089. [DOI] [PubMed] [Google Scholar]
- 139.Jiang LL, Qin X, Zhong XZ, Liu L, Jiang L, Lu Y, Fan LM, He ZG, Chen Q. Glycine-induced cytoprotection is mediated by ERK1/2 and AKT in renal cells with ATP depletion. Eur J Cell Biol. 2011;90(4):333–341. doi: 10.1016/j.ejcb.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 140.Weinberg JM, Venkatachalam MA, Roeser NF, Saikumar P, Dong Z, Senter RA, Nissim I. Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Ren Physiol. 2000;279(5):F927–F943. doi: 10.1152/ajprenal.2000.279.5.F927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Webb TI, Lynch JW. Molecular pharmacology of the glycine receptor chloride channel. Curr Pharm Des. 2007;13(23):2350–2367. doi: 10.2174/138161207781368693. [DOI] [PubMed] [Google Scholar]
- 142.Vandenberg RJ, French CR, Barry PH, Shine J, Schofield PR. Antagonism of ligand-gated ion channel receptors: two domains of the glycine receptor alpha subunit form the strychnine-binding site. Proc Natl Acad Sci USA. 1992;89(5):1765–1769. doi: 10.1073/pnas.89.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lewis TM, Schofield PR, McClellan AML. Kinetic determinants of agonist action at the recombinant human glycine receptor. J Physiol Lond. 2003;549(2):361–374. doi: 10.1113/jphysiol.2002.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tokutomi N, Kaneda M, Akaike N. What confers specificity on glycine for its receptor-site. Br J Pharmacol. 1989;97(2):353–360. doi: 10.1111/j.1476-5381.1989.tb11961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dong Z, Venkatachalam MA, Weinberg JM, Saikumar P, Patel Y. Protection of ATP-depleted cells by impermeant strychnine derivatives: implications for glycine cytoprotection. Am J Pathol. 2001;158(3):1021–1028. doi: 10.1016/S0002-9440(10)64049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kessler M, Terramani T, Lynch G, Baudry M. A glycine site associated with N-methyl-d-aspartic acid receptors: characterization and identification of a new class of antagonists. J Neurochem. 1989;52(4):1319–1328. doi: 10.1111/j.1471-4159.1989.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 147.Snell LD, Johnson KM. Cycloleucine competitively antagonizes the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988;151(1):165–166. doi: 10.1016/0014-2999(88)90713-3. [DOI] [PubMed] [Google Scholar]
- 148.Wang X-N, Wetzels JFM, Arnold PE, Burke TJ, Schrier RW. Strychnine protects against hypoxic injury in freshly isolated rat renal proximal tubules. J Am Soc Nephrol. 1991;2:656. [Google Scholar]
- 149.Miller GW, Schnellmann RG. A novel low-affinity strychnine binding site on renal proximal tubules: role in toxic cell death. Life Sci. 1993;53(15):1203–1209. doi: 10.1016/0024-3205(93)90538-E. [DOI] [PubMed] [Google Scholar]
- 150.Young AB, Snyder SH. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci USA. 1973;70(10):2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283(4):G856–G863. doi: 10.1152/ajpgi.00503.2001. [DOI] [PubMed] [Google Scholar]
- 152.Yamashina S, Konno A, Wheeler MD, Rusyn I, Rusyn EV, Cox AD, Thurman RG. Endothelial cells contain a glycine-gated chloride channel. Nutr Cancer. 2001;40(2):197–204. doi: 10.1207/S15327914NC402_17. [DOI] [PubMed] [Google Scholar]
- 153.Miller GW, Schnellmann RG. A putative cytoprotective receptor in the kidney: relation to the neuronal strychnine-sensitive glycine receptor. Life Sci. 1994;55(1):27–34. doi: 10.1016/0024-3205(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 154.Sarang SS, Miller GW, Grant DF, Schnellmann RG. Expression and localization of the neuronal glycine receptor beta-subunit in human, rabbit and rat kidneys. Nephron. 1999;82(3):254–260. doi: 10.1159/000045410. [DOI] [PubMed] [Google Scholar]
