SUMMARY
Insect ecdysis sequence is composed of pre-ecdysis, ecdysis and post-ecdysis behaviors controlled by a complex cascade of peptide hormones from endocrine Inka cells and neuropeptides in the central nervous system (CNS). Inka cells produce pre-ecdysis and ecdysis triggering hormones (ETH) which activate the ecdysis sequence through receptor-mediated actions on specific neurons in the CNS. Multiple experimental approaches have been used to determine mechanisms of ETH expression and release from Inka cells and its action on the CNS of moths and flies. During the preparatory phase 1–2 days prior to ecdysis, high ecdysteroid levels induce expression of ETH receptors in the CNS and increased ETH production in Inka cells, which coincides with expression of nuclear ecdysone receptor (EcR) and transcription factor cryptocephal (CRC). However, high ecdysteroid levels prevent ETH release from Inka cells. Acquisition of Inka cell competence to release ETH requires decline of ecdysteroid levels and β-FTZ-F1 expression few hours prior to ecdysis. The behavioral phase is initiated by ETH secretion into the hemolymph, which is controlled by two brain neuropeptides - corazonin and eclosion hormone (EH). Corazonin acts on its receptor in Inka cells to elicit low level ETH secretion and initiation of pre-ecdysis, while EH induces cGMP-mediated ETH depletion and consequent activation of ecdysis. The activation of both behaviors is accomplished by ETH action on central neurons expressing ETH receptors A and B (ETHR-A and B). These neurons produce numerous excitatory or inhibitory neuropeptides which initiate or terminate different phases of the ecdysis sequence. Our data indicate that insect ecdysis is a very complex process characterized by two principal steps: 1) Ecdysteroid-induced expression of receptors and transcription factors in the CNS and Inka cells. 2) Release and interaction of Inka cell peptide hormones and multiple central neuropeptides to control consecutive phases of the ecdysis sequence.
Keywords: Manduca, Drosophila, ecdysis, ETH, receptors, GPCR, hormones, neuropeptides
INTRODUCTION
Animal and human programmed behaviors are genetically encoded innate processes necessary for normal development and reproduction. These include feeding, hunting, migrations, nest building, territorial displays, singing, dancing, courtship, mating, birth contractions, etc. Most of these behaviors have characteristic species- or sex-specific patterns and could be restricted to some animal groups. However, the basic principles and mechanisms regulating programmed behaviors may be common and widespread among most animals.
One of the most common innate behaviors is the ecdysis sequence necessary for regular shedding of the old cuticle during embryonic and larval development of nematodes, arthropods and many other invertebrates placed in the clade Ecdysozoa (Aguinaldo et al., 1997). The insect ecdysis sequence is usually composed of three distinct behavioral phases named pre-ecdysis, ecdysis and post-ecdysis. Each of these behaviors is characterized by distinct and specific contractions of skeletal muscles, but the onset of adult ecdysis (also called eclosion) is in some species delayed by a quiescent period for ~10–30 min.
Pre-ecdysis is composed of various consecutive or overlapping movements of the head, thorax, abdomen and their appendages (antennae, wing pads, legs, and prolegs) depending on particular hemi- or holometabolous species and developmental stage. These contractions may last for 1–2 hr and are required for loosening and splitting of the old cuticle. After the pre-ecdysis motor program is completed, animals switch to ecdysis behavior characterized by peristaltic movements of the abdomen and various contractions of legs and/or prolegs for ~10–15 min. These abdominal peristaltic movements are similar in most insects and are necessary for complete shedding of the old cuticle. Post-ecdysis comprises expansion, tanning and hardening of the new cuticle to its final shape and color. The ecdysis sequence is associated with increased activity of heart, swallowing of molting fluid and air, and reabsorption of molting fluid through the new cuticle. At the end of ecdysis, the new cuticle is coated with a water resistant layer secreted from the Verson’s glands (Lane et al., 1986; Horwath and Riddiford, 1988).
Successful performance of the ecdysis sequence requires expression of specific genes and release of multiple regulatory molecules. Ecdysis-related gene expression is controlled by ecdysteroids from prothoracic glands and gonads, while activation and execution of each behavior is under control of peptide hormones (ETHs) produced by Inka cells and neuropeptides within the central nervous system (CNS). Recent findings provide evidence that increased ecdysteroid levels prior to each ecdysis induce gene expression important for synthesis of a new cuticle and production of receptors and peptide hormones in the CNS and Inka cells (Žitňan et al., 1999; Kim et al., 2006a,b). The subsequent humoral signaling between Inka cells and the CNS results in central release of multiple neuropeptides and activation of neuronal circuits for pre-ecdysis and ecdysis. The ecdysis sequence is therefore an excellent model for studying neuroendocrine mechanisms necessary for regulation of an innate behavior.
In this review we will focus on recent findings regarding the ecdysteroid and peptide signaling cascade leading to ecdysis. The history of identification, expression, release and function of ETHs and central neuropeptides in ecdysis have been recently reviewed in detail (Ewer and Reynolds 2002; Truman, 2005; Žitňan and Adams, 2005).
ROLES OF ECDYSTEROIDS AND TRANSCRIPTION FACTORS IN ECDYSIS
Increased ecdysteroid levels control gene expression in the CNS and Inka cells
Ecdysteroids are important regulators of gene expression during the preparatory phase for ecdysis. Freshly ecdysed, feeding and wandering larvae and early pupal stages with low ecdysteroid levels never respond to ETH injections. Likewise, isolated CNS from these stages shows no specific response to ETH treatment. Animals become responsive to these peptide hormones after peak ecdysteroid levels in the hemolymph induce production of a new cuticle at the end of each larval and pupal stages (pharate stages) 1–2 days prior to natural ecdysis (Žitňan et al., 1999; 2002; Žitňanová et al., 2001). This CNS sensitivity can be artificially induced in vivo by injecting 20-hydroxyecdysone (20E) or its synthetic analog tebufenozine (RH-5992) into freshly ecdysed and feeding larvae, or in vitro by treating the isolated CNS with 20E (Žitňanová et al., 2001). These experiments provided evidence that acquisition of sensitivity to ETH in pharate stages requires direct steroid action on the CNS. Real-time PCR and in situ hybridizations showed that the appearance of CNS sensitivity coincides along with peak ecdysteroid levels and expression of ETH receptor (ETHR) mRNA in specific neurons (Kim et al., 2006a,b). Levels of this mRNA decline along ecdysteroid levels as animals approach ecdysis onset and remain at background levels after ecdysis and during feeding stages till the appearance of a new ecdysteroid peak in the hemolymph (Kim et al., 2006a; Cho, Kim, Žitňan and Adams, unpublished). These data indicate that ecdysteroid-induced expression of ETHR is an obligatory step for the appearance of CNS sensitivity to ETH.
