Abstract
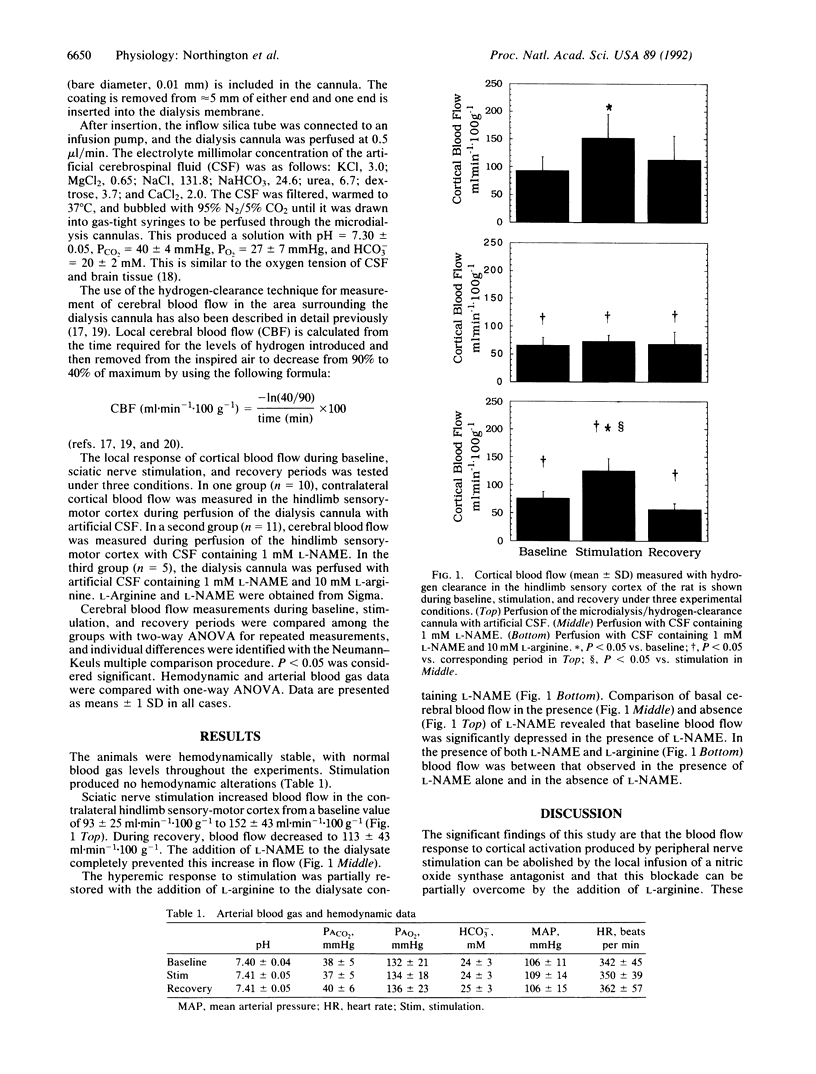
With combined microdialysis and hydrogen clearance techniques for simultaneous local delivery of drugs and blood-flow measurement in the rat hindlimb sensory-motor cortex, we examined the role of nitric oxide in cerebral blood-flow regulation during sciatic nerve stimulation. Infusion of 1 mM nitric oxide synthase antagonist, N eta-nitro-L-arginine methyl ester (L-NAME), blocked the cortical blood-flow response to sciatic nerve stimulation (152 +/- 43 ml.min-1.100 g-1 of tissue in controls and 73 +/- 11 ml.min-1.100 g-1 in the presence of L-NAME; P less than 0.05). Addition of 10 mM L-arginine to the dialysate containing L-NAME partially restored the hyperemic response to nerve stimulation (125 ml.min-1.100 g-1). L-NAME also produced a decrease in baseline cerebral blood flow when compared with the control (66 +/- 14 ml.min-1.100 g-1 vs. 93 +/- 25 ml.min-1.100 g-1). We conclude that nitric oxide from activated neurons participates in the local regulation of cortical blood flow in response to sciatic nerve stimulation and also in the maintenance of basal cortical blood flow.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abman S. H., Chatfield B. A., Hall S. L., McMurtry I. F. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990 Dec;259(6 Pt 2):H1921–H1927. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- Bellan J. A., Minkes R. K., McNamara D. B., Kadowitz P. J. N omega-nitro-L-arginine selectively inhibits vasodilator responses to acetylcholine and bradykinin in cats. Am J Physiol. 1991 Mar;260(3 Pt 2):H1025–H1029. doi: 10.1152/ajpheart.1991.260.3.H1025. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E., Mintun M. A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988 Jul 22;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Förstermann U., Gorsky L. D., Pollock J. S., Schmidt H. H., Heller M., Murad F. Regional distribution of EDRF/NO-synthesizing enzyme(s) in rat brain. Biochem Biophys Res Commun. 1990 Apr 30;168(2):727–732. doi: 10.1016/0006-291x(90)92382-a. [DOI] [PubMed] [Google Scholar]
- Gally J. A., Montague P. R., Reeke G. N., Jr, Edelman G. M. The NO hypothesis: possible effects of a short-lived, rapidly diffusible signal in the development and function of the nervous system. Proc Natl Acad Sci U S A. 1990 May;87(9):3547–3551. doi: 10.1073/pnas.87.9.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Garthwaite G., Palmer R. M., Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989 Oct 17;172(4-5):413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Southam E., Anderton M. A kainate receptor linked to nitric oxide synthesis from arginine. J Neurochem. 1989 Dec;53(6):1952–1954. doi: 10.1111/j.1471-4159.1989.tb09266.x. [DOI] [PubMed] [Google Scholar]
- González C., Estrada C. Nitric oxide mediates the neurogenic vasodilation of bovine cerebral arteries. J Cereb Blood Flow Metab. 1991 May;11(3):366–370. doi: 10.1038/jcbfm.1991.76. [DOI] [PubMed] [Google Scholar]
- Ibayashi S., Ngai A. C., Howard M. A., 3rd, Meno J. R., Mayberg M. R., Winn H. R. Lack of sympathetic and cholinergic influences on cerebral vasodilation caused by sciatic nerve stimulation in the rat. J Cereb Blood Flow Metab. 1991 Jul;11(4):678–683. doi: 10.1038/jcbfm.1991.120. [DOI] [PubMed] [Google Scholar]