- 155.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol. 2015;26(11):2669–2677. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pan C, Bai XM, Fan LM, Ji Y, Li XY, Chen Q. Cytoprotection by glycine against ATP-depletion-induced injury is mediated by glycine receptor in renal cells. Biochem J. 2005;390:447–453. doi: 10.1042/BJ20050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu Y, Zhang J, Ma B, Li K, Li X, Bai H, Yang Q, Zhu X, Ben J, Chen Q. Glycine attenuates cerebral ischemia/reperfusion injury by inhibiting neuronal apoptosis in mice. Neurochem Int. 2012;61(5):649–658. doi: 10.1016/j.neuint.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 158.Thurman RG, Schemmer P, Zhong Z, Bunzendahl H, Von Frankenberg M, Lemasters JJ. Kupffer cell-dependent reperfusion injury in liver transplantation: new clinically relevant use of glycine. Langenbecks Arch Chir Suppl Kongressbd. 1998;115(185–90):185–190. [PubMed] [Google Scholar]
- 159.Schemmer P, Bradford BU, Rose ML, Bunzendahl H, Raleigh JA, Lemasters JJ, Thurman RG. Intravenous glycine improves survival in rat liver transplantation. Am J Physiol. 1999;276(4 Pt 1):G924–G932. doi: 10.1152/ajpgi.1999.276.4.G924. [DOI] [PubMed] [Google Scholar]
- 160.Wheeler MD, Thurman RG. Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am J Physiol Lung Cell Mol Physiol. 1999;277(5):L952–L959. doi: 10.1152/ajplung.1999.277.5.L952. [DOI] [PubMed] [Google Scholar]
- 161.Spittler A, Reissner CM, Oehler R, Gornikiewicz A, Gruenberger T, Manhart N, Brodowicz T, Mittlboeck M, Boltz-Nitulescu G, Roth E. Immunomodulatory effects of glycine on LPS-treated monocytes: reduced TNF-alpha production and accelerated IL-10 expression. FASEB J. 1999;13(3):563–571. doi: 10.1096/fasebj.13.3.563. [DOI] [PubMed] [Google Scholar]
- 162.Stachlewitz RF, Li X, Smith S, Bunzendahl H, Graves LM, Thurman RG. Glycine inhibits growth of T lymphocytes by an IL-2-independent mechanism. J Immunol. 2000;164(1):176–182. doi: 10.4049/jimmunol.164.1.176. [DOI] [PubMed] [Google Scholar]
- 163.Wheeler M, Stachlewitz RF, Yamashina S, Ikejima K, Morrow AL, Thurman RG. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000;14(3):476–484. doi: 10.1096/fasebj.14.3.476. [DOI] [PubMed] [Google Scholar]
- 164.Wang HD, Lu XX, Lu DX, Qi RB, Wang YP, Fu YM, Wang LW. Glycine inhibits the LPS-induced increase in cytosolic Ca2+ concentration and TNF alpha production in cardiomyocytes by activating a glycine receptor. Acta Pharmacol Sin. 2009;30(8):1107–1114. doi: 10.1038/aps.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Giambelluca MS, Gende OA. Effect of glycine on the release of reactive oxygen species in human neutrophils. Int Immunopharmacol. 2009;9(1):32–37. doi: 10.1016/j.intimp.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 166.Choi JJ, Wang SG, Tung YS, Morrison B, Konofagou EE. Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med Biol. 2010;36(1):58–67. doi: 10.1016/j.ultrasmedbio.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen J, Liu X, Mandel LJ, Schnellmann RG. Progressive disruption of the plasma membrane during renal proximal tubule cellular injury. Toxicol Appl Pharmacol. 2001;171(1):1–11. doi: 10.1006/taap.2000.9105. [DOI] [PubMed] [Google Scholar]
- 168.Schilling WP, Snyder D, Sinkins WG, Estacion M. Palytoxin-induced cell death cascade in bovine aortic endothelial cells. Am J Physiol Cell Physiol. 2006;291(4):C657–C667. doi: 10.1152/ajpcell.00063.2006. [DOI] [PubMed] [Google Scholar]
- 169.Wisnoskey BJ, Estacion M, Schilling WP. Maitotoxin-induced cell death cascade in bovine aortic endothelial cells: divalent cation specificity and selectivity. Am J Physiol Cell Physiol. 2004;287(2):C345–C356. doi: 10.1152/ajpcell.00473.2003. [DOI] [PubMed] [Google Scholar]
- 170.Bergstrom J, Alvestrand A, Furst P. Plasma and muscle free amino-acids in maintenance hemodialysis-patients without protein-malnutrition. Kidney Int. 1990;38(1):108–114. doi: 10.1038/ki.1990.174. [DOI] [PubMed] [Google Scholar]
- 171.Gannon MC, Nuttall JA, Nuttall FQ. The metabolic response to ingested glycine. Am J Clin Nutr. 2002;76(6):1302–1307. doi: 10.1093/ajcn/76.6.1302. [DOI] [PubMed] [Google Scholar]
- 172.Duran MA, Spencer D, Weise M, Kronfol NO, Spencer RF, Oken DE. Renal epithelial amino acid concentrations in mercury-induced and postischemic acute renal failure. Toxicol Appl Pharmacol. 1990;105(2):183–194. doi: 10.1016/0041-008X(90)90180-3. [DOI] [PubMed] [Google Scholar]
- 173.Adibi SA, Morse EL. Enrichment of glycine pool in plasma and tissues by glycine, di-, tri-, and tetraglycine. Am J Physiol. 1982;243(5):E413–E417. doi: 10.1152/ajpendo.1982.243.5.E413. [DOI] [PubMed] [Google Scholar]