However, mechanisms controlling ETHR expression are completely unknown. For example, it is not clear if ETHR expression is mediated directly by 20E and its receptor, or indirectly, through some other unidentified transcription factors. Search for response elements and enhancers in the ETHR promoter region may help to clarify mechanisms involved in ETHR regulation.
More is known about the mechanisms of ecdysteroid action on Inka cells associated with expression and release of ETH. High ecdysteroid levels coincide with expression of nuclear ecdysone receptor (EcR-B1) and markedly increased production of PETH, ETH and their propeptide precursors. Likewise, injection of 20E or its more stable analog tebufenosine caused obvious elevation of Inka cell peptides and precursors (Žitňan et al., 1999; Žitňanová et al., 2001). The promoter region of the eth gene contains a direct repeat of the ecdysone response element (AGGTCA), indicating that eth expression is under direct control of ecdysteroid-EcR complex. However, preliminary studies in Drosophila and in the embryonic Manduca cell line GV1, indicate that although ecdysteroids upregulate eth expression, some additional factors are apparently required (Filippov, Adams, and Gill, unpublished; Žitňan and Adams, 2005).
Experimental data in Drosophila suggest that two of these factors are encoded by neighboring genes cryptocephal (crc) and dimmed (dimm). The CRC is a basic-leucine zipper transcription factor expressed in Inka cells (Hewes et al., 2000; Gauthier and Hewes, 2006). Using in situ hybridization technique, severely suppressed eth transcription was observed in crc mutant larvae, which resulted in ecdysis defects comparable with eth mutants lacking ETH. Previous work suggested that cis-regulatory elements patterning eth expression reside in 380 bp-long upstream region of the eth gene (Park Y. et al., 2002). Additional experiments showed that CRC requires this 382 bp enhancer to induce expression of the eth gene. The presence of 1–2 putative CRC binding sites in the eth upstream region provides further evidence that CRC is directly involved in eth expression (Gauthier and Hewes, 2006). The basic helix-loop-helix gene dimm is also expressed in Inka cells and may participate in ETH synthesis (Hewes et al., 2003). In addition, the dimm is expressed in many peptidergic neurons of the CNS and controls transcription of several neuropeptides including kinin and FMRFamide-related peptides (Hewes et al., 2003).
Decrease of ecdysteroid levels controls secretory competence of Inka cells
While high ecdysteroid levels act through transcription factors to induce expression of the eth gene and consequent accumulation of ETH in Inka cells, they suppress release of these peptides into the hemolymph. Injection of 20E into pharate larvae of Manduca causes a dose-dependent delay of ETH release and ecdysis onset (Žitňan et al., 1999). This ecdysteroid-induced delay of ecdysis onset was observed previously in various insects, but its association with Inka cell competence was not determined (Sláma, 1980; Truman et al., 1983; Robinow et al., 1993). Inka cells are not competent to release ETH till concentrations of ecdysteroid levels decline below 0.1 μg/ml about 6–8 hr prior to ecdysis (Kingan and Adams, 2000). This acquisition of secretory competence in Inka cells of Manduca coincides with expression of the orphan nuclear receptor β-FTZ-F1 (Hiruma and Riddiford, 2001). In Drosophila, β-FTZ-F1 is produced in many tissues shortly before each larval and pupal ecdysis. ftz-f1 mutants fail to ecdyse, while premature expression of the β-FTZ-F1 in wild-type larvae causes fatal defects at ecdysis (Yamada et al., 2000). Therefore, β-FTZ-F1 probably regulates late expression of genes associated with ecdysis. Indeed, RNAi-mediated suppression of β-FTZ-F1 expression in Inka cells blocks ETH release, resulting in accumulation of ETH and ecdysis defects (Cho and Adams, in preparation). These data indicate that β-FTZ-F1 is an important competence factor required for specific tissue responses to declining ecdysteroid levels and acquisition of secretory competence in Inka cells.
ROLES OF NEUROPEPTIDES IN ETH RELEASE
Corazonin and its receptor
Corazonin is a very conserved neuropeptide identified in many diverse insects (Fig. 1; see review Žitňan and Adams, 2005) and corazonin immunoreactivity was detected in brain lateral neurosecretory cells in representatives of most major insect orders, except beetles (Veenstra and Davis, 1993; Cantera et al., 1994; Hansen et al., 2001; Roller et al., 2003). In moths these corazonin lateral cells also express the circadian clock protein period (PER) involved in the regulation of circadian rhythms (Sauman and Reppert, 1996; Wise et al., 2002). Connection between circadian rhythms and ecdysis is indicated by extirpation manipulations, which showed that these cells may be important for photoperiod-dependent induction of diapause occurring after pupal ecdysis (Shiga et al., 2003).
Fig. 1.
Corazonin sequences are very conserved in various insect species (Veenstra, 1989; 1991; 1994; Predel et al., 1999; Tawfik et al., 1999; Hua et al., 2000; Riehle et al., 2002). The gene encoding corazonin appears to be absent in the genome of the beetle Tribolium castaneum.
Further experiments in vivo and in vitro showed that corazonin is associated with ecdysis as a natural releaser of ETH from Manduca and Bombyx Inka cells. Injection of corazonin into isolated abdomens of pharate larvae lacking neurons producing EH and corazonin resulted in ETH release from Inka cells. Bath application of corazonin on isolated epitracheal glands demonstrated that this neuropeptide directly acts on Inka cells to elicit ETH release (Kim et al., 2004). Furthemore, Nothern blots and in situ hybridization techniques revealed high level expression of corazonin receptor in Inka cells of Manduca and Bombyx (Kim et al., 2004; Roller and Žitňan, unpublished). This receptor was identified as a GPCR in Drosophila and Manduca (Cazzamali et al., 2002; Park et al., 2002b; Kim et al., 2004). Corazonin is released into the hemolymph just prior to the initiation of ecdysis sequence and its very low concentrations (25–100 pM) induce slow secretion of ETH from Inka cells. Interestingly, high non-physiological corazonin concentrations (10 nM) suppress Inka cells peptide release, indicating fast receptor desensitization (Kim et al., 2004). These data provide evidence that corazonin controls initial secretory activity of Inka cells in the absence of cGMP (see below).
So far, it is not clear if corazonin controls Inka cell secretion in other insects. Corazonin imunoreactivity and biological activity is absent in albino locust mutants and several beetles (Tanaka, 2000; Roller et al., 2003), but these animals show no defects in ecdysis behaviors. On the other hand, it may initiate ETH release in other insects. Further studies are necessary to demonstrate the general role of corazonin in regulation of ETH secretion from Inka cells.