- Johnson R. D., Justice J. B. Model studies for brain dialysis. Brain Res Bull. 1983 Apr;10(4):567–571. doi: 10.1016/0361-9230(83)90156-9. [DOI] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K. R., Ngai A. C., Winn H. R. Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am J Physiol. 1990 Dec;259(6 Pt 2):H1703–H1708. doi: 10.1152/ajpheart.1990.259.6.H1703. [DOI] [PubMed] [Google Scholar]
- Lassen N. A., Ingvar D. H., Skinhøj E. Brain function and blood flow. Sci Am. 1978 Oct;239(4):62–71. doi: 10.1038/scientificamerican1078-62. [DOI] [PubMed] [Google Scholar]
- Lee T. J., Sarwinski S. J. Nitric oxidergic neurogenic vasodilation in the porcine basilar artery. Blood Vessels. 1991;28(5):407–412. doi: 10.1159/000158887. [DOI] [PubMed] [Google Scholar]
- Leniger-Follert E., Lübbers D. W. Behavior of microflow and local PO2 of the brain cortex during and after direct electrical stimulation. A contribution to the problem of metabolic regulation of microcirculation in the brain. Pflugers Arch. 1976 Oct 15;366(1):39–44. doi: 10.1007/BF02486558. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Ngai A. C., Ko K. R., Morii S., Winn H. R. Effect of sciatic nerve stimulation on pial arterioles in rats. Am J Physiol. 1988 Jan;254(1 Pt 2):H133–H139. doi: 10.1152/ajpheart.1988.254.1.H133. [DOI] [PubMed] [Google Scholar]
- Pasztor E., Symon L., Dorsch N. W., Branston N. M. The hydrogen clearance method in assessment of blood flow in cortex, white matter and deep nuclei of baboons. Stroke. 1973 Jul-Aug;4(4):556–567. doi: 10.1161/01.str.4.4.556. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Southam E., East S. J., Garthwaite J. Excitatory amino acid receptors coupled to the nitric oxide/cyclic GMP pathway in rat cerebellum during development. J Neurochem. 1991 Jun;56(6):2072–2081. doi: 10.1111/j.1471-4159.1991.tb03468.x. [DOI] [PubMed] [Google Scholar]
- Toda N., Okamura T. Role of nitric oxide in neurally induced cerebroarterial relaxation. J Pharmacol Exp Ther. 1991 Sep;258(3):1027–1032. [PubMed] [Google Scholar]
- Van Wylen D. G., Park T. S., Rubio R., Berne R. M. Cerebral blood flow and interstitial fluid adenosine during hemorrhagic hypotension. Am J Physiol. 1988 Nov;255(5 Pt 2):H1211–H1218. doi: 10.1152/ajpheart.1988.255.5.H1211. [DOI] [PubMed] [Google Scholar]
- Van Wylen D. G., Park T. S., Rubio R., Berne R. M. Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J Cereb Blood Flow Metab. 1986 Oct;6(5):522–528. doi: 10.1038/jcbfm.1986.97. [DOI] [PubMed] [Google Scholar]
- Van Wylen D. G., Park T. S., Rubio R., Berne R. M. The effect of local infusion of adenosine and adenosine analogues on local cerebral blood flow. J Cereb Blood Flow Metab. 1989 Aug;9(4):556–562. doi: 10.1038/jcbfm.1989.79. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Wang J. Y., Go V. L. Cortical vasodilatation produced by vasoactive intestinal polypeptide (VIP) and by physiological stimuli in the cat. J Cereb Blood Flow Metab. 1987 Jun;7(3):315–326. doi: 10.1038/jcbfm.1987.69. [DOI] [PubMed] [Google Scholar]
- Young W. H2 clearance measurement of blood flow: a review of technique and polarographic principles. Stroke. 1980 Sep-Oct;11(5):552–564. doi: 10.1161/01.str.11.5.552. [DOI] [PubMed] [Google Scholar]