- 174.Quan H, Athirakul K, Wetsel WC, Torres GE, Stevens R, Chen YT, Coffman TM, Caron MG. Hypertension and impaired glycine handling in mice lacking the orphan transporter XT2. Mol Cell Biol. 2004;24(10):4166–4173. doi: 10.1128/MCB.24.10.4166-4173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shalhoub R, Pitts RF, Glabman S, Dehaas J, Klein J, Webber W, Canessaf M. Extraction of amino acids from and their addition to renal blood plasma. Am J Physiol. 1963;204(2):181–186. [Google Scholar]
- 176.Block WD, Hubbard RW. Amino acid content of rabbit urine and plasma. Arch Biochem Biophys. 1962;96:557–561. doi: 10.1016/0003-9861(62)90336-3. [DOI] [PubMed] [Google Scholar]
- 177.Dennis VW, Brazy PC. Sodium, phosphate, glucose, bicarbonate, and alanine interactions in the isolated proximal convoluted tubule of the rabbit kidney. J Clin Invest. 1978;62(2):387–397. doi: 10.1172/JCI109140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barfuss DW, Schafer JA. Active amino acid absorption by proximal convoluted and proximal straight tubules. Am J Physiol. 1979;236(2):F149–F162. doi: 10.1152/ajprenal.1979.236.2.F149. [DOI] [PubMed] [Google Scholar]
- 179.Parks LD, Barfuss DW. Transepithelial transport and metabolism of glycine in S1, S2, and S3 cell types of the rabbit proximal tubule. Am J Physiol Renal Physiol. 2002;283(6):F1208–F1215. doi: 10.1152/ajprenal.00021.2002. [DOI] [PubMed] [Google Scholar]
- 180.Beck FX, Ohno A, Dorge A, Thurau K. Ischemia-induced changes in cell element composition and osmolyte contents of outer medulla. Kidney Int. 1995;48(2):449–457. doi: 10.1038/ki.1995.313. [DOI] [PubMed] [Google Scholar]
- 181.Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25(2):373–383. doi: 10.1016/S0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 182.Richards DA, Silva MA, Murphy N, Wigmore SJ, Mirza DF. Extracellular amino acid levels in the human liver during transplantation: a microdialysis study from donor to recipient. Amino Acids. 2007;33(3):429–437. doi: 10.1007/s00726-006-0480-1. [DOI] [PubMed] [Google Scholar]
- 183.Yin M, Rusyn I, Schoonhoven R, Graves LM, Rusyn EV, Li X, Li F, Cox AD, Harding TW, Bunzendahl H, Swenberg JA, Thurman RG. Inhibition of chronic rejection of aortic allografts by dietary glycine. Transplantation. 2000;69(5):773–780. doi: 10.1097/00007890-200003150-00017. [DOI] [PubMed] [Google Scholar]
- 184.Vieira CP, De Oliveira LP, Da Re Guerra F, Dos Santos De Almeida M, Marcondes MC, Pimentel ER. Glycine improves biochemical and biomechanical properties following inflammation of the achilles tendon. Anat Rec (Hoboken) 2015;298(3):538–545. doi: 10.1002/ar.23041. [DOI] [PubMed] [Google Scholar]
- 185.Li X, Bradford BU, Wheeler MD, Stimpson SA, Pink HM, Brodie TA, Schwab JH, Thurman RG. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: role for glycine-gated chloride channel. Infect Immun. 2001;69(9):5883–5891. doi: 10.1128/IAI.69.9.5883-5891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alvarado-Vasquez N, Lascurain R, Ceron E, Vanda B, Carvajal-Sandoval G, Tapia A, Guevara J, Montano LF, Zenteno E. Oral glycine administration attenuates diabetic complications in streptozotocin-induced diabetic rats. Life Sci. 2006;79(3):225–232. doi: 10.1016/j.lfs.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 187.Alarcon-Aguilar FJ, Almanza-Perez J, Blancas G, Angeles S, Garcia-Macedo R, Roman R, Cruz M. Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice. Eur J Pharmacol. 2008;599(1–3):152–158. doi: 10.1016/j.ejphar.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 188.Blancas-Flores G, Alarcon-Aguilar FJ, Garcia-Macedo R, Almanza-Perez JC, Flores-Saenz JL, Roman-Ramos R, Ventura-Gallegos JL, Kumate J, Zentella-Dehesa A, Cruz M. Glycine suppresses TNF-alpha-induced activation of NF-kappa B in differentiated 3T3-L1 adipocytes. Eur J Pharmacol. 2012;689(1–3):270–277. doi: 10.1016/j.ejphar.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 189.Garcia-Macedo R, Sanchez-Munoz F, Almanza-Perez JC, Duran-Reyes G, Alarcon-Aguilar F, Cruz M. Glycine increases mRNA adiponectin and diminishes pro-inflammatory adipokines expression in 3T3-L1 cells. Eur J Pharmacol. 