Eclosion hormone
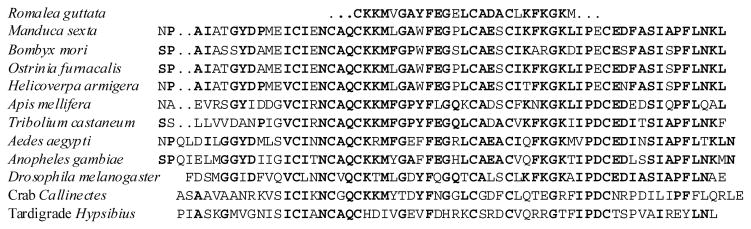
Activity of EH was first demonstrated using brain transplantation experiments to alter timing of adult emergence (also called eclosion) in giant silk moths (Truman and Riddiford, 1970). This brain neuropeptide was isolated and sequenced in moths Manduca and Bombyx (Kono et al., 1997; Kataoka et al., 1987; Marti et al., 1987) and later identified by molecular cloning in Drosophila (Horodyski et al., 1993) or in silico screening in several other insects, crab and tartigrade (Fig. 2).
Fig. 2.
Sequences of eclosion hormone in insects, crab Callinectes sapidus and tartigrade Hypsibius dujardini. Sequences of EH of Manduca, Bombyx and Drosophila were determined from the brain-CC-CA extracts or cDNA clones (Marti et al., 1987; Kataoka et al., 1987; Kono et al., 1987; Horodyski et al., 1989, 1993; Riehle et al., 2002), while remaining sequences are unpublished data obtained by in silico screening of available genomes or cDNA libraries (accession numbers: AF138854, AY822476, CK325798, CV224237, DQ668369, CH901007, XM_001122120, XM_964071). Amino acid homology in unrelated species is indicated by bold letters.
EH role in the ETH release is well established in Manduca. Direct EH action on Inka cells was first indicated by incubation of isolated epitracheal glands with brain extracts, which resulted in ETH release and consequent pre-ecdysis and ecdysis burst patterns in the CNS (Žitňan et al., 1996). EH-induced release of Inka cell peptide hormones was confirmed by in vitro assay using synthetic EH and competent epitracheal glands from pharate pupae 3–4 hr prior to expected ecdysis. Quantitative enzyme immunoassays showed that few preparations respond to low concentrations of EH (10–50 pM) by partial ETH secretion, while higher EH concentrations (100–1000 pM) evoke ETH depletion in most or all Inka cells within 30–45 min of incubation (Kingan et al., 1997; Kim et al., 2004). EH-induced Inka cell secretion is always associated with cGMP elevation (Kingan et al., 1997). Mechanisms of EH-evoked release of ETH include mobilization of intracellular calcium, as well as cGMP production through a unique NO-insensitive guanylate cyclase (MsGC-β3) and phospholipase C (Kingan et al., 2001; Morton and Simpson, 2002). The exact time of EH release and its hemolymph levels have not been determined. Indirect evidence for EH release is indicated by loss of EH immunostaining in neurohaemal proctodeal nerves and appearance of cGMP in Inka cells 20–30 min after the initiation of larval pre-ecdysis (Novicki and Weeks, 1996; Ewer et al., 1997).
In Drosophila and other insects the peripheral role of EH is less clear. Most knockout flies lacking EH-producing neurons display defects in tracheal air filling (McNabb et al., 1997), but show relatively normal ecdysis associated with ETH depletion indicating that additional factors (e.g. corazonin?) control Inka cell secretion (Clark et al., 2004; Kim et al., 2006).
ETH, ETHR AND PEPTIDE REGULATION OF ECDYSIS
Roles of Inka cell peptide hormones in activation of the ecdysis sequence
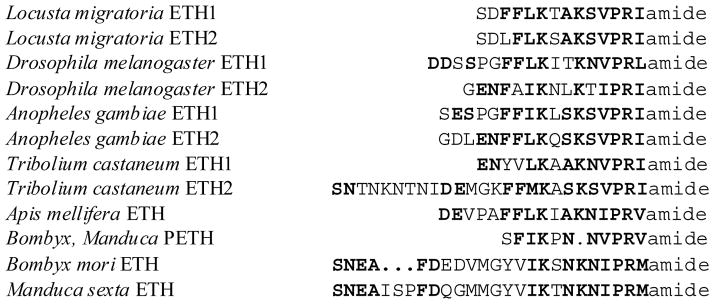
Epitracheal glands were first discovered by Ikeda (1913) in B. mori. Using transmission electron microscopy and immunohistochemical techniques nine pairs of these glands were found attached to the tracheal system near each spiracle in other moths (Žitňan, 1989; Akai, 1992; Žitňan et al., 1996, Klein et al., 1999). Moth epitracheal glands are composed of four cells: large endocrine Inka cell, smaller narrow cell with possible endocrine function, as well as exocrine and duct cells (Klein et al., 1999; Žitňanová et al., 2001). Similar glands are only found in higher holometabolous insects (Diptera, Lepidoptera and some Coleoptera and Hymenoptera). All other examined hemimetabolous and holometabolous insects have a large number of Inka cells scattered throughout the tracheal system (O’Brien and Taghert, 1998; Žitňan et al., 2003). Inka cells produce peptide hormones (PETH, ETH) which were identified biochemically from epitracheal gland extracts or deduced from the cDNA or genomic DNA of several unrelated species (Fig. 3; Park et al., 1999; Žitňan et al, 1996; 1999; 2002; 2003).
Fig. 3.
Amino acid sequences of ecdysis triggering hormones (Žitňan et al, 1996; 1999, 2002, 2003; Park Y. et al., 1999; Riehle et al., 2002; Clynen et al., 2006). Putative Tribolium ETHs were deduced from the genome database. Sequence homology/similarity is indicated by bold letters.
PETH and ETH are released from Inka cells into the hemolymph to activate specific neuronal circuits in the CNS involved in regulation of the ecdysis sequence. In Manduca larvae, PETH controls synchronous dorso-ventral contractions (pre-ecdysis I), while ETH induces posterior-ventral and proleg contractions (pre-ecdysis II) and peristaltic movements (ecdysis). Pharate pupae and adults fail to show any obvious response to PETH injection, but ETH induces behaviors comparable with natural ecdysis or eclosion sequence (Žitňan et al., 1996, 1999; Žitňanová et al., 2001). In Bombyx, both peptide hormones induce the entire behavioral sequence in pharate larvae, pupae and adults (Adams and Žitňan, 1997; Žitňan et al., 2002). Application of PETH or ETH on the isolated CNS elicits specific burst patterns corresponding to pre-ecdysis or ecdysis contractions (Žitňan et al., 1996, 1999, 2002). These data showed that Inka cell peptide hormones have a unique ability to activate their target neurons in the CNS through the blood-brain barrier.