2008;587(1–3):317–321. doi: 10.1016/j.ejphar.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 190.Tastesen HS, Keenan AH, Madsen L, Kristiansen K, Liaset B. Scallop protein with endogenous high taurine and glycine content prevents high-fat, high-sucrose-induced obesity and improves plasma lipid profile in male C57BL/6J mice. Amino Acids. 2014;46(7):1659–1671. doi: 10.1007/s00726-014-1715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ruiz-Ramirez A, Ortiz-Balderas E, Cardozo-Saldana G, Diaz-Diaz E, El-Hafidi M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin Sci (Lond) 2014;126(1):19–29. doi: 10.1042/CS20130164. [DOI] [PubMed] [Google Scholar]
- 192.Sekhar RV, McKay SV, Patel SG, Guthikonda AP, Reddy VT, Balasubramanyam A, Jahoor F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34(1):162–167. doi: 10.2337/dc10-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Nguyen D, Hsu JW, Jahoor F, Sekhar RV. Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J Clin Endocrinol Metab. 2014;99(1):169–177. doi: 10.1210/jc.2013-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.El Hafidi M, Perez I, Zamora J, Soto V, Carvajal-Sandoval G, Banos G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am J Physiol Regul Integr Comp Physiol. 2004;287(6):R1387–R1393. doi: 10.1152/ajpregu.00159.2004. [DOI] [PubMed] [Google Scholar]
- 195.El Hafidi M, Perez I, Banos G. Is glycine effective against elevated blood pressure? Curr Opin Clin Nutr Metab Care. 2006;9(1):26–31. doi: 10.1097/01.mco.0000196143.72985.9a. [DOI] [PubMed] [Google Scholar]
- 196.Cruz M, Maldonado-Bernal C, Mondragon-Gonzalez R, Sanchez-Barrera R, Wacher NH, Carvajal-Sandoval G, Kumate J. Glycine treatment decreases proinflammatory cytokines and increases interferon-gamma in patients with type 2 diabetes. J Endocrinol Invest. 2008;31(8):694–699. doi: 10.1007/BF03346417. [DOI] [PubMed] [Google Scholar]
- 197.Lustgarten MS, Price LL, Phillips EM, Fielding RA. Serum glycine is associated with regional body fat and insulin resistance in functionally-limited older adults. PLoS ONE. 2013;8(12):e84034. doi: 10.1371/journal.pone.0084034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rose ML, Madren J, Bunzendahl H, Thurman RG. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis. 1999;20(5):793–798. doi: 10.1093/carcin/20.5.793. [DOI] [PubMed] [Google Scholar]
- 199.Amin K, Li J, Chao WR, Dewhirst MW, Haroon ZA. Dietary glycine inhibits angiogenesis during wound healing and tumor growth. Cancer Biol Ther. 2003;2(2):173–178. doi: 10.4161/cbt.2.2.280. [DOI] [PubMed] [Google Scholar]
- 200.Bruns H, Petrulionis M, Schultze D, Al Saeedi M, Lin S, Yamanaka K, Ambrazevicius M, Strupas K, Schemmer P. Glycine inhibits angiogenic signaling in human hepatocellular carcinoma cells. Amino Acids. 2014;46(4):969–976. doi: 10.1007/s00726-013-1662-2. [DOI] [PubMed] [Google Scholar]
- 201.Melendez-Hevia E, de Paz-Lugo P, Cornish-Bowden A, Cardenas ML. A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J Biosci. 2009;34(6):853–872. doi: 10.1007/s12038-009-0100-9. [DOI] [PubMed] [Google Scholar]
- 202.Nissim I, Weinberg JM. Glycine attenuates Fanconi syndrome induced by maleate or ifosfamide in rats. Kidney Int. 1996;49(3):684–695. doi: 10.1038/ki.1996.97. [DOI] [PubMed] [Google Scholar]
- 203.Bannai M, Kawai N. New therapeutic strategy for amino acid medicine: glycine improves the quality of sleep. J Pharmacol Sci. 2012;118(2):145–148. doi: 10.1254/jphs.11R04FM. [DOI] [PubMed] [Google Scholar]
- 204.Evins AE, Fitzgerald SM, Wine L, Rosselli R, Goff DC. Placebo-controlled trial of glycine added to clozapine in schizophrenia. Am J Psychiatry. 2000;157(5):826–828. doi: 10.1176/appi.ajp.157.5.826. [DOI] [PubMed] [Google Scholar]
- 205.Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 1999;56(1):29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- 206.Yin M, Zhong Z, Connor HD, Bunzendahl H, Finn WF, Rusyn I, Li X, Raleigh JA, Mason RP, Thurman RG. Protective effect of glycine on renal injury induced by ischemia-reperfusion in vivo. Am J Physiol Renal Physiol. 2002;282(3):F417–F423. doi: 10.1152/ajprenal.00011.2001. [DOI] [PubMed] [Google Scholar]
- 207.Wetzels JFM, Yu L, Shanley PF, Burke TJ, Schrier RW. Infusion of glycine does not attenuate invivo ischemic acute-renal-failure in the rat. J Lab Clin Med. 1993;121(2):263–267. [PubMed] [Google Scholar]
- 208.Collentine GE., Jr On the efficacy and safety of glycine administered by vein. J Lab Clin Med. 1948;33(12):1555–1562. [PubMed] [Google Scholar]
- 209.Croner RS, Kulu Y, Hoerer E, Peters V, Schmidt-Mader B, Schemmer P, Herfarth C, Klar E. Intravenous glycine after cecal ligation and puncture has no effect on impaired hepatic microperfusion, leukocyte adhesion, and mortality in septic rats. Microvasc Res. 2005;69(1–2):71–78. doi: 10.1016/j.mvr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 210.Collins JW, Macdermott S, Bradbrook RA, Keeley FX, Jr, Timoney AG. A comparison of the effect of 1.5 % glycine and 5 % glucose irrigants on plasma serum physiology and the incidence of transurethral resection syndrome during prostate resection. BJU Int. 2005;96(3):368–372. doi: 10.1111/j.1464-410X.2005.05633.x. [DOI] [PubMed] [Google Scholar]
- 211.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457(7231):910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 212.Chaneton B, Hillmann P, Zheng L, Martin ACL, Maddocks ODK, Chokkathukalam A, Coyle JE, Jankevics A, Holding FP, Vousden KH, Frezza C, O’Reilly M, Gottlieb E. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature. 2012;491(7424):458–462. doi: 10.1038/nature11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, Shelton LM, Gui DY, Kwon M, Ramkissoon SH, Ligon KL, Kang SW, Snuderl M, Vander Heiden MG, Sabatini DM. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520(7547):363–367. doi: 10.1038/nature14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Labuschagne CF, van den Broek NJ, Mackay GM, Vousden KH, Maddocks OD. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 2014;7(4):1248–1258. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 215.Johannesen J, Lie M, Kiil F. Effects of glycine and glucagon on glomerular filtration and renal metabolic rates. Am J Physiol. 1977;233:F61–F66. doi: 10.1152/ajprenal.1977.233.1.F61. [DOI] [PubMed] [Google Scholar]
- 216.Heyman SN, Brezis M, Epstein FH, Spokes K, Rosen S. Effect of glycine and hypertrophy on renal outer medulla hypoxic injury in ischemia reflow and contrast nephropathy. Am J Kidney Dis. 1992;19:578–586. doi: 10.1016/S0272-6386(12)80838-9. [DOI] [PubMed] [Google Scholar]
- 217.Deng A, Thomson SC. Renal NMDA receptors independently stimulate proximal reabsorption and glomerular filtration. Am J Physiol Renal Physiol. 2009;296(5):F976–F982. doi: 10.1152/ajprenal.90391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Arora S, Kaur T, Kaur A, Singh AP. Glycine aggravates ischemia reperfusion-induced acute kidney injury through N-Methyl-d-Aspartate receptor activation in rats. Mol Cell Biochem. 2014;393(1–2):123–131. doi: 10.1007/s11010-014-2052-0. [DOI] [PubMed] [Google Scholar]
- 219.Zager RA, Venkatachalam MA. Potentiation of ischemic renal injury by amino acid infusion. Kidney Int. 1983;24(5):620–625. doi: 10.1038/ki.1983.202. [DOI] [PubMed] [Google Scholar]
- 220.Mishra RC, Tripathy S, Quest D, Desai KM, Akhtar J, Dattani ID, Gopalakrishnan V. l-Serine lowers while glycine increases blood pressure in chronic l-NAME-treated and spontaneously hypertensive rats. J Hypertens. 2008;26(12):2339–2348. doi: 10.1097/HJH.0b013e328312c8a3. [DOI] [PubMed] [Google Scholar]
- 221.Bienholz A, Petrat F, Wenzel P, Ickerott P, Weinberg JM, Witzke O, Kribben A, de Groot H, Feldkamp T. Adverse effects of alpha-ketoglutarate/malate in a rat model of acute kidney injury. Am J Physiol Renal Physiol. 2012;303(1):F56–F63. doi: 10.1152/ajprenal.00070.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zager RA, Johnson AC, Naito M, Bomsztyk K. Maleate nephrotoxicity: mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol Renal Physiol. 2008;294(1):F187–F197. doi: 10.1152/ajprenal.00434.2007. [DOI] [PubMed] [Google Scholar]