In Drosophila larvae ETH1 activates tracheal inflation and the entire ecdysis sequence, whereas ETH2 induces only ecdysis behavior after a delay corresponding to pre-ecdysis behaviors (Park et al., 2002a). Both peptides induce the entire ecdysis sequence in pharate pupae and adults, but ETH1 is 10 times more potent than ETH2 (Park et al., 1999; Kim et al., 2006b; see review Žitňan and Adams, 2005).
Molecular genetic approaches in Drosophila demonstrated the essential roles of ETH1,2 in tracheal air filling and ecdysis sequence. Micro-deletion in the eth locus resulted in considerably delayed tracheal air filling (1,5 hr) and severe behavioral defects leading to lethality at the first ecdysis. These defects were rescued by ETH1,2 injection at appropriate time indicating that these peptide hormones are necessary humoral signals for activation of the ecdysis sequence (Park et al., 2002a).
Roles of central neuropeptides and ETH receptors in ecdysis
Central roles of EH and CCAP have been well established in Manduca. Immunohistochemical and electrophysiological techniques showed that EH-producing ventromedial (VM) cells in the brain respond to ETH by increased activity followed by central release of EH from axons running along the entire CNS (Hewes and Truman, 1991; Ewer et al., 1997; Gammie and Truman, 1997; 1999). Central release of EH is associated with cGMP production in a specific network of neurons 27/704 and L2,5 (Ewer et al., 1994, 1997a; Fuse et al., 2002) and these neurons show cGMP elevation in response to ETH and EH treatment in vitro (Gammie and Truman, 1999; Žitňan and Adams, 2000). The presence of ETHR-A in the VM cells (Kim et al., 2006a) clearly suggests that ETH is acting through EH to elicit cGMP production and excitability of 27/704 neurons. However, initial cGMP elevation in Manduca SG and TG1-3 occurs prior to EH release (Ewer et al., 1994; Žitňan and Adams, 2000), and isolated abdomens of Bombyx larvae show normal cGMP elevation in 27/704 homologs without apparent EH release (Žitňan et al., 2002). These data indicate that cGMP elevation in the CNS requires some additional factors.
In moths a network of 27/704 neurons produce neuropeptides CCAP and MIPs in abdominal ganglia (Ewer et al., 1997; Davis et al., 1993, 2003). The appearance of cGMP and decrease in CCAP- and MIP-immunohistochemical staining in axonal arborizations of 27/704 neurons indicates that these neuropeptides are released at ecdysis (Gammie and Truman, 1997; Davis et al., 2003). Incubation of the isolated CNS with CCAP alone results in ecdysis bursts in anterior abdominal ganglia (Gammie and Truman, 1997; Žitňan and Adams, 2000), whereas a mixture of CCAP and MIPs induces characteristic ecdysis burst patterns in the entire CNS (Kim et al., 2006a). These data demonstrate that a mixture of CCAP and MIPs controls the ecdysis behavior.
Further evidence for neuropeptide modulation of the ecdysis sequence comes from studies examining expression and function of ETHR. The first ETHR was identified in Drosophila as a G protein-coupled receptor encoded by the ethr gene CG5911 (Fig. 4; Iversen et al., 2002; Park et al., 2003a). Alternative splicing of its 3-prime exon results in expression of two receptor subtypes (ETHR-A and ETHR-B; Fig. 2). Another ETHR homolog encoding two subtypes (ETHR-A and ETHR-B) was cloned in Manduca (Kim et al., 2006a). Pharmacological and physiological analyses in vitro indicate that each receptor subtype may have specific roles in activation of different phases of the ecdysis sequence. In situ hybridization technique with DNA probes specific for either ETHR-A or ETHR-B showed that each subtype is expressed in a completely different subset of CNS neurons (Kim et al., 2006a,b). Interestingly, most identified ETHR-A neurons produce various neuropeptides, which are probably released upon initial ETH action on the CNS. In Manduca these neuropeptides include kinins, CRF-like and calcitonin-like diuretic hormones (DH), allatostatins, EH, crustacean cardioactive peptide (CCAP), myoinhibitory peptides (MIPs), neuropeptide F (NPF), small neuropeptide F (sNPF) and bursicon (Kim et al., 2006b). Electrophysiological experiments with the isolated CNS indicate that a cocktail of kinins and CRF-like DHs controls pre-ecdysis, EH participates in activation of ecdysis neurons, while a mixture of CCAP and MIPs is responsible for execution of the ecdysis motor program (Kim et al., 2006a).
Fig. 4.
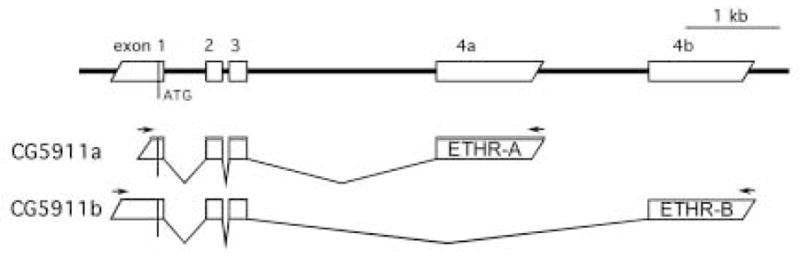
Two subtypes of the ETH receptor (ETHR-A and ETHR-B). From Park Y. et al., 2003.
Drosophila ETHR-A neurons produce kinin, FMRFamides, EH, CCAP, MIPs and bursicon. Intracellular calcium levels were monitored in each group of ETHR-A neurons by expressing GFP-based Ca2+ sensor (GCaMP). The dynamics of cellular calcium levels indicated increasing neural activity, which possibly mirrored neuropeptide release from ETHR-A neurons in response to ETH application. The decrease of staining in EH- and CCAP-immunoreactive axons also indicated the release of these peptides at larval ecdysis and adult eclosion (Baker et al., 1999; Park et al., 2003). Interestingly, each ensemble of neurons showed a specific response corresponding to different behavioral phases. For example, activity of thoracic FMRFamide neurons and their neurohemal endings corresponded to early pre-ecdysis, while EH neurons reached peak activity at ecdysis. A more complex response was observed in neurons 27/704 in different regions of the CNS. Various thoracic and abdominal neurons 27/704 expressing either CCAP alone, CCAP/MIPs, or CCAP/MIPs/bursicon became active during different phases of the ecdysis sequence (Kim et al., 2006b). Thoracic neurons expressing CCAP alone showed peak activity prior to ecdysis, while abdominal neurons expressing CCAP/MIPs/bursicon or CCAP/MIPs were active during post-ecdysis movements (Kim et al., 2006b).