- 223.Heyman SN, Rosen S, Silva P, Spokes K, Egorin MJ, Epstein FH. Protective action of glycine in cisplatin nephropathy. Kidney Int. 1991;40:273–279. doi: 10.1038/ki.1991.210. [DOI] [PubMed] [Google Scholar]
- 224.Thurman RG, Zhong Z, Mv Frankenberg, Stachlewitz RF, Bunzendahl H. Prevention of cyclosporin-induced nephrotoxicity with dietary glycine. Transplantation. 1997;63:1661–1667. doi: 10.1097/00007890-199706150-00021. [DOI] [PubMed] [Google Scholar]
- 225.Zhong Z, Connor HD, Yin M, Moss N, Mason RP, Bunzendahl H, Forman DT, Thurman RG. Dietary glycine and renal denervation prevents cyclosporin A-induced hydroxyl radical production in rat kidney. Mol Pharmacol. 1999;56(3):455–463. doi: 10.1124/mol.56.3.455. [DOI] [PubMed] [Google Scholar]
- 226.Zhong Z, Jones S, Thurman RG. Glycine minimizes reperfusion injury in a low-flow, reflow liver perfusion model in the rat. Am J Physiol. 1996;270(2 Pt 1):G332–G338. doi: 10.1152/ajpgi.1996.270.2.G332. [DOI] [PubMed] [Google Scholar]
- 227.Yin M, Ikejima K, Arteel GE, Seabra V, Bradford BU, Kono H, Rusyn I, Thurman RG. Glycine accelerates recovery from alcohol-induced liver injury. J Pharmacol Exp Ther. 1998;286(2):1014–1019. [PubMed] [Google Scholar]
- 228.Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, Burchardt ER, Rippe RA, Thurman RG. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G200–G207. doi: 10.1152/ajpgi.2001.281.1.G200. [DOI] [PubMed] [Google Scholar]
- 229.Froh M, Zhong Z, Walbrun P, Lehnert M, Netter S, Wiest R, Conzelmann L, Gabele E, Hellerbrand C, Scholmerich J, Thurman RG. Dietary glycine blunts liver injury after bile duct ligation in rats. World J Gastroenterol. 2008;14(39):5996–6003. doi: 10.3748/wjg.14.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yamanouchi K, Eguchi S, Kamohara Y, Yanaga K, Okudaira S, Tajima Y, Kanematsu T. Glycine reduces hepatic warm ischaemia-reperfusion injury by suppressing inflammatory reactions in rats. Liver Int. 2007;27(9):1249–1254. doi: 10.1111/j.1478-3231.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 231.Ito K, Ozasa H, Noda Y, Koike Y, Arii S, Horikawa S. Effect of non-essential amino acid glycine administration on the liver regeneration of partially hepatectomized rats with hepatic ischemia/reperfusion injury. Clin Nutr. 2008;27(5):773–780. doi: 10.1016/j.clnu.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 232.Hafez T, Sheth H, Glantzounis G, Parkes H, Seifalian A, Fuller B, Davidson B. Glycine protects bile physiology and biliary-specific liver cell metabolism from ischemia-reperfusion injury: a H-1 NMR study. Cell preservation technology. 2008;6(3):173–180. doi: 10.1089/cpt.2008.0006. [DOI] [Google Scholar]
- 233.Duenschede F, Westermann S, Riegler N, Miesner I, Erbes K, Ewald P, Kircher A, Schaefer H, Schneider J, Schad A, Dutkowski P, Kiemer AK, Junginger T. Different protection mechanisms after pretreatment with glycine or alpha-lipoic acid in a rat model of warm hepatic ischemia. Eur Surg Res. 2006;38(6):503–512. doi: 10.1159/000096061. [DOI] [PubMed] [Google Scholar]
- 234.Hoffmann K, Buchler MW, Schemmer P. Supplementation of amino acids to prevent reperfusion injury after liver surgery and transplantation - Where do we stand today? Clinical Nutrition. 2011;30(2):143–147. doi: 10.1016/j.clnu.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 235.Wang YS, Yan YH, Zou XF. Protective effect of glycine on liver injury during liver transplantation. Chin Med J (Engl) 2010;123(14):1931–1938. [PubMed] [Google Scholar]
- 236.Stachlewitz RF, Seabra V, Bradford B, Bradham CA, Rusyn I, Germolec D, Thurman RG. Glycine and uridine prevent d-galactosamine hepatotoxicity in the rat: role of Kupffer cells. Hepatology. 1999;29(3):737–745. doi: 10.1002/hep.510290335. [DOI] [PubMed] [Google Scholar]
- 237.Schneider L, Hackert T, Longerich T, Hartwig W, Fritz S, Krych R, Fortunato F, Gebhard MM, Werner J. Effects of gadolinium chloride and glycine on hepatic and pancreatic tissue damage in alcoholic pancreatitis. Pancreas. 2010;39(4):502–509. doi: 10.1097/MPA.0b013e3181bd6470. [DOI] [PubMed] [Google Scholar]
- 238.Rentsch M, Puellmann K, Sirek S, Iesalnieks I, Kienle K, Mueller T, Bolder U, Geissler E, Jauch KW, Beham A. Benefit of Kupffer cell modulation with glycine versus Kupffer cell depletion after liver transplantation in the rat: effects on postischemic reperfusion injury, apoptotic cell death graft regeneration and survival. Transpl Int. 2005;18(9):1079–1089. doi: 10.1111/j.1432-2277.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 239.Bruns H, Watanpour I, Gebhard MM, Flechtenmacher C, Galli U, Schulze-Bergkamen H, Zorn M, Buchler MW, Schemmer P. Glycine and taurine equally prevent fatty livers from Kupffer cell-dependent injury: an in vivo microscopy study. Microcirculation. 2011;18(3):205–213. doi: 10.1111/j.1549-8719.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 240.Zhong X, Li X, Qian L, Xu Y, Lu Y, Zhang J, Li N, Zhu X, Ben J, Yang Q, Chen Q. Glycine attenuates myocardial ischemia-reperfusion injury by inhibiting myocardial apoptosis in rats. J Biomed Res. 2012;26(5):346–354. doi: 10.7555/JBR.26.20110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang Y, Lv SJ, Yan H, Wang L, Liang GP, Wan QX, Peng X. Effects of glycine supplementation on myocardial damage and cardiac function after severe burn. Burns. 2013;39(4):729–735. doi: 10.1016/j.burns.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 242.Ascher E, Hanson JN, Cheng W, Hingorani A, Scheinman M. Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery. 2001;129(2):231–235. doi: 10.1067/msy.2001.112594. [DOI] [PubMed] [Google Scholar]
- 243.Crinnion JN, Homer-Vanniasinkam S, Hatton R, Parkin SM, Gough MJ. Role of neutrophil depletion and elastase inhibition in modifying skeletal muscle reperfusion injury. Cardiovasc Surg. 1994;2(6):749–753. [PubMed] [Google Scholar]
- 244.Gohrbandt B, Fischer S, Warnecke G, Avsar M, Sommer SP, Haverich A, Strueber M. Glycine intravenous donor preconditioning is superior to glycine supplementation to low-potassium dextran flush preservation and improves graft function in a large animal lung transplantation model after 24 h of cold ischemia. J Thorac Cardiovasc Surg. 2006;131(3):724–729. doi: 10.1016/j.jtcvs.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 245.Sommer SP, Sommer S, Sinha B, Leyh RG. Glycine preconditioning to ameliorate pulmonary ischemia reperfusion injury in rats. Interact CardioVasc Thorac Surg. 2012;14(5):521–525. doi: 10.1093/icvts/ivs008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA. 2000;97(6):2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gusev EI, Skvortsova VI, Dambinova SA, Raevskiy KS, Alekseev AA, Bashkatova VG, Kovalenko AV, Kudrin VS, Yakovleva EV. Neuroprotective effects of glycine for therapy of acute ischaemic stroke. Cerebrovasc Dis. 2000;10(1):49–60. doi: 10.1159/000016025. [DOI] [PubMed] [Google Scholar]
- 248.Oda M, Kure S, Sugawara T, Yamaguchi S, Kojima K, Shinka T, Sato K, Narisawa A, Aoki Y, Matsubara Y, Omae T, Mizoi K, Kinouchi H. Direct correlation between ischemic injury and extracellular glycine concentration in mice with genetically altered activities of the glycine cleavage multienzyme system. Stroke. 2007;38(7):2157–2164. doi: 10.1161/STROKEAHA.106.477026. [DOI] [PubMed] [Google Scholar]
- 249.Tanabe M, Nitta A, Ono H. Neuroprotection via strychnine-sensitive glycine receptors during post-ischemic recovery of excitatory synaptic transmission in the hippocampus. J Pharmacol Sci. 2010;113(4):378–386. doi: 10.1254/jphs.10150FP. [DOI] [PubMed] [Google Scholar]
- 250.Selin AA, Lobysheva NV, Vorontsova ON, Tonshin AA, Yaguzhinsky LS, Nartsissov YR. Mechanism underlying the protective effect of glycine in energetic disturbances in brain tissues under hypoxic conditions. Bull Exp Biol Med. 2012;153(1):44–47. doi: 10.1007/s10517-012-1638-3. [DOI] [PubMed] [Google Scholar]
- 251.Chen Z, Hu B, Wang F, Du L, Huang B, Li L, Qi J, Wang X. Glycine bidirectionally regulates ischemic tolerance via different mechanisms including NR2A-dependent CREB phosphorylation. J Neurochem. 2015;133(3):397–408. doi: 10.1111/jnc.12994. [DOI] [PubMed] [Google Scholar]
- 252.Wheeler MD, Rose ML, Yamashima S, Enomoto N, Seabra V, Madren J, Thurman RG. Dietary glycine blunts lung inflammatory cell influx following acute endotoxin. Am J Physiol Lung Cell Mol Physiol. 2000;279(2):L390–L398. doi: 10.1152/ajplung.2000.279.2.L390. [DOI] [PubMed] [Google Scholar]