Molecular genetic tools were used to ablate specific peptidergic neurons involved in regulation of the ecdysis sequence. Neuroendocrine events and behavioral defects were carefully examined in these transgenic knockout flies and compared with controls (McNabb et al., 1997; Clark et al., 2004; Kim et al., 2006b). Pupation of animals with ablated thoracic FMRFamide-producing ETHR-A neurons was normal, except pre-ecdysis contractions appeared weaker (Kim et al., 2006b). Likewise, ablation of EH-producing neurons caused only subtle alterations in duration of larval and pupal ecdysis sequence, but affected tracheal inflation, adult eclosion and post-eclosion behaviors (McNabb et al., 1997; Clark et al., 2004; Kim et al., 2006b).
Deletion of the CCAP neurons resulted in stage-specific defects in ecdysis. Larval pre-ecdysis was considerably prolonged, but animals successfully ecdysed as controls. On the other hand, pupal ecdysis was severely affected; most animals died due to loss of ecdysis and post-ecdysis contractions required for head eversion and extension of appendages (Park et al., 2003; Kim et al., 2006b). Emerging adults showed defects in abdominal inflation, but ecdysis motor program was not affected as observed during larval ecdyses (Park et al., 2003). Since most of CCAP neurons also produce MIPs and bursicon (Dewey et al., 2004; Kim et al., 2006b), defects in abdominal inflation is likely related to bursicon function. Since CCAP and MIPs apparently regulate the ecdysis motor program in Manduca (Gammie and Truman, 1999; Žitňan and Adams, 2000), the results in Drosophila are rather surprising and the physiological significance of CCAP central release at ecdysis must be futher examined (Clark et al., 2004). Considerable differences between regulation of the ecdysis sequence in moths and flies suggest species-specific functional adaptations of various neuropeptides and neuronal circuits.
Functions of other neuropeptides in remaining ETHR-A neurons are not clear at the moment. We propose that ETHR-A neurons expressing sNPF and MIPs in the TAG may be involved in termination of the ecdysis behavior, while thoracic neurons 27/704 expressing bursicon (Dewey et al., 2004; Luo et al., 2005) control post-ecdysis. Although most ETHR-B neurons have not been identified, they are probably involved in regulation of specific pre-ecdysis subunits and delay of ecdysis onset.
CONCLUSIONS AND PERSPECTIVES
We conclude that the ecdysis sequence and probably other innate behaviors are composed of two essential phases: preparatory and behavioral. The preparatory phase is associated with stage-specific transcriptional activity mediated by steroids and multiple transcription factors. In particular, steroids induce expression of essential receptors in target cells and production of regulatory molecules in neuroendocrine organs, as well as control competence of these organs to release their active molecules at appropriate time. The behavioral phase is characterized by ETH activation of peptidergic networks within the CNS to initiate, execute and terminate each behavioral step. ETHs possess the unique ability to activate CNS neurons through the blood-barrier and therefore function as general releasing hormones for multiple unrelated neuropeptides derived at least from 10 genes. These neuropeptides are subsequently released within the CNS to regulate specific behavioral phases and into the hemolymph to control activity of peripheral organs associated with these behaviors. During the ecdysis sequence a cocktail of different neuropeptides is usually co-released from a specific set of neurons to regulate a particular behavioral subunit. On the other hand, the same neuropeptide could be released from distinct neurons at different time to regulate various functions during development.
Most known neuropeptides expressed by ETHR neurons show activity in several established in vitro assays using hindgut, heart, Malpighian tubules, endocrine and exocrine organs (for reviews see: Gäde et al., 1997; Coast et al., 2005). Based on these effects, they have been implicated in various functions, but in many cases their true physiological roles remain enigmatic. Recent experiments indicate that many of these neuropeptides are involved in regulation of the ecdysis sequence programmed within the CNS and associated functions in peripheral organs (e.g. myotropic activities or regulation of water/ion balance during and after molting fluid reabsorption). However, timing of release and hemolymph levels of most neuropeptides are unknown and mechanisms of their action on target organs in vivo is poorly understood. Further studies are apparently needed to pinpoint mechanisms underlying neuropeptide modulation of each behavioral phase and to identify physiological roles of all ETHR neurons and active molecules they produce. De-orphanization of all neuropeptide receptors and characterization of their temporal and spatial expression in target organs are essential steps in understanding basic regulatory principles of animal behaviors and physiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams ME, Zitnan D. Identification of ecdysis-triggering hormone in the silkworm Bombyx mori. Biochem Biophys Res Comm. 1997;230:188–191. doi: 10.1006/bbrc.1996.5915. [DOI] [PubMed] [Google Scholar]
- Aguinaldo AMA, Turbeville JM, Linford LS, Rivera MC, Garey JR, Raff RA, Lake JA. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature. 1997;378:489–493. doi: 10.1038/387489a0. [DOI] [PubMed] [Google Scholar]
- Akai H. Ultrastructure of epitracheal gland during larval-pupal molt of Bombyx mori. Cytologia. 1992;57:195–201. [Google Scholar]
- Baker JD, McNabb SL, Truman JW. The hormonal coordination of behavior and physiology at adult ecdysis in Drosophila melanogaster. J Exp Biol. 1999;202:3037–3048. doi: 10.1242/jeb.202.21.3037. [DOI] [PubMed] [Google Scholar]
- Cantera R, Veenstra JA, Nassel DR. Postembryonic development of corazonin-containing neurons and neurosecretory cells in the blowfly, Phormia terraenovae. J Comp Neurol. 1994;350:559–572. doi: 10.1002/cne.903500405. [DOI] [PubMed] [Google Scholar]
- Cazzamali G, Saxild N, Grimmelikhuijzen C. Molecular cloning and functional expression of a Drosophila corazonin receptor. Biochem Biophys Res Comm. 2002;298:31–36. doi: 10.1016/s0006-291x(02)02398-7. [DOI] [PubMed] [Google Scholar]
- Clark AC, del Campo ML, Ewer J. Neuroendocrine Control of Larval Ecdysis Behavior in Drosophila: Complex Regulation by Partially Redundant Neuropeptides. J Neurosci. 2004;24:4283–4292. doi: 10.1523/JNEUROSCI.4938-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynen E, Huybrechts J, Verleyen P, De Loof A, Schoofs L. Annotation of novel neuropeptide precursors in the migratory locust based on transcript screening of a public EST database and mass spectrometry. BMC Genomics. 2006;7:201. doi: 10.1186/1471-2164-7-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coast GM, Garside CS. Neuropeptide control of fluid balance. In Insects Ann NY Acad Sci. 2005;1040:1–8. doi: 10.1196/annals.1327.001. [DOI] [PubMed] [Google Scholar]
- Davis NT, Homberg U, Dircksen H, Levine RB, Hildebrand JG. Crustacean cardioactive peptide-immunoreactive neurons in the hawkmoth Manduca sexta and changes in their immunoreactivity during postembryonic development. J Comp Neurol. 1993;338:612–627. doi: 10.1002/cne.903380410. [DOI] [PubMed] [Google Scholar]
- Davis NT, Blackburn MB, Golubeva EG, Hildebrand JG. Localization of myoinhibitory peptide immunoreactivity in Manduca sexta and Bombyx mori, with indications that the peptide has a role in molting and ecdysis. J Exp Biol. 2003;206:1449–1460. doi: 10.1242/jeb.00234. [DOI] [PubMed] [Google Scholar]
- Dewey EM, McNabb SL, Ewer J, Kuo GR, Takanishi CL, Truman JW, Honegger HW. Identification of the gene encoding bursicon, an insect neuropeptide responsible for cuticle sclerotization and wing spreading. Curr Biol. 2004;14:1208–1213. doi: 10.1016/j.cub.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Ewer J, De Vente J, Truman JW. Neuropeptide induction of cyclic GMP increases in the insect CNS: resolution at the level of single identifiable neurons. J Neurosci. 1994;14:7704–7712. doi: 10.1523/JNEUROSCI.14-12-07704.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer J, Gammie SC, Truman JW. Control of insect ecdysis by a positive-feedback endocrine system: roles of eclosion hormone and ecdysis triggering hormone. J Exp Biol. 1997;200:869–881. doi: 10.1242/jeb.200.5.869. [DOI] [PubMed] [Google Scholar]
- Ewer J, Reynolds S. Neuropeptide control of molting in insects. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 3. Elsevier Science; Boston: 2002. pp. 1–92. [Google Scholar]
- Ewer J, Truman JW. Invariant association of ecdysis with increases in cyclic 3′,5′-guanosine monophosphate immunoreactivity in a small network of peptidergic neurons in the hornworm, Manduca sexta. J Comp Physiol A Sensory, Neural, and Behavioral Physiology. 1997;181:319–330. doi: 10.1007/s003590050118. [DOI] [PubMed] [Google Scholar]
- Gäde G. Hormonal regulation in insects: Facts, gaps and future directions. Physiological Reviews. 1997;77:963–1032. doi: 10.1152/physrev.1997.77.4.963. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Truman JW. Neuropeptide hierarchies and the activation of sequential motor behaviors in the hawkmoth, Manduca sexta. J Neurosci. 1997;17:4389–4397. doi: 10.1523/JNEUROSCI.17-11-04389.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Truman JW. Eclosion hormone provides a link between ecdysis-triggering hormone and crustacean cardioactive peptide in the neuroendocrine cascade that controls ecdysis behavior. J Exp Biol. 1999;202:343–352. doi: 10.1242/jeb.202.4.343. [DOI] [PubMed] [Google Scholar]
- Gauthier SA, Hewes RS. Transcriptional regulation of neuropeptide and peptide hormone expression by the Drosophila dimmed and cryptocephal genes. J Exp Biol. 2006;209:1803–1815. doi: 10.1242/jeb.02202. [DOI] [PubMed] [Google Scholar]
- Hansen IA, Sehnal F, Meyer SR, Scheller K. Corazonin gene expression in the waxmoth Galleria mellonella. Insect Mol Biol. 2001;10:341–346. doi: 10.1046/j.0962-1075.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- Hewes RS, Truman JW. The roles of central and peripheral eclosion hormone release in the control of ecdysis behavior in Manduca sexta. J Comp Physiol A Sensory Neural & Behavioral Physiology. 1991;168:697–708. doi: 10.1007/BF00224359. [DOI] [PubMed] [Google Scholar]
- Hewes RS, Schaefer AM, Taghert PH. The cryptocephal gene (ATF4) encodes multiple basic-leucine zipper proteins controlling molting and metamorphosis in Drosophila. Genetics. 2000;155:1711–1723. doi: 10.1093/genetics/155.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewes RS, Park D, Gauthier SA, Schaefer AM, Taghert PH. The bHLH protein Dimmed controls neuroendocrine cell differentiation in Drosophila. Development. 2003;130:1771–1781. doi: 10.1242/dev.00404. [DOI] [PubMed] [Google Scholar]
- Hiruma K, Riddiford LM. Regulation of transcription factors MHR4 and betaFTZ-F1 by 20-hydroxyecdysone during a larval molt in the tobacco hornworm, Manduca sexta. Dev Biol. 2001;232:265–274. doi: 10.1006/dbio.2001.0165. [DOI] [PubMed] [Google Scholar]
- Honegger HW, Market D, Pierce LA, Dewey EM, Kostron B, Wilson M, Choi D, Klukas KA, Mesce KA. Cellular localization of bursicon using antisera against partial peptide sequences of this insect cuticle-sclerotizing neurohormone. J Comp Neurol. 2002;452:163–177. doi: 10.1002/cne.10357. [DOI] [PubMed] [Google Scholar]
- Horodyski FM, Ewer J, Riddiford LM, Truman JW. Isolation, characterization and expression of the eclosion hormone gene of Drosophila melanogaster. European J Biochem. 1993;215:221–228. doi: 10.1111/j.1432-1033.1993.tb18026.x. [DOI] [PubMed] [Google Scholar]