- 253.Ikejima K, Iimuro Y, Forman DT, Thurman RG. A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol. 1996;271(1 Pt 1):G97–G103. doi: 10.1152/ajpgi.1996.271.1.G97. [DOI] [PubMed] [Google Scholar]
- 254.Yang S, Koo DJ, Chaudry IH, Wang P. Glycine attenuates hepatocellular depression during early sepsis and reduces sepsis-induced mortality. Crit Care Med. 2001;29(6):1201–1206. doi: 10.1097/00003246-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 255.Grotz MR, Pape HC, van Griensven M, Stalp M, Rohde F, Bock D, Krettek C. Glycine reduces the inflammatory response and organ damage in a two-hit sepsis model in rats. Shock. 2001;16(2):116–121. doi: 10.1097/00024382-200116020-00006. [DOI] [PubMed] [Google Scholar]
- 256.Zhong Z, Enomoto N, Connor HD, Moss N, Mason RP, Thurman RG. Glycine improves survival after hemorrhagic shock in the rat. Shock. 1999;12(1):54–62. doi: 10.1097/00024382-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 257.Gundersen Y, Vaagenes P, Os O, Pillgram-Larsen J, Sundnes KO, Opstad PK. Capacity of glycine to modulate early inflammatory disturbances after serious gunshot injuries in the pig. Scand J Clin Lab Invest. 2007;67(2):143–153. doi: 10.1080/00365510600995226. [DOI] [PubMed] [Google Scholar]
- 258.Aziz M, Jacob A, Wang P. Revisiting caspases in sepsis. Cell Death Dis. 2014;5:e1526. doi: 10.1038/cddis.2014.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Zager RA, Johnson AC, Lund S, Randolph-Habecker J. Toll-like receptor (TLR4) shedding and depletion: acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol. 2007;292(1):F304–F312. doi: 10.1152/ajprenal.00237.2006. [DOI] [PubMed] [Google Scholar]
- 260.Zager RA, Johnson AC, Hanson SY. Proximal tubular cytochrome c efflux: determinant, and potential marker, of mitochondrial injury. Kidney Int. 2004;65(6):2123–2134. doi: 10.1111/j.1523-1755.2004.00638.x. [DOI] [PubMed] [Google Scholar]
- 261.Zager RA, Johnson AC, Hanson SY. Radiographic contrast media-induced tubular injury: evaluation of oxidant stress and plasma membrane integrity. Kidney Int. 2003;64(1):128–139. doi: 10.1046/j.1523-1755.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 262.Zager RA. Plasma membrane cholesterol: a critical determinant of cellular energetics and tubular resistance to attack. Kidney Int. 2000;58(1):193–205. doi: 10.1046/j.1523-1755.2000.00154.x. [DOI] [PubMed] [Google Scholar]
- 263.Aki T, Egashira N, Yamauchi Y, Hama M, Yano T, Itoh Y, Yamada T, Oishi R. Protective effects of amino acids against gabexate mesilate-induced cell injury in porcine aorta endothelial cells. J Pharmacol Sci. 2008;107(3):238–245. doi: 10.1254/jphs.08053FP. [DOI] [PubMed] [Google Scholar]
- 264.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X(7) receptor differentially couples to distinct release pathways for IL-1 beta in mouse macrophage. J Immunol. 2008;180(11):7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 265.Feldkamp T, Park JS, Pasupulati R, Amora D, Roeser NF, Venkatachalam MA, Weinberg JM. Regulation of the mitochondrial permeability transition in kidney proximal tubules and its alteration during hypoxia-reoxygenation. Am J Physiol Renal Physiol. 2009;297(6):F1632–F1646. doi: 10.1152/ajprenal.00422.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Park JS, Pasupulati R, Feldkamp T, Roeser NF, Weinberg JM. Cyclophilin D and the mitochondrial permeability transition in kidney proximal tubules after hypoxic and ischemic injury. Am J Physiol Renal Physiol. 2011;301(1):F134–F150. doi: 10.1152/ajprenal.00033.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Reeves WB. Effects of chloride channel blockers on hypoxic injury in rat proximal tubules. Kidney Int. 1997;51(5):1529–1534. doi: 10.1038/ki.1997.210. [DOI] [PubMed] [Google Scholar]
- 268.Schulz R, Gorge PM, Gorbe A, Ferdinandy P, Lampe PD, Leybaert L. Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection. Pharmacol Therapeut. 2015;153:90–106. doi: 10.1016/j.pharmthera.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]