- Horodyski FM, Riddiford LM, Truman JW. Isolation and expression of the eclosion hormone gene from the tobacco hornworm, Manduca sexta. Proc Natl Acad Sci USA. 1989;86:8123–8127. doi: 10.1073/pnas.86.20.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwath KL, Riddiford LM. Stage and segment specificity of the secretory cell of the dermal glands of the tobacco hornworm, Manduca sexta. Dev Biol. 1988;130:365–373. doi: 10.1016/0012-1606(88)90442-3. [DOI] [PubMed] [Google Scholar]
- Hua Y, Ishibashi J, Saito H, Tawfik AI, Sakakibara M, Tanaka Y, Derua R, Waelkens E, Baggerman G, De Loof A, Schoofs L, Tanaka S. Identification of [Arg7] corazonin in the silkworm, Bombyx mori and the cricket, Gryllus bimaculatus, as a factor inducing dark color in an albino strain of the locust, Locusta migratoria. J Insect Physiol. 2000;46:853–860. doi: 10.1016/s0022-1910(99)00173-0. [DOI] [PubMed] [Google Scholar]
- Ikeda E. Kimon Rimensen. In: Ikeda E, editor. Experimental Anatomy and Physiology of Bombyx mori. Meibundo; Tokyo: 1913. pp. 242–243. [Google Scholar]
- Iversen A, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJ. Molecular identification of the first insect ecdysis triggering hormone receptors. Biochem Biophys Res Comm. 2002;299:924–931. doi: 10.1016/s0006-291x(02)02798-5. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Troetschler RG, Kramer SJ, Cesarin BJ, Schooley DA. Isolation and primary structure of the eclosion hormone of the tobacco hornworm, Manduca sexta. Biochem Biophys Res Comm. 1987;146:746–750. doi: 10.1016/0006-291x(87)90592-4. [DOI] [PubMed] [Google Scholar]
- Kim Y-J, Spalovska-Valachova I, Cho K-H, Žitňanová I, Park Y, Adams ME, Žitňan D. Corazonin receptor signaling in ecdysis initiation. Proc Natl Acad Sci USA. 2004;101:6704–6709. doi: 10.1073/pnas.0305291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-J, Žitňan D, Cho K-H, Mizoguchi A, Schooley D, Adams ME. Endocrine programming of an innate behavior through central release of peptide cotransmitters. Proc Natl Acad Sci USA. 2006;103:14211–14216. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-J, Žitňan D, Galizia G, Cho K-H, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Kingan TG, Gray W, Zitnan D, Adams ME. Regulation of ecdysis-triggering hormone release by eclosion hormone. J Exp Biol. 1997;200:3245–3256. doi: 10.1242/jeb.200.24.3245. [DOI] [PubMed] [Google Scholar]
- Kingan TG, Adams ME. Ecdysteroids regulate secretory competence in Inka cells. J Exp Biol. 2000;203:3011–3018. doi: 10.1242/jeb.203.19.3011. [DOI] [PubMed] [Google Scholar]
- Kingan TG, Cardullo RA, Adams ME. Signal transduction in eclosion hormone-induced secretion of ecdysis-triggering hormone. J Biol Chem. 2001;276:25136–25142. doi: 10.1074/jbc.M102421200. [DOI] [PubMed] [Google Scholar]
- Klein C, Kallenborn HG, Radlicki C. The ‘Inka cell’ and its associated cells: ultrastructure of the epitracheal glands in the gypsy moth, Lymantria dispar. J Insect Physiol. 1999;45:65–73. doi: 10.1016/s0022-1910(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Kono T, Nagasawa H, Isogai A, Fugo H, Suzuki A. Amino acid sequence of eclosion hormone of the silkworm Bombyx mori. Agr Biol Chem. 1987;51:2307–2308. [Google Scholar]
- Lane S, Riddiford LM, Truman JW, Conitz J. Development of the prepupal Verson’s gland of the tobacco hornworm, Manduca sexta, and its hormonal control. J Exp Zool. 1986;240:83–94. doi: 10.1002/jez.1402400111. [DOI] [PubMed] [Google Scholar]
- Lavorgna G, Karim FD, Thummel CS, Wu C. Potential role for a FTZ-F1 steroid receptor superfamily member in the control of Drosophila metamorphosis. Proc Natl Acad Sci USA. 1993;90:3004–3008. doi: 10.1073/pnas.90.7.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi PK, Emmal SA, Park Y, Tublitz NJ. Identification, sequence and expression of a crustacean cardioactive peptide (CCAP) gene in the moth Manduca sexta. J Exp Biol. 2001;204:2803–2816. doi: 10.1242/jeb.204.16.2803. [DOI] [PubMed] [Google Scholar]
- Marti T, Takio K, Walsh KA, Terzi G, Truman JW. Microanalysis of the amino acid sequence of the eclosion hormone from the tobacco hornworm Manduca sexta. FEBS Letters. 1987;219:415–418. doi: 10.1016/0014-5793(87)80263-6. [DOI] [PubMed] [Google Scholar]
- McNabb SL, Baker JD, Agapite J, Steller H, Riddiford LM, Truman JW. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron. 1997;19:813–23. doi: 10.1016/s0896-6273(00)80963-0. [DOI] [PubMed] [Google Scholar]
- Mendive FM, VanLoy T, Claeysen S, Poels J, Williamson M, Hauser F, Grimmelikhuijzen CJP, Vassart G, Vanden Broeck J. Drosophila molting neurohormone bursicon is a heterodimer and the natural agonist of the orphan receptor DLGR2. FEBS letters. 2005;579:2171–2176. doi: 10.1016/j.febslet.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Morton DB, Simpson PJ. Cellular signaling in eclosion hormone action. J Insect Physiol. 2002;48:1–13. doi: 10.1016/s0022-1910(01)00157-3. [DOI] [PubMed] [Google Scholar]
- Novicki A, Weeks JC. The initiation of pre-ecdysis and ecdysis behaviors in larval Manduca sexta: The roles of the brain, terminal ganglion and eclosion hormone. J Exp Biol. 1996;199:1757–1769. doi: 10.1242/jeb.199.8.1757. [DOI] [PubMed] [Google Scholar]
- O’Brien MA, Taghert PH. A peritracheal neuropeptide system in insects: release of myomodulin-like peptides at ecdysis. J Exp Biol. 1998;201:193–209. doi: 10.1242/jeb.201.2.193. [DOI] [PubMed] [Google Scholar]
- Park JH, Schroeder AJ, Helfrich-Forster C, Jackson FR, Ewer J. Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development. 2003;130:2645–2656. doi: 10.1242/dev.00503. [DOI] [PubMed] [Google Scholar]
- Park Y, Žitňan D, Gill SS, Adams ME. Molecular cloning and biological activity of ecdysis-triggering hormones in Drosophila melanogaster. FEBS Letters. 1999;463:133–138. doi: 10.1016/s0014-5793(99)01622-1. [DOI] [PubMed] [Google Scholar]
- Park Y, Filippov V, Gill SS, Adams ME. Deletion of the ecdysis-triggering hormone gene leads to lethal ecdysis deficiency. Development. 2002a;129:493–503. doi: 10.1242/dev.129.2.493. [DOI] [PubMed] [Google Scholar]
- Park Y, Kim YJ, Adams ME. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci USA. 2002b;99:11423–11428. doi: 10.1073/pnas.162276199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Kim YJ, Dupriez V, Adams ME. Two subtypes of ecdysis-triggering hormone receptor in Drosophila melanogaster. J Biol Chem. 2003;278:17710–17715. doi: 10.1074/jbc.M301119200. [DOI] [PubMed] [Google Scholar]
- Predel R, Kellner R, Gade G. Myotropic neuropeptides from the retrocerebral complex of the stick insect, Carausius morosus (Phasmatodea: Lonchodidae) European J Entomol. 1999;96:275–278. [Google Scholar]
- Riehle MA, Garczynski SF, Crim JW, Hill CA, Brown MR. Neuropeptides and peptide hormones in Anopheles gambiae. Science. 2002;298:172–175. doi: 10.1126/science.1076827. [DOI] [PubMed] [Google Scholar]
- Robinow S, Talbot WS, Hogness DS, Truman JW. Programmed cell death in the Drosophila CNS is ecdysone-regulated and coupled with a specific ecdysone receptor. Development. 1993;119:1251–1259. doi: 10.1242/dev.119.4.1251. [DOI] [PubMed] [Google Scholar]
- Roller L, Tanaka Y, Tanaka S. Corazonin and corazonin-like substances in the central nervous system of the Pterygote and Apterygote insects. Cell Tiss Res. 2003;312:393–406. doi: 10.1007/s00441-003-0722-4. [DOI] [PubMed] [Google Scholar]
- Sauman I, Reppert SM. Circadian clock neurons in the silkmoth Antheraea pernyi: novel mechanisms of Period protein regulation. Neuron. 1996;17:889–900. doi: 10.1016/s0896-6273(00)80220-2. [DOI] [PubMed] [Google Scholar]
- Sláma K. Homeostatic function of ecdysteroids in ecdysis and oviposition. Acta Entmol Bohemoslovaca. 1980;77:145–168. [Google Scholar]
- Shiga S, Davis NT, Hildebrand JG. Role of neurosecretory cells in the photoperiodic induction of pupal diapause of the tobacco hornworm Manduca sexta. J Comp Neurol. 2003;462:275–285. doi: 10.1002/cne.10683. [DOI] [PubMed] [Google Scholar]
- Tanaka S. Induction of darkening by corazonins in several species of Orthoptera and their possible presence in ten orders. Appl Entomol Zool. 2000;35:509–517. [Google Scholar]
- Tawfik AI, Tanaka S, De Loof A, Schoofs L, Baggerman G, Waelkens E, Derua R, Milner Y, Yerushalmi Y, Pener MP. Identification of the gregarization-associated dark-pigmentotropin in locusts through an albino mutant. Proc Natl Acad Sci USA. 1999;96:7083–7087. doi: 10.1073/pnas.96.12.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman JW, Riddiford LM. Neuroendocrine control of ecdysis in silk moths. Science. 1970;167:1624–1626. doi: 10.1126/science.167.3925.1624. [DOI] [PubMed] [Google Scholar]
- Truman JW, Rountree DB, Reiss SE, Schwartz LM. Ecdysteroids regulate the release and action of eclosion hormone in the tobacco hornworm, Manduca sexta (L.) J Insect Physiol. 1983;29:895–900. [Google Scholar]
- Truman JW. Hormonal control of insect ecdysis: endocrine cascades for coordinating behavior with physiology. Vitamins and Hormones. 2005;73:1–30. doi: 10.1016/S0083-6729(05)73001-6. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Isolation and structure of corazonin, a cardioactive peptide from the American cockroach. FEBS Letters. 1989;250:231–234. doi: 10.1016/0014-5793(89)80727-6. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Presence of corazonin in three insect species, and isolation and identification of [His7]corazonin from Schistocerca americana. Peptides. 1991;12:1285–1289. doi: 10.1016/0196-9781(91)90208-7. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Isolation and structure of the Drosophila corazonin gene. Biochem Biophys Res Comm. 1994;204:292–296. doi: 10.1006/bbrc.1994.2458. [DOI] [PubMed] [Google Scholar]
- Veenstra JA, Davis NT. Localization of corazonin in the nervous system of the cockroach Periplaneta americana. Cell Tissue Res. 1993;274:57–64. doi: 10.1007/BF00327985. [DOI] [PubMed] [Google Scholar]
- Wise S, Davis NT, Tyndale E, Noveral J, Folwell MG, Bedian V, Emery IF, Siwicki KK. Neuroanatomical studies of period gene expression in the hawkmoth, Manduca sexta. J Comp Neurol. 2002;447:366–380. doi: 10.1002/cne.10242. [DOI] [PubMed] [Google Scholar]
- Yamada M, Murata T, Hirose S, Lavorgna G, Suzuki E, Ueda H. Temporally restricted expression of transcription factor betaFTZ-F1: significance for embryogenesis, molting and metamorphosis in Drosophila melanogaster. Development. 2000;127:5083–5092. doi: 10.1242/dev.127.23.5083. [DOI] [PubMed] [Google Scholar]
- Žitňan D. PhD Dissertation. Slovak Academy of Sciences; Bratislava, Slovakia: 1989. Regulatory peptides and peptidergic organs of insects. [Google Scholar]
- Žitňan D, Kingan TG, Hermesman J, Adams ME. Identification of ecdysis-triggering hormone from an epitracheal endocrine system. Science. 1996;271:88–91. doi: 10.1126/science.271.5245.88. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Ross LS, Zitnanova I, Hermesman JL, Gill SS, Adams ME. Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence. Neuron. 1999;23:523–535. doi: 10.1016/s0896-6273(00)80805-3. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Adams ME. Excitatory and inhibitory roles of central ganglia in initiation of the insect ecdysis behavioural sequence. J Exp Biol. 2000;203:1329–1340. doi: 10.1242/jeb.203.8.1329. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Hollar L, Spalovska I, Takac P, Žitňanová I, Gill SS, Adams ME. Molecular cloning and function of ecdysis-triggering hormones in the silkworm Bombyx mori. J Exp Biol. 2002;205:3459–3473. doi: 10.1242/jeb.205.22.3459. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Žitňanová I, Spalovska I, Takac P, Park Y, Adams ME. Conservation of ecdysis-triggering hormone signalling in insects. J Exp Biol. 2003;206:1275–1289. doi: 10.1242/jeb.00261. [DOI] [PubMed] [Google Scholar]
- Žitňan D, Adams ME. Neuroendocrine regulation of insect ecdysis. In: Gilbert LI, Iatrou K, Gill SS, editors. Coprehensive Molecular Insect Science. Vol. 3. 2005. pp. 1–60. [Google Scholar]
- Žitňanová I, Adams ME, Zitnan D. Dual ecdysteroid action on the epitracheal glands and central nervous system preceding ecdysis of Manduca sexta. J Exp Biol. 2001;204:3483–3495. doi: 10.1242/jeb.204.20.3483. [DOI] [PubMed] [Google Scholar]